Abstract
Glutaric aciduria type 1 is an autosomal recessive disorder caused by deficiency of glutaryl-coenzyme A dehydrogenase, with accumulation of glutaric acid, 3-hydroxyglutaric acid and glutaconic acid. Increased blood glutarylcarnitine levels are the basis for identification of affected infants by newborn screening. Despite the highly variability, this disease usually presents with an acute encephalitis-like encephalopathy in infancy or childhood after a period of normal development. The characteristic neurological sequel is a complex movement disorder due to acute bilateral striatal injury. Frequently, the only abnormality preceding the first episode is a progressive macrocephaly. Although neuroimaging findings are quite variable, the widening of the Sylvian fissures combined with abnormalities of the basal ganglia in a child with macrocephaly should raise the suspicion of this diagnosis. We describe two patients in whom macrocephaly was the only presenting symptom and whose diagnosis was suggested by the brain MRI findings. Our purpose is to illustrate the clinical value of neuroimaging in the diagnosis of glutaric aciduria type 1 even before the onset of neurologic symptoms, which is particularly important if newborn screening is not available.
Keywords: glutaric aciduria type 1, expanded neonatal screening, macrocephaly, neuroimaging
Introduction
Glutaric aciduria type 1 (GA-1) (OMIM 232670) is an autosomal recessive disorder of lysine, hydroxylysine and tryptophan catabolism, caused by deficiency of glutaryl-coenzyme A (CoA) dehydrogenase (GCDH)1. It has an estimated prevalence of 1 in 100 000 newborns2. GA-1 is characterized by accumulation of glutaric acid, 3-hydroxyglutaric acid, glutaconic acid and glutarylcarnitine3,4. Glutarylcarnitine detection is the basis of newborn screening for GA-14. The diagnosis is confirmed by enzyme assay and/or molecular analysis of the GCDH gene5.
GA-1 is a disease with highly variable clinical manifestations. It usually presents with an acute encephalitis-like encephalopathy in infancy or childhood after a period of normal development. Untreated, approximately 90% of the patients will develop an acute crisis, often precipitated by gastroenteritis, intercurrent febrile illness, immunization or surgical intervention between three and 36 months of age6,7. The characteristic neurological sequel is a complex movement disorder, due to bilateral striatal injury3,8-10. Dystonia is the dominant extrapyramidal symptom, usually superimposed on axial hypotonia7,9,10. Some patients may additionally develop spasticity or akinetic-rigid parkinsonism11,12.
A few patients, with insidious-onset13 and late-onset14 types of GA-1, have neurological disease without encephalopathic crisis. Frequently, the first disease manifestation is a developing macrocephaly, which may already be present at birth3,8,15 and even in the prenatal period16,17.
Neuroimaging findings are highly variable in GA-1, although the combinations of some features should raise the suspicion of this diagnosis. Computed tomography (CT) scan of the brain discloses enlarged CSF spaces in most untreated patients, even if asymptomatic8.
Brain magnetic resonance imaging (MRI) is the modality of choice to investigate suspected patients. Atrophy or hypoplasia of the frontotemporal regions of cerebral hemispheres, with enlarged anterior temporal fossa subarachnoid spaces, and cyst-like dilation of the Sylvian fissures, with “batwing” or “box-like” fissures, are often early findings15,18. After an acute event of decompensation or following a chronic course, neurotoxicity of the basal ganglia, particularly the lentiform nuclei, becomes evident15,19.
GA-1 is a treatable condition. A restrictive lysine and tryptophan diet, supplemented with L-carnitine and eventually, riboflavin, and a specific emergency treatment during intercurrent illness has significantly improved the outcome of pre-symptomatically diagnosed children 20-22 Therefore, newborn screening for GA-1 has been implemented in some countries5,7,23,24.
We describe two GA-1 patients illustrating the relevance of brain MRI for the diagnosis.
Case Reports
We retrospectively reviewed the clinical files of two GA-1 patients, evaluated for isolated macrocephaly, whose diagnosis was suggested by brain RMI.
Case 1
This girl was the third child of non-consanguineous Caucasian parents, born at 38 weeks by caesarean section after an uneventful pregnancy. At birth, her body weight was 3410 g (P50-75), length 51.5 cm (P75) and head circumference (HC) 36.2 cm (P95). Her two brothers were healthy. There was a family history of diabetes mellitus type 2.
