Abstract
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system, predominantly affecting the white matter, but also the grey matter. Aim of this study was to detect MS lesions with double inversion recovery (DIR), fluid-attenuated inversion recovery (FLAIR) and T2-weighted magnetic resonance (MR) techniques and determine the sensitivity of these techniques, and the correlation between the number of lesions and expanded disability state scale (EDSS) scores. Thirty-four patients with MS (20 females and 14 males) were included in this study. DIR and conventional MR (T2-A, FLAIR) sequences were obtained. Lesions were counted and classified as belonging to one of seven anatomical regions: cortical, juxtacortical, deep grey matter, deep white matter, mixed white matter-grey matter, periventricular white matter and infratentorial. The correlation between lesion number and EDSS scores was investigated. DIR images showed more intracortical and mixed white matter-grey matter lesions in comparison with both FLAIR and T2 sequences (p=0, p=0 respectively). There was a significant difference between mean lesion numbers at the juxtacortical region, obtained with DIR and T2-weighted images (p = 0.002). The total number of lesions obtained with all methods was similar. DIR brain imaging had the highest sensitivity in the detection of cortical and mixed white matter - grey matter lesions, compared with FLAIR and T2 sequences. In addition, the lesions obtained with DIR images were more easily visualized.
Keywords: adult, brain, magnetic resonance imaging, multiple sclerosis
Introduction
Multiple sclerosis (MS) is the most common chronic inflammatory demyelinating disease of the central nervous system, resulting in both physical and neurocognitive disability12. MS typically affects the white matter, but recent clinical autopsy studies have also reported changes in the grey matter3,4. Magnetic resonance imaging (MRI) has played a very important role in elucidating the pathophysiology, diagnosis and treatment of MS. T1- and T2-weighted conventional MRI images reveal islands of demyelinating plaques, more frequently found in white matter than grey matter. Autopsy studies have also demonstrated inflammatory and pathological changes in MS, which are not present in regions of demyelinating plaques but appear as islands in MRI sequences and are present almost throughout the brain, encompassing both the white and grey matter and appearing macroscopically normal. Thus, the inability to demonstrate such changes using conventional MRI sequences reflects the technical insufficiency of these classical techniques1,5-10. Conventional magnetic resonance imaging (MRI) does not show any histopathological characteristics apart from an association between specific T2-weighted images of hyperintense lesions (demyelinating plaques) and inflammation with gadolinium uptake. These considerations have necessitated the development of new techniques to obtain a better understanding of MS pathophysiology. Such novel techniques include fluid–attenuated inversion recovery (FLAIR) and double inversion recovery (DIR). Although FLAIR has been used in a routine clinical setting, DIR is more frequently used in experimental studies, although it has recently started to enter clinical use.
The FLAIR sequence is a sequence that suppresses the signal of cerebrospinal fluid (CSF) with a reverse cycle (inversion recovery) pulse and a high time Echo (TE values increase) T2-weight11,12. This sequence increases the contrast of supratentorial lesions, in particular lesions that arise in juxtaposition to the CSF compared to the spin echo (SE) and turbo spin echo (TSE)12. However, some lesions appear in T2-weighted SE sequences, but not in FLAIR sequences, since their T2 relaxation times are different13,14. The DIR sequence was developed by Redpath and Smith15. It differs from FLAIR in its utilization of a second inversion pulse. These images have hybrid features of FLAIR and a short time inversion recovery (STIR)16. Both the white and grey matter play a clear role in the physical and neurocognitive disability of MS patients. However, a sufficient correlation has not been demonstrated between conventional MRI findings and disability, which is mostly due to the inability to demonstrate all of the histopathological changes present in MS in vivo using classical MR images. In particular, lesions located at the junction of the grey matter-CSF, grey matter-white matter (juxtacortical areas) and white matter-CSF could not be visualized in conventional MR sequences because of the signal suppression of the grey matter by signals from both the white matter and CSF as well as the absence of sufficient contrast. Signals from the CSF are suppressed in FLAIR sequences so that lesions in the parenchymal areas, which are in juxtaposition to the CSF, become more prominent. However, this approach still cannot reveal lesions in the junction of the grey and white matter. In addition, signals from the white matter should be suppressed to detect these lesions. Because the DIR technique can better accomplish this suppression, it is thought that MS plaques located in the grey matter are more easily delineated using DIR7,8,17-20. The disturbances in consciousness, cognitive and psychic changes and epileptic fits observed in MS patients in clinical practice cannot be explained by the changes in white matter observed in conventional MR sequences. This is most likely due to the inability of classical MR techniques to detect grey matter lesions. Thus, the detection of grey matter lesions will play an important role in our understanding of both the physical and neurocognitive disability observed in MS patients1,5,7-10.
We aimed to detect grey matter lesions not adequately observed using conventional MRI in patients with MS. If present, we sought to determine the relationship between these lesions and the expanded disability status scale (EDSS) scores.
Material and Methods
This study was approved by the ethics committee of our hospital, and informed consent was obtained from all participating patients. A total of 34 patients with relapsing–remitting multiple sclerosis (RRMS) were evaluated and treated in our clinic.
A detailed neurological examination of all patients was performed. Conventional cranial and cervical MRI and DIR images were obtained from patients with RRMS after a remission of three months, and their functional capacities were evaluated with the EDSS. The presence of a neurological disturbance, according to a subjective patient report or objective observation, of at least 24 hours in duration was considered a new attack. The patients were discharged after treatment of the attack, and follow-up evaluations were performed. Patients who did not exhibit a new attack within three months of follow-up (i.e., who were in remission for three months) were asked to return to the clinic, and a new MRI was performed and an EDSS score was determined.
