Abstract
Atherosclerosis is a chronic, inflammatory disease affecting large and medium arteries and is considered to be a major underlying cause of cardiovascular disease (CVD). Although the development of pharmacotherapies to treat CVD has resulted in a decline in cardiac mortality in the past few decades, CVD is estimated to be the cause of one in three global deaths. Nutraceuticals are natural nutritional compounds that are beneficial for the prevention or treatment of disease and, therefore, represent a possible therapeutic avenue for the treatment of atherosclerosis. The purpose of this review is to highlight potential nutraceuticals for use as anti-atherogenic therapies, with evidence from in vitro, in vivo, clinical, and observational studies.
In 2015, the WHO reported that approximately one third of global deaths were attributable to a cardiovascular disease (CVD)-related event1. Atherosclerosis, an inflammatory disorder of the vasculature, is the primary cause of CVD-related events, including myocardial infarction (MI) and stroke. Given the increase in prevalence of obesity and diabetes in developing countries, the global incidence of CVD is predicted to increase and impose a greater economic burden on the health-care services around the world.
Under normal healthy conditions, the metabolism and transport of cholesterol, including influx and efflux within cells, is highly regulated. The development of atherosclerosis can begin when these homeostatic mechanisms become unbalanced in favour of either increased cholesterol influx or decreased efflux. Within the blood, there are several lipoproteins that each has a different function in lipid transportation. LDL is one of the most important lipoproteins found in the bloodstream and its function is to transport cholesterol from the liver to the peripheral tissues2. LDL particles enter cells primarily by receptor-mediated endocytosis using the LDL receptor (LDLr). In order to maintain a balance in cholesterol metabolism, HDL transports excess cholesterol from the peripheral tissues back to the liver for excretion via the bile system by a process known as reverse cholesterol transport2. However, only 5% of the biliary cholesterol is excreted in faeces as the rest is reabsorbed in the intestine2. Given that high LDL-cholesterol and low HDL-cholesterol levels have been associated with reduced endothelial function, increased LDL-cholesterol and HDL-cholesterol levels are thought to be pro-atherogenic and anti-atherogenic, respectively3. Therefore, strategies for treating atherosclerosis should be aimed at lowering plasma LDL levels and increasing serum HDL levels.
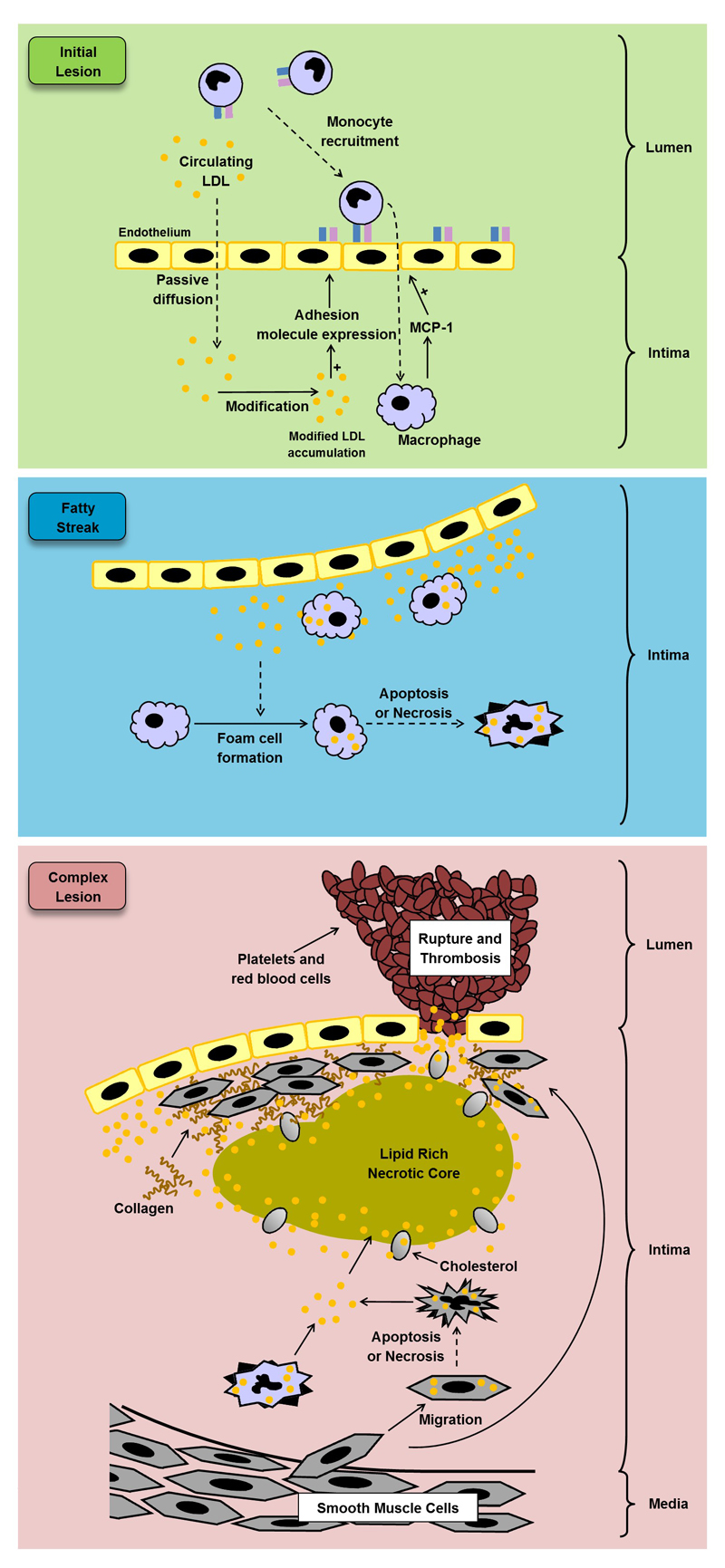
Research on mouse models in the past twenty years have improved our understanding of the pathophysiology of atherosclerosis. Mice do not naturally develop atherosclerosis, but LDLr-deficient and apolipoprotein E (ApoE)-deficient mouse models are prone to atherosclerotic lesion formation on a high-fat or high-cholesterol diet and are able to mimic several aspects of the disease seen in humans. Atherosclerosis is often characterised by the build-up of fatty deposits and the formation of plaques in the walls of large and medium arteries, followed by a strong immunological response to the fatty deposit accumulation (Figure 1). This initial fatty deposit build-up in the intima of arteries, often referred to as a fatty streak, is composed of ApoB containing lipoproteins, in particular LDL and other lipoprotein remnants2. This trapped LDL can then become oxidised to form oxidised LDL (oxLDL)2. The presence of oxLDL within the intima of the artery triggers an inflammatory response in the neighbouring endothelium cells which start producing pro-inflammatory cytokines and chemokines2,4. The roles of different cytokines and chemokines in atherosclerosis have been extensively reviewed elsewhere and these can be generally classified as either pro-inflammatory or anti-inflammatory4,5.
Figure 1. Formation of an atherosclerotic plaque.
The expression of pro-inflammatory genes, including ICAM-1 and MCP-1, is triggered by the build-up of modified LDL in the neighbouring endothelial cells during the development of the initial lesion. Circulating monocytes are then recruited to the modified LDL accumulation and migrate into the intima and differentiate into macrophages. Once in the walls of the artery, the macrophages are able to take up the modified LDL and become lipid-laden foam cells, which can accumulate and form a fatty streak. During complex lesion formation, foam cell lysis by apoptosis and necrosis leads to the formation of a necrotic core, and together with defective efferocytosis, leads to the amplification of the inflammatory response. SMCs begin to migrate from the media to the intima and the ECM produced by them forms fibrous cap and stabilises the plaque. SMCs also transform to foam cells. During later stages of the complex lesion the plaque can become unstable owing to the inflammatory response, resulting in an inhibition of ECM formation, particularly collagen production by SMCs. The remaining ECM can then start to be degraded by proteases released by macrophages, resulting in an unstable lesion that can rupture and lead to thrombosis. These events can cause a myocardial infarction or stroke, depending on the location of plaque formation. ECM, Extracelular matrix; ICAM-1, Intercellular adhesion molecule-1; LDL, Low density lipoprotein; MCP-1, Macrophage chemoattractant protein-1; SMCs, Smooth muscle cells.
Once monocytes have migrated into the intima of the arteries, they become exposed to macrophage colony-stimulating factor and differentiate into macrophages, a process that is associated with increased expression of scavenger receptors (SRs) on their cell surface2. The uptake of LDL via the LDLr is controlled by a negative feedback loop, whereas oxLDL uptake via SRs, such as MSR1 and CD36, is unregulated6. Pro-inflammatory cytokines are capable of inducing foam cell formation by altering the expression of key genes implicated in the regulation of cholesterol metabolism and transport including APOE, ABCA1, ACAT1, and MSR12,4,5,7,8. Foam cells subsequently begin to accumulate and form an initial lesion that matures into an atherosclerotic plaque2,4,5,8.
During maturation of the atherosclerosis lesion, the accumulated foam cells begin to undergo apoptosis and necrosis, causing them to release their fatty contents into the intima of the arteries. The apoptotic cells and the fatty contents accumulate to form a lipid-rich necrotic core. During the latter stages of plaque progression, macrophages, endothelial cells, and T cells stimulate the proliferation and migration of vascular smooth muscle cells from the media to the intima of arteries, resulting in the formation of a fibrous cap over the lipid core2,9. The fibrous cap is then strengthened by the extracellular matrix (ECM) produced by the vascular smooth muscle cells2,10. Given that the fibrous cap stabilises the lesion, the balance of ECM deposition and degradation is critical in dictating the clinical progression of atherosclerosis. ECM degrading enzymes are released particularly from macrophages that are undergoing apoptosis, shifting the balance towards ECM degradation and increasing the risk of a plaque rupture11. Clinical symptoms of plaque development are usually not observable until the plaque ruptures. Upon rupture, platelet aggregation rapidly occurs, which can quickly impede or obstruct blood flow though the artery2, resulting in a coronary event. Statins are the most commonly used cholesterol-lowering agents. Statins reduce circulating LDL-cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-CoA reductase (HMG CoA reductase), the enzyme involved in the rate limiting step during cholesterol biosynthesis12. However, patients taking statins still harbour a discernible residual risk of a CVD-related event and a small proportion of patients are unable to achieve target plasma cholesterol levels, despite receiving the maximum recommended dosage of statin2,13. Furthermore, high-dose statin therapy is associated with side effects such as muscle pain and hepatic abnormalities14,15. Therefore, new therapeutics are needed that can either be taken alone or in combination with statins2,13. Despite emerging therapies such as ezetimibe16–18 and antibodies targeting proprotein convertase subtilisin/kexin type 9 (PCSK9)19–22 and certain pro-inflammatory cytokines23 being explored, further research should be carried out on alternative approaches that limit inflammation and other pro-atherogenic changes in atherosclerosis.
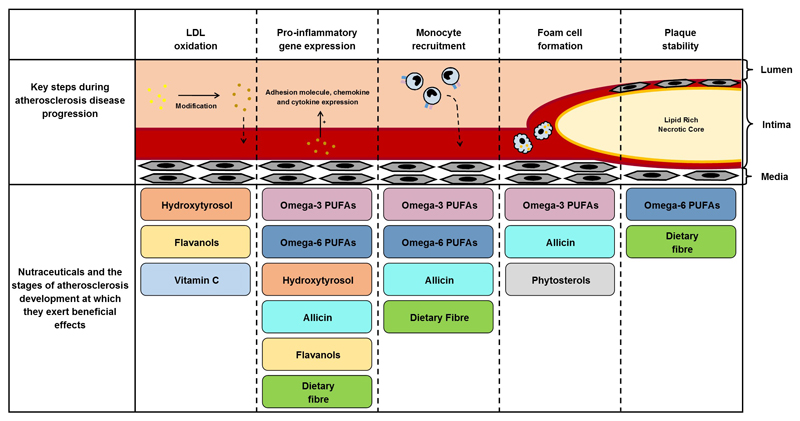
One potential therapeutic avenue being explored for the prevention of atherosclerosis is natural products, known as nutraceuticals that are thought to have anti-inflammatory properties. Nutraceuticals can be classified as either functional foods or dietary supplements with health benefits beyond their basic nutritional value. Diets that are rich in fruit, vegetables, fish, cereal grains or olive oil have all been associated with cardiovascular health benefits24–26. The aim of this review is to assess the key nutraceutical components in these diets and to discuss their possible uses for the prevention of atherosclerosis development with evidence from both pre-clinical and clinical studies found within the current literature. Figure 2 provides a summary of the stages of atherosclerosis development at which different nutraceuticals exert their potential beneficial effects.
Figure 2. The stages of atherosclerosis development at which different nutraceuticals exert their potential beneficial effects.
There are several major steps involved in the development of atherosclerosis including LDL oxidation, pro-inflammatory gene expression, monocyte migration, foam cell formation, and plaque stability. This figure highlights the stages at which the major nutraceuticals discussed in this review could aid in reducing atherosclerosis disease progression. LDL, Low density lipoprotein; PUFAs, Polyunsaturated fatty acids.
Omega-3 polyunsaturated fatty acids
Polyunsaturated fatty acids (PUFAs) are capable of regulating blood pressure and clotting, and are involved in the formation of eicosanoids, mediators that can modulate the inflammatory response25. PUFAs contain two or more carbon-carbon double bonds and can be classified as either omega-6 or omega-3 depending on the position of the carbon-carbon double bond closest to the methyl terminus of the molecule25. Dietary intake of PUFAs is vital as they cannot be synthesised in vivo; fish oils, flax seeds and nuts are a rich source of omega-3 PUFAs25, whereas vegetable oils and animal fat are the major source of omega-6 PUFAs25.
The cardiovascular health benefits of omega-3 PUFAs have been shown through several epidemiological and clinical studies over the past 60 years27,28. The American Heart Association (AHA) recommend eating two portions of oily fish every week, where one portion is defined as at least 100g29. The AHA also advise individuals who are unable to boost their omega-3 intake through diet alone to discuss with their doctor about the possibility of taking omega-3 supplements29. An epidemiological study published in 1980 found a reduced incidence of CVD-related events that could be attributed to lower serum cholesterol levels in the Inuit population of Greenland, despite their diet being rich in saturated fats (in the form of fish and whale meat) and low in fruit and vegetables30. The omega-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are both known to exert cardiovascular health benefits.
The omega-3:omega-6 PUFAs ratio is generally considered as a major determinant of CVD-related events. Consumption of omega-6 PUFAs in Europe, particularly linoleic acid (LA), has increased by approximately 50% in the past 20 years, correlating with the increased rates of inflammatory-based diseases, particularly CVD31. While the ideal dietary intake of omega-3:omega-6 PUFAs is 1:4, the actual ratio consumed is considered to be closer to 1:15 in developed countries, owing to the increased consumption of omega-6-rich vegetable oils32. Furthermore, diets high in omega-6 PUFAs and deficient in omega-3 PUFAs have been linked to increased production of pro-inflammatory eicosanoids33. Although diets high in omega-6 PUFAs have generally been linked to increased susceptibility of oxLDL formation ex vivo34, there is growing evidence that some are also capable of exerting anti-inflammatory effects and reducing atherosclerosis development35 (addressed below in detail under omega-6 polyunsaturated fatty acids).
Many in vitro and in vivo studies have shown that omega-3 PUFAs are capable of attenuating several key steps involved in atherosclerotic plaque development. Omega-3 PUFAs can reduce the expression of key pro-atherogenic markers in both murine and human macrophages stimulated with pro-inflammatory cytokines36,37. The attenuation of monocyte migration to the plaque has also been demonstrated in both in vitro and in vivo assays after omega-3 PUFA supplementation38. Furthermore, omega-3 PUFA treatment has been shown to both reduce the expression of genes implicated in the uptake of LDL and increase the expression of genes involved in cholesterol efflux39, which might explain in part the observation of reduced cholesterol uptake40 and accelerated cholesterol efflux41 in vitro.
