Abstract
Repeated evidence has demonstrated that combined primer-booster immunization regimens can improve both secreted and humoral immune responses to antigens derived from viral, bacterial, and parasitic pathogens. For the present work, we evaluated the synergic serum immunoglobulin G (IgG) and fecal IgA antibody responses elicited in BALB/c mice who were intramuscularly primed with a DNA vaccine, pRECFA, followed by oral boosting with an attenuated Salmonella enterica serovar Typhimurium vaccine (HG3) strain, with both vaccines encoding the structural subunit (CfaB) of the CFA/I fimbriae produced by human-derived enterotoxigenic Escherichia coli (ETEC) strains. The immunological properties of the vaccine regimen were evaluated according to the order of the administered vaccines, the nature of the oral antigen carrier, the age of the vaccinated animals, the interval between the priming and boosting doses, and the amount of injected DNA. The production of gamma interferon and the IgG2a subclass in serum indicated that mice immunized with the primer-booster regimen developed prevailing type 1 T-cell-dependent immune responses. The synergic effect of the vaccine regimen on the induced antibody responses was also revealed by its ability to block the adhesive properties of CFA/I fimbriae expressed by live bacteria, as shown by the inhibition of Caco-2 cell and human erythrocyte binding. Moreover, DBA2 newborn mice were protected from lethal challenges with a CFA/I+ ETEC strain after the incubation of live bacteria with serum samples harvested from mice who were subjected to the primer-booster regimen. We propose, therefore, that the DNA primer-Salmonella booster regimen represents an alternative for the development of vaccines requiring both mucosal and systemic antibody responses for immunological protection.
The stimulation of mammalian immune systems by the administration of DNA vaccines encoding heterologous antigens has been repeatedly demonstrated to efficiently activate humoral and cellular immune responses to various infectious agents and tumors (9, 19). Nonetheless, it is also well known that one of the main limitations of genetic vaccines is their limited capacity to induce specific secreted antibody responses at intestinal or respiratory epithelia of animals that are immunized via parenteral routes. Consequently, several approaches have been designed to circumvent the limited mucosal immunogenicity of DNA vaccines, such as direct delivery of DNA to mucosal sites (23, 26, 30), incorporation of DNA into liposomes or biodegradable polymers (22, 25), coadministration of plasmids expressing cytokine or costimulatory molecules (12, 42), in vivo transfection mediated by attenuated bacterial vectors (8, 13), and primer-booster immunization regimens (11, 41).
So far, most primer-booster immunization strategies based on DNA vaccines have targeted the induction of cellular and humoral systemic immune responses and have usually employed a DNA vaccine for priming and recombinant viruses or purified proteins for boosting (24, 28, 34, 36, 37, 40). The direct mucosal delivery of viral vectors can enhance both systemic and secreted immune responses in mice who are primed parenterally with DNA vaccines encoding the same target antigens (11, 41). However, the performance of recombinant bacteria, as either the priming or boosting component, in combined immunization regimens including a DNA vaccine has not been evaluated thoroughly.
Attenuated enteric bacteria, such as Salmonella enterica, represent logical alternatives for boosting both systemic and secreted immune responses in animals that were previously primed with DNA vaccines encoding the same antigen. Attenuated Salmonella strains can colonize the intestinal mucosa and efficiently target the carried heterologous antigens to the gut-associated lymphoid tissue (GALT), leading to enhanced humoral and cellular mucosal immune responses (29). Attenuated S. enterica serovar Typhimurium strains can also transiently invade the intestinal epithelia and proliferate at internal tissues and organs, triggering effective systemic immune responses such as the production of antibodies and the activation of other T-cell-dependent responses. Previous attempts to use a recombinant Salmonella vaccine strain to prime or boost immune responses in mice who were subjected to a vaccine regimen including a DNA vaccine have been limited to the administration of the bacterial strain via the parenteral route and the evaluation of systemic responses to the encoded protein, a protective antigen derived from Mycobacterium tuberculosis (31). Based on these studies, the induced antibody responses elicited in mice subjected to the primer-booster regimen did not show any significant improvement over those attained by animals vaccinated with only with one vaccine type, irrespective of the vaccine administration order (31).
Enterotoxigenic Escherichia coli (ETEC) is a common cause of acute infantile diarrhea in developing countries and in travelers who visit such areas (4). Colonization of the small intestine, the first step in the establishment of the diarrheic disease, is a major ETEC virulence-associated feature and is the target of vaccines aiming for the generation of secreted immunoglobulin A (IgA) responses that are able to block the attachment of bacteria to intestinal epithelial cells (21). Colonization factor antigen I (CFA/I) represents one of the best-studied fimbriae expressed by human-derived ETEC strains and has a widespread occurrence in areas of endemicity (14). Antibodies binding to specific epitopes at the major structural fimbrial subunit, the CfaB protein, have been shown to inhibit the adhesive properties of CFA/I+ ETEC strains (5, 35). Nonetheless, despite the magnitude of the disease burden in developing countries, no ETEC vaccine for human use has been licensed, and this need therefore represents a priority for most developing countries and for the World Health Organization (36).