All of the patients' MR sequences were obtained with a 1.5 T super-conductive magnet (Philips Achieva) with a standard head coil of 8-16 channels. Transverse T2-weighted (TR: 5291 ms; TE: 110 ms, matrix: 300×212, NEX: 2, slice thickness: 5 mm, section width: 1 mm; exposure time: 1.10 min) and FLAIR (TR: +1000 ms, TE: 120 mS, TI: 2000 ms, matrix: 264×149, NEX: 2, slice thickness: 5 mm, section width: 1 mm, exposure time: 1.30 min) sequences were obtained, according to the conventional MRI protocol. Three-dimensional DIR images were obtained with the following technical features: TR, +1000 ms; TE, 120 ms; TI, 2000 ms; matrix, 264×149; NEX, 2; slice thickness, 5 mm; section width, 1 mm; and exposure time, 1.30 min. Radiological evaluations of the obtained sequences were performed by two physicians, a neuroradiologist and a neurologist, blinded for the clinical findings and paraclinical test results. All of the hyperintense signals observed in the T2, FLAIR and DIR images were considered lesions. In contrast, the hyperintense signals originating from the sinuses or major vessels or from signals extending as an extra-cortical strip were considered to artifacts and not lesions. The detected lesions were divided according to seven anatomical regions: cortical, juxtacortical, deep white matter, deep grey matter, mixed white matter-grey matter, periventricular white matter, and infratentorial regions. The lesion numbers according to the regions were determined. In addition, T2-weighted spinal images of all patients were obtained. The evaluation of a spinal lesion was classified as “present” or “absent” and not as a lesion count.
The statistical analysis was performed with the Statistical Package for Social Sciences (SPSS) version 15.0 software. Two independent group t tests were performed on the data, according to parametric test pre-conditions. In addition, a Mann Whitney U-test was performed on the data, which did not meet with parametric test pre-conditions. An independent one-way analysis of variance and the Kruskal Wallis test were used to compare variables with three or more groups. The Tukey test was used in multiple comparisons of variables, which were significant in the one-way analysis of variance. The Dunn test was used following the Kruskal Wallis test results. Repeated measurements were analyzed with an analysis of variance of dependent groups, and the Friedman test was used if p?0.05, which was considered the statistical significance level.
Results
Thirty-four consecutive patients with RRMS were included in this study. There were 20 (58.8%) female and 14 (41.2%) male patients, with a mean age of 38 ± 20 years. The upper and lower age limits for the female and male patients were 18 to 49 years and 20 to 58 years, respectively. Spinal lesions were observed in 22 out of 34 MS patients (64.7%) (9 out of 14 male patients [64%] and 13 out of 20 female patients [65%]). Demographic, clinical and radiological characteristics and the EDSS scores of the MS patients are presented in Table 1.
Table 1.
Some demographic, clinical and radiological characteristics and EDSS scores of patients with multiple sclerosis.
| Minimum | Maximum | Mean±Std. Deviation | |
|---|---|---|---|
| Patient age (year) | 18 | 58 | 32±10.42 |
| Duration of illness (year) | 1 | 5 | 2.35±1.67 |
| EDSS score | 0 | 7.5 | 2.24±1.72 |
| Lesion number with T2 | 6 | 60 | 29.41±15.89 |
| Lesion number with FLAIR | 8 | 101 | 40.27±24.81 |
| Lesion number with DIR | 6 | 81 | 39.82±23.72 |
T2, FLAIR and DIR images of MS lesions of one patient are shown in Figures 1 to 5. The lesion distributions in the seven anatomical regions and the mean lesion numbers obtained by T2-weighted, FLAIR and DIR images of MS patients are shown in Table 2. The mean number of lesions in the cortical region with DIR was significantly higher than the mean number of lesions detected with T2 and FLAIR (p = 0.00 and p = 0.00, respectively). The mean number of lesions detected with DIR in the juxtacortical region was significantly higher than the mean number of lesions detected with T2 (p = 0.002). The mean number of lesions detected in the deep white matter with T2 and FLAIR were higher than the mean number of lesions detected with DIR (p = 0.022 and p = 0.027, respectively). The mean number of lesions detected with DIR in the mixed white matter-grey matter was significantly higher than the mean number of lesions detected with T2 and FLAIR (p = 0.000 and p = 0.000, respectively). The mean number of lesions detected in the periventricular white matter with FLAIR was significantly higher than the mean number of lesions detected with DIR (p = 0.000). The mean number of lesions detected by T2 in the infratentorial region was significantly higher than the mean number of lesions detected with DIR (p = 0.001). There was no significant difference between the mean number of lesions detected in the infratentorial region with DIR or FLAIR (p = 0.802). The statistical comparisons between the different groups are shown in Table 3.
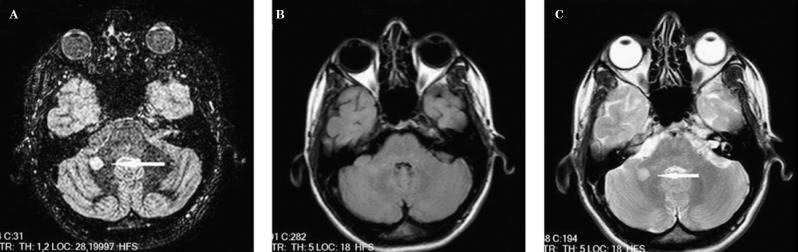
Figure 1.
