Abstract
Hematopoietic stem cells (HSCs) sustain blood formation throughout life. Pathways regulating maintenance of adult HSCs are largely unknown. Here we report that the Ets-related transcription factor Tel/Etv6, the product of a locus frequently involved in translocations in leukemia, is a selective regulator of HSC survival. Following inactivation of Tel/Etv6, HSCs are lost in the adult bone marrow but their progeny are unaffected and transiently sustain blood formation. Accordingly, absence of Tel/Etv6 after lineage commitment is ostensibly without consequence except for unexpected impairment of maturation of megakaryocytes. Thus, we establish Tel/Etv6 as a selective and essential regulator of postembryonic HSCs.
Keywords: Ets, hematopoietic stem cell, megakaryocyte, Cre/loxP, Tel/Etv6
Most blood cells have short lifespans and require constant renewal from a small population of hematopoietic stem cells (HSCs). HSCs have two defining properties: the capacity for differentiation and stepwise maturation into all known blood lineages, and generation of additional HSCs through the process of self-renewal (Orkin 2000; Kondo et al. 2003). During development, the site of blood formation in the embryo changes sequentially from yolk sac to fetal liver to bone marrow (Orkin 2000). After birth, the bone marrow is the dominant site of hematopoiesis and the number of HSCs is maintained in steady-state by continuous low-level turnover throughout adult life (Cheshier et al. 1999; Kondo et al. 2003). Although genes essential for initial specification of HSCs in the embryo, as well as for their propagation during embryonic development, have been identified (Tsai et al. 1994; Shivdasani et al. 1995; Okuda et al. 1996; Orkin 2000; Kumano et al. 2003), experiments thus far have not shown any of these regulators of embryonic hematopoiesis to be essential for maintaining HSC levels and function after hematopoiesis is established in the bone marrow. In fact, adult HSC function is remarkably resistant to loss of Scl (Mikkola et al. 2003), Aml1/runx1 (Ichikawa et al. 2004), Notch1 (Radtke et al. 1999), and Integrin β1 (Brakebusch et al. 2002), each of which is essential for embryonic hematopoiesis. Similarly, loss of β-catenin (Cobas et al. 2004) and HoxB4 (Brun et al. 2004), factors that expand HSC numbers on forced expression (Sauvageau et al. 1995; Reya et al. 2003), fails to affect adult bone marrow HSCs. Thus, the molecular control of adult HSCs remains largely undefined.
The Tel/Etv6 locus, which encodes an Ets-related transcriptional repressor, is a frequent target of diverse chromosomal translocations in human leukemias (Golub et al. 1994). Embryos with a conventional knockout (KO) of the Tel/Etv6 gene die by day 11 of embryonic development (E11) due to vascular abnormalities. Blood formation in the embryo is largely unperturbed (Wang et al. 1997). Yet, studies using chimeric mice from Tel/Etv6-deficient embryonic stem (ES) cells suggested a requirement in bone marrow hematopoiesis (Wang et al. 1998). Here, we used inducible and lineage-specific gene disruption strategies to define the role of Tel/Etv6 in adult hematopoiesis.
Results and Discussion
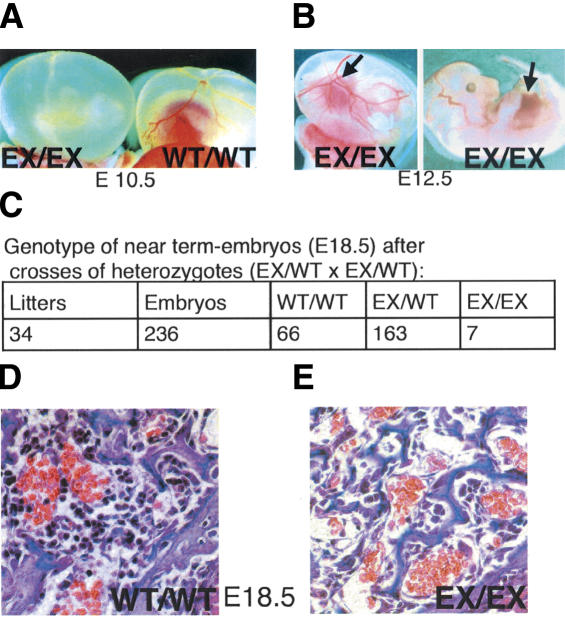
We engineered mice harboring a conditional (floxed) Tel/Etv6 allele in which recognition sequences (loxP sites) for Cre recombinase flank exon 7, which encodes a portion of the protein critical for DNA-binding (Supplementary Fig. 1). Homozygous floxed mice were healthy and had normal Tel/Etv6 protein levels, indicating that the unexcised allele was fully functional (Supplementary Fig. 2; data not shown). Likewise, mice heterozygous for the excised allele (generated by Cre-mediated recombination in the germ line) were normal (data not shown). As anticipated from the prior knockout, intercrossing of heterozygotes failed to yield viable homozygous excised pups. Analysis of embryonic development confirmed that ∼95% of homozygous embryos died by E11 with failure of yolk sac angiogenesis (Fig. 1A). The remaining homozygous embryos survived past this stage and exhibited normal fetal liver hematopoiesis (Fig. 1B,C; data not shown). They were not born alive, but appeared normal when delivered by C-section on E18.5 (Fig. 1C). Pathologic examination revealed markedly decreased bone marrow hematopoiesis (Fig. 1D). Diminished hematopoiesis within the bone marrow in embryos that escaped death due to the yolk sac vascular abnormalities is consistent with our prior finding of impaired adult hematopoiesis in the absence of Tel/Etv6 function, as deduced from chimera analysis (Wang et al. 1998). (Mutants that survived longer then E11.5 were not observed during the original analysis of the conventional KO allele, but were subsequently found with the conventional KO when analyzed in the same mixed background as the excised conditional described here, suggesting that both disrupted alleles are indistinguishable; data not shown).
