Abstract
Netherton syndrome (NS) is a human autosomal recessive skin disease caused by mutations in the SPINK5 gene, which encodes the putative proteinase inhibitor LEKTI. We have generated a transgenic mouse line with an insertional mutation that inactivated the mouse SPINK5 ortholog. Mutant mice exhibit fragile stratum corneum and perinatal death due to dehydration. Our analysis suggests that the phenotype is a consequence of desmosomal fragility associated with premature proteolysis of corneodesmosin, an extracellular desmosomal component. Our mouse mutant provides a model system for molecular studies of desmosomal stability and keratinocyte adhesion, and for designing therapeutic strategies to treat NS.
Keywords: Spink5, LektI, proteinase inhibitor, Netherton Syndrome, desmosomes, Cdsn
Netherton Syndrome (NS) (MIM 256500) is a severe, recessively inherited skin disease in humans with high neonatal lethality. It is characterized by ichthyosiform erythroderma, atopic dermatitis, bamboo hair, skin barrier defects, and elevated IgE levels in survivors (Krafchik and Toole 1983; Judge et al. 1994). Mutations have been identified in the SPINK5 (serine proteinase inhibitor Kazal type 5) gene of NS patients (Chavanas et al. 2000; Sprecher et al. 2001; Walley et al. 2001; Bitoun et al. 2002; Komatsu et al. 2002). SPINK5 encodes LEKTI (lympho-epithelial Kazal-type-related inhibitor), which is a putative proteinase inhibitor that contains an N-terminal signal peptide and 15 domains with high internal homology (Magert et al. 1999). Each domain has four conserved cysteines. Domains 2 and 15 possess two additional cysteines, which make them typical Kazal-type proteinase inhibitor domains (Magert et al. 1999). LEKTI exhibits proteinase inhibitor activity in vitro (Magert et al. 1999; Komatsu et al. 2002; Walden et al. 2002; Mitsudo et al. 2003). In NS patients, loss or reduction of LEKTI activity is presumed to result in elevated proteolytic activity in the suprabasal epidermis, leading to erythroderma and skin-barrier defects. However, the specific proteins that are targeted for degradation in these patients have not been identified. We describe here a Spink5 mutant mouse line that shows severe skin defects associated with desmosomal fragility, and thus, provides insights into the molecular pathogenesis of NS and a novel model system for studies of keratinocyte adhesion.
Results and Discussion
Transgenic mouse line OVE1498 was generated by coinjection of a tyrosinase-tagged (Yokoyama et al. 1990) Sleeping Beauty transposon (Ivics et al. 1997) (termed pT-Tybs-3′E) along with PGK2-SB10 (Ivics et al. 1997) (see Supplementary Fig. S1) into inbred FVB/N embryos. The transgenic founder and its transgenic F1 offspring were phenotypically normal and showed no evidence for transposition of the transgene(s) (data not shown). When transgenic F1 mice were intercrossed, ∼25% of the newborn offspring developed severe skin blistering and water barrier defects, leading to death within several hours after birth (Fig. 1A). This observation suggested that this transgenic line carried a recessive lethal insertional mutation.
Figure 1.
Insertional inactivation of the Spink5 gene in mice. (A) Mutant phenotype. A typical OVE1498 neonatal homozygote (MT) is shown on the left. The newborn mutant has a severe skin disorder with peeling of the stratum corneum and onset of dehydration. (WT) Wild-type littermate. (B) Schematic drawing of the transgenic integration site in OVE1498. Integration was accompanied by deletion of all exons of the Spink5 gene on mouse chromosome 18. The chromosomal locations of the predicted start and stop codons of mSpink5, the integration junctions, and the closest upstream and downstream genes (Ttid [NM_021484] and loc225443 [XM_129009]) are indicated (Ensemble mouse genome database). Primers used for genotyping are denoted with red arrows.
To identify the transgenic integration site, we used an inverse PCR approach (Ochman et al. 1988) to amplify genomic sequences flanking the right arm of the tyrosinase-tagged transgene (see Supplementary Fig. S2). Sequences linked to the transgene-matched mouse genomic sequences from contig NT_078853 on chromosome 18B3 (data not shown). The junction between transgenic and genomic sequences was located 6 kb downstream from the stop codon of mouse gene 2310065D10Rik (NCBI accession no. XM_283487).