LDLr deficient mice fed on a high fat diet to mimic human atherosclerosis were supplemented with fish oil for 16 weeks in a study by Brown and colleagues38. The fish oil-treated mice had a significant decrease in plasma cholesterol levels and atherosclerotic plaque size compared with the control group38, attributable to a 50% reduction in monocyte migration into the atherosclerotic lesion. However, the same study reported no differences in lesion size or monocyte migration after fish oil supplementation in ApoE-deficient mice38. In a separate study, investigators fed LDLr-deficient mice with a high fat diet for 8 weeks before switching to a normal diet with or without 5% EPA for an additional 4 weeks42. EPA supplementation increased plasma HDL levels and caused the plaque to regress by 20.9%. Furthermore, the expression of several pro-inflammatory factors including IFN-γ, IL-12, tumour necrosis factor (TNF)-α, and intercellular adhesion molecule (ICAM)-1, were all significantly reduced in the atherosclerotic plaques in the EPA-treated mice42.
The importance of the ratio of omega-3 to omega-6 has been demonstrated in an ApoE -deficient mouse model that also expressed a fat-1 gene from Caenorhabditis elegans43. Fat-1 transgenic mice are able to metabolise omega-6 into omega-3 PUFAs using an omega-3 fatty acid desaturase and, therefore, should have an approximate 1:1 ratio of omega-3 fatty acids to omega-6 fatty acids43. After being fed a high-fat diet for 14 weeks, the apoE−/−/fat-1 mice had smaller atherosclerotic lesions and reduced expression of IFN-γ and monocyte chemoattractant protein-1 (MCP-1; also known as C-C motif chemokine 2) compared with the apoE−/− littlermates43. However, no differences in the plasma levels of LDL, HDL, or cholesterol were observed between the two groups. Together, these preclinical data support the use of EPA and DHA dietary supplementation to repress pro-inflammatory eicosanoid production and reduce the incidence of CVD.
Relevant clinical data in humans
The cardiovascular benefit of omega-3 PUFAs has also been demonstrated in humans. A meta-analysis published in 2015 reported that increased EPA and DHA consumption, through either supplementation or consumption of enriched foods, was associated with decreased blood triacylglycerol levels in healthy patients or in patients with marginal hyperlipidaemia44. Omega-3 PUFAs have very few detrimental side effects, and have been shown to be beneficial for individuals suffering from hypertriglycaeridemia45. Furthermore, a cohort of 600 men with CVD receiving fish oil supplementation showed reduced markers of atherothrombotic risk46. A study involving 160 Japanese patients found that low serum levels of DHA correlated with reduced endothelial function, as measured by flow-mediated dilatation47. This observation confirms the results seen in an earlier study that reported an improvement in endothelial function and arterial stiffness, as measured by flow-mediated dilatation and pulse wave velocity respectively in 29 participants after 12 weeks of daily omega-3 PUFA intake48.
Over the past 30 years there have been three well known trials performed to assess the cardiovascular benefits of EPA and DHA supplementation: the DART trial49, GISSI-Prevenzione trial50, and the JELIS trial51. The DART trial, published in 1989, recruited 2,033 men who had recently suffered a MI and randomly allocated them to receive advice or no advice on each of three dietary factors: reduced fat intake to increase the ratio of polyunsaturated fat to saturated fat; increased omega-3 PUFAs intake either in the form of oily fish or fish capsules; and increased cereal fibre intake49. After a 2-year follow up, patients who were advised to increase omega-3 PUFAs in their diet showed a significant 29% reduction in mortality compared with those who did not49, which was largely attributable to a reduction in CVD-related events. No differences were found in the mortality of patients allocated to receive the other dietary advice49.
Investigators of the subsequent GISSI-Prevenzione trial,50 published in 1999, recruited 11,324 patients with recent MI and randomly assigned them to receive omega-3 supplements (1g daily), vitamin E, both, or none for 3.5 years. The primary endpoint of the study was a composite of death, nonfatal MI, and stroke. After 6 months, the study found no clinically important changes in the serum levels of total cholesterol, LDL-cholesterol, and HDL-cholesterol50. However, one year after initial treatment, patients who had received omega-3 PUFA supplementation, but not vitamin E, showed a 15% reduction in the primary endpoint of the study. Furthermore, sudden cardiac death was 45% lower in the treatment group compared to the control group50. Together, these two large trials support the use of omega-3 PUFAs in the context of MI.
The benefit of adding EPA to statin therapy has been evaluated in several trials. In a study published in 2007, a total of 18,645 patients with hypercholesterolaemia recruited for the JELIS trial51 were randomly assigned to statin therapy combined with EPA supplementation, or statin-only therapy. After an average follow up of 4.6 years, a 19% relative reduction in major CVD-related events was observed in patients receiving EPA and statin, compared with the statin-only group. However, EPA supplementation did not increase serum HDL levels or reduce serum LDL levels51. In another study published in 2016, 95 patients who had been receiving statin treatment for a minimum of 6 months were randomised to receive EPA supplementation (1,800 mg/day) or no additional treatment for 6 months52. Compared with the statin-only therapy group, the atherosclerotic plaques of patients who had received EPA had fibrous caps with increased collagen content as well as a reduction in lipid volume, indicating increased plaque stability52. Furthermore, patients receiving EPA showed reduced levels of pro-inflammatory cytokines, including MCP-152. These clinical trials provide support that increasing omega-3 PUFA levels, especially EPA and DHA, alone or in combination with statin therapy can substantially reduce an individual’s risk of a major CVD-related event.
Despite the promising results discussed thus far, the benefits of omega-3 PUFA supplementation on cardiovascular health remain inconclusive, given the conflicting results in the literature. In 2014, a meta-analysis that included five trials enrolling 396 participants found no significant reduction in CVD-related events in individuals with peripheral arterial disease53. A systematic review that included 48 randomised controlled trials (36,913 individuals) and 41 cohort studies also did not detect any significant reductions in CVD-related mortality in patients receiving omega-3 supplementation for 6 months54. A meta-analysis that specifically focused on patients with a history of CVD was also unable to identify any substantial protective effects of omega-3 PUFA supplementation in 14 randomised double blind trials that recruited 20,485 participants55. A further meta-analysis also failed to demonstrate any association between omega-3 supplementation and mortality risk after evaluation of 20 randomised clinical studies that included 68,680 individuals in total56. However, care must be taken when interpreting the results of clinical trials owing to the heterogeneity within the designs of the studies53. One difference between the trials is whether omega-3 PUFAs were given alone or in combination with statins, which together might have exerted synergistic effects. In addition, dose and intervention time period differs between the trials. Furthermore, one key difference between the trials is the populations used. The consumption of omega-3 PUFAs is approximately 15 times lower among Western populations compared with the Japanese population57,58, which might affect studies that use omega-3 PUFA dietary supplementation. All of these factors are likely to affect the outcomes of the trials, and result in the inconsistent results found within the clinical trials and meta-analysis.
Two trials are currently ongoing that use omega-3 PUFA supplementation: the REDUCE-IT59 and STREGTH60. The REDUCE-IT trial, which is expected to be completed in 2017, has an estimated enrolment of 8,000 participants and is designed to investigate the effect of Vascepa® (icosapent ethyl), a purified ethy ester of EPA for the treatment of hyperglyceridaemia59. The primary aim of the REDUCE-IT trial is to evaluate whether Vascepa® and statins are able to further reduce the incidence of CVD-related events compared with statin-only treatment. The secondary aim of the study is to evaluate its effects on serum lipid and lipoprotein levels59. The STREGTH trial, which is scheduled to be completed in 2019, is designed to assess the effect of combined statin and Epanova® (ω-3 carboxylic acids) therapy in an estimated 13,000 individuals60. The main aim of the STREGTH trial is to assess whether Epanova® can reduce the number of CVD-related events compared with those who received the statin-only treatment. These two new large clinical trials will hopefully be able to provide more insight into whether omega-3 PUFA supplementation can reduce the residual risk of CVD present in users of statin.
Omega-6 polyunsaturated fatty acids
Although a high intake of omega-6 PUFAs is traditionally thought to promote inflammation and contribute to the pathogenesis of many diseases, including CVD, not all omega-6 PUFAs are associated with detrimental effects. The AHA currently recommend that omega-6 PUFAs should generally make up 5% to 10% of the energy intake of an individual’s diet, provided other AHA dietary and lifestyle guidelines are followed, as it is thought that lowering omega-6 intake any further is more likely to increase the risk of suffering a CVD-related event rather than decrease it61. One key omega-6 PUFA that is considered to have anti-atherogenic effects is dihomo-γ-linolenic acid (DGLA) that can be metabolised after consumption into prostaglandin E1 (PGE1), a potent anti-atherogenic compound35. Pre-treating murine macrophages with DGLA resulted in a dose-dependent increase in prostaglandin levels, primarily PGE1 and prostaglandin D1, following lipopolysaccharide stimulation62. PGE1 has been shown to improve atherosclerotic plaque stability by increasing the thickness of fibrous cap in a dose-dependent manner in rabbits with a vulnerable plaque induced by balloon injury and a high cholesterol diet63. The right balance of omega-3 and omega-6 PUFAs is essential for optimal cardiovascular health, as they are capable of interacting and influencing the metabolism of one another35. DGLA can increase the metabolism of EPA into prostaglandin I3, a potent vasodilator and platelet anti-aggregator64, whereas EPA inhibits DGLA conversion to arachidonic acid, resulting in higher tissue levels of DGLA35. This is subsequently metabolised into a variety of products, in particular PGE1. ApoE deficient mice receiving a 0.5% DGLA diet for 6 months showed a significant increase in vasodilatation and a reduction in mRNA levels of ICAM-1 and vascular cell adhesion molecule (VCAM)-165. DGLA supplementation was also associated with a decrease in plaque size, exemplified through a reduction in lipid accumulation, monocyte and macrophage number, and migration of vascular smooth muscle cells65. Furthermore, diets that are enriched in γ-linolenic acid (GLA), a precursor of DGLA during omega-6 metabolism, have also been shown to reduce blood pressure in spontaneously hypertensive rats66.
Relevant clinical data in humans
Several observational studies have shown a reduction in omega-6 PUFAs in patients with atherosclerosis. Luostarinena and colleagues compared the make-up of fatty acids in the phospholipid fraction of human coronary arteries between aged-matched patients who died of ischaemic heart disease and patients who died of other non-cardiovascular causes67, and found a reduced proportion of both omega-3 and omega-6 PUFAs in those who had died from a CVD-related event. In a separate study, the lipid profile of 668 aortic plaques from 30 men who died of ischaemic heart disease were analysed and compared with their undisrupted plaques68. The concentration of all fatty acids was significantly increased at the edge of disrupted plaques compared with the center; however, the proportion of omega-6 PUFAs as a percentage of total fatty acid concentration was significantly lower, suggesting possible oxidation of PUFAs.
Low serum levels of GLA has been correlated with peripheral arterial disease in a cohort of 474 participants69. Treatment of 120 individuals suffering from lower limb atherosclerosis with a combination of GLA and EPA also significantly improved their blood pressure after 2 years compared to those receiving the placebo70. Additionally there was a small but non-significant reduction in the number of non-fatal CVD-related events70. Furthermore a smaller study has observed a decrease in serum triacylglycerol, total cholesterol and LDL levels as well as an increase in serum HDL levels following daily GLA consumption for 4 months in hyperlipidemic patients71. By contrast, an epidemiological study involving 2,206 Japanese men found that increased serum levels of omega-6 PUFAs was associated with increased arterial stiffness, in addition to higher serum C-reactive protein (CRP) levels72. An additional study involving 501 participants also linked increased serum levels of omega-6 PUFAs with increased arterial stiffness73, whereas a smaller randomised, double-blind trial reported that daily DGLA administration for 4 weeks did not exert any anti-thrombotic effects74. Given these mixed findings, whether DGLA or GLA can contribute to the prevention of atherosclerosis or reduce the risk of a CVD-event in individuals who already have atherosclerosis remains controversial, emphasising the need for further studies.
Although DGLA and GLA are products of linoleic acid metabolism, linoleic acid supplementation has not always convincingly been associated with cardioprotective effects. Linoleic acid did not improve arterial stiffness, blood pressure, serum lipid concentrations, or serum CRP levels after 6 months of supplementation in overweight individuals75. However recent re-evaluation of the Minnesota Coronary Experiment (MCE), performed in 1968 involving 9570 participants, found that replacing saturated fat with linoleic acid reduced serum cholesterol levels76. Despite lowering serum cholesterol levels, linoleic acid supplementation was unable to reduce the risk of a CVD-related event76. In contrast, an epidemiological study involving 1,813 individuals found an association between higher tissue levels of linoleic acid and a decreased risk of MI77. However, given that linoleic acid is metabolised into GLA and DGLA, this higher linoleic acid tissue level might actually represent increased GLA and DGLA formation. DGLA and GLA might thus be more suitable for use as nutraceuticals than linoleic acid.
Together, the data presented suggests that DGLA or its precursor GLA as a nutraceutical might be as effective as EPA and DHA supplementation for preventing atherosclerotic development, owing to their direct actions or the need to maintain an optimal ratio of omega-3 to omega-6 PUFA. However, the observation that omega-6 PUFAs might be associated with increased arterial stiffness is concerning, and requires further investigation.
Hydroxytyrosol
The Mediterranean diet has long been associated with reduced incidence of CVD-events78. Individuals living in countries within the Mediterranean basin consume a greater amount of olive oil compared with those elsewhere around the world. Several epidemiological studies have reported a correlation between increased levels of olive oil in the diet and a lower risk of developing atherosclerosis and other CVD26,79. Numerous polyphenol compounds in olive oil exert anti-inflammatory effects, including oleuropein, tyrosol and hydroxytyrosol. Oleuropein has been shown to reduce reactive oxygen species (ROS)-mediated expression of matrix metalloproteinase (MMP)-9 and cyclooxygenase 2 (COX-2) in human umbilical vein endothelial cells (HUVEC)80. Furthermore, oleuropein and hydroxytyrosol have been shown to inhibit lipopolysaccharide-induced expression of VCAM-1, ICAM-1, and E-selectin in a dose-dependent manner in HUVEC81. However, oleuropein undergoes almost complete degradation during olive ripening and, therefore, is unlikely to contribute to the cardiovascular health benefits associated with the Mediterranean diet82. By contrast, hydroxytyrosol levels increase throughout the ripening process82 and therefore it is often considered as one of the major anti-atherogenic polyphenol compounds in olive oil.
Numerous in vitro and in vivo studies have assessed the use of hydroxytyrosol as a nutraceutical for atherosclerosis. Co-incubation of hydroxytyrosol with pro-inflammatory cytokines in HUVEC in vitro resulted in a significant reduction in the expression of cell surface adhesion molecules such as VCAM-1 and ICAM-1 compared with incubation with cytokines alone83. Furthermore, hydroxytyrosol has been shown to reduce the production of several pro-inflammatory markers in cultures of primary human monocytes84. A murine study involving 32 Wistar rats that were fed olive oil-based diets for 6 weeks demonstrated that a phenol-enriched olive oil was able to significantly increase plasma HDL levels85. The same study also showed that the non-enriched virgin olive oil did not significantly alter HDL levels, indicating that cardioprotective effects of the olive oil was dependent on the phenol compounds85. However, neither the virgin olive oil nor the enriched olive oil was able to reduce plasma LDL levels. A subsequent study involving 60 Wistar rats did observe a decrease in total cholesterol and plasma LDL-cholesterol levels in those fed both virgin olive oil and cholesterol, compared with rats fed with only cholesterol after 4 weeks86. Hydroxytyrosol was also able to reduce atherosclerotic plaque size and improve antioxidant status in hyperlipaemic rabbits fed an atherogenic diet87.