She sought medical attention at 13 months of age, when her HC grew above P95. Interestingly, the mother complained that she could not pull clothes over the child's head and, against medical instructions, maintained exclusive breast milk until nine months of age, when vegetable soup was initiated. When first observed in our Hospital at 15 months of age because of macrocephaly and failure to thrive, she was still on maternal milk. Slight motor and language delay was noticed, iron deprivation suspected (haemoglobin 10.6 g/dL, mean corpuscular volume 74.8 fL, mean corpuscular haemoglobin 24.5 pg) and the need for a “normal” diet reinforced.
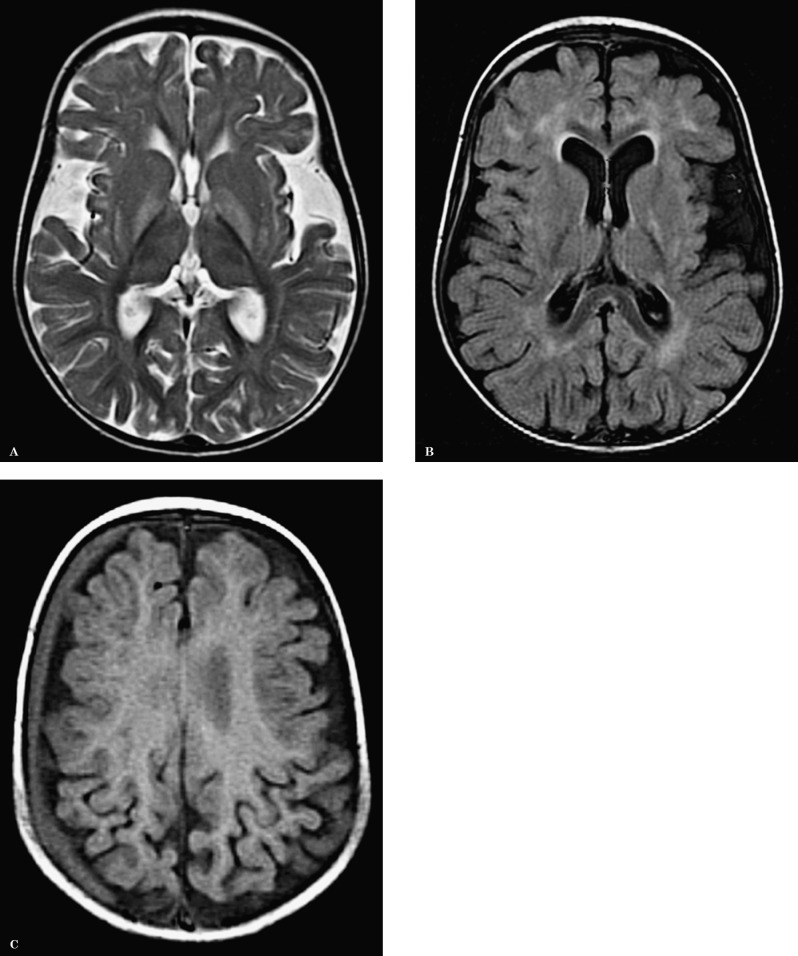
Brain MRI disclosed pronounced widening of the Sylvian fissures, symmetrical abnormal increased signal intensity on T2 and FLAIR-weighted images of the basal ganglia, particularly in the globus pallidus. Several foci of abnormal signal intensity were present in the periventricular, deep and subcortical white matter. A right hemispheric subdural chronic haematoma was noted (Figure 1). The neuroimaging findings suggested a diagnosis of glutaric aciduria type I.
Figure 1.
A) Axial T2-weighted image show enlarged Sylvian fissures and abnormal hyperintensity of the basal ganglia, more prominent in the globus pallidus. B) Axial FLAIR image also shows several foci of abnormal increased signal in the white matter. C) Axial T1-weighted image: an additional finding in this child is a subdural right hemispheric haematoma.
Diagnosis was confirmed by elevated urinary excretion of glutaric acid (4305 μmol/mmol creatinine, normal <5.3) and 3-hydroxyglutaric acid (102 μmol/mmol creatinine, normal <4.2), with marked hypocarnitaemia (2 μmol/L, reference range 30-50).
Treatment was initiated at 17 months of age with low lysine and tryptophan diet, riboflavin, carnitine and iron supplements. Treatment compliance and evolution were favourable with HC at P75-90. Her global development quotient was 96 at 2.6 years of age, when assessed by the Griffiths Development Scale25. She was lost to follow-up after three years of age as her family emigrated to the UK.