PCR walking was used to identify the other integration junction (see Supplemental Material). The left junction was found to be 3.8 kb upstream of the start codon of the 2310065D10Rik gene. Integration of the transgenic DNA was accompanied by a deletion of 66.8 kb in mouse chromosome 18, including the entire coding region of 2310065D10Rik, but excluding any exons from the predicted adjacent genes (Fig. 1B). The adjacent genes (Loc225443 and Ttid) are expressed at normal levels by RT-PCR analysis in the mutant newborns (Supplementary Fig. S3).
The human cDNA with the highest homology (68%) to 2310065D10Rik is the SPINK5 cDNA. SPINK5 maps to chromosome 5q32, which is syntenic to mouse chromosome 18B3. SPINK5 has 33 exons and 2310065D10Rik has 32 exons. The positions of the exon-intron junctions are well conserved between mouse and human (see Supplementary Fig. S4). On the basis of the homology of the genes and the similarity of the mutant phenotypes, we propose that 2310065D10Rik and the protein encoded by this gene should be named Spink5 and LektI, respectively, and we will refer to them as such. The protein sequence of LektI is 58% identical and 71% homologous to human LEKTI. The two proteins have a similar domain organization, including two Kazal-type domains, domains 2 and 15 (Supplementary Fig. S4). Domain 6 of LEKTI is completely absent from LektI. In addition, there are 30 extra amino acids between domains 13 and 14 in LektI (Supplementary Fig. S4). We also compared LektI with the putative rat LektI protein (rLektI), which is encoded by Loc361319 (XM_341607) (Supplementary Fig. S4).
Spink5 expression in perinatal skin was analyzed by RT-PCR (Fig. 2A). Expression was detected in wild-type prenatal (embryonic days 16.5-18.5 [E16.5-E18.5]) and neonatal (P0) skin (Fig. 2A). No transcript was amplified from homozygous mutant skin. By in situ hybridization, Spink5 transcripts were detected specifically in the upper spinous and granular layers of newborn skin (Fig. 2B). The transcript was also detected in hair follicles of adult skin (Fig. 2B). This expression pattern is similar to that reported for human SPINK5 (Komatsu et al. 2003).
Figure 2.
Expression analysis of Spink5. (A) RT-PCR using E16.5 to P0 wild-type skin cDNA (wild type, WT) and P0 mutant skin cDNA (MT). HPRT was used as a positive control. (B) In situ hybridization on wild-type P0 and adult skin. Spink5 transcripts were detected in the granular cells (GR) of P0 skin and adult skin, and also in adult hair follicles (hf). The dashed lines indicate the boundary between dermis and epidermis. (SC) Stratum corneum; (GR) granular layer; (SP) spinous layer; (BL) basal layer; (hf) hair follicle; (de) dermis. Bar, 50 μM.
On the basis of histological sections, it appeared that skin development in Spink5-/- mouse embryos was not significantly altered at E15.5 (data not shown). By E17.5, however, the stratum corneum (SC) had begun to peel off and focal detachment of granular cells was observed (data not shown). At birth (P0), detachment of the SC was widespread (Fig. 3A). The spinous layer in mutant skin appeared to be normal. To determine whether the Spink5-/- mutation caused significant changes in epidermal gene expression, we analyzed a battery of skin differentiation markers by immunohistochemistry on P0 skin. Keratin 14, keratin 5, keratin 1, loricrin, filaggrin, desmoglein1, and desmoglein2 (Dsg1, Dsg2) and desmocollin 1 (Dsc1) (Cheng and Koch 2004) were expressed in a normal pattern in Spink5-/- skin (data not shown). Expression of keratin 6, a stress marker, was elevated in the mutant granular layer (data not shown). Although elevated expression of keratin 6 can indicate epidermal hyperplasia, BrdU incorporation was restricted to the basal keratinocytes and occurred at comparable levels in Spink5-/- embryos (E19) and in wild-type controls (data not shown).
Figure 3.