A lesion in an infratentorial location, which can be seen in the DIR image (A) and T2-weighted images (C) but is not well depicted in the FLAIR image (B).
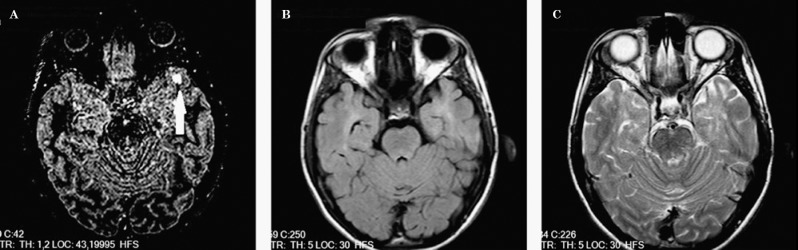
Figure 2.
A lesion of mixed white matter–grey matter location, which is seen in the DIR image (A) but cannot be seen in FLAIR (B) or T2-weighted images (C).
Figure 3.
Two lesions of juxtacortical location prominent in the DIR (A) and FLAIR (B) images.
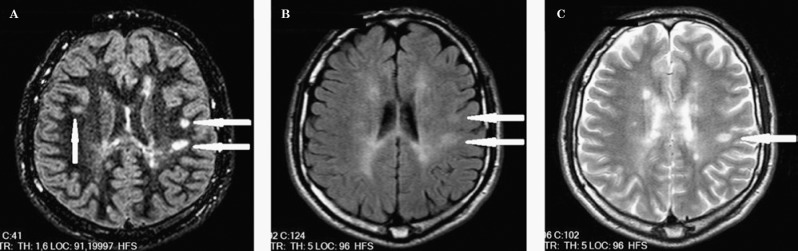
Figure 4.
Three juxtacortical lesions can be seen in the DIR image (A), but only two can be seen in FLAIR (B), and only one in T2-weighted images (C). The periventricular lesion is not prominent in FLAIR (B) or T2-weighted images (C).
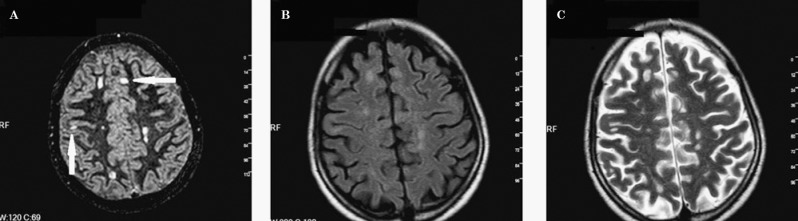
Figure 5.
Cortical lesions are more prominently seen in the DIR image (A) than in FLAIR (b) and T2-weighted images (C).
Table 2.
Lesions distributions in seven anatomical regions and mean lesion numbers obtained by T2-weighted, FLAIR and DIR images of MS patients.
| Z | T2 | T2 | T2 | T2 | T2 | T2 | T2 | T2 T | F | F | F | F | F | F | F | FT | DIR | DIR | DIR | DIR | DIR | DIR | DIR | DIR T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | JC | DWM | DGM | MWMGM | PVWM | İT | C | JC | DWM | DGM | MWMGM | PVWM | İT | C | JC | DWM | DGM | MWMGM | PVWM | İT | ||||
| 1 | 0 | 1 | 2 | 1 | 0 | 2 | 4 | 10 | 0 | 1 | 2 | 1 | 0 | 2 | 2 | 8 | 3 | 1 | 2 | 1 | 0 | 2 | 2 | 11 |
| 2 | 0 | 2 | 30 | 2 | 1 | 8 | 2 | 45 | 6 | 16 | 10 | 0 | 0 | 17 | 1 | 50 | 31 | 6 | 15 | 0 | 1 | 11 | 1 | 65 |
| 3 | 1 | 2 | 1 | 1 | 1 | 5 | 3 | 14 | 1 | 1 | 2 | 1 | 0 | 5 | 0 | 10 | 10 | 4 | 1 | 1 | 0 | 2 | 0 | 18 |
| 4 | 0 | 1 | 2 | 0 | 0 | 3 | 2 | 8 | 2 | 3 | 3 | 0 | 0 | 6 | 1 | 15 | 1 | 1 | 0 | 1 | 0 | 3 | 0 | 6 |
| 5 | 0 | 8 | 11 | 2 | 0 | 18 | 2 | 41 | 2 | 12 | 18 | 1 | 0 | 16 | 1 | 50 | 21 | 24 | 10 | 1 | 6 | 17 | 2 | 81 |
| 6 | 0 | 0 | 3 | 0 | 0 | 4 | 1 | 8 | 0 | 0 | 2 | 0 | 0 | 6 | 0 | 8 | 3 | 0 | 1 | 0 | 0 | 2 | 0 | 6 |
| 7 | 0 | 4 | 4 | 0 | 0 | 5 | 0 | 13 | 2 | 8 | 8 | 0 | 0 | 6 | 0 | 24 | 8 | 6 | 11 | 0 | 2 | 2 | 0 | 29 |
| 8 | 0 | 4 | 6 | 0 | 0 | 8 | 4 | 22 | 3 | 6 | 0 | 0 | 1 | 8 | 1 | 19 | 6 | 2 | 4 | 0 | 2 | 9 | 1 | 24 |
| 9 | 0 | 7 | 17 | 0 | 4 | 29 | 2 | 59 | 14 | 24 | 31 | 0 | 0 | 30 | 2 | 101 | 22 | 13 | 11 | 2 | 4 | 25 | 0 | 77 |
| 10 | 0 | 17 | 11 | 0 | 0 | 13 | 5 | 46 | 6 | 21 | 14 | 1 | 0 | 28 | 2 | 72 | 24 | 16 | 7 | 1 | 4 | 7 | 1 | 60 |
| 11 | 1 | 6 | 7 | 1 | 0 | 8 | 2 | 25 | 2 | 4 | 6 | 1 | 0 | 4 | 1 | 18 | 8 | 6 | 5 | 0 | 1 | 7 | 0 | 27 |
| 12 | 4 | 10 | 18 | 0 | 0 | 26 | 2 | 60 | 6 | 7 | 23 | 0 | 0 | 39 | 2 | 77 | 9 | 6 | 24 | 0 | 2 | 25 | 2 | 68 |