Case 2
This girl was the first child of non-consanguineous Caucasian parents. Prenatal ultrasound had disclosed progressive macrocephaly at 30 weeks of gestation. The delivery, after full term pregnancy, was spontaneous vaginal. Birth weight was 3250 g (P25-50), length, 51 cm (P50-75) and HC, 39 cm (> P95). The neonatal cerebral ultrasound showed a thinning of the corpus callosum and abnormal opercularization pattern.
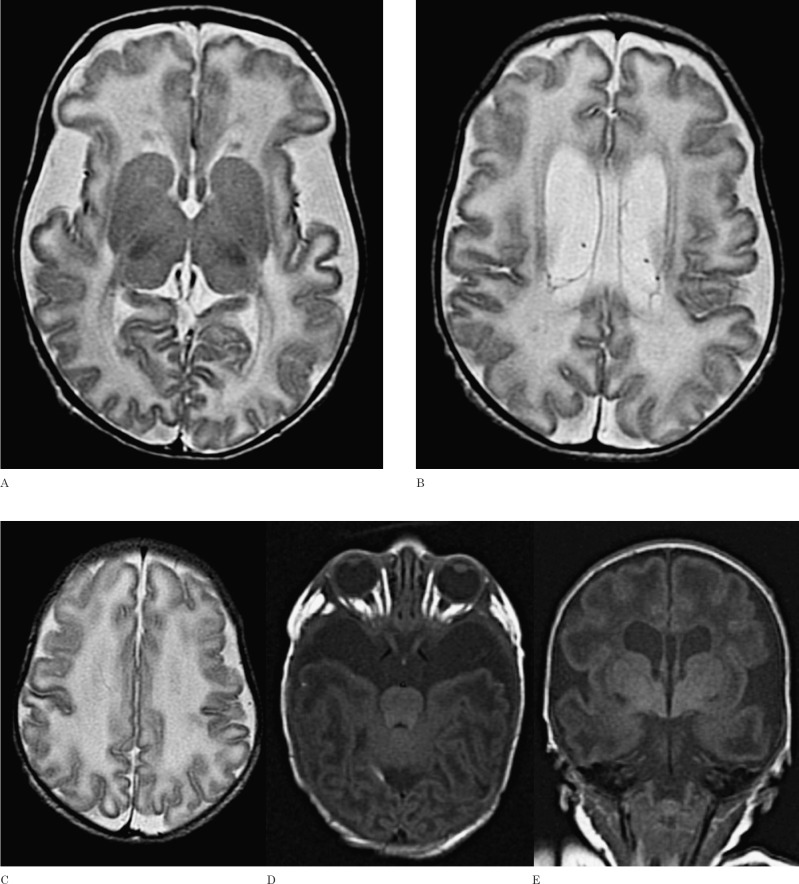
Brain MRI at the eighth day of life revealed severe widening of Sylvian fissures and anterior temporal CSF spaces. Myelination was delayed and the ventricular system enlarged (Figure 2). No grey matter abnormalities were seen. The findings of wide Sylvian fissures and myelination delay in an infant with macrocephaly were suggestive of GA-I. At the age of three months she was referred to our hospital because of macrocephaly and poor head control.
Figure 2.
Axial T2-weighted images (A-C), axial and coronal T1-weighted images (D, E) show wide Sylvian and anterior temporal CSF spaces and myelination delay in this 8-day-old neonate. In this case the basal ganglia are normal. A small subependymal cyst in the posterior part of the body of the left lateral ventricle is shown (B).
Elevated urinary excretion of glutaric acid (5382 μmol/mmol creatinine) and 3-hydroxy-glutaric acid (239 μmol/mmol creatinine) and a homozygous R402W mutation in the GCDH gene was disclosed.
On low lysine and tryptophan diet, riboflavin, carnitine and iron supplements since the age of three months, she has developed appropriately. Intellectual quotient was 91 when assessed by the Wechsler Intelligence Scale for Children26 at six years of age, with HC 56 cm (>P95).
Discussion
Glutaric aciduria type 1 can be suspected based on clinical presentation or neuroradiology findings.
Like the cases discussed, macrocephaly is noted at or shortly after birth or even during pregnancy in most GA-1 patients (75%) 3,8,15,16,17,27 In fact patient 1 showed a slight relative macrocephaly at birth, which was progressive, attracting her mother's attention. In patient 2, HC overgrowth was detected during the third trimester of the pregnancy, although GA-1 was considered only after birth, in the sequence of brain MRI. A few asymptomatic patients have been diagnosed in the course of a diagnostic work-up for macrocephaly. Although macrocephaly is a non-specific sign, with a low predictive value for GA-1, it becomes relevant when associated with typical brain lesions detected at MRI7,15.