Histology and epidermal barrier assay on Spink5-/- mice. (A) Histology of P0 Spink5-/- (MT) and wild-type (WT) mouse skin. The stratum corneum has detached and is not seen in the mutant skin. In addition, granular cells are seen prematurely scaling off from the mutant skin (arrows). (SC) Stratum corneum; (GR) granular layer; (SP) spinous layer; (BL) basal layer; (hf) hair follicle; (de) dermis. Bar, 50 μM. (B) Epidermal barrier assays by X-gal staining. Wild-type and Spink5-/- embryos and neonates were incubated with X-gal. Skin without a functional epidermal water barrier stains blue.
Because newborn Spink5-/- mice were typically found as dehydrated corpses within 24 h after birth, we assayed for changes in skin barrier development by X-gal staining (Hardman et al. 1998). This procedure uses incubation at an acidic pH to detect endogenous β-glycosidase activity. At E17.5 and E19, mutant skin was largely unstained, which showed evidence for skin-barrier formation. Nevertheless, focal lack of barrier function (i.e., blue staining) was observed in the articular regions such as the knees, elbows, cheeks, and neck (Fig. 3B). After birth, Spink5-/- mice stained blue over their entire body surface, demonstrating complete loss of barrier function (Fig. 3B). These results are reminiscent of clinical observations in neonatal patients with severe NS, where skin lesions occur preferentially in articular regions and aggravate soon after birth with generalized exfoliative erythroderma (Komatsu et al. 2002; Muller et al. 2002).
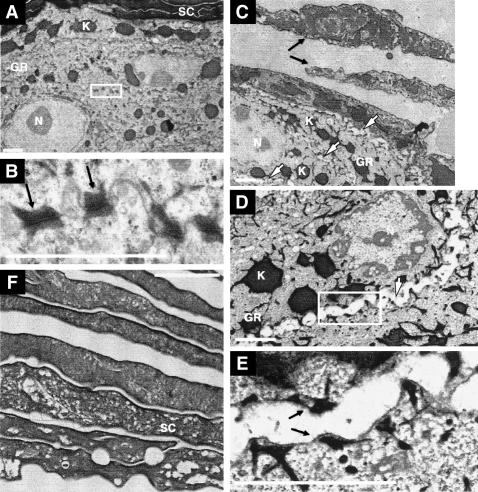
Transmission electron microscopy on skin samples from newborn Spink5-/- and wild-type littermates revealed abnormal cornification in the mutant mice (Fig. 4C). Specifically, we observed loosely interconnected corneocytes that contained abnormal low electron-dense vesicles in their cytoplasm (Fig. 4F), a phenomenon observed in human NS samples as well (Muller et al. 2002). Furthermore, we found a distinctive architectural loss of cell-cell adhesion (acantholysis) in the granular layer of mutant epidermis (Fig. 4D). Half desmosomes with attached keratin filaments were visible at the plasma membranes of most granular cells (Fig. 4E). This desmosomal defect is reminiscent of the ultrastructural lesions observed in the skin of pemphigus foliaceus (PF) patients, who suffer from suprabasal intraepidermal blistering due to the presence of Dsg1 auto-antibodies (Stanley 2000; Stanley et al. 2001).
Figure 4.
Transmission electron microscopy on P0 wild-type (A,B) and Spink5-/- (C-F) skin. (A) Stratum corneum (SC) and granular layer (GR) of wild-type mice. The granular cells are compact and highly interconnected. (N) Nucleus; (K) keratohyalin granules. (B) Higher magnification of the boxed region from A. Typical desmosomes with keratin intermediate filaments attached to the cytoplasmic plaque (black arrows) are shown. (C) GR of Spink5-/- mice. Detaching, incompletely cornified corneocytes are visible (black arrows). Note the acantholysis evident in the GR (white arrows). (D) Subcorneal acantholysis of Spink5-/- mice (white arrow). (E) A higher magnification of the boxed region from D. Half desmosomes with attached keratin intermediate filaments are visible on the bottom and roof of the blister (black arrows). (F) Spink5-/- corneocytes with abnormal vesicles. Bars, 2 μm.