Members of our laboratory previously failed to induce secreted IgA responses in mice who were parenterally immunized with DNA vaccines encoding the CFA/I fimbria structural subunit (1). However, the priming of animals with an intramuscularly (i.m.) delivered DNA vaccine encoding the CfaB subunit followed by oral boosting with a recombinant S. enterica serovar Typhimurium vaccine strain expressing the same antigen has been shown to enhance both systemic and secreted antibody responses to the ETEC antigen (27). For this work, we evaluated some properties of the immune responses elicited in mice that were subjected to the DNA primer-Salmonella booster immunization regimen and evaluated aspects that affected the systemic and secreted antibody responses to the target antigen, both quantitatively and qualitatively. The present results show that the combined immunization regimen can also be an alternative for the development of vaccines against enteric pathogens.
MATERIALS AND METHODS
Bacterial and mouse strains.
The S. enterica serovar Typhimurium SL3261 strain, a nonreverting aromatic-dependent (aroA) vaccine strain, was kindly supplied by B. A. Stocker (Stanford University). The ETEC 258909-3 strain (CFA/I+ O128:H?, heat-stable enterotoxin/heat-labile enterotoxin) and its isogenic nonfimbriate derivative (258909-3 M) were gifts from A. M. Svennerholm at the University of Gothenburg, Gothenburg, Sweden. The Salmonella HG3 strain was routinely cultivated in liquid Luria-Bertani (LB) broth at 37°C with agitation, while the ETEC strains were grown on Casamino Acids-yeast extract agar plates at 37°C, as previously described (1). Specific-pathogen-free isogenic female BALB/c and DBA2 mice were supplied by the Isogenic Mouse Breeding Facility of the Biomedical Sciences Institute at the University of São Paulo. All procedures were performed in accordance with the principles of the Brazilian code for the use of laboratory animals and were approved by the Ethics Committee on Use of Laboratory Animals of the Biomedical Sciences Institute of the University of São Paulo.
Preparation of Salmonella-ETEC vaccine strain.
The S. enterica serovar Typhimurium SL3261 strain was transformed with plasmid pCFA-1, which encodes the mature CfaB protein under control of the tac promoter (17). The recombinant bivalent Salmonella-ETEC vaccine strain, named strain HG3, was obtained after transformation with an F plasmid carrying a copy of the lacIq gene, which allowed induction of the CfaB protein upon the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to the growth medium (18). The HG3 strain was cultivated at 37°C in LB broth in the presence of 0.5 M IPTG for 4 h, harvested by centrifugation, washed once, suspended in phosphate-buffered saline (PBS) to a final concentration of 2 × 1010 CFU/ml, and inoculated on the same day of preparation. CfaB expression by the S. enterica serovar Typhimurium HG3 strain was confirmed for each administered dose. When required, both the Salmonella and ETEC strains were inactivated by suspension in 70% ethanol, an overnight incubation at 4°C before washing, and a final suspension in sterile PBS.
Preparation of DNA vaccine.
Plasmid pRECFA, kindly supplied by G. Cohen (University of Pennsylvania), was derived from pRE4 (7) and encodes a hybrid CfaB protein fused with the herpes simplex virus (HSV) type 1 glycoprotein D (gD) under the control of a Rous sarcoma virus (RSV) promoter (1). pRECFA and pRE4 were purified by equilibrium density centrifugation in a CsCl gradient, which was repeated twice, and then were precipitated with ethanol and suspended in PBS (1).
Immunization regimen and sample collection.
Groups of five 4- to 6-week-old female BALB/c mice were inoculated i.m. twice with 50 μg of pRECFA or pRE4 at each hind limb tibialis anterior muscle (100 μg/dose) at intervals of 2 weeks. The standard immunization regimen employed two peroral (p.o.) boosting doses with 1010 CFU of the HG3 strain, given 1 week apart, starting 2 weeks after the last DNA immunization. The same procedure was repeated for experiments with live or inactivated ETEC bacteria. For some experiments, the interval between the last DNA dose and the first HG3 dose was increased up to 52 weeks. For another set of experiments, the amount of priming DNA was reduced to 50, 10, or 1 μg per dose. To evaluate the role of mouse age on the induced antibody responses, we vaccinated 7-day- to 52-week-old mice with the first DNA dose of the standard immunization regimen. Control immunization groups were vaccinated with two i.m. doses of pRECFA, two p.o. doses of the HG3 strain, or two priming i.m. doses of pRE4 followed by two p.o. doses of the HG3 strain. All immunization experiments were repeated at least twice, except those based on intervals of 16 and 52 weeks between the last priming DNA dose and the first booster with the Salmonella HG3 strain. Two weeks after the last immunization with the HG3 strain, the mice were bled by puncturing the retro-orbital plexus, and feces were collected for one night. Individual blood samples were tested for anti-CfaB antibody responses, pooled, and then stored at −20°C for further testing. Fecal pellets were first freeze-dried and then were stored at −20°C. Before testing, 15 pellets (approximate 0.6 g) were homogenized in 500 μl of PBS and centrifuged at 10,000 × g for 10 min at 4°C. The supernatants were collected for determinations of CfaB-specific IgA levels. Anti-CfaB total IgG, IgG1, IgG2a, and IgA titers were determined by enzyme-linked immunosorbent assays (ELISAs) with purified CFA/I fimbriae.