Increasing dietary intake of hydroxytyrosol might be a strategy to increase serum HDL levels, as well as decreasing serum oxLDL levels. However, this approach might not be effective for those already on a low-cholesterol diet. ApoE-deficient mice that were given a standard chow diet and daily hydroxytyrosol supplementation for 10 weeks showed larger atherosclerotic lesions compared with the control group88, in addition to a decrease in ApoA1 levels, and an increase in total cholesterol levels, with no changes in plasma HDL levels. These results indicate that hydroxytyrosol might actually enhance atherosclerosis development in those on a low-cholesterol diet. Given that the majority of patients at risk of CVD are likely to already be on a low-cholesterol diet, further in vivo studies are required to understand the effects of hydroxytyrosol supplementation when taken in combination with a low-cholesterol diet.
Relevant clinical data in humans
Many clinical trials have been performed to investigate the potential health benefits of hydroxytyrosol supplementation. The randomized, crossover, controlled EUROLIVE study involving 200 healthy male individuals that were assigned to receive olive oil with low, medium, or high phenolic content found a linear relationship between the phenolic content of olive oil and an increase in serum HDL levels, which resulted in a decrease in the ratio of total cholesterol to HDL cholesterol89. This increase in HDL was also accompanied by a decrease in triacylglycerol levels, as well as a reduction in the markers of oxidative stress89. Consistent with this finding, two additional studies have shown that hydroxytyrosol is also capable of decreasing serum oxLDL concentration in a dose-dependent manner in both healthy individuals and patients with coronary heart disease (CHD)90,91. Hydroxytyrosol has also been shown to exert anti-inflammatory effects in 28 patients with stable CHD who received a daily dose of virgin olive oil (50ml) for 21 days92. Daily intake of virgin olive oil intake reduced IL-6 and CRP levels, key markers of inflammation and predictors of CVD92. Furthermore, a randomised, controlled, double-blind, crossover study involving 13 prehypertensive or hypertensive individuals found that olive oil enriched with its own polyphenols significantly improved endothelial function and decreased oxLDL levels compared with non-enriched olive oil alone93.
The PREDIMED study, involving 7447 participants considered to be at high CVD risk, found that receiving a Mediterranean diet supplemented with either extra-virgin olive oil or nuts for 5 years significantly reduced an individual’s risk of suffering a CVD-related event compared to those on the low-fat control diet94. However it should be noted that there was no difference in the total number of CVD-related events between the olive oil and nut diet receiving groups94. Earlier analysis of the PREDIMED study in 187 asymptomatic high CVD risk patients, identified a significant reduction in the intima-media thickness in those with an initial baseline of 0.9 mm or thicker after one year on a Mediterranean diet supplemented with either olive oil or nuts95. However no changes in the intima-media thickness were observed in patients whose baseline was less than 0.9 mm, indicating a possible role for the use of the Mediterranean diet in order to reduce subclinical atherosclerosis in those at a greater initial risk95. The PREDIMED study would have benefited from a Mediterranean diet without supplementation group to fully determine whether olive oil and nut supplementation exerted additional cardiovascular protectives compared to the base diet.
Another trial randomly assigned 90 participants into three treatment groups, patient’s regular diet, Mediterranean diet and virgin olive oil (328 mg/kg polyphenols) or Mediterranean diet and washed virgin olive oil (55 mg/kg polyphenols) for 3 months96. A significant reduction in serum levels of LDL, HDL and total cholesterol were observed after 3 months when compared to baseline levels in those receiving the Mediterranean plus virgin olive oil diet with no changes in total cholesterol:HDL or LDL:HDL ratios96. The serum cholesterol levels of those receiving the Mediterranean and washed olive oil were also not significantly altered when compared to baseline, however both Mediterranean dietary interventions significantly reduced serum CRP levels when compared to their respective baselines96. Furthermore, the Mediterranean diet with virgin olive oil significantly reduced the expression of several pro-atherogenic genes, including IFN-γ, compared to the control group96. Additionally a trial involving 52 participants who received polyphenol enriched olive oil for 4 months showed signs of significant improvement in endothelium function as well as a decrease in several inflammatory markers, including serum ICAM-1 levels and monocyte number97. However it should be noted that some individuals also received an olive oil enriched with epigallocatechin 3-gallate, however it did not provide any additional benefits when compared to the polyphenol enriched oil group97. These clinical studies together highlight the potential anti-atherogenic properties of hydroxytyrosol.
Allicin
Allicin (diallyl thiosulfinate) is a natural organic sulphur-containing compound found in garlic (Allium sativum). When fresh garlic is crushed, alliin is converted into allicin by the enzyme alliinase. The newly formed allicin is highly unstable and rapidly breaks down into several smaller polysulphides which are able to form hydrogen sulphide (H2S) in a thiol-dependent manner in cells98. The anti-atherogenic and anti-inflammatory health benefits of garlic are attributable to this formation of H2S.
The benefits of treatment with H2S donors (compounds capable of being broken down into H2S) have been observed in both in vitro and in vivo studies. The treatment of lipopolysaccharide-stimulated murine macrophages with sulphur-containing compounds originating from garlic has been shown to attenuate the expression of several pro-inflammatory cytokines, including IL-1β, IL-6, and TNFα99. The anti-inflammatory abilities of H2S donors have also been observed in vivo using murine models, which demonstrate inhibition of leukocyte adherence to the endothelium, indicative of a reduction in the inflammatory response100. In addition to diminishing the initial inflammatory response, H2S has also been shown to attenuate p38 mitogen-activated protein kinase activation and caspase-3 cleavage, which results in accelerated resolution of inflammation by stimulating the short-term survival of neutrophils101.
Potent antioxidant effects have been associated with H2S, with many studies showing that H2S donors are capable of reducing lipopolysaccharide-stimulated inducible nitric oxide synthase and cyclooxygenase (COX)-2 expression, which consequentially diminishes ROS production in vitro99,102. Furthermore, H2S donors have been shown to reduce foam cell formation by attenuating the expression of MSR1, sterol O-acyltransferase 1 (also known as ACAT1), and CD36 in human monocyte-derived macrophages, possibly through the ATP-sensitive K+ channel (KATP), and mitogen-activated protein kinase 1 and 3 pathways103. ACS14 (2-acetyloxybenzoic acid 4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl ester) is a novel H2S-releasing aspirin that has been used to study the effects of H2S donors on atherosclerotic plaque development in ApoE-deficient mice104. Mice supplemented with ACS14 developed smaller atherosclerotic lesions compared with mice receiving the equivalent dose of regular aspirin, possibly attributable to reduced monocyte migration into the plaque104. Administration of pure allicin in ApoE-deficient and LDLr-deficient mice has also been shown to reduce plaque size by approximately 69% and 57%, respectively, after 8 weeks compared with placebo105.
Relevant clinical data in humans
The benefits of garlic supplementation have also been observed in clinical studies. In a study involving 152 participants, high-dose dietary garlic supplementation (900 mg garlic powder/day) for 48 months significantly attenuated lesion volume by 6–18%106. A subsequent preliminary study showed that treatment with statin plus aged garlic extract was capable of slowing the rate of atherosclerotic development by reducing coronary calcification compared with statin-only therapy107. A meta-analysis of 45 trials found that garlic supplementation was also able to reduce serum levels of LDL, triacylglycerol and cholesterol after 1-3 months but not after 6 months108. The study also found that garlic was unable to significantly improve blood pressure. The effect of garlic supplementation on clinical outcomes was not analysed due to the lack of robust, long-term trials, stressing the need for large clinical trials to fully evaluate the potential of garlic as a nutraceutical. Notably, a randomised clinical study involving 192 participants found no differences in LDL or HDL levels between patients receiving garlic in three different forms (aged garlic extract, raw garlic or garlic powder) and the patients receiving placebo109. However, allicin might exert its cardioprotective effects via other mechanisms, such as reducing ROS production and attenuating pro-inflammatory gene expression, rather than directly altering the ratio of LDL to HDL in the bloodstream.
Phytosterols
Phytosterols are steroid compounds found in plant sources and are similar in structure to cholesterol. Diets rich in phytosterols have long been associated with reduced plasma-LDL levels110,111. Phytosterols are thought to exert their cardioprotective effects by competing with cholesterol in the lumen of the intestine during dietary and biliary cholesterol uptake111. Murine macrophages treated with phytosterols in vitro have shown changes in the expression of genes implicated in cellular cholesterol homeostasis, including an increase in ABCA1 and a decrease in LDLR112. Furthermore, phytosterols also increase cholesterol efflux in response to ApoA1 and HDL in human THP-1 macrophages, consistent with the observed changes in gene expression112.
Phytosterols have also been shown to mediate strong anti-inflammatory effects in vivo. This effect has been demonstrated in ApoE-deficient mice fed a high-fat diet supplemented with 2% phytosterols for 2 weeks, and then injected with ovalbumin to trigger an inflammatory response to a foreign antigen113. The spleen cells from phytosterol-treated mice showed reduced production of pro-inflammatory cytokines IL-6 and TNF-α, and increased production of the anti-inflammatory cytokine IL-10 compared with the spleen cells from mice on the control diet113. In addition, atherosclerotic lesion size was 60% smaller in mice on the phytosterol-enriched diet113. After 14 weeks on a diet supplemented with 2% phytosterols, ApoE-deficient mice showed alterations in the expression of 132 genes, including several hepatic genes associated with the regulation of sterol metabolism114. The changes in gene expression in this study may provide a greater insight into how phytosterols mechanistically exert their cardiovascular protective effects. However, further studies are required to link specific altered gene expression patterns to the anti-atherogenic properties of phytosterols. In a separate study, the atherosclerotic lesions of ApoE-deficient mice fed a high-fat diet supplemented with a 2% phytosterols mixture for 20 weeks were reduced by approximately 50% compared with the high-fat diet-only control group115. Furthermore, phytosterol supplementation was also associated with reduced hepatic lipase activity and plasma fibrinogen concentrations, in addition to a small increase in HDL-cholesterol levels115. Consistent with these findings, later studies also reported smaller atherosclerotic lesions and lower plasma LDL-levels in ApoE-deficient mice fed a 2% phytosterol-supplemented diet after 12 and 14 weeks116,117.
Relevant clinical data in humans
An epidemiological study involving 22,256 participants found a correlation between diets with high levels of phytosterols and low levels of serum LDL, supporting the role of phytosterols in LDL lowering111. Consistent with this finding, a study involving 233 participants demonstrated a significant reduction in serum LDL levels with 12 weeks of phytosterol supplementation, though no changes in flow-mediated dilatation or pulse-wave velocity was found118. Recent meta-analysis, involving 20 randomised control trials and 1308 participants, found an association between regular phytosterol intake and reduced serum LDL levels119. However, the study failed to find any significant correlation between phytosterol consumption and plasma CRP levels119, highlighting the need for further research to assess the effects of phytosterol dietary supplementation on inflammation. Nevertheless, because of the LDL lowering effects of phytosterols, the European Atherosclerosis Society (EAS) consensus panel has recommended the use of phytosterol supplementation in individuals who are either: at low/intermediate risk of CVD but fail to meet requirements for traditional pharmaceutical therapies; suffering from familial hypercholesterolemia; or unable to achieve target LDL levels while receiving statin therapy120,121.
The type of phytosterol-delivery system has also been shown to impact their LDL lowering properties. For example, one study found that treatment of hypercholesterolemia with phytosterol capsules did not result in a reduction in plasma LDL levels122. However other studies have reported that phytosterol capsules and phytosterol-rich foods do not differ in their LDL lowering properties123. These studies emphasise the need for further trials to evaluate whether the LDL lowering properties of phytosterols are altered by the chosen delivery system.
Despite the reported beneficial effects of phytosterols, other studies have also suggested that high levels of phytosterol in the diet might actually be detrimental and contribute towards atherosclerotic development124. In a study involving 109 postmenopausal women, an increased ratio of phytosterol to cholesterol was associated with a higher risk of developing CHD125. However, many studies claiming that phytosterols can increase the risk of CVD-events lack appropriate controls or fail to match serum LDL levels between cases and controls; therefore, their findings must be taken with caution124. For example, Assmann and colleagues reported that serum phytosterol levels were significantly higher in 159 participants who had suffered from a MI or sudden cardiac death compared with 318 healthy individuals126. However, the study failed to match LDL-cholesterol, total cholesterol, and triacylglycerol levels, in addition to blood pressure levels between the two groups, all of which are risk factors for CVD-events124. Given that the ratio of phytosterol to cholesterol between the two groups was not significantly different, the study fails to provide conclusive evidence that the CVD-events were directly linked to increased phytosterol levels124.
Flavanols
Flavanols, a subclass of flavonoids, are secondary plant metabolites that are commonly found in fruit and vegetables127. Given that a diet rich in fruit and vegetables is linked with cardiovascular health benefits, flavanol supplementation represents a promising avenue as a nutraceutical for the prevention of atherosclerosis24. Catechin is a major flavanol present in green tea and cocoa that has been found to reduce endothelial exocytosis128, a process by which activated endothelial cells are able to release pro-inflammatory cytokines and chemokines, which are usually stored in intracellular endothelial granules, into the extracellular space128. Catechins might therefore have a role in reducing vascular inflammation during the development of atherosclerosis.
ApoE*3-Leiden mice fed a high-fat diet supplemented with 0.1% epicatechin (cis configuration isomer of catechin) for 20 weeks showed attenuation of atherosclerotic lesion area with no effect on plasma lipids129. Furthermore, a microarray analysis also revealed that epicatechin supplementation resulted in 173 genes being differently expressed compared with no supplementation, including 77 that appeared to be inversely regulated129. A substantial number of these 173 genes were involved in cell migration129, highlighting a possible mechanism by which epicatechin is able to reduce lesion size.
Relevant clinical data in humans
An increase in nitric oxide production has also been observed in a small clinical study in which 27 healthy individuals consumed a flavanol-rich diet consisting of cocoa (epicatechin and catechin) for 5 days130. This increase in nitric oxide production was accompanied by an increase in vasodilatation, providing an insight into another mechanism by which flavanols exert their cardioprotective effects130. Other studies have since confirmed the vasodilatory properties of flavanols, in addition to observing a reduction in circulating oxLDL levels after 5 weeks of flavanol supplementation in the form of green tea extract131,132. Daily catechin consumption for 24 weeks has also been shown to significantly reduce circulating LDL levels in obese or near-obese children compared with those who did not receive supplementation133. In addition, the consumption of cocoa flavanol-rich supplements for 30 days improved flow-mediated dilatation in 57 patients with end-stage renal disease134, highlighting its use in a population with endothelial dysfunction at high risk of developing CVD. Furthermore in a trial with 20 patients with congestive heart failure, who were randomly assigned flavanol-rich chocolate or control chocolate for 4 weeks, found that flavanols significantly improved flow-mediated dilation135.
Many other studies have also demonstrated the beneficial effects of cocoa flavanols in healthy individuals. Daily cocoa flavanol supplementation for 30 days resulted in improved vascular function in 100 healthy individuals without a prior history of CVD136. Additionally, the consumption of cocoa flavanols for 14 days improved flow-mediated vasodilation and reduced arterial stiffness in both young and elderly participants137. Furthermore, catechin has been shown to exert anti-inflammatory effects, as consumption of a green tea extract attenuated the levels of several pro-inflammatory mediators, including Fas ligand, IL-6 receptor, IL-8, soluble TNF-receptor 2, and neutrophil-activating peptide138. The ratio of total cholesterol to HDL cholesterol is also significantly reduced in 17 healthy men after daily catechin supplementation for 3 weeks underlining its possible use in the prevention of atherosclerosis139. However the same study found no reductions in other cardiovascular disease risk biomarkers such as blood pressure139. Although another study investigating the intake of daily cocoa flavanol for 4 weeks failed to show a decrease in blood pressure and flow-mediated dilatation in 30 overweight adults, a significant improvement in arterial stiffness was found in the female participants140. The lack of reduction in blood pressure in these studies contradicts the decreases found in the previously mentioned trials130–132. This discrepancy might be attributable to the small number of individuals used in the trials.
Together, these data suggest that flavanols might exert their cardiovascular health benefits by lowering circulating LDL levels and possibly blood pressure, both of which are key risk factors of atherosclerosis development.