The similarities in the desmosomal defects of PF patients and the Spink5-/- mice suggested that the stability of desmosomal proteins might be altered in our mutant mice. Because the Spink5 gene encodes a putative secreted proteinase inhibitor, we tested for an increase in proteinase activity using an in situ zymogram (ISZ) assay. The mutant SC exhibited higher caseinolytic activity than wild-type SC, and increased proteinase activity was also detected in the upper spinous and granular layers of Spink5-/- skin (Fig. 5A). Therefore, the desmosomal defect seen in the mutant epidermis might result from an abnormal increase in proteinase activity that leads to premature degradation of extracellular desmosomal component(s).
Figure 5.
Increased proteolytic activity and corneodesmosin degradation in neonatal Spink5-/- skin. (A) In situ zymogram assay on P0 wild-type (WT) and Spink5-/- mutant (MT) skin for caseinolytic activity. White arrows indicate the boundary of stratum corneum (SC) and granular layer (GR). Phase-contrast images from the same visual fields are shown in the lower left corners of both fluorescence images. Bar, 50 μM. Preincubation with SBTI resulted in the complete absence of green fluorescence for both wild-type and mutant skin (data not shown). (B) Western blot assay on desmosomal proteins from wild-type (WT) and mutant (MT) P0 skin. Desmocollin1 (Dsc1) and desmogleins 1/2 (Dsg1/2) are detected in the detergent-insoluble fraction in both wild-type (WT1, WT2) and mutant (MT1, MT2) skin. No significant reduction in the steady-state level of either protein was detected in the mutant samples. Full-length corneodesmosin (Cdsn) protein (∼75 kDa) was present in the detergent-soluble fraction of both the wild-type and mutant skin. In the insoluble fraction, note the decrease in the 45-60-kDa Cdsn cluster and the increase in the 36-40-kDa cluster in mutant skin. Sizes of the protein bands are indicated on the right. (H) Mouse hemoglobin heavy chain that binds nonspecifically to the CDSN antibody (Montezin et al. 1997).
The major transmembrane desmosomal proteins in the upper spinous and granular layers are Dsg1 and Dsc1 (Garrod et al. 2002b). It is thought that heterophilic interactions between these two types of transmembrane glycoproteins establish desmosome-mediated cell-cell adhesion (Schwarz et al. 1990; Garrod et al. 2002a,b; Cheng and Koch 2004). Another extracellular component with a less well-defined function is corneodesmosin (CDSN) (Simon et al. 1997, 2001). Human CDSN is a 52-56-kDa glycoprotein expressed in the upper spinous and granular layers of normal skin, and in the inner root sheath of hair follicles (Levy-Nissenbaum et al. 2003). This protein is secreted into the intercellular space and becomes an extracellular component of desmosomes in the granular layer and of corneodesmosomes (corneocyte-specific desmosomes) in the SC (Simon et al. 1997, 2001). CDSN has been hypothesized to stabilize desmosomes and to become covalently associated with the cornified cell envelope (CE) (Simon et al. 1997). Furthermore, expression of a chimeric protein consisting of the N-terminal domain of CDSN and the transmembrane domain of E- cadherin promotes cell-cell aggregation in transfection experiments, suggesting that CDSN might function as a homophilic adhesion molecule (Jonca et al. 2002). Within normal skin, CDSN is progressively proteolysed during cornification and desquamation (Simon et al. 1997, 2001). It has been hypothesized that the controlled degradation of CDSN leads to a destabilization of corneodesmosomes during physiological desquamation (Simon et al. 1997, 2001).
To determine whether Dsg1, Dsc1, or Cdsn (mouse ortholog of human CDSN, XP_111684), were affected in newborn Spink5-/- skin, we used Western blotting with Triton X-100 (TX 100)-soluble and TX 100-insoluble protein extracts (Fig. 5B). The TX-100-insoluble fraction contains proteins integrated into mature desmosomes. Dsc1 and Dsg1 are mainly present in the TX 100-insoluble fraction (Pasdar and Nelson 1988). Using Dsc1 and Dsg1/2 antibodies (Cheng et al. 2004), we did not detect a significant reduction in the steady-state level of Dsc1 or Dsg1/2 in either the TX 100-soluble or TX 100-insoluble fractions of Spink5-/- skin (Fig. 5B).