Figure 1.
Germ-line excision of a floxed Tel/Etv6 allele causes either midembryonic or perinatal lethality with hypocellular bone marrow. (A) Most progeny homozygous for germ-line-excised Tel/Etv6 (EX/EX) die at approximately E10.5 with absent yolk sac angiogenesis; wild-type (WT/WT) yolk sac at the same stage is shown for comparison. (B) A few homozygous excised embryos survive longer; they have normal yolk sac angiogenesis and exhibit red livers indicative of active hematopoiesis (arrows) on E12.5. (C) Homozygous excised embryos are not born alive (not shown) but at E18.5 (C-section), ∼3% of pups that appear normal are homozygous for the excised allele. (D) Pathological examination of E18.5 homozygotes (n = 3) and their wild-type littermates (n = 3) revealed markedly reduced bone marrow hematopoiesis in the mutants (cross-sections through the humeral head, 60×).
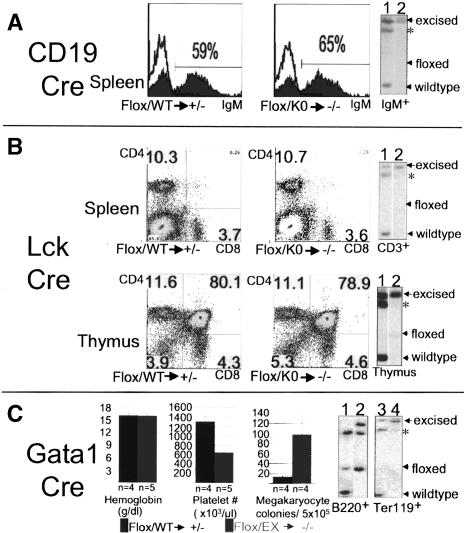
Tel/Etv6 is widely expressed within and outside the hematopoietic system (Wang et al. 1997). To examine its role in the adult blood system, we used Cre-expressing mouse strains that excise selectively in committed lineages by using lineage-specific promoters; that is, CD19-Cre: B lineage; Lck-Cre: T cells; Gata1-Cre: erythroid/megakaryocytic lineages (Fig. 2). Matings were performed to generate mice harboring a Cre allele in addition to one floxed and one disrupted Tel/Etv6 allele. By this strategy only a single excision event per cell results in complete loss of Tel/Etv6 without potential ambiguity from heterozygosity (Flox/KO → Cre → -/-). Controls bore a wild-type allele in addition to the floxed allele, and therefore retained function after Cre-mediated recombination (Flox/WT → Cre → +/-). Remarkably, Tel/Etv6 was not essential for the maintenance of any of these mature lineages (Fig. 2A-C). Thus, IgM+ cells in the spleen were not affected by excision by CD19-Cre (Fig. 2A; data not shown). Additionally, excision in proB-cell lines generated from floxed mice failed to implicate Tel/Etv6 in their proliferation (Supplementary Fig. 2). Likewise, excision by Lck-Cre did not alter the frequencies of T cells in thymus and spleen (Fig. 2B). Gata1-Cre efficiently excised in Ter119+ (erythroid) precursor cells in the bone marrow, but this did not lead to anemia or changes in the frequency or morphology of erythroid precursors (Fig. 2C; data not shown). However, platelet counts were ∼50% reduced compared to controls and megakaryocyte colony-forming cells were fivefold increased, findings consistent with a terminal defect in megakaryocyte maturation, partially compensated for by increased progenitors. This requirement for Tel/Etv6 in the megakaryocyte lineage is intrinsic, as absence of recombination in B220+ B cells from the bone marrow indicated that there was no excision in HSCs (Fig. 2C). With the exception of the megakaryocyte lineage, therefore, Tel/Etv6 function is dispensable for progenitors and precursors.
Figure 2.