Vitamin C and E
Given that the human body is unable to store vitamin C (also known as ascorbic acid), it is vital that foods rich in vitamin C, such as oranges, orange juice, broccoli and blackcurrants, form part of the daily diet. Increased intake of vitamin C has long been associated with a decrease in the prevalence of coronary artery disease141. Numerous in vivo studies have shown that vitamin C supplementation can improve endothelial function142,143. ApoE-deficient mice supplemented with 1% vitamin C for 26 to 28 weeks were found to have restored endothelial nitric oxide synthase activity and increased tetrahydrobiopterin levels in the aorta compared with the control mice142. A later study in ApoE-deficient mice fed a high-fat diet supported these results by demonstrating that chronic treatment with vitamin C inhibited endothelial dysfunction of the carotid artery induced by hypercholesterolaemia143. Despite such promise, the use of vitamin C as a nutraceutical for the prevention of atherosclerosis remains controversial because many studies have failed to show any benefit on plaque lesions or lipid profiles. Dietary supplementation with a cocktail of anti-oxidants (vitamin E, vitamin C, and β-carotene) in ApoE deficient mice did not reduce lesion size or alter plasma lipid profile144. However, this study involved older mice (20 weeks old), whereas the previously mentioned positive mouse studies were performed in much younger mice (4–5 weeks142,143), suggesting that age might influence the cardiovascular health benefits of vitamin C supplementation in ApoE-deficient mice. Consistent with this observation, vitamin C and E supplementation in ApoE-deficient mice aged 50–60 weeks failed to significantly reduce angiotensin II induced plaque rupture145. By contrast, vitamin E supplementation in 26-week-old ApoE-deficient mice prevented angiotensin II mediated plaque rupture146.
Relevant clinical data in humans
Lower serum vitamin C levels have been linked with a greater risk of a CVD-event in humans147. Vitamin C can exert its cardiovascular health benefits by mitigating inflammatory and oxidative stresses mediated by a high-fat and high-carbohydrate diet, by preventing endotoxin increase and Toll-like receptor expression148. Others have reported that vitamin C is able to improve vasodilatation in patients with coronary artery disease149 and in smokers150, thereby resulting in reduced blood pressure, and consequently reduced risk of CVD-related events. In 2014, a meta-analysis based on 44 clinical trials found a positive association between vitamin C supplementation and improved endothelial function in patients with atherosclerosis151. In addition, the ASAP trial, involving 520 participants, showed a significant attenuation in the progression of atherosclerosis in men following a treatment with a combined supplementation of vitamin C and E twice a day for 3 years152. However, individual supplementation with vitamin C or E failed to reduce intima-media thickness, and the combined supplementation did not reduce atherosclerosis progression in women152. Pooled analysis of 9 studies by Knekt et al.153 found an association between high vitamin C supplementation and a reduced risk of CVD-related event. However, the same analysis also found that high vitamin E intake was not associated with any cardiovascular protective effects153. By contrast, the CHAOS trial involving 2,002 patients with established atherosclerosis found that daily vitamin E supplementation reduced the risk of suffering a non-fatal MI compared to those receiving the placebo after 1 year154. However the study also found that vitamin E supplementation was unable to reduce CVD-related deaths154. Furthermore, the treatment of 30 hypertensive men with a combined vitamin C and E supplement every day for 8 weeks significantly improved arterial stiffness and flow-mediated dilation, as well as reducing their oxidative stress levels155.
Despite numerous positive findings, the inconsistencies in the results assessing vitamin C and vitamin E supplementation are also evident in many other clinical trials. A randomised study that used an initial 2g dose followed by a daily intake of 1g of vitamin C in 20 young adult smokers showed improved vasodilation after the first 2 hours, but there were no sustained beneficial effects after 8 weeks156. In addition, a large-scale study involving 20,536 adults in the UK with either coronary artery disease, peripheral occlusive arterial disease, or diabetes that were randomly assigned a daily dietary supplement containing either vitamin E, vitamin C, β-carotene, or placebo reported no observable benefits in terms of all-cause mortality or CVD-events at the 5-year follow up157. Furthermore, several studies have failed to demonstrate any cardiovascular protective effects following vitamin E consumption. The previously mentioned GISSI-Prevenzione trial found that daily consumption of vitamin E (300 mg) was not associated with a reduced risk of CVD-related events50. The HOPE study, which involved 9,541 participants considered to be at high risk of a CVD-related event, was also unable to find any significant reductions in cardiovascular deaths following daily vitamin E consumption for 4.5 years158. On the other hand, the VEAPS trial observed a decrease in plasma oxLDL levels and a reduction in the vulnerability of LDL to oxidation in 353 individuals following daily vitamin E supplementation for 3 years159. However, this trial also demonstrated that vitamin E supplementation was unable to reduce the intima-media thickness compared to the placebo159, indicating that it was unable to prevent atherosclerosis development. In conclusion, although both vitamin C and E were once considered ideal nutraceuticals for the prevention of atherosclerosis owing to their antioxidative and vasodilatory properties, they have not been proven to be consistently effective in long-term prevention of CVD. This position is consistent with the AHA whose advisory panel in 2004 recommended against using vitamin supplements to reduce the risk of CVD-related events160.
Dietary fibre
Dietary fibre can be fermented by the gut microbiota in the intestine to produce a variety of short chain fatty acids that are capable of exerting anti-atherogenic properties. Butyrate is a key short chain fatty acid produced during fibre fermentation that has been shown to prevent inflammation161,162. Butyrate treatment of murine macrophages stimulated with lipopolysacchardie have reduced pro-inflammatory cytokine production, including IL-1β, IL-6, and TNF-α, and attenuated nitric oxide production162. Furthermore, HUVEC treated with butyrate for 24 hours resulted in increased ICAM-1 expression, but no changes in VCAM-1 expression163,164. However, preincubation of HUVEC with butyrate attenuated TNF-α induced expression of VCAM-1, which correlated with a decrease in monocyte adhesion to endothelial cells164.
In vivo studies have also demonstrated the benefits of butyrate in atherosclerosis. ApoE-deficient mice fed a chow diet supplemented with 1% butyrate for 10 weeks developed smaller and more stable lesions compared to the control mice165. Lesions were reduced by approximately 50% owing to attenuated monocyte and macrophage migration towards the site of the plaque, together with lower levels of VCAM-1 and MCP-1 expression in the lesion165. Furthermore, the lesions in the butyrate-supplemented mice were composed of more ECM compared with the control mice, which is an indicator of increased plaque stability.
Relevant clinical data in humans
The relationship between increased fibre intake and reduced cardiovascular disease has been well established. A 6-year follow-up study involving 39,876 female participants found higher fibre intake was associated with a lower risk of MI and CVD after adjusting for age and other treatments received166. However, this relationship was no longer found to be significant after controlling for other confounding variables. Another study involving 46,032 men found that increased dietary intake of fibre was significantly linked with reduced risk of peripheral arterial disease over a 12 year follow up, even after adjusting for all other factors167. Increased dietary fibre has also been correlated with a lower risk of haemorrhagic stroke168. Furthermore, a meta-analysis of 10 cohort studies involving 91,058 men and 245,186 women reported an inverse relationship between increased dietary fibre intake and the risk of suffering a CVD- event169. For every 10g increase of dietary fibre per day, there was a 14% and 27% decrease in the risk of suffering a CVD-event and coronary death, respectively, over a 6–10 year follow-up period169. However, addition of fibre to statin and/or ezetimibe treatment did not provide extra cardiovascular health benefits to patients with hypercholesterolaemia, but improved blood glucose levels and reduced BMI170. Notably, given that this study did not include a fibre-only treatment group, it is not possible to delineate the effects of fibre that is independent of the lipid-lowering therapy.
Other less-studied nutraceuticals
Carnosine
Carnosine, a known anti-oxidant171, is a dipeptide formed from histidine and beta-alanine and is commonly found in meat given its abundance in animal proteins. Carnosine has been shown to reduce the glycation of LDL in human monocyte-derived macrophages, resulting in reduced intracellular cholesterol accumulation and attenuated foam cell formation172. This process is important in patients with diabetes as they are at increased risk of developing atherosclerosis. ApoE-deficient mice with diabetes receiving a carnosine dietary supplementation showed an improvement in key indicators of atherosclerotic plaque stability after 20 weeks173. Although carnosine did not reduce plaque size, it stabilised the lesion by increasing the collagen content by 50% and reduce the area of the plaque filled by lipids by 60%173. However, the number of macrophages within the plaque was also increased by approximately 70%,173 an indicator of plaque instability. Furthermore, carnosine supplementation in Sprague-Dawley rats for 6 weeks significantly improved serum HDL levels as well as reducing serum LDL levels, however the levels of total cholesterol and triacylgycerols were unaffected174. The same study also found that carnosine supplementation increased serum levels of superoxide dismutase while simultaneously decreasing plasma malondialdehyde (a marker of lipid peroxidation) levels174. This study highlights carnosine’s strong anti-oxidant properties and may explain how it exerts some of its cardiovascular protective effects. A small double-blind randomised trial also found that carnosine supplementation every day for 12 weeks significantly improved patient’s insulin resistance, however there was no improvement in blood pressure, serum cholesterol or CRP levels175. Carnosine might therefore represent a promising nutraceutical for patients with diabetes at risk of atherosclerosis, but further studies are required to elucidate its effect on plaque stability. Given the lack of in vivo and clinical data directly linking carnosine supplementation with anti-atherogenic effects, its use as a nutraceutical for patients with atherosclerosis should remain limited, until sufficient clinical data has been gathered.
Coenzyme CoQ10
Coenzyme Q10 (CoQ10), an antioxidant that is present in many food sources, has an important role in the electron transport chain within the mitochondria. Given that CoQ10 and cholesterol synthesis share the same intermediate steps in their respective biosynthetic pathways, patients receiving statin treatment also experience a reduction in CoQ10176. In an in vivo study involving ApoE-deficient mice receiving CoQ10 dietary supplementation for 4 weeks, CoQ10 treatment attenuated LDL oxidation and reduced foam cell formation177. These effects were achieved by enhancing the reverse cholesterol transport process via the microRNA miR-378, resulting in increased cholesterol efflux from the cell and decreasing the formation of foam cells177. Furthermore, the sizes of the plaques from the mice receiving CoQ10 were significantly smaller compared with the control group. By contrast, in another in vivo study involving ApoE-deficient mice, CoQ10 supplementation for 15 weeks was unable to reduce lesion size in cigarette smoke-enhanced atherosclerotic development178. The ability of CoQ10 to increase cholesterol efflux has also been observed in human monocyte-derived macrophages ex vivo179. In a small study with 20 healthy participants, who were either given placebo or CoQ10 supplements twice a day for 1 week, CoQ10 consumption significantly increased cholesterol efflux from macrophages, which correlated with an increase in the expression of the ABCG1 gene implicated in the promotion of cholesterol efflux179.
In patients with multiple sclerosis, CoQ10 supplementation was linked with a reduction in the plasma levels of the pro-inflammatory markers such as TNF-α, IL-6, and MMP-9, but did not alter anti-inflammatory markers such as IL-4 and transforming growth factor-β180. A meta-analysis of five trials involving a total of 194 participants concluded that CoQ10 supplementation significantly improved endothelial function181. In addition, daily dose of CoQ10 for 8 weeks in participants with left ventricular systolic dysfunction improved flow-mediated dilatation182. However, CoQ10 did not lower blood pressure or serum CRP levels182. CoQ10 supplementation for 12 weeks also failed to improve arterial stiffness or serum levels of oxLDL and CRP in obese percipients183. A random, double-blind FAITH trial involved 65 fire-fighters considered to have a high CVD risk (occupational stress) taking a daily combined CoQ10 and garlic supplement for a year184,185. The study found that the combined supplement was able to significantly reduce serum CRP levels as well as improve both pulse wave velocity and endothelium function compared to the placebo184,185. However, as the study did not include a garlic or CoQ10 only group, it is not possible to conclude whether the cardiovascular protective effects were due to one of the nutraceuticals or the combined supplement. Overall, given that statin therapy reduces its de novo synthesis, CoQ10 might be a promising nutraceutical to take in combination with statins to further reduce atherosclerotic development. However, the lack of consistent studies demonstrating the benefit of CoQ10 supplementation for prevention of atherosclerosis has limited its use as a nutraceutical at present.
Curcumin
Curcumin is the active component of turmeric and is the dietary pigment which gives curry its orange colour. Curcumin has been shown to reduce phorbol-12-myristate-13-acetate (PMA) and lipopolysaccharide-induced expression of key proatherogenic cytokines such as MCP-1, IL-1β, and TNF-α in primary human monocytes186. Further in vitro studies have also demonstrated that curcumin is also capable of mediating the polarisation of the anti-inflammatory M2 phenotype in murine macrophages187. An in vivo study involving rabbits fed a diet containing lard and cholesterol found that LDL was less susceptible to oxidation in those receiving turmeric extract for 7 weeks188. In addition, 30-day turmeric supplementation in high-fat fed rabbits resulted in a smaller fatty streak compared with the untreated control189. Furthermore, a reduction in atherosclerotic lesion size has also been observed in ApoE and LDLr double knockout mice after a daily dose of 0.3 mg of curcumin for 4 months190. Lesion area was reduced by approximately 50% compared with the control group190.
The benefit of curcumin in patients at risk of atherosclerosis has also been described. A randomised double-blind trial involving 240 individuals with type 2 diabetes reported a decrease in CVD risk with 6 months of curcumin dietary supplementation, exemplified through a lower pulse wave velocity and improved metabolic profile191. Furthermore, the use of curcumin for 8 weeks improved flow-mediated dilatation in 32 postmenopausal women192. Interestingly the same study also found that the improvement in flow-mediated dilatation was similar to those who did not receive supplement, but who exercised for 8 weeks instead192. A major limitation to using curcumin as a nutraceutical is its poor bioavailability, owing to inadequate absorption in the gut and as it is rapidly broken down and quickly excreted from the body193. Several strategies are being pursued in an attempt to increase the bioavailability of curcumin, including the use of liposomal curcumin, nanoparticles, and a curcumin phospholipid complex193.
Lycopene
Lycopene is the carotenoid that gives tomatoes their bright red colour. Several epidemiological studies have found an association between diets rich in lycopene and a reduced incidence of CVD194,195, leading to several studies to further investigate its potential cardioprotective effects. Lycopene might exert its anti-atherogenic effects by inhibiting de novo cholesterol synthesis, as demonstrated in vitro using murine macrophages196. By contrast, another in vitro study reported that LDL isolated from human donors which was then enriched with lycopene before being co-incubated with human endothelial cells actually increased its susceptibility to oxidation197.
In a randomised clinical study involving 144 participants with subclinical atherosclerosis, a combined dietary supplementation of 20mg lutein (another carotenoid with potential cardioprotective effects) and 20mg lycopene for 12 months significantly reduced the thickness of the intima and media in the carotid artery198. Given that the combination of lutein and lycopene supplementation was more effective than lutein alone, synergistic effects of lutein and lycopene might exist. Reduced serum levels of lycopene have also been linked with increased arterial stiffness199. Flow-mediated dilatation was also improved in patients receiving combination lycopene and statin therapy compared with statin alone200. However, no changes in dilatation were observed in healthy participants given the lycopene supplementation, possibly indicating an additive or synergistic effect of lycopene when taken in combination with statins, and highlighting a potential role as a secondary prevention nutraceutical200. In the same study, arterial stiffness, CRP serum levels, and blood pressure levels were also unchanged by lycopene in either the healthy participants or patients with CVD200. Another clinical trial involving 225 healthy participants also found that lycopene supplementation did not reduce blood pressure or improve arterial stiffness201. The possible dual effect of lycopene and statin therapy requires further investigation in studies with a larger cohort.