In vitro transfection of mammalian cells.
BHK-21 cells were transfected with either pRECFA or pRE4 by the use of Lipofectamine in Dulbecco's modified Eagle's medium (Invitrogen) under conditions suggested by the manufacturer. The cells were harvested 48 h after transfection, whole-cell extracts were suspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and boiled for 5 min, and the proteins were sorted by SDS-PAGE followed by Western blotting.
SDS-PAGE and Western blotting.
SDS-PAGE was performed according to standard procedures in a Mini Protean II vertical electrophoresis unit (Bio-Rad). The samples were boiled in an equal amount of sample buffer (0.625 M Tris [pH 6.8], 10% [vol/vol] glycerol, 2% [wt/vol] SDS, and 5% mercaptoethanol in distilled water) for 5 min and then applied to 15% (wt/vol) acrylamide gels. The gels were run at 120 V, and the sorted proteins were transferred to nitrocellulose sheets (0.45-μm pore size; Sigma) at 200 mA for 1 h under previously described buffer conditions (1). After an overnight block with 1% (wt/vol) bovine serum albumin-PBS at 4°C, the sheets were incubated at room temperature for 1 h with MAb84, a CfaB-specific mouse monoclonal antibody (MAb) that is able to recognize an unexposed linear epitope, followed by an additional 1-h incubation with diluted (1:3,000) horseradish peroxidase-conjugated rabbit anti-mouse IgG (Sigma). The membranes were developed by use of an ECL kit (Amersham) and were exposed to Kodak-X Omat films for 1 min.
Detection of specific antibody responses.
ELISAs were performed as previously described (1), with purified heat-denatured CFA/I fimbriae as the solid-phase bound antigen. Briefly, 96-well MaxiSorp plates (Nunc) were coated with purified heat-denatured CFA/I fimbriae (0.1 μg/well), and after blocking, the plates were incubated for 1 h with serially diluted mouse sera or fecal extracts. After being washed, the plates were incubated with diluted (1:3,000) peroxidase-conjugated rabbit anti-mouse IgG, IgA (Sigma), IgG1, or IgG2a (Southern Biotechnologies) for an additional hour. The color reactions were made visible by the use of O-phenylenediamine (0.4 mg/ml; Sigma) and H2O2. Absorbance values were measured at 492 nm in a microtiter plate reader (LabSystem), and end-point titers (IgG, IgG1, and IgG2a) were automatically calculated by the Microcal Origin 6.0 Professional program as the reciprocal values of the last dilutions with an optical density of 0.1. Fecal IgA results were expressed as A492 values of serially diluted fecal extracts. Background levels, as determined with preimmunization serum pools, were deducted from the results obtained with the different immunization groups. Determinations of the statistical significance of the antibody titers were performed by use of the end-point titers (serum IgG) or A492 values of the lowest tested dilution (IgA fecal extracts).
Detection of anti-CfaB ASC by enzyme-linked immunospot assay.
Spleens from five immunized BALB/c mice were removed, and single-cell suspensions prepared in RPMI 1640 medium were supplemented with 5% fetal calf serum, 2 mM l-glutamine, 0.05% 2-mercaptoethanol, penicillin (100 U/ml), and streptomycin (100 μg/ml), as previously described (15). A similar procedure was applied to obtain single-cell suspensions of five isolated Peyer's patches (PP) and mesenteric lymph nodes (MLN) from each immunized mouse. Reactions were carried out in Nunc Maxisorp 96-well plates coated overnight (4°C) with purified CFA/I fimbriae (2 μg/well) in 0.05 M carbonate-bicarbonate buffer, pH 9.6. The plates were blocked with 1% gelatin in PBS at 37°C for 2 h. Titrated numbers of cells from spleens, PP, or MLN (107 cells/ml) in RPMI medium with 2% fetal calf serum were cultured for 6 h at 37°C in a 5% CO2 atmosphere. After being washed, the plates were treated with goat anti-mouse IgG or goat anti-mouse IgA conjugated with alkaline phosphatase (Sigma) at a final dilution of 1:3,000. After an incubation for 2 h at 37°C, the plates were washed, and antibody-secreting cells (ASC) were detected after the addition of molten agarose containing 5-bromo-chloro-3-indolyl phosphate (Sigma) diluted in 2-amino 2-methyl 1-propanol (Merck). The experiment was based on duplicate plates for each tested sample, and the total numbers of specific IgG- or IgA-producing ASC were determined by counting the corresponding blue spots under a dissecting microscope.
Detection of cytokines in culture supernatants.
For in vitro cytokine measurements, splenocytes isolated from five BALB/c mice were cultured in 24-well tissue culture plates at a final concentration of 5 × 106 cells/ml in RPMI and then were stimulated with purified CfaB protein (25 μg). Supernatant fluids were harvested after 24 or 72 h of incubation at 37°C in 5% CO2 and were assayed for their interleukin-4 (IL-4) or gamma interferon (IFN-γ) content, respectively. Cytokine assays were performed by two-site sandwich ELISAs using the following MAbs: for IL-4, 11B11 (2 μg/ml) and biotinylated BVD6-24G2 (2 μg/ml); and for IFN-γ, XMG 12 (2 μg/ml) and biotinylated AN 18 (4 μg/ml). The binding of biotinylated MAbs was detected by use of a diluted (1:4,000) streptavidin-biotinylated horseradish peroxidase complex (Sigma) and 2,2′-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) substrate solution (Sigma) in 0.1 M citrate buffer containing hydrogen peroxide. Samples were quantified by comparisons with standard curves of recombinant mouse cytokines. The results are expressed in nanograms of secreted cytokines per milliliter and are arithmetic means of duplicate cultures ± standard deviations.
