Abstract
Spinal chordomas are more often located on the midline and are associated with marked destruction of the vertebral bodies. We report a rare case of large cervical (C2-C3) right lateral paravertebral chordoma extending into the spinal canal through a very enlarged intervertebral foramen. The tumor was initially diagnosed as a mucous adenocarcinoma on a percutaneous needle biopsy. However, the neuroradiological features, including the well-defined tumor margins, the regular and sclerosing lytic bone changes with regular enlargement of the intervertebral C2-C3 foramen, were in favor of a more slowly growing lesion, such as schwannoma or neurofibroma. At surgery a well-demarcated capsulated tumor involving the nerve root was partially resected. Histology was in favor of a low-grade chordoma (Ki-67/MIB-1<1%). Postoperative proton beam therapy was also performed. The differential neuroradiological diagnosis is discussed.
Keywords: chordoma, cervical spine, magnetic resonance
Introduction
Chordoma is a rare low-grade malignant neoplasm derived from the remnants of the embryonic notochord accounting for only 1-4% of all bone malignancies1. Population-based studies suggest an incidence of chordoma of 0.08 per 100,000 with predominance in men and a peak incidence between 50 and 60 years of age2. In addition, an almost equal incidence is reported in the skull base (32%), mobile spine (32.8%) and sacrum (29.2%)2.
Spinal chordomas occur more frequently in the cervical region (20 to 50%) than in the lumbar and thoracic spine3. They are commonly located on the midline and are associated with variable and usually marked destruction of one or more vertebral bodies4-6.
We describe a rare case of large cervical (C2-C3) right paravertebral chordoma initially diagnosed as malignant tumor of glandular origin7. The magnetic resonance (MR) and computed tomography (CT) features and differential radiological diagnosis are discussed.
Case Report
A 76-year-old woman with a history of thyroid gland carcinoma and skin melanoma was observed for cervical and right lateral neck pain. One month before an ultrasound scan of the cervical region as follow-up of the thyroid neoplasm showed a large (40 mm) right paravertebral mass with enlarged lymph nodes. A percutaneous needle biopsy of the mass was in favor of a mucous adenocarcinoma, likely arising from the submandibular gland.
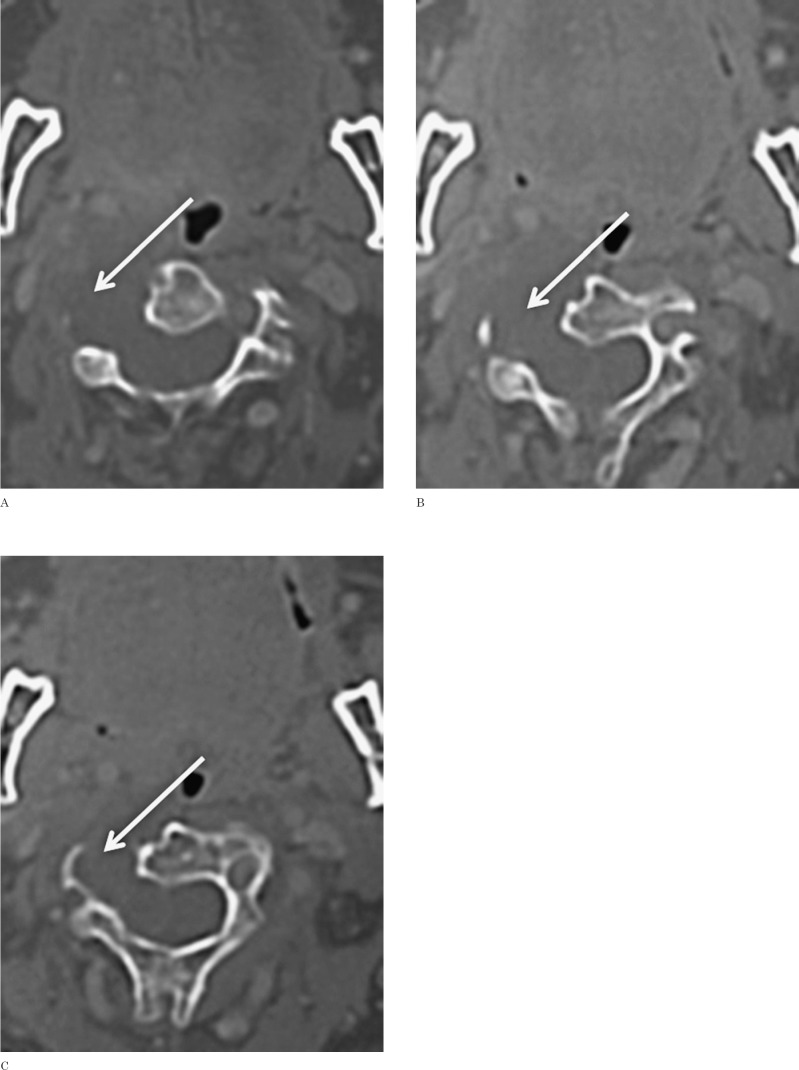
At clinical observation no neurological deficits were disclosed. A CT scan of the neck and cervical spine (Figure 1) showed a large (4 × 3.5 cm) lobulated mass with inhomogeneous hypodensity located in the right paravertebral region at C2-C3 level. The tumor mass caused bone destruction of the right lateral aspect of the C2 and C3 vertebral bodies and the right transverse processes. The tumor extended into the spinal canal through an enlarged intervertebral C2-C3 foramen, causing cord compression. The bone lytic changes showed regular sclerotic margins suggesting a slowly growing lesion. Peripheral inhomogeneous contrast enhancement was noted.