| 13 | 0 | 5 | 6 | 1 | 1 | 4 | 0 | 17 | 1 | 4 | 3 | 1 | 1 | 4 | 0 | 14 | 7 | 3 | 0 | 0 | 0 | 2 | 0 | 12 |
| 14 | 0 | 4 | 8 | 0 | 0 | 10 | 0 | 22 | 0 | 6 | 8 | 0 | 0 | 11 | 0 | 25 | 10 | 3 | 4 | 0 | 1 | 6 | 0 | 24 |
| 15 | 0 | 3 | 9 | 0 | 0 | 14 | 1 | 27 | 2 | 5 | 8 | 0 | 0 | 19 | 0 | 34 | 3 | 7 | 6 | 0 | 1 | 14 | 1 | 32 |
| 16 | 0 | 2 | 5 | 0 | 0 | 7 | 2 | 16 | 0 | 5 | 7 | 0 | 0 | 8 | 0 | 20 | 4 | 4 | 6 | 1 | 3 | 10 | 0 | 28 |
| 17 | 1 | 12 | 5 | 0 | 0 | 40 | 2 | 60 | 2 | 16 | 2 | 0 | 0 | 44 | 0 | 64 | 20 | 25 | 12 | 0 | 2 | 20 | 1 | 80 |
| 18 | 0 | 4 | 9 | 1 | 0 | 13 | 0 | 27 | 8 | 15 | 9 | 1 | 0 | 26 | 0 | 59 | 12 | 6 | 2 | 1 | 0 | 18 | 1 | 40 |
| 19 | 0 | 3 | 3 | 1 | 0 | 15 | 0 | 22 | 0 | 5 | 4 | 0 | 0 | 27 | 0 | 36 | 1 | 2 | 2 | 1 | 1 | 11 | 0 | 18 |
| 20 | 1 | 3 | 7 | 1 | 0 | 15 | 1 | 28 | 1 | 4 | 9 | 1 | 0 | 22 | 1 | 38 | 1 | 6 | 0 | 0 | 1 | 15 | 0 | 23 |
| 21 | 0 | 3 | 8 | 0 | 0 | 31 | 4 | 46 | 2 | 2 | 5 | 0 | 0 | 35 | 0 | 44 | 12 | 11 | 6 | 0 | 4 | 25 | 6 | 64 |
| 22 | 0 | 5 | 9 | 0 | 0 | 29 | 4 | 47 | 1 | 20 | 9 | 0 | 0 | 46 | 1 | 77 | 9 | 6 | 4 | 0 | 4 | 40 | 1 | 64 |
| 23 | 1 | 9 | 6 | 2 | 0 | 15 | 2 | 35 | 5 | 8 | 11 | 1 | 0 | 31 | 1 | 57 | 17 | 13 | 3 | 1 | 0 | 24 | 0 | 58 |
| 24 | 0 | 13 | 2 | 1 | 0 | 18 | 6 | 40 | 4 | 17 | 7 | 1 | 0 | 17 | 1 | 47 | 16 | 17 | 8 | 1 | 3 | 14 | 3 | 62 |
| 25 | 0 | 0 | 1 | 0 | 0 | 11 | 2 | 14 | 1 | 1 | 0 | 0 | 0 | 10 | 0 | 12 | 3 | 1 | 0 | 0 | 0 | 12 | 0 | 16 |
| 26 | 3 | 16 | 10 | 0 | 0 | 20 | 0 | 49 | 7 | 28 | 9 | 1 | 0 | 38 | 0 | 83 | 8 | 13 | 8 | 1 | 0 | 28 | 2 | 60 |
| 27 | 0 | 1 | 2 | 0 | 0 | 9 | 2 | 14 | 0 | 0 | 4 | 0 | 0 | 11 | 2 | 17 | 5 | 1 | 3 | 0 | 0 | 5 | 2 | 16 |
| 28 | 0 | 7 | 8 | 0 | 0 | 13 | 0 | 28 | 2 | 15 | 7 | 0 | 0 | 27 | 0 | 51 | 5 | 9 | 1 | 0 | 1 | 12 | 0 | 28 |
| 29 | 0 | 1 | 1 | 0 | 0 | 3 | 1 | 6 | 0 | 2 | 2 | 0 | 0 | 11 | 1 | 16 | 4 | 2 | 0 | 0 | 0 | 6 | 0 | 12 |
| 30 | 3 | 9 | 5 | 0 | 0 | 11 | 3 | 31 | 7 | 37 | 2 | 0 | 0 | 21 | 3 | 70 | 19 | 20 | 4 | 0 | 9 | 11 | 1 | 64 |
| 31 | 0 | 6 | 4 | 0 | 0 | 34 | 1 | 45 | 0 | 5 | 5 | 0 | 0 | 41 | 0 | 51 | 9 | 7 | 2 | 0 | 0 | 27 | 1 | 46 |
| 32 | 0 | 12 | 5 | 0 | 0 | 16 | 1 | 34 | 3 | 8 | 9 | 0 | 0 | 16 | 0 | 36 | 7 | 17 | 6 | 0 | 1 | 16 | 1 | 48 |
| 33 | 0 | 2 | 1 | 0 | 0 | 12 | 1 | 16 | 0 | 2 | 1 | 0 | 0 | 18 | 1 | 22 | 10 | 2 | 0 | 0 | 0 | 11 | 1 | 24 |
| 34 | 1 | 6 | 4 | 0 | 0 | 13 | 1 | 25 | 2 | 13 | 1 | 0 | 0 | 27 | 1 | 44 | 17 | 11 | 8 | 0 | 4 | 23 | 0 | 63 |
| T | 16 | 188 | 230 | 14 | 7 | 482 | 63 | 1000 | 92 | 321 | 241 | 11 | 2 | 677 | 25 | 1369 | 345 | 271 | 176 | 13 | 57 | 462 | 30 | 1354 |