Next, we probed Western blots with an antibody (F28-F27) generated against human CDSN (Montezin et al. 1997). In humans, this antibody recognizes three clusters of bands as follows: a full-length cluster (52-56 kDa), which is mainly found in the keratinosomes and present in the detergent-soluble pool; an intermediate-size cluster (40-48 kDa), which primarily exists in the detergent-insoluble fraction and is thought to contribute to desmosome stabilization; and a small-size cluster (33-36 kDa), which is normally present in the uppermost SC, and probably lacks adhesive properties (Montezin et al. 1997; Simon et al. 1997, 2001). In mouse skin, F28-F27 also recognizes three Cdsn clusters, but the sizes are slightly different from those observed in humans, that is, 75 kDa, 45-60 kDa, and 36-40 kDa, respectively (Montezin et al. 1997). The intermediate-sized 45-60-kDa cluster seen in wild-type skin was nearly absent in Spink5-/- skin, and instead, nearly all of the insoluble Cdsn was 36-40 kDa, which could be caused by inappropriately premature proteolysis (Fig. 5B). On the basis of these results, we hypothesize that in the absence of LektI, the premature proteolysis of Cdsn/CDSN in Spink5-/- mice/NS patients may contribute significantly to desmosomal fragility, epidermal detachment, and skin barrier defects.
CDSN degradation and dissolution of corneodesmosome-mediated adhesion are normal processes in the stratum corneum. In our mouse model, as well as human NS patients, absence of LektI/LEKT1 leads to a premature activation of proteinases involved in this process. Serine proteinases SCCE (stratum corneum trypsin-like enzyme) (Hansson et al. 1994) and SCTE (stratum corneum chymotrypsin-like enzyme) (Brattsand and Egelrud 1999), which are synthesized in granular cells and secreted into the intercellular space, can both digest human CDSN-producing proteolytic products of 30-33 kDa (Simon et al. 2001; Caubet et al. 2004). They may cooperate with LEKTI to regulate the CDSN stability in the suprabasal epidermis.
Mutations that produce truncated forms of CDSN have been described in humans (Levy-Nissenbaum et al. 2003). These mutations lead to a dominant hair disorder (hypotrichosis simplex of the scalp), implying that CDSN plays a role in hair-follicle growth and maintenance (Levy-Nissenbaum et al. 2003). So far, no homozygous CDSN/Cdsn mutants have been reported in humans or mice.
In summary, our results show a link between NS skin defects and impaired desmosome function in the subcorneal epidermis, and provide a possible molecular mechanism for the pathogenesis of NS. Spink5-/- mice mimic severe NS, and can be used to design and test perinatal therapies for treatment of the disease. In addition, our mouse can be used to study desmosomal maturation and stabilization in the suprabasal layers of the epidermis.
Materials and methods
Genotyping of mutant allele by PCR
Tail and yolk sack DNA samples were used as PCR templates. Primers R3 (CCACTGGGAATGTGATGAAAGAAATAAAAGC), R-WT-s (GGCATATAGTGTGTTTGGAGC), and R-WT-as (GGCATCCATTAGTTTATCTCTTACTCCAG) were designed based on the right insertional junction (Fig. 1B). R3 and R-WT-as generate a 272-bp band from the mutant allele. R-WT-s and R-WT-as produce a 247-bp band from the wild-type allele.
RT-PCR
Total RNA from wild-type and mutant dorsal skin was extracted with the RNeasy Mini Kit (QIAGEN). First-strand cDNA was synthesized using the Superscript cDNA first strand synthesis kit (Invitrogen). Spink5-exon3-s (TGGTACACTGACGTGTCCCAAAG) and Spink5-exon7-as (GGATTGGATCACTTTCCCGTGT) primers were used to amplify a 434-bp Spink5 cDNA from wild-type samples. HPRT-s (ATGACCTAGATTTGTTTTGTATACC) and HPRT-as (GTAGCTCTTCAGTCTGATAAAATCTAC) primers were used to amplify hypoxanthine-guanine phosphoribosyltransferase (HPRT) cDNA as a positive control.
Epidermal permeability assay
Embryos or neonates were briefly washed with 0.9% NaCl and immediately immersed into acidic X-gal mix (100 mM phosphate buffer at pH 4.3, 3 mM K3Fe(CN)6, 3 mM K4Fe(CN)6, 2 mM MgCl2 1 mg/mL X-gal), then incubated for 8 h at room temperature in the dark (Hardman et al. 1998). To avoid damage to the neonatal epidermis, we separated pups from their mothers immediately after birth. In the absence of a mature epidermal barrier, X-gal penetrates into the cells of the epidermis where it is hydrolyzed to produce a blue color.