Tel/Etv6 is dispensable for selected lineage-committed hematopoietic cells. (A) CD19-Cre mediated excision in the B-lymphoid lineage does not affect frequencies of IgM+ B cells in the spleen assessed by FACS analysis (control, left histogram; lineage-specific mutant, right histogram); in each case, germ-line genotype of mice ([WT] wild-type; [Flox] floxed; [KO] conventional knockout) and expected functional status of both alleles in cells excised by Cre (following arrow) are indicated below. Results from representative animals are shown (n = 5). Right panel shows Southern blot analysis of sorted IgM+ B cells from spleen. Note complete excision of the floxed allele in both control (lane 1) and lineage-specific mutant (lane 2). (sstarf;) Band shared by floxed and wild-type allele; see Supplementary Figure 1 for complete explanation of band pattern. (B) Lck-Cre-mediated excision in the T-lymphoid lineage does not affect frequencies of CD4+ and CD8+ T cells in the spleen (upper FACS plots) or distribution of subsets of developing T cells in the thymus (lower FACS plots) (n = 4). Southern blots on the right show complete excision in sorted CD3+ T cells from the spleen (upper) and subtotal excision in the thymus (lower) of both controls (lane 1) and lineage-specific mutants (lane 2). (C) Gata1-Cre-mediated excision in erythroid and megakaryocytic lineages reveals terminal megakaryocyte defect but normal steady-state erythropoiesis. Bar graphs on the left show no difference in hemoglobin levels but ∼50% reduction in peripheral blood platelet counts in the lineage-specific mutants compared with controls. Third bar graph shows approximately fivefold elevation of megakaryocyte colonies from mutant bone marrow. Error bars represent standard deviations. Southern blots show the unexcised allele in sorted B220+ B cells from bone marrow (left; control [lane 1], mutant [lane 2]) but only the excised allele in Ter119+ (erythroid) cells (right; control [lane 3], mutant [lane 4]). (Lane 1) Note that control B220+ cells from Gata1-Cre mice lack detectable excised allele, confirming that excision is intrinsic to the megakaryocyte/erythroid lineage and does not occur earlier; that is, in HSCs.
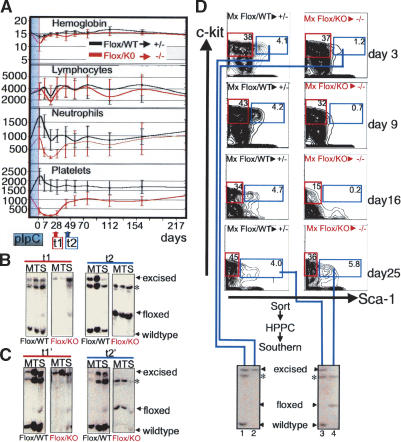
To address a potential requirement in HSCs, we inactivated the floxed Tel/Etv6 allele in a transient and inducible fashion by use of the Mx-Cre transgene, which is activated by interferon-α (IFN-α) or its inducer polyI-polyC (pIpC) (Kuhn et al. 1995). Of note, the IFN-α response itself was not altered by absence of Tel/Etv6 in proB cells ex vivo (Supplementary Fig. 2F). Injections of pIpC into control mice (Flox/WT) resulted in moderate transient changes in peripheral blood counts and in stable but quantitative excision of the floxed allele in hematopoietic tissues (Fig. 3A,B). In conditional mutants (Flox/KO), most blood counts were also preserved (Fig. 3A). Hemoglobin concentration and lymphocyte counts remained indistinguishable from those of controls, and neutrophil counts were only moderately reduced. Platelet counts were reduced dramatically after Tel disruption but, in contrast to findings obtained with Gata1-Cre matings, counts started to recover after ∼4 wk (Fig. 3A). Serial analysis of the excision status detected exclusively excised cells for 3-4 wk after Cre induction (coinciding with the period of low platelets) (Fig. 3B, t1). After ∼5-6 wk, however, excised cells became undetectable in the bone marrow and were replaced by cells harboring the unexcised floxed allele (Fig. 3B, t2). Thus, whereas the excision of the floxed allele was complete and stable when paired with a wild-type allele in control mice, profound selection against the excised allele was observed when paired with an inactive (KO) allele. Similar changes in blood counts and selection against loss of Tel/Etv6 function were observed when unexcised, floxed mutant marrow was first transplanted to wild-type mice and subsequently induced for Cre-mediated excision (Fig. 3C). This provides formal demonstration that the requirement for Tel/Etv6 is intrinsic to the hematopoietic system. Remarkably, the main precursor populations in the bone marrow (B-cell, myeloid, and erythroid) were largely sustained for several weeks despite the absence of detectable Tel/Etv6 (Supplementary Fig. 3). Likewise, B-cell, myeloid, and erythroid colony-forming progenitors were preserved (albeit in somewhat reduced numbers) after complete excision of Tel/Etv6 (Supplementary Fig. 4).
Figure 3.