Figure 1.
CT study. Axial CT images after intravenous administration of contrast material show a destructive hypodense lesion, with no apparent enhancement. The lesion causes markedly lytic destruction of the right lateral aspect of the C2 and C3 vertebral bodies and of the right transverse processes, extending into the spinal canal through a very enlarged intervertebral C2-C3 foramen and causing cord compression.
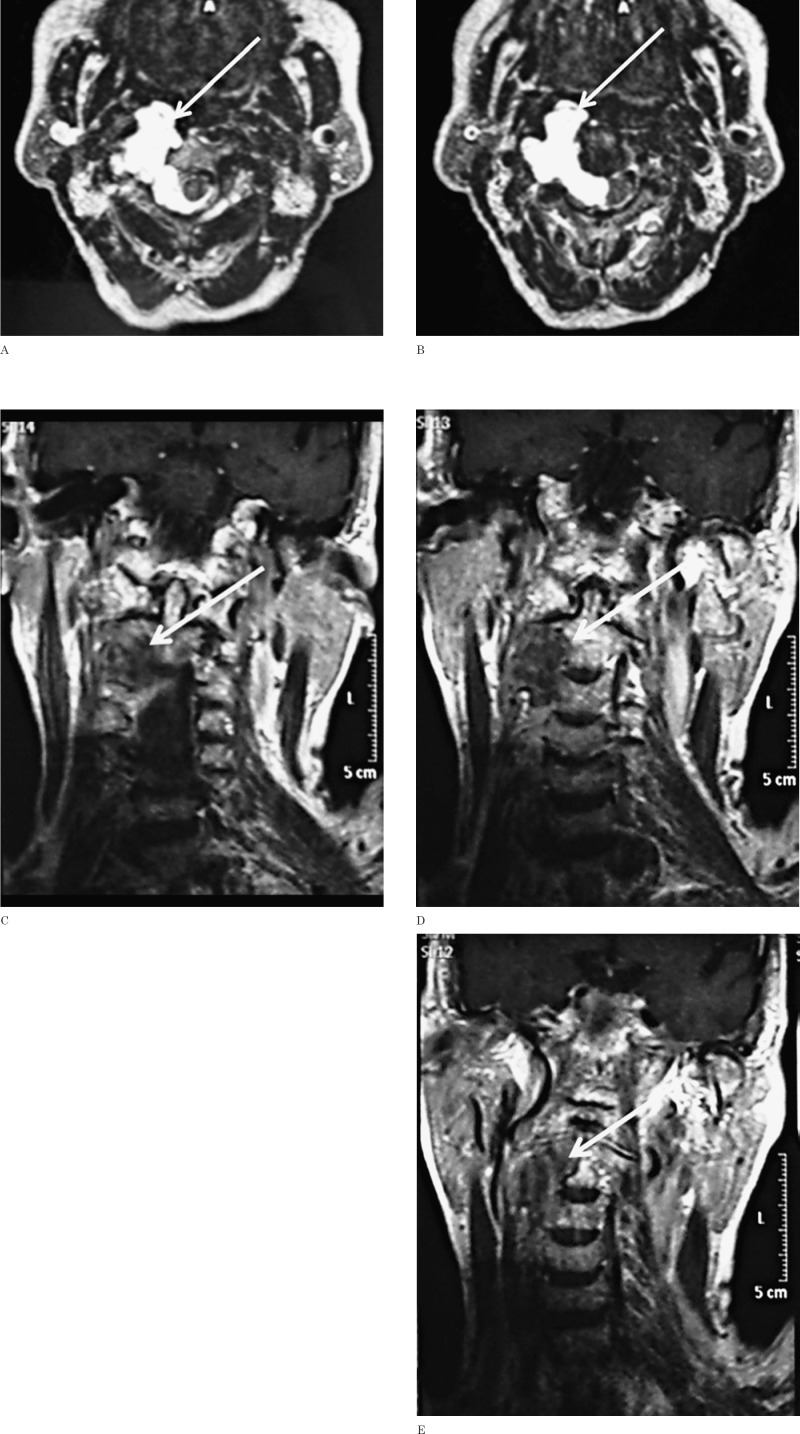
Magnetic resonance of the cervical spine (Figure 2) confirmed the C2-C3 right paravertebral mass with inhomogeneous hypointense signal on T1-weighted images and hyperin-tense on T2-weighted images. Marked inhomogeneous contrast enhancement was noted. The pharynx and the internal carotid artery and jugular vein were displaced forward and laterally and the vertebral artery was displaced laterally. The intraspinal component caused cord compression and contralateral displacement. The most likely preoperative neuroradiological diagnosis was schwannoma or neurofibroma.
Figure 2.
MRI study. A, B) Axial T2-weighted TSE images clearly show the lobulated cervical chordoma with diffuse homogeneous hyperintensity and extension along the right lateral aspect of C2 and C3. The tumor shows anterior and lateral spread to the paravertebral and retropharyngeal spaces. Posteriorly, it widens the right intervertebral C2-C3 foramen and breaks into the epidural space with compression of the cord. C-E) On coronal T1-weighted TSE images after intravenous administration of contrast material the mass exhibits heterogeneous mainly peripheral enhancement.
