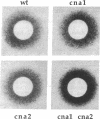
Abstract
Calcineurin, or phosphoprotein phosphatase type 2B (PP2B), is a calmodulin-regulated phosphoprotein phosphatase. We isolated a gene encoding a yeast PP2B homolog (CNA1) by screening a yeast genomic DNA library in the expression vector lambda gt11, first with 125I-labeled yeast calmodulin and then with a human cDNA encoding the catalytic (or A) subunit of calcineurin. The predicted CNA1 gene product is 54% identical to its mammalian counterpart. Using the polymerase chain reaction (PCR) with oligonucleotide primers based on sequences conserved between CNA1 and mammalian PP2B genes, we isolated a second gene, CNA2. CNA2 is identical to PP2Bw, a partial cDNA clone previously described by others as originating from rabbit brain tissue. Our findings demonstrate that a unicellular eukaryote contains phosphoprotein phosphatases of the 2B class. Haploid cells containing a single cna1 or cna2 null mutation, or both mutations, were viable. MATa cna1 cna2 double mutants were more sensitive than wild-type cells or either single mutant to growth arrest induced by the mating pheromone alpha factor and failed to resume growth during continuous exposure to alpha factor. Thus, calcineurin action antagonizes the mating-pheromone response pathway.
Full text
PDF


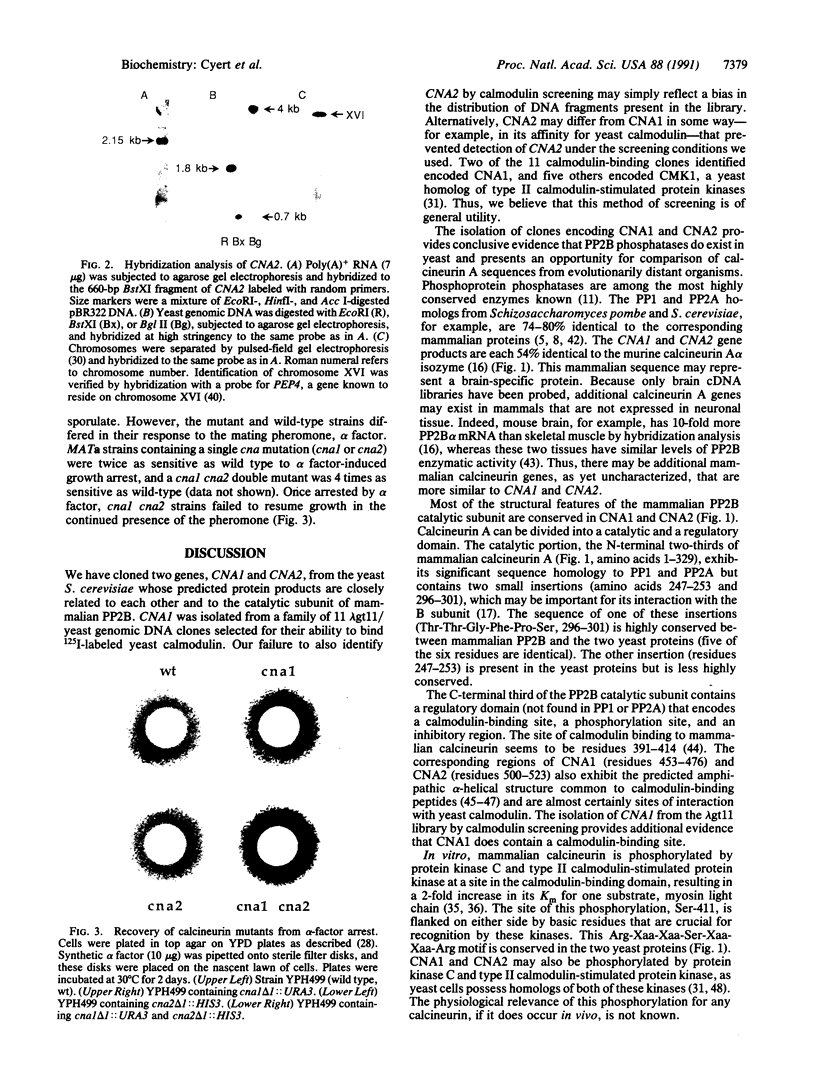

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alani E., Cao L., Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987 Aug;116(4):541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt K. T., Styles C. A., Fink G. R. A suppressor of a HIS4 transcriptional defect encodes a protein with homology to the catalytic subunit of protein phosphatases. Cell. 1989 Feb 24;56(4):527–537. doi: 10.1016/0092-8674(89)90576-x. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- Berndt N., Campbell D. G., Caudwell F. B., Cohen P., da Cruz e Silva E. F., da Cruz e Silva O. B., Cohen P. T. Isolation and sequence analysis of a cDNA clone encoding a type-1 protein phosphatase catalytic subunit: homology with protein phosphatase 2A. FEBS Lett. 1987 Nov 2;223(2):340–346. doi: 10.1016/0014-5793(87)80316-2. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Brewis N. D., Hughes V., Mann D. J. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 1990 Aug 1;268(2):355–359. doi: 10.1016/0014-5793(90)81285-v. [DOI] [PubMed] [Google Scholar]
- Cohen P., Schelling D. L., Stark M. J. Remarkable similarities between yeast and mammalian protein phosphatases. FEBS Lett. 1989 Jul 3;250(2):601–606. doi: 10.1016/0014-5793(89)80804-x. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Cross F., Hartwell L. H., Jackson C., Konopka J. B. Conjugation in Saccharomyces cerevisiae. Annu Rev Cell Biol. 1988;4:429–457. doi: 10.1146/annurev.cb.04.110188.002241. [DOI] [PubMed] [Google Scholar]
- Cyert M. S., Thorner J. Putting it on and taking it off: phosphoprotein phosphatase involvement in cell cycle regulation. Cell. 1989 Jun 16;57(6):891–893. doi: 10.1016/0092-8674(89)90325-5. [DOI] [PubMed] [Google Scholar]
- Dent P., Lavoinne A., Nakielny S., Caudwell F. B., Watt P., Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990 Nov 22;348(6299):302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- Doonan J. H., Morris N. R. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989 Jun 16;57(6):987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Félix M. A., Cohen P., Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990 Mar;9(3):675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerini D., Klee C. B. Cloning of human calcineurin A: evidence for two isozymes and identification of a polyproline structural domain. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9183–9187. doi: 10.1073/pnas.86.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero V., Jr, Russo M. A., Olson N. J., Putkey J. A., Means A. R. Domain organization of chicken gizzard myosin light chain kinase deduced from a cloned cDNA. Biochemistry. 1986 Dec 30;25(26):8372–8381. doi: 10.1021/bi00374a007. [DOI] [PubMed] [Google Scholar]