| O | 0.4 | 5.5 | 6.7 | 0.4 | 0.2 | 14.1 | 1.8 | 29.4 | 2.7 | 9.4 | 7.0 | 0.3 | 0.1 | 19.9 | 0.7 | 40.2 | 10.1 | 7.9 | 5.1 | 0.3 | 1.6 | 13.5 | 0.8 | 39.8 |
| ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± |
| SD | 0.9 | 4.4 | 5.8 | 0.6 | 0.7 | 9.5 | 1.5 | 15.8 | 3.1 | 8.8 | 6.5 | 0.4 | 0.2 | 12.1 | 0.8 | 24.8 | 7.4 | 6.7 | 5.1 | 0.5 | 2.0 | 9.3 | 1.2 | 23.7 |
PN: Patient number, C: cortical lesion, JC: juxtacortical lesion, DWM: deep white matter lesion, DGM: deep grey matter lesion, MWMGM: mixed white matter - grey matter lesion, PWM: periventricular white matter lesion, İT: infratentorial lesion, F: FLAIR, T2: T2 total lesion number, F: FLAIR total lesion number, DIR: Double inversion recovery, T: separate and total sum of all lesions in every regions. 0±SD: Mean ± standard deviation,
Table 3.
Statistical comparisons of T2, FLAIR and DIR sequences according to lesion locations.
| C | JC | DWM | DGM | MWMGM | PVWM | İT | TOTAL | |
|---|---|---|---|---|---|---|---|---|
| DIR - T2 | p=0a | p=0.002a | p=0.022a | p=0.813b | p=0.00a | p=0.398b | p=0.001a | p=0.126b |
| DIR –FLAIR | p=0.000a | p=0.231b | p=0.027a | p=0.527b | p=0.000a | p=0.000a | p=0.802b | p=0.996b |
C: cortical lesion, JC: juxtacortical lesion, DWM: deep white matter lesion, DGM: deep grey matter lesion, MWMGM: mixed white matter-grey matter lesion; PWM: periventricular white matter lesion, İT: infratentorial lesion;
statistically significant,
not statistically significant.
There was a significant correlation between the number of periventricular white matter lesions in the T2-weighted images and the EDSS scores (r = 0.463, p = 0.006). In addition, there was a significant correlation between the number of lesions detected with DIR in the juxtacortical and periventricular white matter and the EDSS scores (r = 0.398; p = 0.02; r = 0.465, p = 0.006, respectively). Although a significant correlation was detected between the total number of lesions in the T2 images and the EDSS scores (r = 0.383, p = 0.025), no significant correlation was detected between the total number of lesions in the FLAIR images and the EDSS scores or between the total number of lesions in the DIR images and the EDSS scores. The relationships between the number of lesions in the seven different locations for each sequence and the EDSS scores as well as the total number of lesions and the EDSS scores are shown in Table 2.
Discussion
This study obtained brain imaging sequences of patients with MS using both conventional (T2, FLAIR) and DIR. We investigated the number of lesions with each sequence and the relationship between the number of lesions in each sequence and the EDSS scores.