At surgery through a posterior cervical approach and right C2-C3 hemilaminectomy a right extradural whitish paravertebral mass of hard consistency was exposed. The right C3 root was wholly enveloped by the tumor capsule, suggesting a neurofibroma. The posterior part of the tumor extending to the spinal canal was removed to decompress the spinal cord, then the intratumoral mucous component was evacuated. However, because of the tenacious adherence of the residual mass to the vertebral and carotid arteries and pharynx, no further resection was decided.
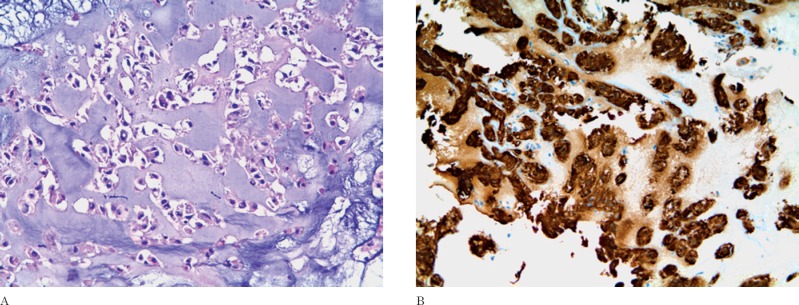
Histology revealed a tumor composed of often vacuolized epithelioid cells surrounded by a myxoid background (Figure 3). The immuno-histochemical staining was positive for EMA, CK AE1-AE3 and S-100 protein. The Ki-67/ MIB-1 was <1%. The histological diagnosis was in favor of a chordoma. No postoperative complications or neurological deficits occurred. The residual tumor was treated by proton beam therapy.
Figure 3.
Histology. A) Epithelioid cells with discrete cell borders in a myxoid background (hematoxylin-eosin, original magnification ×20). B) Immunoreactivity for cytokeratin AE1-AE3 (original magnification ×20).
Discussion
The present case is noteworthy for both the tumor localization and the problems of the correct preoperative neuroradiological diagnosis.
Most chordomas of the spine occur on the midline and present as a soft tissue mass with variable and often extensive bone destruction and extension into the spinal space8-11. CT is important to assess the bony involvement and soft tissue extension, tumor calcification, any residual ossified fragments and the status of the cortical bone. The bone destruction may be irregular or may present reactive sclerosis (40-60% of the cases)4,12.