- Hanley R. M., Means A. R., Ono T., Kemp B. E., Burgin K. E., Waxham N., Kelly P. T. Functional analysis of a complementary DNA for the 50-kilodalton subunit of calmodulin kinase II. Science. 1987 Jul 17;237(4812):293–297. doi: 10.1126/science.3037704. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., King M. M., Soderling T. R. Regulatory interactions of calmodulin-binding proteins: phosphorylation of calcineurin by autophosphorylated Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1988 Sep;85(18):7001–7005. doi: 10.1073/pnas.85.18.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Perrino B. A., Soderling T. R. Identification of an autoinhibitory domain in calcineurin. J Biol Chem. 1990 Feb 5;265(4):1924–1927. [PubMed] [Google Scholar]
- Hashimoto Y., Soderling T. R. Regulation of calcineurin by phosphorylation. Identification of the regulatory site phosphorylated by Ca2+/calmodulin-dependent protein kinase II and protein kinase C. J Biol Chem. 1989 Oct 5;264(28):16524–16529. [PubMed] [Google Scholar]
- Herskowitz I., Jensen R. E. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 1991;194:132–146. doi: 10.1016/0076-6879(91)94011-z. [DOI] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Hubbard M. J., Klee C. B. Functional domain structure of calcineurin A: mapping by limited proteolysis. Biochemistry. 1989 Feb 21;28(4):1868–1874. doi: 10.1021/bi00430a066. [DOI] [PubMed] [Google Scholar]
- Iida H., Yagawa Y., Anraku Y. Essential role for induced Ca2+ influx followed by [Ca2+]i rise in maintaining viability of yeast cells late in the mating pheromone response pathway. A study of [Ca2+]i in single Saccharomyces cerevisiae cells with imaging of fura-2. J Biol Chem. 1990 Aug 5;265(22):13391–13399. [PubMed] [Google Scholar]
- Ingebritsen T. S., Stewart A. A., Cohen P. The protein phosphatases involved in cellular regulation. 6. Measurement of type-1 and type-2 protein phosphatases in extracts of mammalian tissues; an assessment of their physiological roles. Eur J Biochem. 1983 May 2;132(2):297–307. doi: 10.1111/j.1432-1033.1983.tb07362.x. [DOI] [PubMed] [Google Scholar]
- Ito A., Hashimoto T., Hirai M., Takeda T., Shuntoh H., Kuno T., Tanaka C. The complete primary structure of calcineurin A, a calmodulin binding protein homologous with protein phosphatases 1 and 2A. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1492–1497. doi: 10.1016/0006-291x(89)91148-0. [DOI] [PubMed] [Google Scholar]
- James P., Maeda M., Fischer R., Verma A. K., Krebs J., Penniston J. T., Carafoli E. Identification and primary structure of a calmodulin binding domain of the Ca2+ pump of human erythrocytes. J Biol Chem. 1988 Feb 25;263(6):2905–2910. [PubMed] [Google Scholar]
- Jones J. S., Prakash L. Yeast Saccharomyces cerevisiae selectable markers in pUC18 polylinkers. Yeast. 1990 Sep-Oct;6(5):363–366. doi: 10.1002/yea.320060502. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Giri P. R., Higuchi S., Tamura J., Dixon S. C., Marietta C. A., Amorese D. A., Martin B. M. Cloning and characterization of molecular isoforms of the catalytic subunit of calcineurin using nonisotopic methods. J Biol Chem. 1990 Jul 5;265(19):11312–11319. [PubMed] [Google Scholar]