Interestingly, only a few previous studies have examined this topic and have reported contrasting results. Moraal et al.20 compared the lesion burden detected with DIR, FLAIR and T2-weighted imaging and found that the highest number of intracortical lesions was detected with DIR. In addition, they suggested that the number of intracortical lesions detected using both DIR and FLAIR techniques was significantly higher, but they did not detect a significant difference between the DIR and FLAIR images. Our study found a higher number of intracortical lesions detected with DIR compared to both FLAIR and T2. In line with with our study, Geurts et al.18 examined the number of intracortical lesions in MS patients and detected a higher number of lesions with DIR sequences than both FLAIR and T2 sequences, where the gain was 152% and 538%, respectively. Moreover, Calabrese et al. (24) investigated the number of intracortical lesions with DIR in 380 patients, including 163 RRMS, 101 SPMS and 116 clinical isolated syndrome patients. They found intracranial lesions (ICL) in 58% of these patients. The number of ICLs was higher in patients with SPMS compared to patients with clinical isolated syndrome and patients with RRMS. Patients with ICLs also exhibited higher EDSS scores. There was a significant correlation between the number of ICLs and the EDSS scores. Moreover, there was also a significant association between ICLs and male gender. Our study only found a significant correlation between juxtacortical lesions and the EDSS scores. Calabrese et al.24 also indicated that grey matter (i.e., cortical) lesions showed different inflammatory characteristics from white matter lesions and that these lesions resolved faster and displayed active remyelination that stabilized over time. In addition, they indicated that grey matter damage may contribute to the development of disability. It is well-established that ICLs are more easily detected in postmortem tissues by immunohistological examination, although very few of these lesions may be revealed using conventional MRI sequences. The employment of DIR sequences should not be omitted in the detection of intracortical lesions. Although this sequence shows fewer ICLs than immunohistological methods, the sensitivity of DIR may provide additional biological information, which cannot be obtained through ex vivo investigations.
Moraal et al.20 also reported the ability to detect the highest number of juxtacortical lesions with T2-weighted images and the detection of the lowest number of lesions with DIR images. However, there were no statistically significant differences between these two groups. Geurts et al.18 reported the detection of the highest number of juxtacortical lesions with T2 and the detection of the lowest number of lesions with FLAIR. In our study, we visualized the highest number of juxtacortically located lesions with FLAIR images and the lowest number with T2, but no significant difference between the number of lesions obtained with DIR and FLAIR was observed, although DIR detected significantly more lesions than T2. Moreover, Wattjes et al.19 reported that the highest number of juxtacortical lesions was detected with FLAIR and that the lowest number was detected with T2 sequences. There was no statistically significant difference between DIR and FLAIR or T2. Although our results are consistent with the findings of Wattjes et al.19, we also reveal that DIR detected significantly more lesions than T2.
Moraal et al.20 reported the detection of the highest number of mixed white matter-grey matter lesions with DIR and no differences between the detection of the mixed white matter-grey matter lesion burden with DIR and FLAIR. However, the number of lesions detected using these two methods was significantly higher than that revealed with T2-weighted images. In a study conducted by Geurts et al.18, the highest number of mixed white matter–grey matter lesions was found with DIR; the lowest, with T2. In line with with this study, we also detected the highest number of mixed white matter–grey matter lesions with DIR sequences; the number of lesions detected was significantly higher with DIR than with T2 or FLAIR. Wattjes et al.19 also found that DIR visualized the highest number of mixed white matter–grey matter lesions and that T2 revealed the lowest number, but no statistically significant difference in the number of lesions was detected with either DIR and FLAIR or DIR and T2. We detected 57 lesions with DIR, two lesions with FLAIR and seven lesions with T2. Moraal et al.20 also detected the highest number of lesions with DIR, which was significantly higher than that visualized with T2. However, in their study, the number of lesions detected with FLAIR was lower than that detected by T2.
Three-dimensional DIR imaging has a few advantages, including higher sensitivity for intracortical lesions and an enhanced ability to discriminate between mixed white matter–grey matter, juxtracortical and pure intracortical lesions. Although T2-weighted MRI is a sensitive technique, the discrimination between these three lesion types may prove difficult with T2. Our study found a significantly higher number of both juxtacortical and mixed white matter–grey matter lesions with DIR. Although DIR is superior to T2 within these two regions, 89.1% of the total number of lesions in these three regions were found to be juxtacortical, and 3.3% of the lesions were observed in the mixed white matter-grey matter. In comparison, with DIR, these percentages were 40.2% and 8.4%, respectively. These results suggest that some of the lesions, which were assessed as juxtacortical with T2, may be classified as mixed lesions with DIR. Because the difference in contrast between the white and grey matter is minimal, it is difficult to differentiate mixed white matter–grey matter lesions from purely intracortical or juxtacortical lesions. The contrast obtained with 3D DIR sequences is thought to be sensitive in its ability to identify cortical lesions. Although DIR is sensitive, Calabrese et al.24 reported that DIR detected a lower number of cortical lesions compared with histopathological methods and may have specifically missed subpial lesions. Furthermore, DIR sequences may be affected by current artifacts, which mask its ability to visualize the lesions.
Moraal et al.20 also reported that the highest number of lesions was detected with FLAIR images in the periventricular white matter region and that no significant difference was observed between FLAIR and DIR. However, both DIR and FLAIR revealed more lesions than T2-weighted images. In contrast, Geurts et al.18 and Wattjes et al.19 reported the ability to visualize the highest number of periventricular lesions with DIR. Our study detected the highest number of periventricular lesions with FLAIR and found that DIR did not detect more lesions than T2.
Moraal et al.20 found the highest number of lesions and a statistically significant difference in the deep white matter with FLAIR and the lowest number with DIR. However, they did not report a significant difference between T2 and DIR within this region. Geurts et al.18 reported the highest number of deep white matter lesions with FLAIR and the lowest number with T2. Wattjes et al.19 also reported visualization of the highest number of deep white matter lesions with FLAIR, equal numbers with DIR and T2, and no statistically significant differences in the number of lesions detected between DIR and FLAIR or between DIR and T2. Similarly, we detected the highest number of lesions with FLAIR and the lowest number with DIR. Furthermore, we also found that the number of lesions detected was significantly higher with FLAIR and T2 than with DIR.