The typical widening of Sylvian fissures, in association with macrocephaly, is suggestive of GA-115. In some patients no imaging abnormalities are noticed, whereas in others only or mainly enlarged CSF spaces are seen. Some cases show a predominance of striatal lesions, while others have a combination of striatal and white matter abnormalities.
Brain imaging performed shortly after birth, as in patient 2, usually shows widening of the Sylvian fissures. This is due to abnormal brain growth during intrauterine life and is therefore hypoplasia rather than atrophy. The reduced amount of brain tissue within an enlarged head has been called micrencephalic macrocephaly. In case of micrencephaly and enlargement of the CSF spaces, physiologic inertial cerebral forces, and tearing of bridging veins or fenestrations in the arachnoid membrane can lead to fluid accumulation within the subdural space, explaining the presence of subdural hygromas or haematomas, as was the case in patient 1, sometimes following minor head trauma15.
During the acute stage, oedema within the putamen and caudate are manifest by increased signal intensity on T2-weighted imaging 15,19 Diffusion restriction reflects cytotoxic oedema and disturbed oxidative metabolism in the acute stage. With time, neuronal loss and astrogliosis lead to atrophy of the putamen and caudate nucleus, leaving persistent T2 hyper-intensity. Additional findings include hyperin-tense signal on T2-weighted images involving the dentate nuclei. Lesions in other deep grey matter nuclei have been described sporadically12,16. In fact, patient 1 presented an abnormal signal of the globus pallidus bilaterally, without significant involvement of the putamen or nucleus caudatus. Twomey et al. published a series of 20 patients with GA-1, in which 14 presented abnormal signal of the globus pallidus, being an isolated finding in four of them, with otherwise normal basal ganglia28. Other authors have suggested that pallidal MRI abnormalities are not coupled with the acute encephalopathic crises, in contrast to striatal lesions12. Regardless of encephalopathic crises, the presence of pallidal abnormalities correlated with the presence of white matter abnormalities12, possibly reflecting that the pallidum is a myelin-rich grey matter structure8.
MRI of patient 1 also disclosed several foci of abnormal signal in the periventricular and subcortical white matter. The white matter signal changes reflect the combination of neurotoxic effects of metabolic by-products and dysmyelination and/or demyelination15. When present, white matter abnormalities are usually extensive, symmetrical, most marked in the frontal and occipital periventricular regions and in the centrum semiovale. The arcuate fibres and corpus callosum are usually spared8. As the disease progresses, generalized cerebral atrophy, ventricular dilatation, and basal ganglia atrophy become more conspicuous. Deviations from normal maturation of the developing brain, such as immature gyral pattern and myelination delay, have also been described12. Patient 2, a full-term neonate, also revealed myelination delay.
In our cases, the widening of the Sylvian fissures in patients with macrocephaly, favoured the diagnosis of GA-1. In patient 1, the abnormal high signal of the globus pallidus, white matter signal changes and the presence of a chronic subdural haematoma, and in patient 2, the enlargement of the pretemporal middle cranial fossa subarachnoid spaces, enlargement of the ventricular system and the myelination delay reinforced the suspicion.
In patients suspected of GA-1, a specific diagnostic work-up should include quantitative analysis of glutaric acid and 3-hydroxyglutaric acid in urine or blood, GCDH gene mutation analysis, and/or enzyme analysis7. Based on the urinary excretion of glutaric acid, individuals with GA-1 fall into two biochemically different subgroups: high excretors and low excre-tors1,6,7.
Early clinical diagnosis is hampered by the lack of characteristic or even pathognomonic signs and symptoms before an encephalopathic crisis or irreversible neurological damage occurs7. Therefore, GA-1 has been included in the disease panel of newborn screening in some countries5,7,23,24. The Portuguese Neonatal Screening Programme, which was expanded to other disorders besides phenylketonuria and hypothyroidism in 2005, includes GA-129,30. The cases presented were diagnosed before that date.
Newborn screening for GA-1 is based on increased glutarylcarnitine levels in blood, quantified by tandem mass spectrometry (MS-MS) analysis4. The majority of the rare patients excreting normal or only minimally elevated levels of glutaric acid (low excretors), that might have normal levels of glutarylcarnitine in blood spots, can be identified by expanded newborn screening, though some patients may be missed1,2,15. So, if an infant or child presents with clinical signs or symptoms, or neuroradiological findings of GA-1, a diagnostic evaluation should be undertaken, regardless of whether the child was previously evaluated in a newborn screening programme7.