In situ hybridization
A Spink5 cDNA fragment generated by RT-PCR with Spink5-exon3-s and Spink5-exon7-as primers was subcloned into the pGEM-T easy vector (Promega) and used as a template for generating an antisense RNA Probe. Digoxygenin-UTP, T7 RNA polymerase, and the Dig Nucleic Acid Detection Kit (Roche) were used to label the probe and to detect hybridization. (The nonradioactive in situ Protocol is described online at http://www.roche-applied-science.com/prod_inf/manuals/InSitu/pdf/ISH_149-163.pdf.)
In situ zymogram
BODIPYL FL casein, green fluorescein conjugate (EnzChek Proteinase Assay Kit; Molecular Probes) was used for in situ zymography (ISZ) (Kheradmand et al. 2002). Briefly, the intramolecularly quenched substrate (BODIPYL FL casein) was reconstituted according to the manufacturer's recommendations and was diluted 1:1 in 1% agar immediately before addition to cryostat sections of newborn skin, followed by an incubation for up to 24 h at 37°C. Fluorescence intensity was monitored every 2 h forthe first 10 h, then at 24 h. Skin sections from wild-type and Spink5-/- mice were photographed at equal time points and exposure time. For inhibition of serine proteinase activity, cryostat sections of the skin were incubated with 100 mM of soybean trypsin inhibitor (SBTI, Boehringer Mannheim).
Western blots
P0 dorsal skin was pulverized in liquid nitrogen and extracted for 15 min in lysis buffer (10 mM Tris-Cl at pH 7.4, 5 mM EDTA, 100 mM NaCl, 1% Triton X-100 with Complete Proteinase Inhibitors Cocktail from Roche). Soluble and insoluble fractions were separated by centrifugation. The insoluble pellets were dissolved with 5× Laemmli buffer (0.05 M Tris-Cl, 4% SDS, 8% glycerol, with 20% β-Mercaptoethanol) at 100°C for 20 min. Equal amounts of protein (determined using the Protein Assay Dye Reagent from Bio-Rad) were fractionated on 4%-15% gradient SDS-polyacrylamide gels (Bio-Rad), then transferred to PVDF membranes (Bio-Rad). The following primary antibodies were used: Dsg3.10 (1:800; from RDI, detects Dsg1/2), Dsc1 antibody (Cheng et al. 2004) (1:800), and corneodesmosin antibody F28-F27 (1:1000, from Dr. Guy Serre, University of Toulouse III, Toulouse, France).
Acknowledgments
We thank Dr. Guy Serre for generously sharing the CDSN antibody. We thank Kusal Mihindukulasuriya for his excellent technical support. We thank Drs. Brendan Lee and Jianming Xu for equipment. We also thank Dr. Dennis R. Roop for reviewing this manuscript and valuable discussions and suggestion. This research was supported by NIH grants AR45316, AR47898 and EY10448.
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1232104.
Corresponding authors.