Resveratrol
Although increased alcohol consumption is associated with hypertension and elevated plasma cholesterol levels, the phenomena known as the ‘French paradox’ has been used to explain why the incidence of CVD is lower in France, despite a similar westernised diet high in fat and carbohydrates202. Resveratrol is a natural phenol commonly found in the skin of grapes and is considered to be one of the key active compounds responsible for these cardiovascular protective effects. Resveratrol has also been shown to reduce foam cell formation by inhibiting oxLDL uptake as well as increasing cholesterol efflux in human THP-1 macrophages203. This increase in efflux corresponded to an elevation in the expression of key proteins involved in the regulation of cholesterol efflux203. ApoE*3-Leiden.CETP mice fed a high-cholesterol diet with a 0.01% dietary supplementation of resveratrol were also found to have smaller atherosclerotic lesions by approximately 50% compared with control mice, in addition to improved lesion stability due to increased ratio of collagen to macrophages204. However, the cardioprotective benefits were similar between the reservatrol-only and statin-only groups, and the combination of treatments was unable to provide any benefit204.
After adjusting for other risk factors, one epidemiological study concluded that the higher average alcohol consumption in France (particularly wine) was attributable to a lower incidence of CVD202. The GISSI-Prevenzione trial also found an association between daily consumption of wine and a reduced risk of a CVD-event and all-cause mortality205. Although resveratrol might be useful in the prevention of atherosclerosis, it might not enhance the anti-atherogenic effects of statins. Large clinical trials involving statin-only treatment versus statin-plus-resveratrol treatment are required to determine its potential as a nutraceutical.
Berberine
Berberine is a cholesterol-lowering plant alkaloid known for its anti-inflammatory and anti-diabetic effects206. In vitro studies have reported that berberine can attenuate the expression of lipopolysaccharide-induced pro-inflammatory genes such as MCP-1, iNOS, IL-1β, and IL-6 in mouse macrophages207. Furthermore, berberine is capable of reducing macrophage migration208, indicating a potential role for retarding the progression of atherosclerotic development. oxLDL accumulation within human macrophages is also reduced, owing to an upregulation of expression of ABCA1, a key gene implicated in the promotion of cholesterol efflux, after berberine treatment209. Although the expression of SRs were unaffected, the capability of berberine to increase cholesterol efflux from human macrophages might make it a possible nutraceutical for reducing foam cell formation. ApoE-deficient mice fed a western diet and berberine for 8 weeks developed less atherosclerotic lesions compared with those on the control diet, in addition to a reduction in the levels of ICAM-1 and VCAM-1, and decreased oxidative stress210. In addition, high-fat fed obese mice treated with berberine for 36 days were found to have lower levels of serum total cholesterol211, which correlates with an earlier study in human hepatic cells that described a reduction in PCSK9 expression after berberine treatment212. As PCSK9 is an inhibitor of LDLr expression, berberine treatment was also found to increase the mRNA levels of LDLr212. These data highlight a possible mechanism by which berberine can exert its cardioprotective effects.
Berberine has also been shown to reduce serum cholesterol levels in several clinical studies. In a study involving 91 Chinese patients with hypercholesterolaemia, berberine supplementation twice a day for 3 months reduced serum levels of total cholesterol and LDL cholesterol, but did not alter serum HDL levels213. In a separate study, berberine treatment twice a day for 3 months lowered serum total cholesterol and LDL levels and increased serum HDL levels in 144 patients who were considered to have a low cardiovascular risk214. The effect of berberine treatment in combination with statins has also been assessed215. Participants with hypercholesterolaemia received either berberine, statins, or a combination of the two for 2 months. Both statins and berberine were individually able to lower serum total cholesterol and LDL-cholesterol levels. Furthermore, the combination of the two therapies provided an additive effect, reducing total cholesterol and LDL further compared with the individual therapies215. A daily combination of berberine, red yeast rice, and policosanol for 6 weeks in 50 individuals was also effective in reducing serum levels of both total cholesterol and LDL, as well as improving flow-mediated dilatation216. Together, these studies show that berberine has the potential of being used as cholesterol-lowering nutraceutical either to prevent the development of atherosclerosis or to be taken in combination with statins to enhance LDL-lowering capability.
Limitations and future directions
The potential cardioprotective effects of all the nutraceuticals mentioned in this review from either preclinical studies or human studies are summarised in Table 1 and 2, respectively. One of the major challenges involved in nutraceutical research is identifying whether the cardioprotective effects of an individual’s diet is attributable to a specific compound or as a result of a combination of elements. Therefore, when a potential nutraceutical is identified, its effectiveness needs to be assessed using robust randomised, controlled trials before it can be recommended as a dietary supplement. The majority of current clinical trials only compare the nutraceutical to a placebo or a nutraceutical in combination with other pharmaceutical therapies to a placebo. We therefore suggest that future clinical trials focusing on patients with subclinical atherosclerosis should investigate the effect of a nutraceutical alone or in combination with other pharmaceuticals and compare the outcomes to both a placebo group as well as those only receiving pharmaceutical intervention. This would allow the effectiveness of nutraceuticals to be directly compared to pharmaceutical only strategies in addition to identifying any additional/synergistic benefits that may occur from taking a combination.
Table 1. Summary of potential cardiovascular benefits of nutraceuticals in preclinical studies.
| Nutraceutical | Cardiovascular health benefits | References |
|---|---|---|
| Allicin | • Reduced the expression of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in murine macrophages stimulated with lipopolysaccharide | 99 |
| • Decreased the inflammatory response by reducing leukocyte adherence | 100 | |
| • Attenuated the expression of MSR1, ACAT1 and CD36 in human monocyte-derived macrophages, resulting in reduced foam cell formation. | 103 | |
| • Together with supplementation of H2S donors resulted in slowing of atherosclerosis development via reduction of lesion size in ApoE-deficient mice | 104 | |
| Berberine | • Attenuated lipopolysaccharide-induced pro-inflammatory gene expression, including MCP-1, iNOS, IL-1β and IL-6, in mouse macrophages | 207 |
| • Reduced macrophage migration | 208 | |
| • Induced the expression of cholesterol efflux gene ABCA1, resulting in reduced intracellular accumulation of oxidised LDL in human macrophages | 209 | |
| • Reduced serum total cholesterol levels and the number of atherosclerotic lesions in ApoE-deficient mice | 210,211 | |
| Butyrate | • Attenuated nitric oxide and pro-inflammatory cytokine production in lipopolysaccaride-stimulated murine macrophages | 162 |
| • Reduced plaque size by attenuating monocyte and macrophage migration in ApoE-deficient mice | 165 | |
| Carnosine | • Protected against foam cell formation in vitro | 172 |
| • Improved key factors associated with plaque stability in murine diabetes-associated atherosclerosis models | 173 | |
| • Increased serum HDL and reduced those of LDL in rats as well as increasing serum superoxide dismutase levels | 174 | |
| Coenzyme Q10 | • Promoted macrophage reverse cholesterol transport and slowed the development of atherosclerosis, possibly via miR-378 | 177 |
| • Increased cholesterol efflux in human monocyte-derived macrophages, which correlated with increased expression of the cholesterol efflux gene ABCG1 | 179 | |
| Curcumin | • Decreased the production of pro-inflammatory cytokines in primary human monocytes | 186 |
| • Stimulated an anti-inflammatory M2 macrophage phenotype in vitro | 187 | |
| • Reduced oxidative stress and LDL oxidation, in addition to reducing aortic fatty streak development | 188,189 | |
| • Dietary supplementation in ApoE and LDL receptor double knockout mice resulted in smaller atherosclerotic lesions after 4 months | 190 | |
| Flavanols | • Reduced the size of atherosclerotic plaques in ApoE*3-Leiden mice after 20 weeks | 129 |
| • Attenuated endothelial exocytosis (the process of releasing pro-inflammatory cytokines and chemokines into the extracellular space) in HUVEC | 128 | |
| Hydroxytyrosol | • Reduced the expression of the pro-inflammatory adhesion proteins VCAM-1 and ICAM-1 in HUVEC | 83 |
| • Wistar rats fed a diet containing hydroxytyrosol had higher plasma HDL levels and lower plasma LDL levels compared to control rats | 85,86 | |
| • Attenuated atherosclerosis disease development in hyperlipaemic rabbits, shown by smaller atherosclerotic lesions | 87 | |
| Lycopene | • Inhibited LDL oxidation and cholesterol synthesis in vitro | 196 |
| ω-3 PUFAs | • Attenuated the expression of several key atherosclerotic markers in both murine and human macrophages | 36,37 |
| • Increased the expression of cholesterol efflux genes and decreased the expression of LDL-uptake genes | 39 | |
| • Reduced atherosclerotic lesion size and increased plasma HDL levels in LDLr deficient mice | 38,42 | |
| ω-6 PUFAs | • PGE1, a metabolite of DGLA, improved plaque stability in rabbits by increasing the thickness of the fibrous cap | 63 |
| • GLA-enriched diets after 7 weeks reduced blood pressure in hypertensive rats | 66 | |
| • DGLA dietary supplementation decreased the size of atherosclerotic plaque in murine models after 6 months | 65 | |
| Phytosterols | • Increased cholesterol efflux in human THP-1 macrophages | 112 |
| • Decreased the size of the lesions as well as plasma LDL levels in mouse model systems | 113,115–117 | |
| Resveratrol | • Reduced foam cell formation by increasing cholesterol efflux in human THP-1 macrophages | 203 |
| • Reduced atherosclerotic lesions by approximately 50% in ApoE*3-Lieiden.CEPT mice | 204 | |
| Vitamin C | • Improved eNOS activity and enhanced endothelial function | 142,143 |
ACAT-1, Acyl-CoA acyltransferase; ApoE, Apolipoprotein E; CHD, Coronary heart disease; CVD, Cardiovascular disease; DGLA, Dihomo-γ-linolenic acid; DHA, Docosahexaenoic acid; eNOS, Endothelial nitrogen oxide synthase; EPA, Eicosapentaenoic acid; GLA, γ-linolenic acid; H2S, Hydrogen sulphide; HDL, High density lipoprotein; HUVEC, human umbilical vein endothelial cells; ICAM-1, Intercellular adhesion molecule-1; IL, Interleukin; LDL, Low density lipoprotein; LDLr, LDL receptor; LPS, Lipopolysaccharide; MSR1, Macrophage scavenger receptor 1; MCP-1, Monocyte chemotactic protein-1; miR, Micro RNA; MMP, Matrix metalloproteinases; NO, Nitric oxide; oxLDL, Oxidised LDL; PGE1, Prostaglandin E1; PUFAs, Polyunsaturated fatty acids; TNFα, Tumour necrosis factor α; VCAM-1, Vascular cellular adhesion molecule-1.
Table 2. Summary of potential cardiovascular benefits of nutraceuticals in human studies.
| Nutraceutical | Study type | Size | Observations | References |
|---|---|---|---|---|
| Allicin | Clinical | 152 | Reduced lesion volume | 106 |
| Preliminary | 19 | Reduced coronary calcification, resulting in a diminished rate of atherosclerosis development | 107 | |
| Clinical | 192 | No change in LDL and HDL levels | 109 | |
| Meta-analysis | 2,987 | Short term reduction in levels of serum LDL, total cholesterol and triacylglycerols but no long term benefits | 108 | |
| Berberine | Clinical | 91 | Reduced serum total cholesterol and LDL-cholesterol levels. No change in serum HDL-cholesterol levels | 213 |
| Clinical | 144 | Reduced serum total cholesterol, LDL-cholesterol, and HDL-cholesterol levels | 214 | |
| Clinical | 63 | Reduced serum total cholesterol levels and LDL-cholesterol levels. An additive effect was observed when berberine was taken in combination with statins | 215 | |
| Butyrate | Epidemiological | 39,876 | Trend for reduced risk of CVD-events, which were no longer significant after controlling for other confounding variables | 166 |
| Epidemiological | 46,032 | Reduced risk of peripheral arterial disease | 167 | |
| Epidemiological | 78,779 | Reduced risk of haemorrhagic stroke | 168 | |
| Meta-analysis | 336,244 | Reduced risk of a CVD-event | 169 | |
| Clinical | 116 | No cardiovascular benefits | 170 | |
| Carnosine | Clinical | 30 | Improved insulin resistance with no change in blood pressure, serum cholesterol or CRP levels | 175 |
| Coenzyme Q10 | Clinical | 45 | Reduced plasma levels of pro-inflammatory markers, and no changes in plasma levels of anti-inflammatory markers | 180 |
| Meta-analysis | 194 | Improved endothelial function | 181 | |
| Clinical | 56 | Enhanced endothelial function, but no changes in blood pressure and serum CRP levels | 182 | |
| Clinical | 51 | No changes in arterial stiffness, serum oxLDL levels, and serum CRP levels | 183 | |
| Clinical | 65 | CoQ10 and garlic supplementation reduced serum CRP levels, improved arterial stiffness and endothelial function | 184,185 | |
| Curcumin | Clinical | 240 | Reduced arterial stiffness | 191 |
| Clinical | 32 | Improved endothelial function | 192 | |
| Flavanols | Clinical | 27 | Increased vasodilatation | 130 |
| Clinical | 14 | Increased vasodilatation, reduced oxLDL levels | 132 | |
| Clinical | 40 | Reduced circulating LDL levels | 133 | |
| Clinical | 60 | Reduced expression of pro-inflammatory cytokines | 138 | |
| Clinical | 17 | Reduced ratio of total cholesterol to HDL cholesterol, but no change in any other CVD risk biomarkers | 139 | |
| Clinical | 57 | Enhanced endothelial function | 134 | |
| Clinical | 100 | Enhanced endothelial function | 136 | |
| Clinical | 42 | Enhanced endothelial function, reduced arterial stiffness | 137 | |
| Clinical | 30 | Reduced arterial stiffness, but no changes in blood pressure or endothelial function | 140 | |
| Clinical | 20 | Enhanced flow-mediated dilation in patients with congestive heart failure | 135 | |
| Hydroxytyrosol | Epidemiological | 12,763 | Reduced risk of CVD-event | 79 |
| Clinical | 200 | Correlation between phenolic content of olive oils and increased serum HDL levels | 89 | |
| Clinical | 40 | Reduced serum oxLDL levels | 90 | |
| Clinical | 30 | Reduced serum oxLDL levels | 91 | |
| Clinical | 28 | Reduced expression of inflammatory biomarkers | 92 | |
| Clinical | 26 | Improved endothelial function, reduced serum oxLDL levels | 93 | |
| Clinical | 7447 | Reduced risk of CVD-event | 94 | |
| Clinical | 187 | Reduced subclinical atherosclerosis in high risk patients | 95 | |
| Clinical | 90 | Reduced serum LDL, HDL and total cholesterol levels, decreased pro-atherogenic gene expression, but no changes in CRP levels | 96 | |
| Clinical | 52 | Improved endothelial function, reduced inflammatory markers | 97 | |
| Lycopene | Clinical | 144 | Reduced thickness of the intima and media in the carotid artery | 198 |
| Epidemiological | 264 | Low serum levels of lycopene were associated with increased arterial stiffness | 199 | |
| Clinical | 72 | Improved endothelial function in those also receiving statins, but no change in arterial stiffness, serum CRP levels, or blood pressure | 200 | |
| Clinical | 225 | No change in blood pressure or arterial stiffness | 201 | |
| ω-3 PUFAs | Clinical | 600 | Reduced atherothrombotic risk | 46 |
| Clinical | 2,033 | Reduced number of CVD-events | 49 | |
| Clinical | 11,323 | Reduced number of sudden cardiac deaths, but no change in serum total cholesterol, LDL, or HDL levels | 50 | |
| Clinical | 18,645 | Reduced number of major CVD-events, but no change in serum HDL or LDL levels | 51 | |
| Clinical | 95 | Increased plaque stability and reduced levels of pro-inflammatory cytokines | 52 | |
| Meta-analysis | 396 | No change in number of CVD-events | 53 | |
| Meta-analysis | 36,913 | No change in cardiac mortality | 54 | |
| Meta-analysis | 20,485 | No cardiovascular protective effects associated with omega-3 PUFA supplementation | 55 | |
| Meta-analysis | 68,680 | No change in mortality risk | 56 | |
| Epidemiological | 160 | Low serum DHA levels correlated to reduced endothelial function | 47 | |
| Clinical | 29 | Increased endothelial function and reduced arterial stiffness | 48 | |
| ω-6 PUFAs | Epidemiological | 59 | Reduced omega-3 and omega-6 PUFA levels associated with spontaneous CVD death | 67 |
| Epidemiological | 30 | Reduced levels of omega-6 PUFAs found in the shoulder regions of plaques and in ruptured plaques compared to non-ruptured plaques | 68 | |
| Epidemiological | 474 | Reduced serum GLA levels correlated with increased risk of peripheral arterial disease | 69 | |
| Clinical | 120 | Reduced systolic blood pressure | 70 | |
| Clinical | 33 | No anti-thrombotic effects observed | 74 | |
| Epidemiological | 2206 | Increased arterial stiffness and serum CRP levels | 72 | |
| Epidemiological | 501 | Increased arterial stiffness | 73 | |
| Clinical | 12 | Reduced serum LDL, total cholesterol and triacylglycerol levels | 71 | |
| Clinical | 9570 | Reduced serum cholesterol levels, but no reduction in the risk of a CVD-related event | 76 | |
| Phytosterols | Epidemiological | 22,256 | Reduced levels of serum LDL | 111 |
| Epidemiological | 48 | Raised ratio of serum phytosterol to cholesterol associated with higher risk of developing CHD | 125 | |
| Epidemiological | 477 | Increased number of sudden CVD deaths, however, the study failed to match other CVD risk factors between the different groups | 124,126 | |
| Clinical | 233 | No change in endothelium function and arterial stiffness. Reduced serum LDL levels | 118 | |
| Meta-analysis | 1,308 | Reduced levels of serum LDL, but no change in serum CRP levels | 119 | |
| Resveratrol | Epidemiological | 11,282 | Reduced risk of CVD-event | 205 |
| Vitamin C & E | Epidemiological | 19,496 | Vitamin C reduced risk of CVD-event | 147 |
| Epidemiological | 85,118 | Vitamin C reduced prevalence of CAD | 141 | |
| Clinical | 46 | Vitamin C increased vasodilatation. | 149 | |
| Clinical | 20 | Vitamin C increased vasodilatation | 150 | |
| Meta-analysis | 1,129 | Vitamin C increased endothelial function | 151 | |
| Clinical | 20 | Vitamin C had no long-term cardiovascular protective effects | 156 | |
| Clinical | 20,536 | No change in number of CVD-events | 157 | |
| Clinical | 520 | Reduced atherosclerosis progression in men | 152 | |
| Meta-analysis | 293,172 | Vitamin C but not vitamin E was associated with reduced risk of CVD-event | 153 | |
| Clinical | 2,002 | Vitamin E supplementation reduced risk of non-fatal CVD-events, but no change in CVD-related deaths | 154 | |
| Clinical | 30 | Combined vitamin C and E supplementation improved arterial stiffness, flow-mediated dilation and oxidative stress levels | 155 | |
| Clinical | 11,323 | Vitamin E did not reduce risk of CVD-event | 50 | |
| Clinical | 9,541 | Vitamin E did not reduce CVD deaths | 158 | |
| Clinical | 353 | Vitamin E reduced serum oxLDL levels, but no change in intima-media thickness | 159 | |
CAD, coronary artery disease; CHD, Coronary heart disease; CRP, C-reactive protein; CVD, Cardiovascular disease; DHA, Docosahexaenoic acid; GLA, γ-linolenic acid; HDL, High density lipoprotein; LDL, Low density lipoprotein; oxLDL, Oxidised LDL; PUFAs, Polyunsaturated fatty acids.
