Abstract
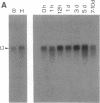
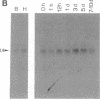
Freshwater planarians (Platyhelminthes, Turbellaria, and Tricladida) are acoelomate, triploblastic, unsegmented, and bilaterally symmetrical organisms that are mainly known for their ample power to regenerate a complete organism from a small piece of their body. To identify potential pattern-control genes in planarian regeneration, we have isolated two homeobox-containing genes, Dth-1 and Dth-2 [Dugesia (Girardia) tigrina homeobox], by using degenerate oligonucleotides corresponding to the most conserved amino acid sequence from helix-3 of the homeodomain. Dth-1 and Dth-2 homeodomains are closely related (68% at the nucleotide level and 78% at the protein level) and show the conserved residues characteristic of the homeodomains identified to data. Similarity with most homeobox sequences is low (30-50%), except with Drosophila NK homeodomains (80-82% with NK-2) and the rodent TTF-1 homeodomain (77-87%). Some unusual amino acid residues specific to NK-2, TTF-1, Dth-1, and Dth-2 can be observed in the recognition helix (helix-3) and may define a family of homeodomains. The deduced amino acid sequences from the cDNAs contain, in addition to the homeodomain, other domains also present in various homeobox-containing genes. The expression of both genes, detected by Northern blot analysis, appear slightly higher in cephalic regions than in the rest of the intact organism, while a slight increase is detected in the central period (5 days) or regeneration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter M., Schier A., Gehring W. J. Homeodomain proteins and the regulation of gene expression. Curr Opin Cell Biol. 1990 Jun;2(3):485–495. doi: 10.1016/0955-0674(90)90132-x. [DOI] [PubMed] [Google Scholar]
- Bürglin T. R., Finney M., Coulson A., Ruvkun G. Caenorhabditis elegans has scores of homoeobox-containing genes. Nature. 1989 Sep 21;341(6239):239–243. doi: 10.1038/341239a0. [DOI] [PubMed] [Google Scholar]
- Bürglin T. R. The yeast regulatory gene PHO2 encodes a homeo box. Cell. 1988 May 6;53(3):339–340. doi: 10.1016/0092-8674(88)90153-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Brönner G., Küttner F., Jürgens G., Jäckle H. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature. 1989 Mar 30;338(6214):432–434. doi: 10.1038/338432a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrmann C., Azpiazu N., Frasch M. A new Drosophila homeo box gene is expressed in mesodermal precursor cells of distinct muscles during embryogenesis. Genes Dev. 1990 Dec;4(12A):2098–2111. doi: 10.1101/gad.4.12a.2098. [DOI] [PubMed] [Google Scholar]
- Field K. G., Olsen G. J., Lane D. J., Giovannoni S. J., Ghiselin M. T., Raff E. C., Pace N. R., Raff R. A. Molecular phylogeny of the animal kingdom. Science. 1988 Feb 12;239(4841 Pt 1):748–753. doi: 10.1126/science.3277277. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Bode H. R. Nucleotide sequence of an actin-encoding gene from Hydra attenuata: structural characteristics and evolutionary implications. Gene. 1989 Dec 7;84(1):55–64. doi: 10.1016/0378-1119(89)90139-x. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- García-Blanco M. A., Clerc R. G., Sharp P. A. The DNA-binding homeo domain of the Oct-2 protein. Genes Dev. 1989 Jun;3(6):739–745. doi: 10.1101/gad.3.6.739. [DOI] [PubMed] [Google Scholar]
- Gehring W. J. Homeo boxes in the study of development. Science. 1987 Jun 5;236(4806):1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- Gehring W. J., Müller M., Affolter M., Percival-Smith A., Billeter M., Qian Y. Q., Otting G., Wüthrich K. The structure of the homeodomain and its functional implications. Trends Genet. 1990 Oct;6(10):323–329. doi: 10.1016/0168-9525(90)90253-3. [DOI] [PubMed] [Google Scholar]
- Guazzi S., Price M., De Felice M., Damante G., Mattei M. G., Di Lauro R. Thyroid nuclear factor 1 (TTF-1) contains a homeodomain and displays a novel DNA binding specificity. EMBO J. 1990 Nov;9(11):3631–3639. doi: 10.1002/j.1460-2075.1990.tb07574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kim Y., Nirenberg M. Drosophila NK-homeobox genes. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7716–7720. doi: 10.1073/pnas.86.20.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting G., Qian Y. Q., Billeter M., Müller M., Affolter M., Gehring W. J., Wüthrich K. Protein--DNA contacts in the structure of a homeodomain--DNA complex determined by nuclear magnetic resonance spectroscopy in solution. EMBO J. 1990 Oct;9(10):3085–3092. doi: 10.1002/j.1460-2075.1990.tb07505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y. Q., Billeter M., Otting G., Müller M., Gehring W. J., Wüthrich K. The structure of the Antennapedia homeodomain determined by NMR spectroscopy in solution: comparison with prokaryotic repressors. Cell. 1989 Nov 3;59(3):573–580. doi: 10.1016/0092-8674(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Treisman J., Gönczy P., Vashishtha M., Harris E., Desplan C. A single amino acid can determine the DNA binding specificity of homeodomain proteins. Cell. 1989 Nov 3;59(3):553–562. doi: 10.1016/0092-8674(89)90038-x. [DOI] [PubMed] [Google Scholar]