References
- Bitoun E., Chavanas, S., Irvine, A.D., Lonie, L., Bodemer, C., Paradisi, M., Hamel-Teillac, D., Ansai, S., Mitsuhashi, Y., Taieb, A., et al. 2002. Netherton syndrome: Disease expression and spectrum of SPINK5 mutations in 21 families. J. Invest Dermatol. 118: 352-361. [DOI] [PubMed] [Google Scholar]
- Brattsand M. and Egelrud, T. 1999. Purification, molecular cloning, and expression of a human stratum corneum trypsin-like serine protease with possible function in desquamation. J. Biol. Chem. 274: 30033-30040. [DOI] [PubMed] [Google Scholar]
- Caubet C., Jonca, N., Brattsand, M., Guerrin, M., Bernard, D., Schmidt, R., Egelrud, T., Simon, M., and Serre, G. 2004. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J. Invest Dermatol. 122: 1235-1244. [DOI] [PubMed] [Google Scholar]
- Chavanas S., Bodemer, C., Rochat, A., Hamel-Teillac, D., Ali, M., Irvine, A.D., Bonafe, J.L., Wilkinson, J., Taieb, A., Barrandon, Y., et al. 2000. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat. Genet. 25: 141-142. [DOI] [PubMed] [Google Scholar]
- Cheng X. and Koch, P.J. 2004. In vivo function of desmosomes. J. Dematol. 31: 171-187. [DOI] [PubMed] [Google Scholar]
- Cheng X., Mihindukulasuriya, K., Den, Z., Kowalczyk, A.P., Calkins, C.C., Ishiko, A., Shimizu, A., and Koch, P.J. 2004. Assessment of splice variant-specific functions of desmocollin 1 in the skin. Mol. Cell Biol. 24: 154-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D.R., Merritt, A.J., and Nie, Z. 2002a. Desmosomal adhesion: Structural basis, molecular mechanism and regulation (Review). Mol. Membr. Biol. 19: 81-94. [DOI] [PubMed] [Google Scholar]
- ____. 2002b. Desmosomal cadherins. Curr. Opin. Cell Biol. 14: 537-545. [DOI] [PubMed] [Google Scholar]
- Hansson L., Stromqvist, M., Backman, A., Wallbrandt, P., Carlstein, A., and Egelrud, T. 1994. Cloning, expression, and characterization of stratum corneum chymotryptic enzyme. A skin-specific human serine proteinase. J. Biol. Chem. 269: 19420-19426. [PubMed] [Google Scholar]
- Hardman M.J., Sisi, P., Banbury, D.N., and Byrne, C. 1998. Patterned acquisition of skin barrier function during development. Development 125: 1541-1552. [DOI] [PubMed] [Google Scholar]
- Ivics Z., Hackett, P.B., Plasterk, R.H., and Izsvak, Z. 1997. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 91: 501-510. [DOI] [PubMed] [Google Scholar]
- Jonca N., Guerrin, M., Hadjiolova, K., Caubet, C., Gallinaro, H., Simon, M., and Serre, G. 2002. Corneodesmosin, a component of epidermal corneocyte desmosomes, displays homophilic adhesive properties. J. Biol. Chem. 277: 5024-5029. [DOI] [PubMed] [Google Scholar]
- Judge M.R., Morgan, G., and Harper, J.I. 1994. A clinical and immunological study of Netherton's syndrome. Br. J. Dermatol. 131: 615-621. [DOI] [PubMed] [Google Scholar]
- Kheradmand F., Rishi, K., and Werb, Z. 2002. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J. Cell Sci. 115: 839-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N., Takata, M., Otsuki, N., Ohka, R., Amano, O., Takehara, K., and Saijoh, K. 2002. Elevated stratum corneum hydrolytic activity in Netherton syndrome suggests an inhibitory regulation of desquamation by SPINK5-derived peptides. J. Invest Dermatol. 118: 436-443. [DOI] [PubMed] [Google Scholar]
- Komatsu N., Takata, M., Otsuki, N., Toyama, T., Ohka, R., Takehara, K., and Saijoh, K. 2003. Expression and localization of tissue kallikrein mRNAs in human epidermis and appendages. J. Invest Dermatol. 121: 542-549. [DOI] [PubMed] [Google Scholar]
- Krafchik B.R. and Toole, J.W. 1983. What is Netherton's syndrome? Int. J. Dermatol. 22: 459-462. [DOI] [PubMed] [Google Scholar]
- Levy-Nissenbaum E., Betz, R.C., Frydman, M., Simon, M., Lahat, H., Bakhan, T., Goldman, B., Bygum, A., Pierick, M., Hillmer, A.M., et al. 2003. Hypotrichosis simplex of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat. Genet. 34: 151-153. [DOI] [PubMed] [Google Scholar]