Another possible strategy to improve the identification of novel nutraceuticals is to include preclinical studies that focus on plaque regression rather than the prevention of atherosclerosis. The outcomes of such studies would be much more translatable to humans, as those requiring medicinal intervention are likely to already have established atherosclerosis. Another limitation of preclinical studies is that the doses of nutraceuticals used are sometimes much higher than those used in clinical trials, meaning the nutraceuticals often show no beneficial effect when they reach the clinical trial phase. Preclinical studies should employ a dose that is physiologically relevant to humans (i.e. a dose that is found within the bloodstream following consumption), rendering the outcomes of mechanistic studies more relevant with clinical studies. Furthermore, such trials need to be designed to also investigate clinically relevant end points as well as surrogate markers, and might consider including using younger participants and investigating markers of atherosclerosis regression. The development of new imaging techniques that allow the measurement of atherosclerotic plaques sizes within arteries or the identification of new biomarkers that predict atherosclerotic development will help in the design of these trials.
A major advantage of nutraceuticals is that they can be taken safely over the life time of an individual, whereas pharmaceutical strategies are only administered once an atherosclerotic risk has been identified and can result in adverse effects with prolonged use. The effects of using multiple nutraceuticals in combination must be examined further to determine whether any synergistic effects takes place and can lead to a greater reduction in atherosclerosis development compared to the individual components. Finally, nutraceuticals should not be seen as alternatives to current atherosclerosis therapies but rather as an additional complementary strategy to ensure both prevention and treatment of atherosclerosis in order to further reduce its global prevalence.
Conclusions
There is growing evidence that nutraceuticals are able to exert cardiovascular protective effects and reduce an individual’s risk of suffering a CVD-related event such as a MI or stroke. Further studies are required to fully evaluate the effectiveness of some of the nutraceuticals mentioned in this review. Such advances in our understanding of nutraceutical actions will lead to the identification of novel treatment and prevention strategies in order to reduce the global prevalence of CVD.
Key points.
Atherosclerosis is a chronic inflammatory disease of the arterial walls and is the primary cause of cardiovascular disease.
Statins therapy are not effective in reducing cholesterol levels in a small proportion of users and prolonged use of statins can increase the risk of adverse effects.
Nutraceuticals are natural compounds derived from food sources that are known be beneficial against disease.
Several nutraceuticals have been shown to potentially exert anti-inflammatory effects making them promising compounds to explore for novel anti-atherogenic therapies.
Although nutraceuticals are showing some promise, large, robust clinical trials are required to determine their full effectiveness in attenuating/regressing atherosclerosis disease progression.
Acknowledgements
Research in our laboratory was supported by grants from the British Heart Foundation (PG/07/031/22716, PG/08/073/25520, PG/10/55/28467 and PG/12/50/29691). We apologise to all the authors whose work could not be cited because of space limitations and thank Dr. Daryn R. Michael for valuable discussions.
Footnotes
Author contributions
Both authors researched data for the article, discussed its content, and wrote, reviewed, and edited the manuscript before submission.
Conflict of Interests: None
References
- 1.WHO. World Health Organisation Fact Sheet 317. 2015 http://www.who.int/mediacentre/factsheets/fs317/en/
- 2.McLaren JE, Michael DR, Ashlin TG, Ramji DP. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog Lipid Res. 2011;50:331–347. doi: 10.1016/j.plipres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Vogel RA. Coronary risk factors, endothelial function, and atherosclerosis: a review. Clin Cardiol. 1997;20:426–432. doi: 10.1002/clc.4960200505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramji DP, Davies TS. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015;26:673–685. doi: 10.1016/j.cytogfr.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley ML, Ramji DP. The influence of dysfunctional signaling and lipid homeostasis in mediating the inflammatory responses during atherosclerosis. Biochim Biophys Acta. 2015;1852:1498–1510. doi: 10.1016/j.bbadis.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Chistiakov DA, Bobryshev YV, Orekhov AN. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med. 2016;20:17–28. doi: 10.1111/jcmm.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaren JE, Ramji DP. Interferon gamma: a master regulator of atherosclerosis. Cytokine Growth Factor Rev. 2009;20:125–135. doi: 10.1016/j.cytogfr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Moss JWE, Ramji DP. Interferon-γ: Promising therapeutic target in atherosclerosis. WJEM. 2015;5:154–159. doi: 10.5493/wjem.v5.i3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiologica. 2015;214:33–50. doi: 10.1111/apha.12466. [DOI] [PubMed] [Google Scholar]
- 10.Katsuda S, Kaji T. Atherosclerosis and extracellular matrix. J Atheroscler Thromb. 2003;10:267–274. doi: 10.5551/jat.10.267. [DOI] [PubMed] [Google Scholar]
- 11.Newby A. Matrix metallproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006;69:614–624. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Haslinger-Loffler B. Multiple effects of HMG-CoA reductase inhibitors (statins) besides their lipid-lowering function. Kidney Int. 2008;74:553–555. doi: 10.1038/ki.2008.323. [DOI] [PubMed] [Google Scholar]
- 13.Leitersdorf E. Cholesterol absorption inhibition: filling an unmet need in lipid-lowering management. Eur Heart J Suppl. 2001;3:E17–E23. doi: 10.1016/S1520-765X(01)90108-7. [DOI] [Google Scholar]
- 14.Parker BA, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127:96–103. doi: 10.1161/circulationaha.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calderon RM, Cubeddu LX, Goldberg RB, Schiff ER. Statins in the treatment of dyslipidemia in the presence of elevated liver aminotransferase levels: A therapeutic dilemma. Mayo Clin Proc. 2010;85:349–356. doi: 10.4065/mcp.2009.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon CP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 17.Tsujita K, et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention the multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol. 2015;66:495–507. doi: 10.1016/j.jacc.2015.05.065. [DOI] [PubMed] [Google Scholar]
- 18.Patel AY, Pillarisetti J, Marr J, Vacek JL. Ezetimibe in combination with a statin does not reduce all-cause mortality. J Clin Med Res. 2013;5:275–280. doi: 10.4021/jocmr1371w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. National Institutes of Health. Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) 2016 [Google Scholar]
- 20.U.S. National Institutes of Health. ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab. 2016 [Google Scholar]
- 21.U.S. National Institutes of Health. The Evaluation of Bococizumab (PF-04950615;RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-1) 2015 [Google Scholar]
- 22.U.S. National Institutes of Health. The Evaluation of Bococizumab (PF-04950615; RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-2) 2015 [Google Scholar]
- 23.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: Moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118:145–156. doi: 10.1161/circresaha.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr. 2012;3:506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 26.Granados-Principal S, Quiles JL, Ramirez-Tortosa CL, Sanchez-Rovira P, Ramirez-Tortosa MC. Hydroxytyrosol: from laboratory investigations to future clinical trials. Nutr Rev. 2010;68:191–206. doi: 10.1111/j.1753-4887.2010.00278.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, O'Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega-3 fatty acids for cardioprotection. Mayo Clin Proc. 2008;83:324–332. doi: 10.4065/83.3.324. [DOI] [PubMed] [Google Scholar]
- 28.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–594. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 29.American Heart Association. Fish and omega-3 fatty acids. 2015 http://www.heart.org/HEARTORG/HealthyLiving/HealthyEating/HealthyDietGoals/Fish-and-Omega-3-Fatty-Acids_UCM_303248_Article.jsp#.V0LhZPkguUl.
- 30.Bang H, Dyerberg J. Lipid metabolism and ischemic heart disease in Greenland Eskimos. Adv Food Nutr Res. 1980;3:1–22. doi: 10.1007/978-1-4757-4448-4_1. [DOI] [Google Scholar]
- 31.Sanders TA. Polyunsaturated fatty acids in the food chain in Europe. Am J Clin Nutr. 2000;71:176S–178S. doi: 10.1093/ajcn/71.1.176s. [DOI] [PubMed] [Google Scholar]
- 32.Simopoulos AP. Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet. 2003;92:1–22. doi: 10.1159/000073788. [DOI] [PubMed] [Google Scholar]
- 33.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 34.Tsimikas S, et al. LDL isolated from Greek subjects on a typical diet or from American subjects on an oleate-supplemented diet induces less monocyte chemotaxis and adhesion when exposed to oxidative stress. Arterioscler Thromb Vasc Biol. 1999;19:122–130. doi: 10.1161/01.atv.19.1.122. [DOI] [PubMed] [Google Scholar]
- 35.Das UN. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008;7:18. doi: 10.1186/1476-511x-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes DA, Southon S, Pinder AC. (n-3) Polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes in vitro. J Nutr. 1996;126:603–610. doi: 10.1093/jn/126.3.603. [DOI] [PubMed] [Google Scholar]
- 37.Miles EA, Wallace FA, Calder PC. Dietary fish oil reduces intercellular adhesion molecule 1 and scavenger receptor expression on murine macrophages. Atherosclerosis. 2000;152:43–50. doi: 10.1016/S0021-9150(99)00446-3. [DOI] [PubMed] [Google Scholar]
- 38.Brown AL, et al. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler Thromb Vasc Biol. 2012;32:2122–2130. doi: 10.1161/ATVBAHA.112.253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song Y, et al. Polyunsaturated fatty acid relatively decreases cholesterol content in THP-1 macrophage-derived foam cell: partly correlates with expression profile of CIDE and PAT members. Lipids Health Dis. 2013;12:111. doi: 10.1186/1476-511X-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLaren JE, Michael DR, Guschina IA, Harwood JL, Ramji DP. Eicosapentaenoic acid and docosahexaenoic acid regulate modified LDL uptake and macropinocytosis in human macrophages. Lipids. 2011;46:1053–1061. doi: 10.1007/s11745-011-3598-1. [DOI] [PubMed] [Google Scholar]
- 41.Lada AT, Rudel LL, St Clair RW. Effects of LDL enriched with different dietary fatty acids on cholesteryl ester accumulation and turnover in THP-1 macrophages. J Lipid Res. 2003;44:770–779. doi: 10.1194/jlr.M200431-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Nakajima K, et al. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1963–1972. doi: 10.1161/atvbaha.111.229443. [DOI] [PubMed] [Google Scholar]
- 43.Wan J-B, et al. Endogenously decreasing tissue n-6/n-3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein E–deficient mice by inhibiting systemic and vascular inflammation. Arterioscler Thromb Vasc Biol. 2010;30:2487–2494. doi: 10.1161/atvbaha.110.210054. [DOI] [PubMed] [Google Scholar]
- 44.Leslie MA, Cohen DJA, Liddle DM, Robinson LE, Ma DWL. A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis. 2015;14 doi: 10.1186/s12944-015-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito MK. Long-chain omega-3 fatty acids, fibrates and niacin as therapeutic options in the treatment of hypertriglyceridemia: A review of the literature. Atherosclerosis. 2015;242:647–656. doi: 10.1016/j.atherosclerosis.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Franzese CJ, et al. Relation of fish oil supplementation to markers of atherothrombotic risk in patients with cardiovascular disease not receiving lipid-lowering therapy. Am J Cardiol. 2015;115:1204–1211. doi: 10.1016/j.amjcard.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Yagi S, et al. Effects of docosahexaenoic acid on the endothelial function in patients with coronary artery disease. J Atheroscler Thromb. 2015;22:447–454. doi: 10.5551/jat.26914. [DOI] [PubMed] [Google Scholar]
- 48.Tousoulis D, et al. Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis. 2014;232:10–16. doi: 10.1016/j.atherosclerosis.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 49.Burr ML, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/S0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 50.Anon. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. doi: 10.1016/S0140-6736(99)07072-5. [DOI] [PubMed] [Google Scholar]
- 51.Yokoyama M, Origasa H, Matsuzaki M. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis [published correction appears in Lancet 2007;370:220] Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 52.Niki T, et al. Effects of the addition of eicosapentaenoic acid to strong statin therapy on inflammatory cytokines and coronary plaque components assessed by integrated backscatter intravascular ultrasound. Circ J. 2016;80:450–460. doi: 10.1253/circj.CJ-15-0813. [DOI] [PubMed] [Google Scholar]
- 53.Enns J, et al. The impact of omega-3 polyunsaturated fatty acid supplementation on the incidence of cardiovascular events and complications in peripheral arterial disease: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2014;14:70. doi: 10.1186/1471-2261-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hooper L, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–760. doi: 10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwak S, Myung S, Lee Y, Seo H, Korean Meta-analysis Study Group, f Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: A meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med. 2012;172:686–694. doi: 10.1001/archinternmed.2012.262. [DOI] [PubMed] [Google Scholar]
- 56.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 57.Iso H, et al. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation. 2006;113:195–202. doi: 10.1161/circulationaha.105.581355. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi M, Sasaki S, Kawabata T, Hasegawa K, Tsugane S. Validity of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I to assess fatty acid intake: comparison with dietary records and serum phospholipid level. J Epidemiol. 2003;13:S64–81. doi: 10.2188/jea.13.1sup_64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.U.S. National Institutes of Health. A Study of AMR101 to Evaluate Its Ability to Reduce Cardiovascular Events in High Risk Patients With Hypertriglyceridemia and on Statin (REDUCE-IT) 2016 [Google Scholar]
- 60.U.S. National Institutes of Health. Outcomes Study to Assess STatin Residual Risk Reduction With EpaNova in HiGh CV Risk PatienTs With Hypertriglyceridemia (STRENGTH) 2016 [Google Scholar]
- 61.Harris WS, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidem. Circulation. 2009;119:902–7. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 62.Kakutani S, Kawashima H, Tanaka T, Shiraishi-Tateishi A, Kiso Y. Uptake of dihomo-γ-linolenic acid by murine macrophages increases series-1 prostaglandin release following lipopolysaccharide treatment. Prostaglandins Leukot Essent Fatty Acids. 2010;83:23–29. doi: 10.1016/j.plefa.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 63.Bai W, Zheng X, Zhou L, Li H. Prostaglandin E1 dose-dependently promotes stability of atherosclerotic plaque in a rabbit model. Can J Physiol Pharmacol. 2012;90:131–139. doi: 10.1139/y11-115. [DOI] [PubMed] [Google Scholar]