Early treatment can prevent significant complications in GA-1 patients, with improved neurologic outcomes compared to those treated only after symptoms emerged1, 7, 20-22, 15, 29. Late treatment is generally not effective in preventing permanent neurologic damage7. GA-1 treatment, highly recommended in asymptomatic patients, consists of a lysine and tryptophan restricted diet, with or without lysine-free, tryptophan reduced amino acid supplements and riboflavin. L-carnitine should be used lifelong to treat or prevent hypocarnitinaemia due to excessive acylcarnitine production7,15,20-22.
Both patients here discussed have normal psychomotor development, at least up to the last evaluation. In fact macrocephaly is not associated with poor neurological outcome1. Even patient 1, who had a slight motor and speech delay at the start of treatment, seems to have fully recovered. In GA-1intellect is relatively preserved. The profound neurological sequelae may lead to death in early childhood. However, some individuals survive for many years. On the other hand, a minority of biochemically affected individuals may remain asymptomatic or experience an insidious onset of mild neurological abnormalities3.
In patients 1 and 2, the evolution has been good in spite of initiating treatment at 17 months and three months of age, respectively. At present newborn screening in Portugal can identify newborns with GA-1, allowing early treatment and prevention of significant complications1,7. Without treatment, 75-90% of all children with GA-1 will develop neurologic disease, but once correctly diagnosed, the encephalopathic crisis and consequent irreversible neurological changes can be avoided1,6. Although not highly specific, brain neuroimaging can be a useful tool in the diagnosis of GA-1, as was evident in the cases here described. Therefore, it is important that radiologists involved in paediatric neuroradiology are aware that neurometabolic disorders, such as GA-1 may initially manifest as macrocephaly and have suggestive lesions on MR. This is particularly important in countries where newborn screening does not include GA-1.
References
- 1.Viau K Ernst SL Vanzo JR et al. , Glutaric acidemia type 1: outcomes before and after expanded newborn screening. Mol Genet Metab. 2012; 106: 430–438. [DOI] [PubMed] [Google Scholar]
- 2.Lindner M Kölker S Schulze A et al. Neonatal screening for glutaryl-CoA dehydrogenase deficiency. J Inherit Metab Dis. 2004; 27: 851–859. [DOI] [PubMed] [Google Scholar]
- 3.Funk CB Prasad AN Frosk P et al. Neuropathological, biochemical and molecular findings in a glutaric acidemia type 1 cohort. Brain. 2005; 128: 711–722. [DOI] [PubMed] [Google Scholar]
- 4.Tortorelli S Hahn SH Cowan TM et al. The urinary excretion of glutarylcarnitine is an informative tool in the biochemical diagnosis of glutaric acidemia type I. Mol Genet Metab. 2005; 84: 137–143. [DOI] [PubMed] [Google Scholar]
- 5.Lindner M Ho S Fang-Hoffmann J et al. , Neonatal screening for glutaric aciduria type I: strategies to proceed. J. Inherit. Metab. Dis. 2006; 29: 378–382. [DOI] [PubMed] [Google Scholar]
- 6.Kölker S Garbade SF Greenberg CR et al. Natural history, outcome, and treatment efficacy in children and adults with glutaryl-CoA dehydrogenase deficiency. Pediatr. Res. 2006; 59: 840–847. [DOI] [PubMed] [Google Scholar]
- 7.Kölker S Christensen E Leonard JV et al. Diagnosis and management of glutaric aciduria type I—revised recommendations. J Inherit Metab Dis. 2011; 34: 677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Knaap MS Valk J. Magnetic resonance of myelination and myelin disorders, 3rd ed. New York: Springer; 2005. pp 294–299. [Google Scholar]
- 9.Hoffmann GF Trefz FK Barth PG et al. Glutaryl-CoA dehydrogenase deficiency: a distinct encephalopathy. Pediatrics. 1991; 88: 1194–1203. [PubMed] [Google Scholar]
- 10.Kyllerman M Skjeldal OH Lundberg M et al. , Dystonia and dyskinesia in glutaric aciduria type I: clinical heterogeneity and therapeutic considerations. Mov Disord. 1994; 9: 22–30. [DOI] [PubMed] [Google Scholar]