Disruption of Tel/Etv6 in adult mice reveals an essential and selective role for survival of HSCs. (A) Peripheral blood during (shaded blue) and after pIpC injection to induce Cre-mediated disruption of the floxed allele. Note the absence of differences in hemoglobin (g/dL) and lymphocyte counts (cell#/μL), moderate transient drop in neutrophil counts (cell#/μL) and drastic but transient drop in platelet count (cell# × 103/μL) in conditional mutants (genotype: Floxed/conventional knockout [Flox/KO]; n = 6-11) compared with controls (genotype: Floxed/wild-type [Flox/WT]; n = 7-11). (B) Southern blot of bone marrow (M), thymus (T), and spleen (S) 3 wk after the final pIpC injection demonstrates complete excision of the floxed allele in controls (Flox/WT) and conditional mutants (Flox/KO). Left panels (t1) show representative results from analysis of six mice in each group from three independent experiments; complete excision of the floxed allele in bone marrow was confirmed as early as 2 d after the final pIpC injection (n = 6, not shown, see Supplementary Fig. 5A). Right panels (t2, 6 wk after Cre induction) show persistent excision in control mice (Flox/WT) but absence of detectable excised allele associated with re-emergence of the floxed allele in conditional mutants (Flox/KO). Representative results from analysis of six mice in each group from three independent experiments are shown; excision in controls was found to be undiminished as late as 18 mo after Cre-induction (n = 3; not shown). (sstarf;) Band shared by floxed and wild-type allele; see Supplementary Figure 1 for complete explanation of band pattern. (C) Excision analysis in experiment performed as above except that mutant marrow had been transplanted into lethally irradiated wild-type recipients 3 mo prior to Cre induction. Longitudinal development of peripheral blood counts (not shown) and excision status after 3 wk (left panel, t1′) and 6 wk (right panel, t2′) following Cre induction was indistinguishable from that in untransplanted mutants. (D) FACS analysis of HSCs (lineage-, c-Kit+, Sca-1+, marked with blue frames) and myeloid progenitors (lin-, c-Kit-, Sca-1+, marked with red frames) at days 3, 9, 16 and 25 after Cre induction. In controls (Flox/WT), HSC frequency remained stable, but in conditional mutants HSCs were decreased at day 3, hardly detectable on days 9 and 16, but re-emerged on day 24 (results are representative of six mice for each time point in three independent experiments). Southern blot (bottom) shows excision in high proliferative potential colonies (HPPCs) from sorted control (lane 1) and mutant (lane 2) HSC populations at day 3. At day 25, complete excision was still observed in controls (lane 3) but in conditional mutants stem cells that had re-emerged exclusively harbored unexcised Tel/Etv6 (lane 4).
We hypothesized that the modest eventual decline in Tel/Etv6 progenitor/precursors could be accounted for entirely by their limited clonal lifespan in the face of a block at the HSC level. Therefore, we assessed the numbers of phenotypic HSCs over time after induction of Cre expression. Figure 3D shows that in control (Flox/WT) mice Lin-, Sca1+, c-Kit+ cells, which include all short-term and long-term repopulating HSCs (Uchida and Weissman 1992), were fully excised and their frequency remained constant after Cre induction. In contrast, in conditional mutant (Flox/KO) mice, HSCs continuously declined in frequency in the first 2 wk until they were virtually undetectable (Fig. 3D, d3, d9, d16). Four weeks after Cre-induction (d25 in Fig. 3D), HSCs had fully recovered. Critically, these HSCs were now found to harbor the unexcised allele. We infer that the rare HSCs that had escaped excision (below detection limit on day 16, Fig. 3D) had expanded rapidly to restore the stem cell compartment, similar to transplant settings where single HSCs are sufficient to rescue hematopoiesis (Osawa et al. 1996). Remarkably, however, HSCs lacking Tel/Etv6 did not survive. The conclusion that adult HSCs require Tel/Etv6 for survival is supported by lack of contribution of Tel/Etv6-/- cells to bone marrow in chimeric mice (Wang et al. 1998) and the bone marrow failure of Tel/Etv6-deficient E18.5 embryos (Fig. 1D).
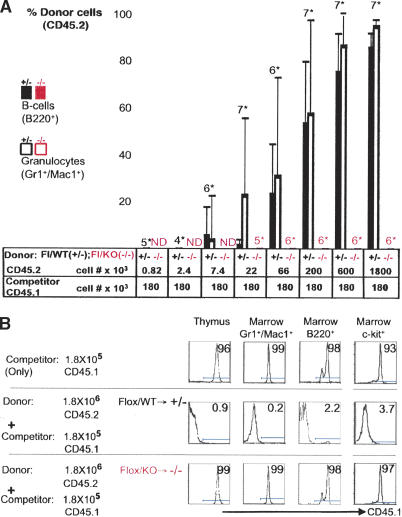
To exclude the possibility that Tel/Etv6 disruption alters the marker profile of HSCs, rather than leads to their loss, we assessed the biological activity of potential HSCs by bone marrow transplantation assays. We transplanted bone marrow cells 3 d after induction of Cre expression. At this time excision of floxed sequences in bone marrow cells appeared nearly complete, and yet the overall cellular composition of the marrow had not changed dramatically (Supplementary Figs. 3, 5). Colony-forming progenitors that had been excised at the Tel/Etv6 locus were abundant (Supplementary Fig. 4); indeed, some excised HSCs were still detectable (Fig. 3D). However, following injection into lethally irradiated mice, Tel/Etv6-deficient bone marrow (Flox/KO → Cre → -/-; 1.8 × 106 cells/recipient) failed to rescue survival of any recipients (0/8), whereas control marrow (Flox/WT → Cre → +/-; 1.8 × 106 cells/recipient) rescued all recipients (8/8) and established donor-derived hematopoiesis for more than 6 mo (data not shown). To specifically assess long-term repopulating HSCs, we performed competitive repopulation assays, in which mice received a small dose of wild-type competitor marrow cells along with a titration of donor marrow in order to ensure survival of the recipient. In this setting, excised control marrow cells contributed to hematopoiesis in a dose-dependent fashion (Fig. 4A,B; Supplementary Fig. 5). Neither mutant nor control marrow gave rise to detectable unexcised hematopoietic cells in recipients, indicating very high efficiency of excision in HSCs (Supplementary Fig. 5). On the other hand, Tel/Etv6-deficient cells failed to contribute to recipient hematopoiesis even at high dose levels of donor cells, confirming that loss of Tel/Etv6 abrogates long-term repopulating HSC activity.