- 64.Juan H, Sametz W. Dihomo-δ-linolenic acid increases the metabolism of eicosapentaenoic acid in perfused vascular tissue. Prosta Leukotr Med. 1985;19:79–86. doi: 10.1016/0262-1746(85)90162-3. [DOI] [PubMed] [Google Scholar]
- 65.Takai S, et al. Anti-atherosclerotic effects of dihomo-γ-linolenic acid in ApoE-deficient mice. J Atheroscler Thromb. 2009;16:480–489. doi: 10.5551/jat.No430. [DOI] [PubMed] [Google Scholar]
- 66.Engler MM. Comparative study of diets enriched with evening primrose, black currant, borage or fungal oils on blood pressure and pressor responses in spontaneously hypertensive rats. Prostaglandins Leukot Essent Fatty Acids. 1993;49:809–814. doi: 10.1016/0952-3278(93)90030-Z. [DOI] [PubMed] [Google Scholar]
- 67.Luostarinene R, Boberg M, Saldeen T. Fatty acid composition in total phospholipids of human coronary arteries in sudden cardiac death. Atherosclerosis. 1993;99:187–193. doi: 10.1016/0021-9150(93)90021-L. [DOI] [PubMed] [Google Scholar]
- 68.Felton CV, Crook D, Davies MJ, Oliver MF. Relation of plaque lipid composition and morphology to the stability of human aortic plaques. Arterioscler Thromb Vasc Biol. 1997;17:1337–1345. doi: 10.1161/01.ATV.17.7.1337. [DOI] [PubMed] [Google Scholar]
- 69.Gautam M, et al. Importance of fatty acid compositions in patients with peripheral arterial disease. PLoS One. 2014;9:e107003. doi: 10.1371/journal.pone.0107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leng GC, et al. Randomized controlled trial of gamma-linolenic acid and eicosapentaenoic acid in peripheral arterial disease. Clin Nutr. 1998;17:265–271. doi: 10.1016/S0261-5614(98)80318-X. [DOI] [PubMed] [Google Scholar]
- 71.Guivernau M, Meza N, Barja P, Roman O. Clinical and experimental study on the long-term effect of dietary gamma-linolenic acid on plasma lipids, platelet aggregation, thromboxane formation, and prostacyclin production. Prostaglandins Leukot Essent Fat Acids. 1994;51:311–316. doi: 10.1016/0952-3278(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 72.Tomiyama H, et al. Relationships among the serum omega fatty acid levels, serum C-reactive protein levels and arterial stiffness/wave reflection in Japanese men. Atherosclerosis. 2011;217:433–436. doi: 10.1016/j.atherosclerosis.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Reinders I, et al. Higher plasma phospholipid n-3 PUFAs, but lower n-6 PUFAs, are associated with lower pulse wave velocity among older adults. J Nutr. 2015;145:2317–2324. doi: 10.3945/jn.115.212282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szczeklik A, Gryglewski RJ, Sladek K, Kostaka-Trabka E, Zmuda A. Dihomo-gamma-linolenic acid in patients with atherosclerosis: effects on platelet aggregation, plasma lipids and low-density lipoprotein-induced inhibition of prostacyclin generation. Thromb Haemost. 1984;51:186–188. [PubMed] [Google Scholar]
- 75.Sluijs I, Plantinga Y, de Roos B, Mennen LI, Bots ML. Dietary supplementation with cis-9,trans-11 conjugated linoleic acid and aortic stiffness in overweight and obese adults. Am J Clin Nutr. 2010;91:175–183. doi: 10.3945/ajcn.2009.28192. [DOI] [PubMed] [Google Scholar]
- 76.Ramsden CE, et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73) BMJ. 2016;353:i1246. doi: 10.1136/bmj.i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smit LA, Baylin A, Campos H. Conjugated linoleic acid in adipose tissue and risk of myocardial infarction. Am J Clin Nutr. 2010;92:34–40. doi: 10.3945/ajcn.2010.29524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kromhout D, et al. Food consumption patterns in the 1960s in seven countries. Am J Clin Nutr. 1989;49:889–894. doi: 10.1093/ajcn/49.5.889. [DOI] [PubMed] [Google Scholar]
- 79.Keys A. Coronary heart disease in seven countries. Nutrition. 1997;13:250–252. doi: 10.1016/s0899-9007(96)00410-8. discussion 249, 253. [DOI] [PubMed] [Google Scholar]
- 80.Scoditti E, et al. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. 2012;527:81–89. doi: 10.1016/j.abb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Carluccio MA, et al. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: Antiatherogenic properties of mediterranean diet phytochemicals. Arterioscler Thromb Vasc Biol. 2003;23:622–629. doi: 10.1161/01.atv.0000062884.69432.a0. [DOI] [PubMed] [Google Scholar]
- 82.Soler-Rivas C, Espín JC, Wichers HJ. Oleuropein and related compounds. J Sci Food Agr. 2000;80:1013–1023. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1013::AID-JSFA571>3.0.CO;2-C. [DOI] [Google Scholar]
- 83.Dell'Agli M, et al. Minor components of olive oil modulate proatherogenic adhesion molecules involved in endothelial activation. J Agric Food Chem. 2006;54:3259–3264. doi: 10.1021/jf0529161. [DOI] [PubMed] [Google Scholar]
- 84.Rosignoli P, Fuccelli R, Fabiani R, Servili M, Morozzi G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J Nutr Biochem. 2013;24:1513–1519. doi: 10.1016/j.jnutbio.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 85.Mangas-Cruz MA, et al. Effects of minor constituents (non-glyceride compounds) of virgin olive oil on plasma lipid concentrations in male Wistar rats. Clin Nutr. 2001;20:211–215. doi: 10.1054/clnu.2000.0382. [DOI] [PubMed] [Google Scholar]
- 86.Gorinstein S, et al. Olive oils improve lipid metabolism and increase antioxidant potential in rats fed diets containing cholesterol. J Agric Food Chem. 2002;50:6102–6108. doi: 10.1021/jf020306k. [DOI] [PubMed] [Google Scholar]
- 87.González-Santiago M, et al. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis. 2006;188:35–42. doi: 10.1016/j.atherosclerosis.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 88.Acin S, et al. Hydroxytyrosol administration enhances atherosclerotic lesion development in apo E deficient mice. J Biochem. 2006;140:383–391. doi: 10.1093/jb/mvj166. [DOI] [PubMed] [Google Scholar]
- 89.Covas M, et al. The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann Intern Med. 2006;145:333–341. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- 90.Fito M, et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: a randomized, crossover, controlled, clinical trial. Atherosclerosis. 2005;181:149–158. doi: 10.1016/j.atherosclerosis.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 91.Gimeno E, et al. Changes in the phenolic content of low density lipoprotein after olive oil consumption in men. A randomized crossover controlled trial. Br J Nutr. 2007:1–8. doi: 10.1017/S0007114507778698. [DOI] [PubMed] [Google Scholar]
- 92.Fito M, et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur J Clin Nutr. 2008;62:570–574. doi: 10.1038/sj.ejcn.1602724. [DOI] [PubMed] [Google Scholar]
- 93.Valls R-M, et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chemistry. 2015;167:30–35. doi: 10.1016/j.foodchem.2014.06.107. [DOI] [PubMed] [Google Scholar]
- 94.Estruch R, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 95.Murie-Fernandez M, et al. Carotid intima-media thickness changes with Mediterranean diet: a randomized trial (PREDIMED-Navarra) Atherosclerosis. 2011;219:158–62. doi: 10.1016/j.atherosclerosis.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 96.Konstantinidou V, et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: a randomized controlled trial. FASEB J. 2010;24:2546–57. doi: 10.1096/fj.09-148452. [DOI] [PubMed] [Google Scholar]
- 97.Widmer RJ, et al. Beneficial effects of polyphenol-rich olive oil in patients with early atherosclerosis. Eur J Nutr. 2013;52:1223–31. doi: 10.1007/s00394-012-0433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 99.Lee DY, et al. Anti-inflammatory activity of sulfur-containing compounds from garlic. J Med Food. 2012;15:992–999. doi: 10.1089/jmf.2012.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zanardo RCO, et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 101.Rinaldi L, et al. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest. 2006;86:391–397. doi: 10.1038/labinvest.3700391. [DOI] [PubMed] [Google Scholar]
- 102.Muzaffar S, et al. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J Vasc Res. 2008;45:521–528. doi: 10.1159/000129686. [DOI] [PubMed] [Google Scholar]
- 103.Zhao ZZ, et al. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp Biol Med (Maywood) 2011;236:169–176. doi: 10.1258/ebm.2010.010308. [DOI] [PubMed] [Google Scholar]
- 104.Zhang H, et al. Effect of S-aspirin, a novel hydrogen-sulfide-releasing aspirin (ACS14), on atherosclerosis in apoE-deficient mice. Eur J Pharmacol. 2012;697:106–116. doi: 10.1016/j.ejphar.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 105.Gonen A, et al. The antiatherogenic effect of allicin: possible mode of action. Pathobiology. 2005;72:325–334. doi: 10.1159/000091330. [DOI] [PubMed] [Google Scholar]
- 106.Koscienly J, et al. The antiatherosclerotic effect of Allium sativum. Atherosclerosis. 1999;144:237–249. doi: 10.1016/S0021-9150(99)00060-X. [DOI] [PubMed] [Google Scholar]
- 107.Budoff M, et al. Inhibiting progression of coronary calcification using Aged Garlic Extract in patients receiving statin therapy: a preliminary study. Preventative Medicine. 2004;39:985–991. doi: 10.1016/j.ypmed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 108.Ackermann RT, et al. Garlic shows promise for improving some cardiovascular risk factors. Arch Intern Med. 2001;161:813. doi: 10.1001/archinte.161.6.813. [DOI] [PubMed] [Google Scholar]
- 109.Gardner C, et al. Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: A randomized clinical trial. Arch Intern Med. 2007;167:346–353. doi: 10.1001/archinte.167.4.346. [DOI] [PubMed] [Google Scholar]
- 110.Katan MB, et al. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78:965–978. doi: 10.4065/78.8.965. [DOI] [PubMed] [Google Scholar]
- 111.Andersson SW, et al. Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: a cross-sectional study. Eur J Clin Nutr. 2004;58:1378–1385. doi: 10.1038/sj.ejcn.1601980. [DOI] [PubMed] [Google Scholar]
- 112.Sabeva NS, et al. Phytosterols differentially influence ABC transporter expression, cholesterol efflux and inflammatory cytokine secretion in macrophage foam cells. J Nutr Biochem. 2011;22:777–783. doi: 10.1016/j.jnutbio.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nashed B, Yeganeh B, HayGlass K, Moghadasian M. Anti-atherogenic effects of dietary plant sterols are associated with inhibition of proinflammatory cytokine production in Apo E-KO mice. J Nutr. 2005;135:2438–2444. doi: 10.1093/jn/135.10.2438. [DOI] [PubMed] [Google Scholar]
- 114.Xu Z, Le K, Moghadasian MH. Long-term phytosterol treatment alters gene expression in the liver of apo E-deficient mice. J Nutr Biochem. 2008;19:545–554. doi: 10.1016/j.jnutbio.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 115.Moghadasian MH, McManus BM, Godin DV, Rodrigues B, Frohlich JJ. Proatherogenic and antiatherogenic effects of probucol and phytosterols in apolipoprotein E-deficient mice: possible mechanisms of action. Circulation. 1999;99:1733–1739. doi: 10.1161/01.CIR.99.13.1733. [DOI] [PubMed] [Google Scholar]
- 116.Yeganeh B, Moshtaghi-Kashanian GR, Declercq V, Moghadasian MH. Combination of dietary phytosterols plus niacin or fenofibrate: effects on lipid profile and atherosclerosis in apo E-KO mice. J Nutr Biochem. 2005;16:222–228. doi: 10.1016/j.jnutbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 117.Moghadasian MH. Dietary phytosterols reduce cyclosporine-induced hypercholesterolemia in apolipoprotein E-knockout mice. Transplantation. 2006;81:207–213. doi: 10.1097/01.tp.0000188177.21406.97. [DOI] [PubMed] [Google Scholar]
- 118.Ras RT, et al. The effect of a low-fat spread with added plant sterols on vascular function markers: results of the Investigating Vascular Function Effects of Plant Sterols (INVEST) study. Am J Clin Nutr. 2015;101:733–741. doi: 10.3945/ajcn.114.102053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rocha VZ, et al. Effects of phytosterols on markers of inflammation: a systematic review and meta-analysis. Atherosclerosis. 2016;248:76–83. doi: 10.1016/j.atherosclerosis.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 120.Gylling H, et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis. 2014;232:346–60. doi: 10.1016/j.atherosclerosis.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 121.Stock J. Focus on lifestyle: EAS Consensus Panel position statement on phytosterol-added foods. Atherosclerosis. 2014;234:142–5. doi: 10.1016/j.atherosclerosis.2014.01.047. [DOI] [PubMed] [Google Scholar]
- 122.Ottestad I, et al. Phytosterol capsules and serum cholesterol in hypercholesterolemia: a randomized controlled trial. Atherosclerosis. 2013;228:421–5. doi: 10.1016/j.atherosclerosis.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 123.Amir Shaghaghi M, Abumweis SS, Jones PJH. Cholesterol-lowering efficacy of plant sterols/stanols provided in capsule and tablet formats: results of a systematic review and meta-analysis. J Acad Nutr Diet. 2013;113:1494–503. doi: 10.1016/j.jand.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 124.Chan Y, et al. Plasma concentrations of plant sterols: Physiology and relationship with coronary heart disease. Nutr Rev. 2006;64:385–402. doi: 10.1111/j.1753-4887.2006.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 125.Rajaratnam RA, Gylling H, Miettinen TA. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J Am Coll Cardiol. 2000;35:1185–1191. doi: 10.1016/S0735-1097(00)00527-1. [DOI] [PubMed] [Google Scholar]
- 126.Assmann G, et al. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the Prospective Cardiovascular Munster (PROCAM) study. NMCD. 2006;16:13–21. doi: 10.1016/j.numecd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 127.Falcone Ferreyra ML, Rius SP, Casati P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci. 2012;3:222. doi: 10.3389/fpls.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamakuchi M, Bao C, Ferlito M, Lowenstein CJ. Epigallocatechin gallate inhibits endothelial exocytosis. Biol Chem. 2008;389:935–941. doi: 10.1515/BC.2008.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morrison M, et al. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB in vivo. Atherosclerosis. 2014;233:149–156. doi: 10.1016/j.atherosclerosis.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 130.Fisher N, Hughes M, Gerhard-Herman M, Hollenberg N. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 131.Velayutham P, Babu A, Liu DM. Green tea catechins and cardiovascular health: An update. Curr Med Chem. 2008;15:1840–1850. doi: 10.2174/092986708785132979#sthash.eT1P4kEn.dpuf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tinahones F, et al. Green tea reduces LDL oxidability and improves vascular function. J Am Coll Nutr. 2008;27:209–213. doi: 10.1080/07315724.2008.10719692. [DOI] [PubMed] [Google Scholar]
- 133.Matsuyama T, Tanaka Y, Kamimaki I, Nagao T, Tokimitsu I. Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity. 2008;101:1338–1348. doi: 10.1038/oby.2008.60. [DOI] [PubMed] [Google Scholar]
- 134.Rassaf T, et al. Vasculoprotective effects of dietary cocoa flavanols in patients on hemodialysis: A double-blind, randomized, placebo-controlled trial. CJASN. 2016;11:108–118. doi: 10.2215/cjn.05560515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Flammer AJ, et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur Heart J. 2012;33:2172–80. doi: 10.1093/eurheartj/ehr448. [DOI] [PubMed] [Google Scholar]