Inhibition of ETEC adherence to Caco-2 cells and hemagglutination (IHA).
Caco-2 cells were maintained in Dulbecco's modified Eagle's medium supplemented with gentamicin (100 μg/ml), kanamycin (50 μg/ml), l-glutamine (2 mM), 100 μM minimum essential medium nonessential amino acid solution (Invitrogen), and 10% fetal calf serum in a humidified atmosphere containing 5% CO2 at 37°C. The in vitro inhibition of ETEC adherence to Caco-2 cell monolayers was determined by the use of heat-inactivated sera (60°C for 1 h) from nonimmune and immunized mice. For evaluations of the inhibitory action of anti-CfaB antibodies, serum or fecal samples were incubated at a final dilution of 1:3 with 107 ETEC bacteria for 1 h at 37°C before being added to Caco-2 cultures. After incorporation of the bacteria, the Caco-2 monolayers were kept at 37°C for 3 h. The cell monolayers were washed with PBS, fixed with methanol, and stained with Giemsa solution to visualize the adherent bacteria by oil immersion light microscopy. The number of Caco-2 cells with bound bacteria in a set of 100 cells was determined for each tested antibody. IHA tests were performed on glass plates with drops (10 μl) of the ETEC 258909-3 strain at an optical density at 600 nm of 0.15 incubated for 10 min with aliquots (10 μl) of twofold serial dilutions of serum pools or filtered fecal extracts harvested from different mouse immunization groups. Aliquots (20 μl) of a 3% suspension of human group A erythrocytes that had been washed in PBS were added to the treated bacterial samples and incubated at room temperature for 30 min in humidified chambers. The results were evaluated by visual inspection, and the maximal dilutions that were able to inhibit the hemagglutination reaction were noted. d-Mannose was added at a concentration of 1% (wt/vol) to the dilution buffer (PBS) for both tests to avoid binding mediated by type 1 fimbriae. The test was repeated independently at least three times. The assay specificities were confirmed with tests performed with the CFA/I-negative ETEC 258909-3 M strain. Serum samples from animals subjected to the primer-booster regimen were diluted with PBS to titers similar to those harvested from mice immunized only with pRECFA to allow a more precise comparison of the antiadhesive properties of the tested antibodies.
Protective role of anti-CfaB antibodies in murine neonatal challenge model.
A neonatal ETEC challenge model (10) was adapted for the human-derived 258909-3 strain. Briefly, bacterial cells cultivated on CFA plates for 18 h at 37°C were gently suspended in PBS to a final concentration of 109 CFU/ml. Bacterial cell aliquots containing 2 × 107 CFU were mixed with anti-CfaB serum samples at a final dilution of 1:25 or 1:50. The mixtures were incubated at room temperature for 1 h and then directly inoculated, in 200-μl aliquots, into the stomachs of neonate mice within the first 48 h of birth by use of a syringe for insulin administration (12.7 by 0.33 mm needle; BD). The inoculated pups were returned to their respective dams and kept under observation for 1 week. All deaths registered between 24 h and 6 days after the challenge were considered for mortality rate determination. Control test groups were inoculated with the 258909-3 strain, which had previously been incubated with a nonimmune serum pool or PBS.
Statistical analyses.
Antibody titers and cytokine production were compared by use of the Student t test, and significance was assessed at P values of <0.05 throughout the study.
RESULTS
Parenteral priming with DNA and oral boosting with a recombinant Salmonella strain confers synergic systemic (serum IgG) and secreted (fecal IgA) antibody responses to ETEC CFA/I fimbriae.