Geurts et al.18 reported a higher number of deep grey matter lesions detected with DIR compared to those detected with both FLAIR and T2 sequences. Moraal et al.20 reported no significant differences between the number of deep grey matter lesions visualized with DIR, FLAIR or T2 images. Our study also failed to find a significant difference between these sequences in the detection of deep grey matter lesions.
Moraal et al.20 found a similar number of lesions in the infratentorial region with DIR, FLAIR and T2 images. Geurts et al.18 reported the detection of more lesions with DIR in the infratentorial region compared to both T2 and FLAIR. Wattjes et al.19 detected the highest number of infratentorial lesions with DIR and the lowest with FLAIR and found a statistically significant difference in the number of lesions detected between DIR and FLAIR and between DIR and T2. However, our findings were not similar to any of the above-mentioned studies. Wattjes et al.19 suggested that the infratentorial lesion burden had an important prognostic value in determining long-term disability. Previous studies have indicated that a short echo duration in DIR sequences may decrease sensitivity in the detection of lesions. In addition, DIR sequences may be affected by current artifacts, which may lead to misclassification of some actual lesions as artifacts. This may be due to differences in the detection of infratentorial lesions with DIR.
Moraal et al.20 also reported the detection of the highest number of lesions with FLAIR and the lowest number with T2 compared with the total lesion burden determined by DIR, FLAIR and T2-weighted imaging. In addition, no significant differences were found between DIR and FLAIR and between DIR and T2, although significant differences were observed between FLAIR and T2. Furthermore, Geurts et al. (18) found that the total number of lesions detected was higher for DIR than for FLAIR or T2. In addition, Wattjes et al.19 revealed that the highest number of lesions was detected with DIR and that the lowest was detected with T2, and a statistically significant difference in the number of lesions detected was observed between DIR and FLAIR and between DIR and T2. We detected the highest number of lesions with DIR and FLAIR and the lowest number with T2 sequences. However, we did not find a significant difference in the number of lesions detected between DIR and FLAIR and between DIR and T2. These results are consistent with those obtained by Moraal et al. but differ from the results of Wattjes et al.19.
Neema et al.25 conducted a study with 97 MS patients and reported a significant association between the hypointensity of deep grey matter and the progression of disability. However, they were unable to find a significant association between the global hyperintense lesion number and clinical progression. This finding suggests that the neurodegenerative and destructive aspects of MS, which include involvement of the deep grey matter, may have a closer association with disability compared with white matter inflammation and demyelination. Furthermore, the T2 hyperintense lesions are insufficient to fully account for the underlying pathology observed in MS and the disclosure of the clinically related but diffuse occult disease.
In a study conducted by Ciccarelli et al.26, the lesion burden detected with FLAIR was 34% greater than that obtained with T2, which resulted in a mild correlation with the EDSS scores. However, this correlation was not more robust than the mild correlation detected between T2 and the EDSS scores.
Calabrese et al.27 also reported a positive correlation between the intracortical lesion number and EDSS scores with the DIR technique. They found that the number and volume of cortical lesions were higher in patients who showed clinical deterioration in the follow-up evaluation compared to the patients who did not. In addition, they reported a correlation between cortical lesion volume and both the EDSS scores and change in EDSS scores over time.
Our study examined the relationships between EDSS scores and the lesion numbers detected in seven anatomical regions with DIR, FLAIR and T2 sequences. We found a positive correlation between the number of lesions detected with DIR in the juxtacortical and periventricular white matter regions and the EDSS scores. However, a correlation was only found between the periventricular white matter lesions detected with T2 and the EDSS scores. We were unable to find a correlation between the lesion numbers detected with FLAIR sequences in seven anatomical regions and the EDSS scores. Of the total number of lesions, a significant correlation with the EDSS scores was found only with the number of cerebral T2 lesions. Thus, the absence of a correlation between the total DIR lesion burden and the EDSS scores may have resulted because 51% of all lesions detected with 3D DIR were classified as cortical and the EDSS, which primarily measures ambulatory capacity, was limited in evaluating cortical function.
Histopathological studies have also demonstrated generalized myelin and axonal involvement in MS, in addition to the lesion regions detected with both DIR and non-conventional MRI sequences. From this perspective, studies investigating only the correlation between the lesion burden and disability, such as our study, are a priori insufficient. Thus, the EDSS would be useful in the primary detection of disability, although it has its limitations.
Taken together, we found that the DIR technique is more sensitive in the detection of a higher number of lesions in patients with MS, the enhancement of imaging these lesions, and the determination of their anatomical localizations compared with conventional MRI techniques (T2, FLAIR). Although we disclosed a significant correlation between the lesions in a few anatomical locations and the EDSS scores, we were unable to find a significant correlation between the total lesion burden and the EDSS scores. Therefore, the DIR technique may be used in routine application to visualize lesions in patients with MS in order to develop practical batteries of tests to detect cognitive losses not readily observed in EDSS scores and are thus frequently missed, which may increase patients' physical disability.
Conclusion
DIR brain imaging had the highest sensitivity in the detection of cortical and mixed white matter-grey matter lesions compared with FLAIR and T2 sequences. In addition, the lesions observed with DIR images were more easily visualized.