MRI is better to delineate the full extension of the tumor, its relationship to the adjacent vascular structures (including cord compression) and the extension along the epidural space4,13,14. The tumor mass is heterogeneous hypo-isointense (compared to bone marrow) on T1W images (depending on the presence of hemorrhage, cystic degeneration, bony fragments, calcifications) and moderately to extremely hy-perintense to both CSF and intervertebral discs on T2W images. The contrast enhancement is variable and heterogeneous.
Chordomas with significant bone destruction may erroneously be confused with other spinal tumors causing lytic bone changes, such as chondrosarcoma and more frequent vertebral metastases, myeloma and lymphoma 4. Chondrosarcoma shows rather similar MR features with respect to chordoma, although it involves the neural arch more frequently than the vertebral body. In addition, the chondroid matrix is often evident as characteristic rings and arcs. Other primary tumors (myeloma and lymphoma)15,16 and spinal metastases17 show a more heterogeneous T2 signal; the finding of multifocal spinal localizations obviously excludes the diagnosis of chordoma.
Several spinal chordomas, as in our case, present as a large paravertebral mass with no or scarce destruction of the vertebral bodies; the tumor may extend along the nerve roots into the epidural space enlarging the neural foramina. In such cases the neuroradiological diagnosis of chordoma is more difficult.
Our patient was referred from a peripheral hospital with the diagnostic suspicion of primary or metastatic mucous adenocarcinoma of glandular origin as a result of needle biopsy under ultrasonic guidance. However, CT and MRI showed a tumor mass with well-defined margins, bony lytic changes in the C2-C3 vertebral bodies with regular and sclerotic margins, and regular enlargement of the right C2-C3 intervertebral foramen. These findings were in favor of a more slowly growing lesion, such as schwannoma or neurofibroma18. The intraoperative evidence of the right C3 root enveloped within the tumor capsule at the level of the enlarged intervertebral foramen supported this diagnostic suggestion.
Large dumbbell schwannomas and neurofibromas may show several neuroradiological features evidenced in the present case, including a mass hypointense in T1 and hyperintense in T2-weighted images, enlarged intervertebral foramen with smooth bone margins, and regular scalloping of the vertebral body19-21. However, the location of the paravertebral tumor mass lateral and anterior to the vertebral body was against a tumor of nerve root origin, which does not show this pattern of growth.
Chordoma exhibits various degrees of histological atypia22, but the relationship between histopathological features and biological behavior is controversial3,23 and the outcome remains unpredictable24. In fact, cervical chordomas are so uncommon that the long-term follow-up has been evaluated only in single case reports.
Although complete surgical resection is the treatment of choice for spinal chordomas5,25-28, it may be obtained in less than half the cases. For patients submitted to incomplete resection favorable results with good tumor control have been demonstrated with the use of hadron-based therapy3,5,29.
References
- 1.Healey JH Lane JM. Chordoma: a critical review of diagnosis and treatment. Orthop Clin North Am. 1989; 20: 417–426. [PubMed] [Google Scholar]
- 2.McMaster ML Goldstein AM Bromley CM et al. Chordoma: incidence and survival patterns in the United States, 1973–1995. CancerCauses Control. 2001; 12:1–11. [DOI] [PubMed] [Google Scholar]
- 3.Walcott BP Nahed BV Mohyeldin A et al. Chordoma: current concepts, management and future direction. Lancet Oncol. 2012; 13: e69–e76. [DOI] [PubMed] [Google Scholar]
- 4.Ross JS Brant-Zawadski M Moore KR (eds). Diagnostic imaging spine. AMIRSYS 2005; IV 1: 46–49. [Google Scholar]
- 5.Jiang L Liu ZJ Liu XG et al. Upper cervical spine chordoma of C2-C3. Eur Spine J. 2009; 18 (3): 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Divitiis O Elefante A de Divitiis E. Historic background of spinal disorders. World Neurosurg. 2013; 79 (1): 91–94. [DOI] [PubMed] [Google Scholar]