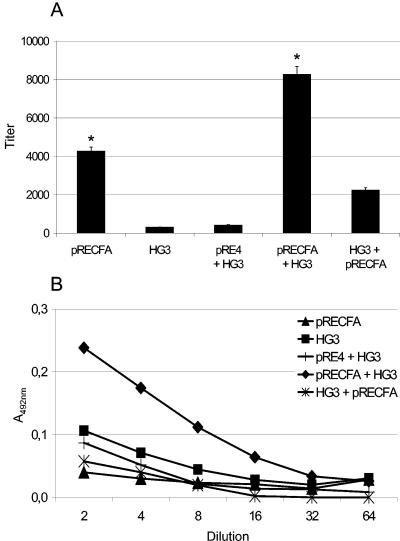
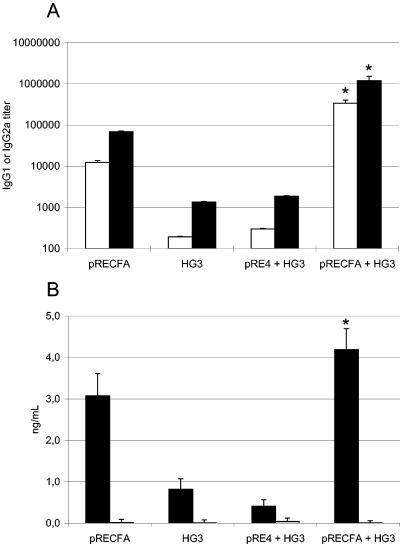
The vaccine regimen used for this study was based on the i.m. delivery of a DNA vaccine, pRECFA, encoding the CfaB subunit genetically fused to the HSV gD1 protein under control of the RSV early promoter (Fig. 1), followed by oral administration of the recombinant S. enterica serovar Typhimurium HG3 strain, which intracellularly expresses the mature nonfused form of the CfaB subunit (Fig. 1). BALB/c mice who were primed with two doses of pRECFA and then received boosters 2 weeks later consisting of two oral doses of the CFA/I-expressing HG3 strain developed serum IgG responses to the commonly encoded CfaB subunit which could not be attained in mice who were immunized with only one vaccine type (Fig. 2). Mice that were immunized with the primer-booster regimen developed CfaB-specific IgG levels which were at least twofold higher than the levels attained in mice that were immunized with two or more i.m. doses of pRECFA (average reverse titers of 8,280 ± 220 for the primer-booster regimen and 4,268 ± 168 for immunization with pRECFA only). The difference between the anti-CfaB serum IgG levels in mice subjected to the primer-booster vaccine procedure and those in mice who were immunized with two doses of the HG3 strain only was even higher (up to 20-fold), which could be attributed to the reduced immunogenicity of this particular Salmonella vaccine strain when delivered via the oral route (17) (Fig. 2A). Mice that were primed with the plasmid vector without the cloned cfaB gene (pRE4) and then boosted with the HG3 strain did not mount any enhanced systemic anti-CfaB serum antibody responses. More relevantly, mice subjected to the priming-boosting immunization regimen elicited enhanced CfaB-specific secreted IgA responses in feces compared to animals who were immunized with pRECFA or the HG3 strain only or who were primed with pRE4 and boosted with the HG3 strain (Fig. 2B). Therefore, under our assay conditions, parenteral administration of the DNA vaccine was able to prime not only systemic but also secreted antibody responses to the encoded antigen, as demonstrated with mice who received oral boosters with the recombinant Salmonella strain. Curiously, an inversion of the immunization regimen, i.e., priming with the orally delivered HG3 strain followed by i.m. boosting with pRECFA, did not result in any synergy of the induced anti-CfaB antibody responses, either in the serum (IgG) or feces (IgA). Indeed, oral priming with the recombinant HG3 strain caused a reproducible antagonistic effect on the induced anti-CfaB antibody responses compared to those in mice who were vaccinated with only the DNA vaccine (Fig. 2A).
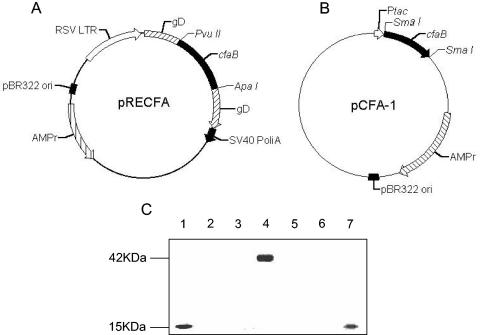
FIG. 1.
Schematic representation of CfaB-encoding plasmids and in vitro expression of the encoded peptides. (A) The pRECFA vaccine was obtained after cloning of the PCR-amplified cfaB gene into the pRE4 vector after PvuII-ApaI double digestion of both the fragment and the plasmid (1). The encoded CfaB protein was expressed as an in-frame sandwich fusion with the HSV-1 gD protein under control of the RSV late promoter. (B) The pCFA-1 plasmid was generated after cloning of a PCR-generated DNA fragment encoding the mature portion (without the signal peptide) of the structural subunit of the CFA/I fimbriae into the SmaI-digested pKK223-3 bacterial expression vector (17, 18). The peptide expressed by transformed Salmonella strains accumulated as an intracellular protein that was unable to form native CFA/I fimbriae. (C) Immunological detection of CfaB expression in whole-cell extracts in a Western blot developed with a specific monoclonal antibody (MAb 84). Samples: 1, purified CFA/I fimbriae isolated from an ETEC strain; 2, BHK-21 whole-cell extract; 3, pRE4-transfected BHK-21 cells; 4, pRECFA-transfected BHK-21 cells; 5, S. enterica serovar Typhimurium SL3261 strain; 6, S. enterica serovar Typhimurium HG3 strain cultivated without inducer (IPTG); 7, S. enterica serovar Typhimurium HG3 cultivated for 4 h in the presence of 0.5 M IPTG. Each lane was loaded with approximately 25 μg of total protein.
FIG. 2.