- Kincaid R. L., Martin B. M. Characterization of the calmodulin-binding domain of calcineurin deduced from a complementary DNA clone. Adv Exp Med Biol. 1989;255:347–358. doi: 10.1007/978-1-4684-5679-0_38. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Ohkura H., Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990 Oct 19;63(2):405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Draetta G. F., Hubbard M. J. Calcineurin. Adv Enzymol Relat Areas Mol Biol. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- Koretzky G. A., Picus J., Thomas M. L., Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature. 1990 Jul 5;346(6279):66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- Kuno T., Takeda T., Hirai M., Ito A., Mukai H., Tanaka C. Evidence for a second isoform of the catalytic subunit of calmodulin-dependent protein phosphatase (calcineurin A). Biochem Biophys Res Commun. 1989 Dec 29;165(3):1352–1358. doi: 10.1016/0006-291x(89)92752-6. [DOI] [PubMed] [Google Scholar]
- Levin D. E., Fields F. O., Kunisawa R., Bishop J. M., Thorner J. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell. 1990 Jul 27;62(2):213–224. doi: 10.1016/0092-8674(90)90360-q. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ishii S., Tokai M., Tsutsumi H., Ohki O., Akada R., Tanaka K., Tsuchiya E., Fukui S., Miyakawa T. The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol Gen Genet. 1991 May;227(1):52–59. doi: 10.1007/BF00260706. [DOI] [PubMed] [Google Scholar]
- Momayezi M., Lumpert C. J., Kersken H., Gras U., Plattner H., Krinks M. H., Klee C. B. Exocytosis induction in Paramecium tetraurelia cells by exogenous phosphoprotein phosphatase in vivo and in vitro: possible involvement of calcineurin in exocytotic membrane fusion. J Cell Biol. 1987 Jul;105(1):181–189. doi: 10.1083/jcb.105.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D., Contopoulou C. R., Kans J. A. Genetic map of Saccharomyces cerevisiae, edition 10. Yeast. 1989 Sep-Oct;5(5):321–403. doi: 10.1002/yea.320050503. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Kinoshita N., Miyatani S., Toda T., Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989 Jun 16;57(6):997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Specific induction of Ca2+ transport activity in MATa cells of Saccharomyces cerevisiae by a mating pheromone, alpha factor. J Biol Chem. 1985 Sep 5;260(19):10482–10486. [PubMed] [Google Scholar]
- Pausch M. H., Kaim D., Kunisawa R., Admon A., Thorner J. Multiple Ca2+/calmodulin-dependent protein kinase genes in a unicellular eukaryote. EMBO J. 1991 Jun;10(6):1511–1522. doi: 10.1002/j.1460-2075.1991.tb07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneke J. E., Blumer K. J., Courchesne W. E., Thorner J. The carboxy-terminal segment of the yeast alpha-factor receptor is a regulatory domain. Cell. 1988 Oct 21;55(2):221–234. doi: 10.1016/0092-8674(88)90045-1. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon A. A., Cohen P. T., Stark M. J. Saccharomyces cerevisiae protein phosphatase 2A performs an essential cellular function and is encoded by two genes. EMBO J. 1990 Dec;9(13):4339–4346. doi: 10.1002/j.1460-2075.1990.tb07883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tash J. S., Krinks M., Patel J., Means R. L., Klee C. B., Means A. R. Identification, characterization, and functional correlation of calmodulin-dependent protein phosphatase in sperm. J Cell Biol. 1988 May;106(5):1625–1633. doi: 10.1083/jcb.106.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler C. D., Haimo L. T. Regulation of organelle transport in melanophores by calcineurin. J Cell Biol. 1990 Nov;111(5 Pt 1):1939–1948. doi: 10.1083/jcb.111.5.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup D. M., Kelly T. J. Purification of replication protein C, a cellular protein involved in the initial stages of simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1989 May;86(10):3584–3588. doi: 10.1073/pnas.86.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz e Silva E. F., Cohen P. T. Isolation of a cDNA likely to encode a novel Ca2+-dependent/calmodulin-stimulated protein phosphatase. Biochim Biophys Acta. 1989 Dec 22;1009(3):293–296. doi: 10.1016/0167-4781(89)90118-8. [DOI] [PubMed] [Google Scholar]