References
- 1.Peterson JW Trapp BD. Neuropathobiology of multiple sclerosis. Neurol Clin. 2005; 23: 107–129. [DOI] [PubMed] [Google Scholar]
- 2.Uludüz D Saip S Siva A. Multipl skleroz'da uzun süreli koruyucu tedaviler. Nöropsikiyatri Arşivi (Archives of Neuropsychiatry). 2008; 45: 26–36. [Google Scholar]
- 3.Bo L Vedeler CA Nyland HI et al. Subpial demiyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003; 62: 723–732. [DOI] [PubMed] [Google Scholar]
- 4.Kutzelnigg A Lassmann H. Cortical lesions and brain atrophy in MS. J Neurol Sci. 2005; 233: 55–59. [DOI] [PubMed] [Google Scholar]
- 5.Bo L Geurts JJG Mork SJ et al. Grey matter pathology in multiple sclerosis. Acta Neurol Scan.d 2006; 113: 48–50. [DOI] [PubMed] [Google Scholar]
- 6.Paty DW Moore GRW. Magnetic resonance imaging changes as living pathology in multiple sclerosis. In: Paty DW Ebers GC (Eds). Multiple Sclerosis. Arizona: CNS; 1997. p. 328–369. [Google Scholar]
- 7.Dolezal O Dwyer MG Horakova D et al. Detection of cortical lesions is dependent on choice of slice thickness in patients with multiple sclerosis. Int Rev Neurobiol. 2007; 79: 475–489. [DOI] [PubMed] [Google Scholar]
- 8.Geurts JJG Bo L Pouwels PJW et al. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. Am Neuroradiol. 2005; 26: 572–577. [PMC free article] [PubMed] [Google Scholar]
- 9.Kidd D Barkhof F McConnell R et al. Cortical lesions in multiple sclerosis. Brain. 1999; 122: 17–26. [DOI] [PubMed] [Google Scholar]
- 10.Lucchinetti CF Parisi J Bruck W. The pathology of multiple sclerosis. Neurol Clin. 2005; 23: 77–105. [DOI] [PubMed] [Google Scholar]
- 11.De Coene B Hajnal JV Gatehouse P et al. MRI of the brain using fluid-attenuated inversion recovery (FLAIR) pulse sequences. Am J Neuroradiol. 1992; 13: 1555–1564. [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmutyazıcıoğlu K Özdemir H Savranlar Ve Ark A. Temporal lop epilepsisinde “double inversion recovery” sekansı: ön sonuçlar. Türk Tanısal ve Girişimsel Radyoloji Dergisi (Diagnostic and Interventional Radiology). 2004; 10: 182–188. [PubMed] [Google Scholar]
- 13.Tubridy N Molyneux PD Moseley IF et al. The sensitivity of thin-slice fast spin echo, fast FLAIR and gadolinium-enhanced T1-weighted MRI sequences in detecting new lesion activity in multiple sclerosis. J Neurol. 1999; 246: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 14.Melhem ER Itoh R. Effect of T1 relaxation time on lesion contrast enhancement in FLAIR MR imaging: a study using computer-generated brain maps. Am J Roentgenol. 2001; 176: 537–539. [DOI] [PubMed] [Google Scholar]
- 15.Redpath TW Smith FW. Use of a double inversion recovery pulse sequence to image selectively grey or white brain matter. Br J Radiol. 1994; 67: 1258–1263. [DOI] [PubMed] [Google Scholar]
- 16.Turetschek K Wunderbaldinger P Bankier AA et al. Double inversion recovery imaging of the brain: initial experience and comparison with fluid attenuated inversion recovery imaging. Magn Reson Imaging. 1998; 16: 127–135. [DOI] [PubMed] [Google Scholar]
- 17.Karagöz E. Multipl skleroz tanısında magnetik rezonans görüntülemenin yeri. T Klin J Med Sci. 2001; 21: 69–76. [Google Scholar]
- 18.Geurts JJG Pouwels PJW Uitdehaag BMJ et al. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion recovery MR imaging. Radiology. 2005; 236: 254–260. [DOI] [PubMed] [Google Scholar]
- 19.Wattjes MP Lutterbey GG Gieseke J et al. Double inversion recovery brain imaging at 3T: diagnostic value in the detection of multiple sclerosis lesions. Am J Neuroradiol. 2007; 28: 54–59. [PMC free article] [PubMed] [Google Scholar]
- 20.Moraal B Roosendaal SD Pouwels PJW et al. Multi-contrast, isotropic, single-slab 3D MR imaging in multiple sclerosis. Eur Radiol. 2008; 18: 2311–2320. [DOI] [PubMed] [Google Scholar]
- 21.Ludbin FD Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996; 46: 907–911. [DOI] [PubMed] [Google Scholar]
- 22.McDonald WI Compston A Edan G et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001; 50: 121–127. [DOI] [PubMed] [Google Scholar]
- 23.Polman CH Reingold SC Edan G et al. Diagnostic criteria for multiple sclerosis. 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 24.Calabrase M De Stefano N Atzori M et al. Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol. 2007; 64: 1416–1422. [DOI] [PubMed] [Google Scholar]
- 25.Neema M Arora A Healy BC et al. Deep gray matter involvement on brain MRI scans is associated with clinical progression in multipl sclerosis. J Neuroimaging. 2009; 19: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciccarelli O Brex PA Thompson AJ et al. Disability and lesion load in MS: a reassessment with MS functional composite score and 3D fast FLAIR. J Neurol. 2002; 249:18–24. [DOI] [PubMed] [Google Scholar]
- 27.Calabrase M Rocca MA Atzori M et al. A 3-year magnetic resonance imaging study of cortical lesions in relapse-onset multiple sclerosis. Ann Neurol. 2010; 67:376–83. [DOI] [PubMed] [Google Scholar]