Systemic (serum IgG) and secreted (fecal IgA) CfaB-specific antibody responses elicited in BALB/c mice subjected to different immunization regimens. Serum IgG (A) and fecal IgA (B) anti-CfaB responses were measured in ELISA plates coated with heat-denatured CfaB protein purified from a CFA/I+ ETEC strain. Sera were collected from the following immunization groups (at least five animals per group): pRECFA (▴), mice immunized with two i.m. doses of pRECFA (100 μg/dose); HG3 (▪), mice immunized with two p.o. doses of IPTG-induced S. enterica serovar Typhimurium HG3 (1010 CFU/dose); pRE4 + HG3 (+), mice immunized with two i.m. doses of the empty vector pRE4 (100 μg/dose) followed by two p.o. doses of the HG3 strain; pRECFA + HG3 (⧫), the complete primer-booster immunization regimen, with mice immunized with two i.m. doses of pRECFA followed by two p.o. doses of the HG3 strain; HG3 + pRECFA (*), inverse primer-booster immunization regimen consisting of two p.o. doses of the HG3 strain followed by two i.m. pRECFA doses. Data corresponding to three determinations are presented as means ± standard errors (SE) of the titers (serum IgG) or of absorbance values at 492 nm of the reactions with diluted fecal extracts (fecal IgA). All values were deducted from the background values obtained with nonimmune sera. All bars marked with an asterisk show statistically significantly different values (P < 0.05) compared to the results of the pRECFA (serum IgG)- or HG3 (fecal IgA)-vaccinated mouse group.
The impact of the primer-booster immunization regimen on the activation of enhanced fecal anti-CfaB IgA responses was further confirmed by the detection of ASC in PP and MLN by an ELISPOT assay. As indicated in Table 1, CfaB-specific IgA-secreting cells in PP and MLN were detected only in mice who were subjected to the DNA primer-Salmonella booster regimen, while no CfaB-specfic ASC were detected in mice who were vaccinated with pRECFA or the HG3 strain only or who were primed with pRE4 and boosted with the HG3 strain. The number of CfaB-specific ASC in spleens of animals subjected to the primer-booster regimen was also higher (13-fold increase [P < 0.05]) than that in spleens of mice who were vaccinated only with pRECFA (Table 1). Taken together, these results indicate that parenteral priming with a DNA vaccine can indeed prime both systemic and secreted antibody responses in mice receiving oral boosters with a recombinant Salmonella strain encoding the same antigen. The available evidence also indicates that under our testing conditions, the order of the priming and boosting doses is essential for the observed synergic effect on the induced antibody responses. Once the effects of the immunization regimen on both systemic and secreted antibody responses were established, additional experiments were performed in order to evaluate some of the conditions affecting the immunogenicity of the primer-booster regimen.
TABLE 1.
Detection of CfaB-specific ASC by ELISPOT assays with mice subjected to different immunization regimens
| Vaccination regimena | No. of CfaB-specific ASC/106 splenocytesb
|
||
|---|---|---|---|
| Spleen | PP | MLN | |
| Nonimmunized | 0 | 0 | 0 |
| pRECFA | 10 ± 0.4 | 0 | 0 |
| HG3 | 0 | 0 | 0 |
| pRE4 + HG3 | 0 | 0 | 0 |
| pRECFA + HG3 | 130 ± 4* | 7 ± 0* | 20 ± 0.8* |
Vaccination regimens: non-immunized mice; pRECFA, mice immunized with two i.m. doses of pRECFA (100 μg/dose); HG3, mice immunized with two p.o. doses (1010 CFU/dose) of the HG3 strain; pRE4 + HG3, mice immunized with two doses of pRE4 followed 2 weeks later by two booster doses with the HG3 strain; pRECFA + HG3, mice subjected to the standard primer-booster immunization regimen, with a 2-week interval between the priming and boosting immunizations.
CfaB-specific ASC were detected after incubation of the cells with alkaline phosphatase-conjugated goat anti-mouse IgG (spleen) or goat anti-mouse IgA (Peyer's patches and mesenteric lymph nodes). All cells were harvested 2 weeks after the last immunization dose either from groups that were vaccinated with a single vaccine type or from groups that were subjected to primer-booster regimens. The results are based on one determination and are expressed as means ± SE. *, statistically significant (P < 0.05) differences compared with corresponding results for mouse groups immunized with pRECFA.
Factors affecting the systemic and secreted antibody responses induced by the primer-booster immunization regimen.
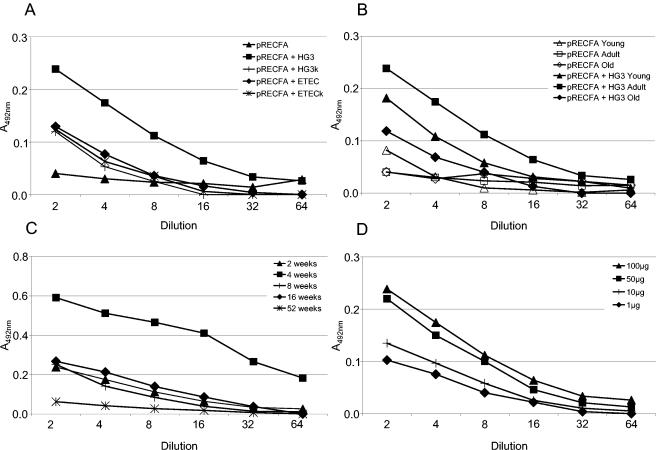
The ability of the DNA priming-Salmonella boosting immunization regimen to elicit enhanced secreted and systemic antibody responses to the CfaB antigen in mice who were subjected to the regimen was evaluated according to four different parameters: (i) the nature of the bacterial vector used to boost the immune responses, (ii) the age of the animals at the time that the vaccine regimen was begun, (iii) the interval between the priming and boosting immunizations, and (iv) the amount of priming DNA vaccine. To investigate the role of the bacterial strain on the induction of antibody responses, we tested the live or ethanol-inactivated CfaB-expressing HG3 strain or a CFA/I+ ETEC strain as boosting antigens in mice who were previously primed with the DNA vaccine. Mice who received oral boosters with the live or inactivated Salmonella HG3 strain or with the live CFA/I+ ETEC strain developed statistically significantly (P < 0.05) different systemic anti-CfaB IgG titers from those in mice who were immunized only with pRECFA, although mice who were boosted with the live HG3 strain consistently yielded higher anti-CfaB serum IgG responses. Regarding the fecal IgA responses, boosting with live HG3 cells was the only stimulus that was able to elicit statistically significant differences in the anti-CfaB IgA levels detected among mice subjected to the primer-booster immunization regimen (see Fig. 4A).