- 136.Sansone R, et al. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: a randomised, controlled, double-masked trial: the Flaviola Health Study. Br J Nutr. 2015;114:1246–1255. doi: 10.1017/s0007114515002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heiss C, et al. Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: a randomized, controlled, double-masked trial. Age. 2015;37:9794. doi: 10.1007/s11357-015-9794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hsu S, et al. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am J Clin Nutr. 2007;86:539–547. doi: 10.1093/ajcn/86.5.1539. [DOI] [PubMed] [Google Scholar]
- 139.Frank J, et al. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J Nutr. 2009;139:58–62. doi: 10.3945/jn.108.096412. [DOI] [PubMed] [Google Scholar]
- 140.West SG, et al. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br J Nutr. 2014;111:653–661. doi: 10.1017/s0007114513002912. [DOI] [PubMed] [Google Scholar]
- 141.Osganian SK, et al. Vitamin C and risk of coronary heart disease in women. J Am Coll Cardiol. 2003;42:246–252. doi: 10.1016/S0735-1097(03)00575-8. [DOI] [PubMed] [Google Scholar]
- 142.d'Uscio LV. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2002;92:88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- 143.Matsumoto T, et al. Protective effect of chronic vitamin C treatment on endothelial function of apolipoprotein E-deficient mouse carotid artery. J Pharm Exp Ther. 2003;306:103–108. doi: 10.1124/jpet.103.049163. [DOI] [PubMed] [Google Scholar]
- 144.Averill MM, et al. Neither antioxidants nor genistein inhibit the progression of established atherosclerotic lesions in older apoE deficient mice. Atherosclerosis. 2009;203:82–88. doi: 10.1016/j.atherosclerosis.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang F, Jones GT, Dusting GJ. Failure of antioxidants to protect against angiotensin II-induced aortic rupture in aged apolipoprotein(E)-deficient mice. Br J Pharmacol. 2007;152:880–890. doi: 10.1038/sj.bjp.0707449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gavrila D, et al. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II–infused apolipoprotein E–deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1671–1677. doi: 10.1161/01.ATV.0000172631.50972.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Khaw K, et al. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European prospective investigation into cancer and nutrition. Lancet. 2001;357:657–663. doi: 10.1016/S0140-6736(00)04128-3. [DOI] [PubMed] [Google Scholar]
- 148.Ghanim H, et al. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr. 2010;91:940–949. doi: 10.3945/ajcn.2009.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Levine GN, et al. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996;93:1107–1113. doi: 10.1161/01.CIR.93.6.1107. [DOI] [PubMed] [Google Scholar]
- 150.Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94:6–9. doi: 10.1161/01.CIR.94.1.6. [DOI] [PubMed] [Google Scholar]
- 151.Ashor AW, Lara J, Mathers JC, Siervo M. Effect of vitamin C on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials. Atherosclerosis. 2014;235:9–20. doi: 10.1016/j.atherosclerosis.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 152.Salonen JT, et al. Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3-year progression of carotid atherosclerosis. J Intern Med. 2000;248:377–86. doi: 10.1046/j.1365-2796.2000.00752.x. [DOI] [PubMed] [Google Scholar]
- 153.Knekt P, et al. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr. 2004;80:1508–20. doi: 10.1093/ajcn/80.6.1508. [DOI] [PubMed] [Google Scholar]
- 154.Stephens NG, et al. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–6. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 155.Plantinga Y, et al. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20:392–7. doi: 10.1016/j.amjhyper.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 156.Raitakari OT, et al. Oral vitamin C and endothelial function in smokers: Short-term improvement, but no sustained beneficial effect. J Am Coll Cardiol. 2000;35:1616–1621. doi: 10.1016/S0735-1097(00)00576-3. [DOI] [PubMed] [Google Scholar]
- 157.Anon. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 158.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 159.Hodis HN. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: The Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–1459. doi: 10.1161/01.CIR.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 160.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–41. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 161.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 162.Liu T, et al. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation. 2012;35:1676–1684. doi: 10.1007/s10753-012-9484-z. [DOI] [PubMed] [Google Scholar]
- 163.Miller SJ, Zaloga GP, Hoggatt AM, Labarrere C, Faulk WP. Short-chain fatty acids modulate gene expression for vascular endothelial cell adhesion molecules. Nutrition. 2005;21:740–748. doi: 10.1016/j.nut.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 164.Menzel T, et al. Butyrate inhibits leukocyte adhesion to endothelial cells via modulation of VCAM-1. Inflamm Bowel Dis. 2004;10:122–128. doi: 10.1097/00054725-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 165.Aguilar EC, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr Metab Cardiovasc Dis. 2014;24:606–613. doi: 10.1016/j.numecd.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 166.Liu S, et al. A prospective study of dietary fiber intake and risk of cardiovascular disease among women. J Am Coll Cardiol. 2002;39:49–56. doi: 10.1016/s0735-1097(01)01695-3. [DOI] [PubMed] [Google Scholar]
- 167.Merchant A, et al. Dietary fiber reduces peripheral arterial disease risk in men. J Nutr. 2003;133:3658–3663. doi: 10.1093/jn/133.11.3658. [DOI] [PubMed] [Google Scholar]
- 168.Oh K, et al. Carbohydrate intake, glycemic index, glycemic load, and dietary fiber in relation to risk of stroke in women. American Journal of Epidemiology. 2005;161:161–169. doi: 10.1093/aje/kwi026. [DOI] [PubMed] [Google Scholar]
- 169.Pereira M, et al. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med. 2004;164:370–376. doi: 10.1001/archinte.164.4.370. [DOI] [PubMed] [Google Scholar]
- 170.Ramos SC, et al. The role of soluble fiber intake in patients under highly effective lipid-lowering therapy. Nutr J. 2011;10:1–8. doi: 10.1186/1475-2891-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kohen R, Yamamoto Y, Cundy KC, Ames BN. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci USA. 1988;85:3175–3179. doi: 10.1073/pnas.85.9.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rashid I, van Reyk D, Davies M. Carnosine and its constituents inhibit glycation of low-density lipoproteins that promotes foam cell formation in vitro. FEBS Letters. 2007;581:1067–1070. doi: 10.1016/j.febslet.2007.01.082. [DOI] [PubMed] [Google Scholar]
- 173.Brown B, et al. Supplementation with carnosine decreases plasma triglycerides and modulates atherosclerotic plaque composition in diabetic apo E−/− mice. Atherosclerosis. 2014;232:403–409. doi: 10.1016/j.atherosclerosis.2013.11.068. [DOI] [PubMed] [Google Scholar]
- 174.Kim MY, Kim EJ, Kim Y-N, Choi C, Lee B-H. Effects of α-lipoic acid and L-carnosine supplementation on antioxidant activities and lipid profiles in rats. Nutr Res Pract. 2011;5:421–8. doi: 10.4162/nrp.2011.5.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.de Courten B, et al. Effects of carnosine supplementation on glucose metabolism: Pilot clinical trial. Obesity. 2016;24:1027–34. doi: 10.1002/oby.21434. [DOI] [PubMed] [Google Scholar]
- 176.Ghirlanda G, et al. Evidence of plasma coq10-lowering effect by HMG-CoA reductase inhibitors: A double-blind, placebo-controlled study. J Clin Pharmacol. 1993;33:226–229. doi: 10.1002/j.1552-4604.1993.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 177.Wang D, et al. Coenzyme Q10 promotes macrophage cholesterol efflux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1–signaling pathway. Arterioscler Thromb Vasc Biol. 2014;34:1860–1870. doi: 10.1161/atvbaha.113.302879. [DOI] [PubMed] [Google Scholar]
- 178.Gairola CG, Howatt DA, Daugherty A. Dietary coenzyme Q10 does not protect against cigarette smoke-augmented atherosclerosis in apoE-deficient mice. Free Radic Biol Med. 2010;48:1535–1539. doi: 10.1016/j.freeradbiomed.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 179.Yan X, et al. Coenzyme Q10 consumption promotes ABCG1-mediated macrophage cholesterol efflux: a randomized, double-blind, placebo-controlled, cross-over study in healthy volunteers. Mol Nutr Food Res. 2015;59:1725–34. doi: 10.1002/mnfr.201500186. [DOI] [PubMed] [Google Scholar]
- 180.Sanoobar M, et al. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: a double blind, placebo, controlled randomized clinical trial. Nutritional Neuroscience. 2015;18:169–176. doi: 10.1179/1476830513Y.0000000106. [DOI] [PubMed] [Google Scholar]
- 181.Gao L, et al. Effects of coenzyme Q10 on vascular endothelial function in humans: A meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221:311–316. doi: 10.1016/j.atherosclerosis.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 182.Dai Y-L, et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: A randomized controlled trial. Atherosclerosis. 2011;216:395–401. doi: 10.1016/j.atherosclerosis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 183.Lee YJ, Cho WJ, Kim JK, Lee DC. Effects of coenzyme Q10 on arterial stiffness, metabolic parameters, and fatigue in obese subjects: a double-blind randomized controlled study. J Med Food. 2011;14:386–390. doi: 10.1089/jmf.2010.1202. [DOI] [PubMed] [Google Scholar]
- 184.Zeb I, et al. Aged garlic extract and coenzyme Q10 have favorable effect on inflammatory markers and coronary atherosclerosis progression: A randomized clinical trial. J Cardiovasc Dis Res. 2012;3:185–90. doi: 10.4103/0975-3583.98883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Larijani VN, et al. Beneficial effects of aged garlic extract and coenzyme Q10 on vascular elasticity and endothelial function: the FAITH randomized clinical trial. Nutrition. 2013;29:71–5. doi: 10.1016/j.nut.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Abe Y, Hashimoto S, Horie T. Curcumin inhibitor of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- 187.Gao S, et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J Mol Cell Cardiol. 2015;85:131–139. doi: 10.1016/j.yjmcc.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 188.Ramirez-Torosa M, et al. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147:371–378. doi: 10.1016/S0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 189.Quiles JL, et al. Curcuma longa extract supplementation reduces oxidative stress and attenuates aortic fatty streak development in rabbits. Arterioscler Thromb Vasc Biol. 2002;22:1225–1231. doi: 10.1161/01.ATV.0000020676.11586.F2. [DOI] [PubMed] [Google Scholar]
- 190.Olszanecki R, et al. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J Physiol Pharmacol. 2005;56:627–635. [PubMed] [Google Scholar]
- 191.Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J Nutr Biochem. 2014;25:144–150. doi: 10.1016/j.jnutbio.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 192.Akazawa N, et al. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr Res. 2012;32:795–799. doi: 10.1016/j.nutres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 193.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 194.Arab L, Steck S. Lycopene and cardiovascular disease. Am J Clin Nutr. 2000;71:1691S–1695S. doi: 10.1093/ajcn/71.6.1691S. discussion 1696S–1697S. [DOI] [PubMed] [Google Scholar]
- 195.Rao AV, Agarwal S. Role of antioxidant lycopene in cancer and heart disease. J Am Coll Nutr. 2000;19:563–569. doi: 10.1080/07315724.2000.10718953. [DOI] [PubMed] [Google Scholar]
- 196.Fuhrman B, Elis A, Aviram M. Hypercholesterolemic effect of lycopene and β-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophage. Biochem Biophys Res Commun. 1997;233:658–662. doi: 10.1006/bbrc.1997.6520. [DOI] [PubMed] [Google Scholar]
- 197.Dugas TR, Morel DW, Harrison EH. Impact of LDL carotenoid and alpha-tocopherol content on LDL oxidation by endothelial cells in culture. J Lipid Res. 1998;39:999–1007. [PubMed] [Google Scholar]
- 198.Zou Z-Y, et al. Effects of lutein and lycopene on carotid intima–media thickness in Chinese subjects with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2014;111:474–480. doi: 10.1017/S0007114513002730. [DOI] [PubMed] [Google Scholar]
- 199.Kim OY, et al. Independent inverse relationship between serum lycopene concentration and arterial stiffness. Atherosclerosis. 2010;208:581–586. doi: 10.1016/j.atherosclerosis.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 200.Gajendragadkar PR, et al. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: a randomised controlled trial. PLoS One. 2014;9:e99070. doi: 10.1371/journal.pone.0099070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Thies F, et al. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: a randomized controlled trial. Am J Clin Nutr. 2012;95:1013–1022. doi: 10.3945/ajcn.111.026286. [DOI] [PubMed] [Google Scholar]
- 202.Richard JL. [Coronary risk factors. The French paradox] Arch Mal Coeur Vaiss. 1987;80:17–21. [PubMed] [Google Scholar]
- 203.Voloshyna I, Hai O, Littlefield M, Carsons S, Reiss A. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARγ and adenosine. Eur J Pharmacol. 2013;698:299–309. doi: 10.1016/j.ejphar.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 204.Berbée JFP, et al. Resveratrol protects against atherosclerosis, but does not add to the antiatherogenic effect of atorvastatin, in APOE*3-Leiden.CETP mice. J Nutr Biochem. 2013;24:1423–1430. doi: 10.1016/j.jnutbio.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 205.Levantesi G, et al. Wine consumption and risk of cardiovascular events after myocardial infarction: results from the GISSI-Prevenzione trial. Int J Cardiol. 2013;163:282–287. doi: 10.1016/j.ijcard.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 206.Pirillo A, Catapano AL. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis. 2015;243:449–461. doi: 10.1016/j.atherosclerosis.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 207.Jeong HW, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 208.Cheng W-E, et al. Berberine reduces Toll-like receptor-mediated macrophage migration by suppression of Src enhancement. Eur J Pharmacol. 2015;757:1–10. doi: 10.1016/j.ejphar.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 209.Lee T-S, et al. Anti-atherogenic effect of berberine on LXRα-ABCA1-dependent cholesterol efflux in macrophages. J Cell Biochem. 2010;111:104–110. doi: 10.1002/jcb.22667. [DOI] [PubMed] [Google Scholar]
- 210.Wang Q, et al. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: The role of uncoupling protein 2. PLoS ONE. 2011;6:e25436. doi: 10.1371/journal.pone.0025436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hu Y, Davies GE. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia. 2010;81:358–366. doi: 10.1016/j.fitote.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 212.Li H, et al. Hepatocyte nuclear factor 1α plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J Biol Chem. 2009;284:28885–28895. doi: 10.1074/jbc.M109.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kong W, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 214.Derosa G, et al. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opin Biol Ther. 2013;13:475–482. doi: 10.1517/14712598.2013.776037. [DOI] [PubMed] [Google Scholar]
- 215.Kong W-J, et al. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism. 2008;57:1029–1037. doi: 10.1016/j.metabol.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 216.Affuso F, Ruvolo A, Micillo F, Saccà L, Fazio S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. NMCD. 2010;20:656–661. doi: 10.1016/j.numecd.2009.05.017. [DOI] [PubMed] [Google Scholar]