- 11.Kyllerman M Skjeldal O Christensen E et al. Long-term follow-up, neurological outcome and survival rate in 28 Nordic patients with glutaric aciduria type 1. Eur J Paediatr Neurol. 2004; 8: 121–129. [DOI] [PubMed] [Google Scholar]
- 12.Harting I Neumaier-Probst E et al. Dynamic changes of striatal and extrastriatal abnormalities in glutaric aciduria type I. Brain. 2009; 132: 1764–1782. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann GF Athanassopoulos S Burlina AB et al. Clinical course, early diagnosis, treatment, and prevention of disease in glutaryl-CoA dehydrogenase deficiency. Neuropediatrics. 1996; 27: 115–123. [DOI] [PubMed] [Google Scholar]
- 14.Bähr O Mader I Zschocke J et al. Adult onset glutaric aciduria type I presenting with a leukoencephalopathy. Neurology. 2002; 59: 1802–1804. [DOI] [PubMed] [Google Scholar]
- 15.Hedlund GL Longo N Pasquali M. Glutaric acidemia type 1”. Am J Med Genet C Semin Med Genet. 2006; 15, 142C (2): 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellerio C Marignier S Roth P et al. Prenatal cerebral ultrasound and MRI findings in glutaric aciduria Type 1: a de novo case. Ultrasound Obstet Gynecol. 2008; 31 (6): 712–714. [DOI] [PubMed] [Google Scholar]
- 17.Lin SK Hsu SG Ho ES et al. Glutaric aciduria (type I): prenatal ultrasonographic findings. Ultrasound Obstet Gynecol. 2002; 20 (3): 305–307. [DOI] [PubMed] [Google Scholar]
- 18.Neumaier-Probst E Harting I Seitz A et al. Neuroradiological findings in glutaric aciduria type I (glutaryl-CoA dehydrogenase deficiency). J Inherit Metab Dis. 2004; 27: 869–876. [DOI] [PubMed] [Google Scholar]
- 19.Desai NK Runge VM Crisp DE et al. Magnetic resonance imaging of the brain in glutaric acidemia type I: a review of the literature and a report of four new cases with attention to the basal ganglia and imaging technique. Invest Radiol. 2003; 38: 489–496. [DOI] [PubMed] [Google Scholar]
- 20.Monavari AA Naughten ER. Prevention of cerebral palsy in glutaric aciduria type I by dietary management. Arch Dis Child. 2000; 82: 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strauss KA Puffenberger EG Robinson DL et al. Type I glutaric aciduria, part 1: natural history of 77 patients. Am J Med Genet. 2003; 121C: 38–52. [DOI] [PubMed] [Google Scholar]
- 22.Köllker S Garbade SF Boy N et al. Decline of acute encephalopathic crises in children with glutaryl-CoA dehydrogenase deficiency identified by newborn screening in Germany. Pediatr Res. 2007; 62: 357–363. [DOI] [PubMed] [Google Scholar]
- 23.Chace DH Kalas TA Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. 2003; 49: 1797–1817. [DOI] [PubMed] [Google Scholar]
- 24.Bijarnia S Wiley V Carpenter K et al. Glutaric aciduria type I: outcome following detection by newborn screening. J Inherit Metab Dis. 2008; 31: 503–507. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths R. The abilities of young children: a comprehensive system of mental measurement for the first eight years of life. North Dean, United Kingdom: Test Agency Ltd.; 1970. [Google Scholar]
- 26.Wechsler D. Escala de Inteligência de Wechsler para Crianças (WISCIII). Manual. Lisboa: Edição revista português ed. CEGOC-TEA; 2003. [Google Scholar]
- 27.Righini A Fiori L Parazzini C et al. Early prenatal magnetic resonance imaging of glutaric aciduria type 1: case report. J Comput Assist Tomogr. 2010; 34 (3): 446–448. [DOI] [PubMed] [Google Scholar]
- 28.Twomey E Naughten E Donoghue et al. , “Neuroimaging findings in glutaric aciduria type 1”. Pediatric Radiology. 2003; 33 (12): 823–830. [DOI] [PubMed] [Google Scholar]
- 29.Vilarinho L Rocha H Marcão A et al. Diagnóstico precoce: resultados preliminares do rastreio metabólico alargado. Acta Pediatr Port. 2006; 37: 186–191. [Google Scholar]
- 30.Garcia P Martins E Diogo L et al. Outcome of three cases of untreated maternal glutaric aciduria type I. Eur J Pediatr. 2008; 167 (5): 569–573. [DOI] [PubMed] [Google Scholar]