FIG. 4.
Factors affecting secreted anti-CfaB fecal IgA responses elicited in mice subjected to the DNA priming-Salmonella boosting immunization regimen. (A) Nature of the bacterial carrier given to pRECFA-primed mice. Mice were boosted p.o. with live (▪, pRECFA + HG3) or ethanol-inactivated (+, pRECFA + HG3 k) S. enterica serovar Typhimurium HG3 or with live (⧫, pRECFA + ETEC) or ethanol-inactivated (*, pRECFA + ETEC k) ETEC 258909-3. As a control, CfaB-specific fecal IgA titers for a mouse group that was immunized with two doses of pRECFA are indicated (▴, pRECFA). (B) Age-dependent CfaB-specific antibody responses elicited in mice who were first immunized with pRECFA at an age of 1 week (▴, young), 6 weeks (▪, adult), or 52 weeks (⧫, old) and then boosted with two doses of the HG3 strain given 2 weeks apart (pRECFA + HG3). As controls, age-matched mouse groups (pRECFA) were immunized with two i.m. doses (100 μg/dose) of pRECFA, as indicated by the open symbols. (C) Interval between the last priming dose with pRECFA and the first oral booster with the CfaB-expressing HG3 strain. CfaB-specific fecal IgA levels detected in mouse groups that were subjected to intervals of 2 (▴), 4 (▪), 8 (+), 16 (⧫), and 52 (*) weeks are indicated. (D) Amount of DNA required to prime CfaB-specific fecal IgA responses in mice receiving oral boosters with the HG3 strain. The amount of DNA inoculated in each dose was 100 μg (▴), 50 μg (▪), 10 μg (+), or 1 μg (⧫). Experiments were performed with pools of fecal extract collected from different immunization groups. The results shown are based on a representative experiment and are expressed as A492 values for twofold serial dilutions. Statistically significant results (P > 0.05) with regard to mouse groups primed with pRE4 and boosted with the HG3 strain at the lowest tested dilutions were obtained for the following groups: A, pRECFA + HG3; B, pRECFA + HG3 adult and pRECFA + HG3 young; C, all groups except the one with an interval of 52 weeks; D, mouse groups that were immunized with 100 or 50 μg of pRECFA.
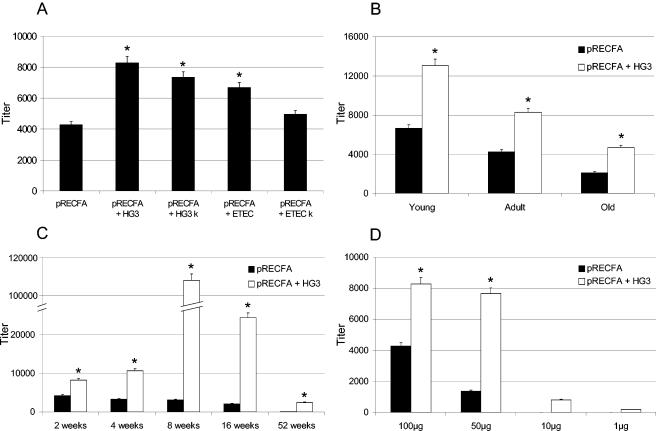
The ability to activate both systemic and secreted antibody responses to the CfaB antigen was age dependent both for animals subjected to the primer-booster regimen and for those who were vaccinated i.m. with pRECFA. As shown in Fig. 3B, mice who were first immunized with pRECFA 1 week after birth developed higher serum IgG responses than 4- or 52-week-old animals subjected to the primer-booster regimen. On the other hand, 4-week-old mice developed the highest anti-CfaB IgA levels in fecal extracts compared to the other mouse groups that were subjected to the primer-booster regimen (Fig. 4B). However, regardless of age, all mouse groups subjected to the primer-booster regimen developed higher anti-CfaB serum IgG or fecal IgA levels than age-matched groups immunized only with pRECFA or with the HG3 strain, respectively (Fig. 3B and 4B; also data not shown).
FIG. 3.