Figure 4.
Bone marrow transplantation following disruption of Tel/Etv6 confirms absence of HSC activity. (A) Analysis of CD45 isotype variants on blood granulocytes (Gr1+Mac1+) and B cells (B220+) 3 mo after transplantation into lethally irradiated recipients demonstrates absence of competitive repopulation activity in bone marrow after Tel/Etv6 disruption. Bone marrow donors had received pIpC to induce Cre until 3 d prior to transplant. Control marrow (Flox/WT → +/-; CD45.2) competed efficiently with a low constant dose of wild-type marrow (“competitor”, CD45.1) in a dose-dependent fashion, whereas conditional mutant marrow (Flox/KO → -/-; CD45.2) failed to compete. (Bars represent averages on groups of mice; numberssstarf; indicate mice/group; error bars show standard deviation; ND indicates not done; one representative of two experiments is shown). (B) CD45 isotype analysis of thymocytes, bone marrow myeloid cells (Gr1+Mac1+), B cells (B220+), and progenitors (c-Kit+) from representative recipients in the competitive repopulation assay shown in A. Note that CD45.2+ control marrow (middle) efficiently prevents contribution of wild-type “competitor” cells (CD45.1, given alone in top row), whereas conditional mutant marrow (CD45.2, bottom) completely fails to prevent “competitor” engraftment. See Supplementary Figure 5 for excision analysis 3 mo after engraftment.
Our data reveal that bone marrow hematopoiesis is entirely dependent on continuous expression of Tel/Etv6. Yet, Tel/Etv6 does not function as master regulator in the sense that it directs a hematopoietic differentiation program in a wide spectrum of hematopoietic cells. Rather, it has two independent roles in the context of specific, narrow stages of hematopoietic differentiation. First, Tel/Etv6 controls the survival of HSCs so that its disruption indirectly affects the majority of all hematopoietic cells which have limited clonal lifespans and eventually will extinguish without constant regeneration from HSCs. Second, Tel/Etv6 is required late in the development of the megakaryocyte lineage, where it presumably acts in concert with transcriptional regulators previously implicated in megakaryopoiesis (Nfe2, Scl, Gfi-1B, Aml1, Gata1, Fli-1, and Fog1; Italiano and Shivdasani 2003) and/or by directly binding ETS motifs known to be critical in megakaryocytic promoters (Wang et al. 2002).
Tel/Etv6 is the first regulator that is selectively required for the survival of adult HSCs. Other transcription factors or components of growth factor pathways are necessary in different facets of HSC biology. For instance, Scl is essential for specification of hematopoietic fate (Shivdasani et al. 1995), and Aml1/runx-1 is required for emergence of HSCs during embryogenesis (Okuda et al. 1996). Yet, neither is stringently required in HSCs once they are formed (Mikkola et al. 2003; Ichikawa et al. 2004). Likewise, Notch1 (Radtke et al. 1999; Kumano et al. 2003) and Notch2 (Saito et al. 2003), which are both expressed in HSCs, and RBP-J (Han et al. 2002), downstream mediator of Notch genes, have been inactivated conditionally in mouse bone marrow without compromising HSCs. Moreover, HoxB4 and β-catenin, both of which induce stem cell expansion on forced expression (Sauvageau et al. 1995; Reya et al. 2003), are dispensable for HSC function (Brun et al. 2004; Cobas et al. 2004). Recently, Bmi-1 (Lessard and Sauvageau 2003; Park et al. 2003) and p21Cip1WAF1 (Cheng et al. 2000) and autocrine production of vascular endothelial growth factor (VEGF) (Gerber et al. 2002) have been shown to be required for proper maintenance of adult HSCs, but these have widespread roles within and outside of the hematopoietic system. Neither p21Cip1WAF1 nor Bmi-1 are strictly required for HSC survival: Marrow from p21-/- mice can be transplanted (Cheng et al. 2000) and in Bmi-1-/- mice phenotypic HSCs are present, even if functionally compromised (van der Lugt et al. 1994; Park et al. 2003).