- 7.Pinto A Caranci F Romano L et al. Learning from errors in radiology: a comprehensive review. Semin Ultrasound CT MR. 2012; 33 (4): 379–382. [DOI] [PubMed] [Google Scholar]
- 8.Wippold FJ 2nd Koeller KK Smirniotopoulos JG. Clinical and imaging features of cervical chordoma. AJR Am J Roentgenol. 1999; 172: 1423–1426. [DOI] [PubMed] [Google Scholar]
- 9.Soo MY. Chordoma: review of clinic-radiological features affecting survival. Australas Radiol. 2001; 45: 427–434. [DOI] [PubMed] [Google Scholar]
- 10.Smolder D Wang X Drevelengas A et al. Value of MRI in the diagnosis of non-clival non-sacral chordoma. Skeletal Radiol. 2003; 32: 343–350. [DOI] [PubMed] [Google Scholar]
- 11.Papagelopoulos PJ Mavrogenis AF Galanis EC et al. Chordoma of the spine: clinicopathological features, diagnosis and treatment. Orthopedics. 2004; 27: 1256–1263. [DOI] [PubMed] [Google Scholar]
- 12.Murphey MD Andrews CL Flemming DJ et al. From the archives of the AFIP. Primary tumors of the spine: radiological pathological correlation. Radiographics. 1996; 16: 1131–1158. [DOI] [PubMed] [Google Scholar]
- 13.Atlas SW. Magnetic resonance imaging of the brain and spine. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 14.Caranci F Brunese L Reginelli A et al. Neck neoplastic conditions in the emergency setting: role of multi-detector computed tomography. Semin Ultrasound CT MR. 2012; 33 (5): 443–448. [DOI] [PubMed] [Google Scholar]
- 15.Caranci F Cirillo M Piccolo D et al. A rare case of intraosseous lipoma involving the sphenoclival region. Neuroradiology Journal. 2012; 25 (6): 680–683. [DOI] [PubMed] [Google Scholar]
- 16.de Divitiis O Elefante A. Cervical spinal brucellosis: a diagnostic and surgical challenge. World Neurosurg. 2012; 78 (3–4): 257–259. [DOI] [PubMed] [Google Scholar]
- 17.Elefante A Peca C Del Basso De Caro ML et al. Symptomatic spinal cord metastasis from cerebral oligodendroglioma. Neurol Sci. 2012; 33 (3): 609–613. [DOI] [PubMed] [Google Scholar]
- 18.Striano P Elefante A Coppola A et al. Dramatic response to levetiracetam in post-ischaemic Holmes' tremor. J Neurol Neurosurg Psychiatry. 2007; 78 (4): 438–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphey MD Smith WS Smith SE et al. Imaging of musculoskeletal neurogenic tumors: radiologic pathologic correlation. Radiographics. 1999; 19: 1253–1280. [DOI] [PubMed] [Google Scholar]
- 20.Woodruff JM Kourea HP Louis DN et al. Neurofibroma. In: Kleihnes P Cavenee WK (eds): Tumors of the Nervous System, 167–168, IARC Press, 2000. [Google Scholar]
- 21.Abul-Kasim K Thurnher MM McKeever P et al. Intradural spinal tumors: current classification and MRI features. Neuroradiology. 2008; 50: 301–314. [DOI] [PubMed] [Google Scholar]
- 22.Chugh R Tawbi H Lucas DR et al. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007; 12: 1344–1350. [DOI] [PubMed] [Google Scholar]
- 23.Crapanzano JP Ali SZ Ginsberg MS et al. Chordoma: a cytologic study with histologic and radiologic correlation. Cancer. 2001; 93: 40–51. [PubMed] [Google Scholar]
- 24.Bergh P Kindblom LG Gunterberg B et al. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000; 88: 2122–2134. [DOI] [PubMed] [Google Scholar]
- 25.Carpentier A Polivka M Blanquet A et al. Suboccipital and cervical chordomas: the value of aggressive treatment at first presentation of the disease. J Neurosurg. 2002; 97: 1070–1077. [DOI] [PubMed] [Google Scholar]
- 26.Bjornsson J Wold LE Ebersold MJ et al. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer. 1993; 71: 735–740. [DOI] [PubMed] [Google Scholar]
- 27.Boriani S Chevalley F Weinstein JN et al. Chordoma of the spine above the sacrum. Treatment and outcome in 21 cases. Spine. 1996; 21: 1569–1577. [DOI] [PubMed] [Google Scholar]
- 28.Klekamp J Samii M. Spinal chordomas — Results of a treatment over a 17-year period. Acta Neurochir (Wien). 1996; 138: 514–519. [DOI] [PubMed] [Google Scholar]
- 29.Hug EB Slater JD. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. Neurosurg Clin N Am. 2000; 11: 627–638. [PubMed] [Google Scholar]