- Magert H.J., Standker, L., Kreutzmann, P., Zucht, H.D., Reinecke, M., Sommerhoff, C.P., Fritz, H., and Forssmann, W.G. 1999. LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J. Biol. Chem. 274: 21499-21502. [DOI] [PubMed] [Google Scholar]
- Mitsudo K., Jayakumar, A., Henderson, Y., Frederick, M.J., Kang, Y., Wang, M., El Naggar, A.K., and Clayman, G.L. 2003. Inhibition of serine proteinases plasmin, trypsin, subtilisin A, cathepsin G, and elastase by LEKTI: A kinetic analysis. Biochemistry 42: 3874-3881. [DOI] [PubMed] [Google Scholar]
- Montezin M., Simon, M., Guerrin, M., and Serre, G. 1997. Corneodesmosin, a corneodesmosome-specific basic protein, is expressed in the cornified epithelia of the pig, guinea pig, rat, and mouse. Exp. Cell Res. 231: 132-140. [DOI] [PubMed] [Google Scholar]
- Muller F.B., Hausser, I., Berg, D., Casper, C., Maiwald, R., Jung, A., Jung, H., and Korge, B.P. 2002. Genetic analysis of a severe case of Netherton syndrome and application for prenatal testing. Br. J. Dermatol. 146: 495-499. [DOI] [PubMed] [Google Scholar]
- Ochman H., Gerber, A.S., and Hartl, D.L. 1988. Genetic applications of an inverse polymerase chain reaction. Genetics 120: 621-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasdar M. and Nelson, W.J. 1988. Kinetics of desmosome assembly in Madin-Darby canine kidney epithelial cells: Temporal and spatial regulation of desmoplakin organization and stabilization upon cell-cell contact. I. Biochemical analysis. J. Cell Biol. 106: 677-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M.A., Owaribe, K., Kartenbeck, J., and Franke, W.W. 1990. Desmosomes and hemidesmosomes: Constitutive molecular components. Annu. Rev. Cell Biol. 6: 461-491. [DOI] [PubMed] [Google Scholar]
- Simon M., Montezin, M., Guerrin, M., Durieux, J.J., and Serre, G. 1997. Characterization and purification of human corneodesmosin, an epidermal basic glycoprotein associated with corneocyte-specific modified desmosomes. J. Biol. Chem. 272: 31770-31776. [DOI] [PubMed] [Google Scholar]
- Simon M., Jonca, N., Guerrin, M., Haftek, M., Bernard, D., Caubet, C., Egelrud, T., Schmidt, R., and Serre, G. 2001. Refined characterization of corneodesmosin proteolysis during terminal differentiation of human epidermis and its relationship to desquamation. J. Biol. Chem. 276: 20292-20299. [DOI] [PubMed] [Google Scholar]
- Sprecher E., Chavanas, S., DiGiovanna, J.J., Amin, S., Nielsen, K., Prendiville, J.S., Silverman, R., Esterly, N.B., Spraker, M.K., Guelig, E., et al. 2001. The spectrum of pathogenic mutations in SPINK5 in 19 families with Netherton syndrome: Implications for mutation detection and first case of prenatal diagnosis. J. Invest Dermatol. 117: 179-187. [DOI] [PubMed] [Google Scholar]
- Stanley J.R. 2000. The pathophysiology of pemphigus. J. Dermatol. Sci. 24: 155-157. [DOI] [PubMed] [Google Scholar]
- Stanley J.R., Nishikawa, T., Diaz, L.A., and Amagai, M. 2001. Pemphigus: Is there another half of the story? J. Invest Dermatol. 116: 489-490. [DOI] [PubMed] [Google Scholar]
- Walden M., Kreutzmann, P., Drogemuller, K., John, H., Forssmann, W.G., and Hans-Jurgen, M. 2002. Biochemical features, molecular biology and clinical relevance of the human 15-domain serine proteinase inhibitor LEKTI. Biol. Chem. 383: 1139-1141. [DOI] [PubMed] [Google Scholar]
- Walley A.J., Chavanas, S., Moffatt, M.F., Esnouf, R.M., Ubhi, B., Lawrence, R., Wong, K., Abecasis, G.R., Jones, E.Y., Harper, J.I., et al. 2001. Gene polymorphism in Netherton and common atopic disease. Nat. Genet. 29: 175-178. [DOI] [PubMed] [Google Scholar]
- Yokoyama T., Silversides, D.W., Waymire, K.G., Kwon, B.S., Takeuchi, T., and Overbeek, P.A. 1990. Conserved cysteine to serine mutation in tyrosinase is responsible for the classical albino mutation in laboratory mice. Nucleic Acids Res. 18: 7293-7298. [DOI] [PMC free article] [PubMed] [Google Scholar]