Because HSCs are capable of extensive proliferative expansion and share with leukemia cells the potentially harmful property of self-renewal, their numbers and survival must be tightly controlled. As Tel/Etv6 activity is most likely modulated by phosphorylation similar to Yan (Lai and Rubin 1992), its closest relative in Drosophila, we speculate that Tel/Etv6 mediates a continuous survival signal specifically within the hematopoietic niche in the bone marrow. Very likely, Tel/Etv6 lies downstream in a pathway initiated by interaction of surface receptors on HSCs and ligands within the marrow microenvironment. The apparent dispensability of Tel/Etv6 for hematopoiesis during embryonic development may simply reflect that a survival signal is not necessary, as embryonic hematopoiesis is transient and not sustained. Elucidating the molecular pathway(s) by which Tel/Etv6 preserves HSCs may explain the unique capability of the bone marrow microenvironment to sustain this rare cell type, and will likely be crucial for developing novel strategies for maintaining and/or expanding HSCs ex vivo.
Materials and methods
Generation of a conditional Tel/Etv6 allele
A 14-kb clone from a 129/sv genomic library containing exons 6, 7, and 8 was modified to generate the targeting vector (Supplementary Fig. 1A). The 5′ loxP site was introduced by blunt end ligation of a synthetic adapter (CACTCGAGATCGATATAACTTCGTATAATGTATGCTATACGAAGTTATTAACTAGTGGCC) → 5′-Spe1-loxP-Xho1-Apa1-3′) into the unique Apa1 site between exons 6 and 7. Subsequently, a loxP-flanked neomycin resistance gene was introduced into a unique EcoRV site between exons 7 and 8, and a thymidine kinase gene was cloned into the pBlueScript backbone outside of the homology region. The final vector was linearized with Pvu1, ligated to hairpins, and introduced into CJ7 ES cells by electroporation. Subsequently, ES cells that had integrated the plasmid in the correct location after homologous recombination were enriched by neomycin selection and gancyclovir counterselection. Clones with correct integration were identified by Southern blot using both 5′ and 3′probes outside of the homology region (Supplementary Fig. 1B). A targeted ES-cell clone was injected into C57/BL6 blastocysts to generate chimeras for germ-line transmission. Floxed-ΔNeo mice (Supplementary Fig. 1A, fourth from the top) were derived from floxed-Neo+ mice (Supplementary Fig. 1A, fifth from the top) fortuitously while intercrossing with lineage-specific Cre mice, but regardless of the presence of the neomycin resistance gene, the floxed allele was fully functional and efficiently excised by Cre. For description and references of Cre-expressing mouse strains, see Supplemental Material.
Genotyping and excision analysis
For detailed explanation and illustration of Southern blot strategy used to assessTel/Etv6 gene status and targeting, please see Supplementary Figure 1. For DNA extraction, tissues were minced or bone marrow was flushed from femurs with PBS using a 22G needle and incubated in lysis solution (100 mM Tris HCL at pH 8.0, 5 mM EDTA, 0.2% SDS, 200 mM NaCL, and 100 μg/mL Proteinase K) at 55°C for 24-48 h, followed by phenol and phenol/chloroform extractions and precipitation in isopropanol. 5′ and 3′ probes were used to confirm the correct targeting, as shown in Supplementary Figure 1A,B. For genotyping of mice harboring the conditional Tel/Etv6 allele and analysis of the excision status of the floxed allele, a cloned fragment combining the PCR-amplified coding regions for exon 6 and exon 7 was used. Probes for neomycin and Cre were used to detect the conventional Tel/Etv6 knockout allele and mouse strains harboring Cre, respectively.
Embryology
Embryos at defined timepoints of development were obtained by C-section after mating of heterozygous excised mice (Fig. 1). Time of conception was determined by daily inspection of female mice for the occurrence of vaginal plugs; the day of visualization of this plug was counted as E0.5. Findings on all embryos were documented; tissue was fixed in Bouin's solution and stored, and mutants were identified by Southern blot analysis. For histological analysis, tissue was embedded in paraffin, sectioned at 6 μm, and stained with hematoxylin-eosin.
Flow cytometry
MoFlow or FACS-Calibur flow cytometers were used for analysis and sorting. Where appropriate, red cells were lysed by brief exposure to ammonium buffer (NH4CL 0.15 M/KHCO3 0.01 M/Na2 EDTA 0.1 mM), and nonspecific binding was reduced by preincubation with unconjugated antibody to FcγII/III (2.4G2). Dead cells were excluded by propidium iodide (Molecular Probes), TO-PRO1 (Molecular Probes), or by forward/side light-scatter profile. Antibody conjugates and matched isotype controls were obtained from PharMingen or eBioscience. IgM (R6-60, FITC), CD4 (RM4-5, PE), CD8 (53-6, PerCP), CD3ε (145-2C11, FITC), Ter119 PE, B220 (RA3-6B2, FITC), Gr1 (RB6-8C5, APC), Mac1 (M1/70, PE). HSC-stain: lineage markers CD4 (RM4-5), CD8 (53-6), CD3ε (145-2C11), TCRβ (H54-597), B220 (RA3-6B2), CD19 (6D5), IgM (R6-60), Gr1 (RB6-8C5), Mac-1 (M1/70), Ter119 (all FITC); CD117 (c-kit, 2B8, APCCy7); Sca-1 (E13-161, PE). CD45 isotype analysis: B220 (RA3-6B2, APC), Gr1 (RB6-8C5, APC), Mac1 (M1/70,PE), CD117 (c-kit, 2B8, APC), CD45.1 (104, FITC), CD45.2 (A20, PE).
Blood counts
Blood samples (250 μL) were obtained by tail vein nicking or retro-orbital sinus puncture, anticoagulated in EDTA, and analyzed using an automated system (Advia 120, Bayer).
Administration of pIpC
Poly(I)-poly(C) (Sigma P-0913) was diluted in endotoxin-free D-PBS (Gibco) at a concentration of 2 mg/mL and passed through a 0.22-μm filter (Millipore) before intraperitoneal injection. Mice received seven doses (every other day) of ∼25 μg/g body weight. The day of the last injection of pIpC was designated day 0.
Bone marrow transplantation
Bone marrow transplantations were performed by intravenous injection of bone marrow cells from 6-10-week-old donor mice (C57BL6/129 mixed) and/or competitor bone marrow into recipients that had received 1200 cGy of radiation as a split dose. Recipients were C57BL6x129 F1 (101043, Jackson). The source of competitor bone marrow cells for competitive repopulation assays was B6.SJL (002014, Jackson)
Acknowledgments
We thank Dr. Lichun Wang for help with targeting the Tel/Etv6 locus, and Dr. Christoph Klein and Dr. Richard Mulligan for help with retroviral gene transfer and the vector CMMP. We thank Dr. Klaus Rajewsky for Mx-Cre and CD19-Cre mice, and Dr. J. Marth for Lck-Cre mice. We appreciate the expert assistance of Maris Handley, Herb Levine, Joyce A. Lavecchio, Melanie Hamblen, Carol Browne, Shelley Galusha and Aimee Williams. We thank Dr. Heather Rooke and Dr. Charles Roberts for critical reading of the manuscript. H.H. was supported by a Special Fellowship of the Leukemia Society of America and by an NCI Career Development award (1 KO8 CA096701-01). This work was in part supported by a Center of Excellence grant in Molecular Hematology from the NIH. S.H.O. is an Investigator with the Howard Hughes Medical Institute.
Supplemental material is available at http://www.genesdev.org.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.1239604.
References
- Brakebusch C., Fillatreau, S., Potocnik, A.J., Bungartz, G., Wilhelm, P., Svensson, M., Kearney, P., Korner, H., Gray, D., and Fassler, R. 2002. β1 integrin is not essential for hematopoiesis but is necessary for the T cell-dependent IgM antibody response. Immunity 16: 465-477. [DOI] [PubMed] [Google Scholar]
- Brun A.C., Bjornsson, J.M., Magnusson, M., Larsson, N., Leveen, P., Ehinger, M., Nilsson, E., and Karlsson, S. 2004. Hoxb4 deficient mice have normal hematopoietic development but exhibit a mild proliferation defect in hematopoietic stem cells. Blood. 103(11): 4126-4133. [DOI] [PubMed] [Google Scholar]
- Cheng T., Rodrigues, N., Shen, H., Yang, Y., Dombkowski, D., Sykes, M., and Scadden, D.T. 2000. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287: 1804-1808. [DOI] [PubMed] [Google Scholar]
- Cheshier S.H., Morrison, S.J., Liao, X., and Weissman, I.L. 1999. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc. Natl. Acad. Sci. 96: 3120-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobas M., Wilson, A., Ernst, B., Mancini, S.J., MacDonald, H.R., Kemler, R., and Radtke, F. 2004. β-catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 199: 221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber H.P., Malik, A.K., Solar, G.P., Sherman, D., Liang, X.H., Meng, G., Hong, K., Marsters, J.C., and Ferrara, N. 2002. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417: 954-958. [DOI] [PubMed] [Google Scholar]
- Golub T.R., Barker, G.F., Lovett, M., and Gilliland, D.G. 1994. Fusion of PDGF receptor β to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 77: 307-316. [DOI] [PubMed] [Google Scholar]
- Han H., Tanigaki, K., Yamamoto, N., Kuroda, K., Yoshimoto, M., Nakahata, T., Ikuta, K., and Honjo, T. 2002. Inducible gene knockout of transcription factor recombination signal binding protein-J. reveals its essential role in T versus B lineage decision. Int. Immunol. 14: 637-645. [DOI] [PubMed] [Google Scholar]
- Ichikawa M., Asai, T., Saito, T., Yamamoto, G., Seo, S., Yamazaki, I., Yamagata, T., Mitani, K., Chiba, S., Hirai, H., et al. 2004. AML-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nat. Med. 10: 299-304. [DOI] [PubMed] [Google Scholar]
- Italiano J.E. and Shivdasani, R.A. 2003. Megakaryocytes and beyond: The birth of platelets. J. Thromb. Haemost. 1: 1174-1182. [DOI] [PubMed] [Google Scholar]
- Kondo M., Wagers, A.J., Manz, M.G., Prohaska, S.S., Scherer, D.C., Beilhack, G.F., Shizuru, J.A., and Weissman, I.L. 2003. Biology of hematopoietic stem cells and progenitors: Implications for clinical application. Annu. Rev. Immunol. 21: 759-806. [DOI] [PubMed] [Google Scholar]
- Kuhn R., Schwenk, F., Aguet, M., and Rajewsky, K. 1995. Inducible gene targeting in mice. Science 269: 1427-1429. [DOI] [PubMed] [Google Scholar]
- Kumano K., Chiba, S., Kunisato, A., Sata, M., Saito, T., Nakagami-Yamaguchi, E., Yamaguchi, T., Masuda, S., Shimizu, K., Takahashi, T., et al. 2003. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18: 699-711. [DOI] [PubMed] [Google Scholar]
- Lai Z.C. and Rubin, G.M. 1992. Negative control of photoreceptor development in Drosophila by the product of the yan gene, an ETS domain protein. Cell 70: 609-620. [DOI] [PubMed] [Google Scholar]
- Lessard J. and Sauvageau, G. 2003. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 423: 255-260. [DOI] [PubMed] [Google Scholar]
- Mikkola H.K., Klintman, J., Yang, H., Hock, H., Schlaeger, T.M., Fujiwara, Y., and Orkin, S.H. 2003. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature 421: 547-551. [DOI] [PubMed] [Google Scholar]
- Okuda T., van Deursen, J., Hiebert, S.W., Grosveld, G., and Downing, J.R. 1996. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84: 321-330. [DOI] [PubMed] [Google Scholar]
- Orkin S.H. 2000. Diversification of haematopoietic stem cells to specific lineages. Nat. Rev. Genet. 1: 57-64. [DOI] [PubMed] [Google Scholar]
- Osawa M., Hanada, K., Hamada, H., and Nakauchi, H. 1996. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273: 242-245. [DOI] [PubMed] [Google Scholar]
- Park I.K., Qian, D., Kiel, M., Becker, M.W., Pihalja, M., Weissman, I.L., Morrison, S.J., and Clarke, M.F. 2003. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423: 302-305. [DOI] [PubMed] [Google Scholar]
- Radtke F., Wilson, A., Stark, G., Bauer, M., van Meerwijk, J., MacDonald, H.R., and Aguet, M. 1999. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10: 547-558. [DOI] [PubMed] [Google Scholar]
- Reya T., Duncan, A.W., Ailles, L., Domen, J., Scherer, D.C., Willert, K., Hintz, L., Nusse, R., and Weissman, I.L. 2003. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423: 409-414. [DOI] [PubMed] [Google Scholar]
- Saito T., Chiba, S., Ichikawa, M., Kunisato, A., Asai, T., Shimizu, K., Yamaguchi, T., Yamamoto, G., Seo, S., Kumano, K., et al. 2003. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity 18: 675-685. [DOI] [PubMed] [Google Scholar]
- Sauvageau G., Thorsteinsdottir, U., Eaves, C.J., Lawrence, H.J., Largman, C., Lansdorp, P.M., and Humphries, R.K. 1995. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes & Dev. 9: 1753-1765. [DOI] [PubMed] [Google Scholar]
- Shivdasani R.A., Mayer, E.L., and Orkin, S.H. 1995. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature 373: 432-434. [DOI] [PubMed] [Google Scholar]
- Tsai F.Y., Keller, G., Kuo, F.C., Weiss, M., Chen, J., Rosenblatt, M., Alt, F.W., and Orkin, S.H. 1994. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371: 221-226. [DOI] [PubMed] [Google Scholar]
- Uchida N. and Weissman, I.L. 1992. Searching for hematopoietic stem cells: Evidence that Thy-1.1lo Lin- Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J. Exp. Med. 175: 175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lugt N.M., Domen, J., Linders, K., van Roon, M., Robanus-Maandag, E., te Riele, H., van der Valk, M., Deschamps, J., Sofroniew, M., van Lohuizen, M., et al. 1994. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes & Dev. 8: 757-769. [DOI] [PubMed] [Google Scholar]
- Wang L.C., Kuo, F., Fujiwara, Y., Gilliland, D.G., Golub, T.R., and Orkin, S.H. 1997. Yolk sac angiogenic defect and intra-embryonic apoptosis in mice lacking the Ets-related factor TEL. EMBO J. 16: 4374-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.C., Swat, W., Fujiwara, Y., Davidson, L., Visvader, J., Kuo, F., Alt, F.W., Gilliland, D.G., Golub, T.R., and Orkin, S.H. 1998. The TEL/ETV6 gene is required specifically for hematopoiesis in the bone marrow. Genes & Dev. 12: 2392-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Crispino, J.D., Letting, D.L., Nakazawa, M., Poncz, M., and Blobel, G.A. 2002. Control of megakaryocyte-specific gene expression by GATA-1 and FOG-1: Role of Ets transcription factors. EMBO J. 21: 5225-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]