Factors affecting serum anti-CfaB IgG responses elicited in mice subjected to the DNA priming-Salmonella boosting immunization regimen. (A) Nature of the bacterial carrier given to mice primed with pRECFA. Mice were boosted p.o. with live (HG3) or ethanol-inactivated (HG3 k) S. enterica serovar Typhimurium HG3 or with live (ETEC) or ethanol-inactivated (ETEC k) ETEC 258909-3. As a control, the mean CfaB-specific serum IgG titer of mice immunized with two doses of pRECFA is indicated (pRECFA). (B) Age-dependent CfaB-specific antibody responses elicited in mice who were first immunized with pRECFA at the age of 1 week (young), 6 weeks (adult), or 52 weeks (old). Mice were immunized with two i.m. doses (100 μg/dose) of pRECFA (black bars) or with the complete primer-booster regimen (white bars). (C) Interval between the last priming dose with pRECFA and the first oral booster with the CfaB-expressing HG3 strain. CfaB-specific antibody levels detected for mouse groups subjected to the primer-booster regimens with intervals of 2, 4, 8, 16, and 52 weeks between the priming and boosting doses are indicated by white bars. As controls, anti-CfaB antibody titers detected in sera of mice who were vaccinated only with two doses of pRECFA and then bled at the same periods as those subjected to the complete primer-booster regimen are indicated by black bars. (D) Amount of DNA required to prime CfaB-specific antibody responses in mice receiving oral boosters with the HG3 strain. The amount of DNA inoculated in each dose is indicated (100, 50, 10, or 1 μg). Data corresponding to one representative determination are presented as means ± SE of the titers. All bars marked with an asterisk show statistically significantly different values (P < 0.05) compared to the results obtained with pRECFA-vaccinated mouse groups that were subjected to the same testing conditions.
We also determined the optimal interval between parenteral priming with pRECFA and the first booster dose with the recombinant Salmonella HG3 strain for the specific systemic and secreted antibody responses to the encoded antigen. An interval of 8 weeks between the second pRECFA dose and the first Salmonella booster resulted in a maximal synergistic effect on the systemic anti-CfaB IgG responses, which were approximately 30-fold higher than the IgG levels attained in mice who were immunized with only pRECFA and 10-fold higher than those detected in mice subjected to a regimen with a 2-week interval (Fig. 3C). On the other hand, maximal anti-CfaB fecal IgA responses were detected in animals who received boosters 4 weeks after the second DNA immunization, and these were at least threefold higher than the anti-CfaB IgA levels attained in mice subjected to an immunization regimen with an interval of 2, 8, or 16 weeks between the priming doses and the first booster with Salmonella (Fig. 4C). It should be noted that the synergic effects of the primer-booster immunization regimen on the induced anti-CfaB serum IgG responses were detected even for an interval of 52 weeks between priming and the first booster dose, even though the final titer values were much lower than those attained in animals who were vaccinated with shorter intervals between the priming and boosting doses.
Mice who were primed with two doses consisting of 50 μg (each) of pRECFA mounted anti-CfaB serum IgG and fecal IgA responses similar to those by animals who were primed with 100 μg of DNA. Further reductions in the amount of DNA (10 or 1 μg) used in the priming doses significantly reduced both the systemic and secreted CfaB-specific antibody responses elicited in mice subjected to the primer-booster immunization regimen (Fig. 3D and 4D).
Mice subjected to the primer-booster immunization regimen developed a prevailing Th1-type cell response pattern.
An analysis of the serum IgG subclass responses revealed that mice subjected to the primer-booster immunization regimen developed a predominant IgG2a response, with an IgG1/IgG2a ratio of 0.29. Mice who were vaccinated with pRECFA (IgG1/IgG2a = 0.18) or the HG3 strain (IgG1/IgG2a = 0.14) only or who were primed with pRE4 and boosted with the HG3 strain (IgG1/IgG2a = 0.15) developed similar IgG2a-dominated serum antibody responses (Fig. 5A). To further confirm the involvement of Th1-type cells on the observed antibody responses, we measured the amounts of IFN-γ and IL-4 secreted by CD4+ T cells present in spleen homogenates harvested from mice who were subjected to different immunization regimens and stimulated with purified CfaB protein. As expected, enhanced IFN-γ levels were detected in the culture supernatants of splenocytes isolated from mice who were subjected to the primer-booster immunization regimen, while IL-4 was not detectable under our assay conditions (Fig. 5B). Taken together, these results indicate that mice subjected to the pRECFA primer-Salmonella booster immunization regimen developed prevailing Th1-type cell responses.
FIG. 5.
Activation of Th1-type responses in mice subjected to the primer-booster immunization regimen. (A) Determination of anti-CfaB IgG1 (white bars) and IgG2a (black bars) subclasses in serum samples collected from mice who were immunized only with pRECFA (pRECFA) or the CfaB-expressing HG3 strain (HG3), primed with pRE4 and boosted with the HG3 strain (pRE4 + HG3), or subjected to the complete primer-booster regimen (pRECFA + HG3). (B) Determination of IFN-γ (black bars) and IL-4 (white bars) production in spleen homogenates harvested from mice who were immunized only with pRECFA (pRECFA) or the CfaB-expressing HG3 strain (HG3), primed with pRE4 and boosted with the HG3 strain (pRE4 + HG3), or subjected to the complete primer-booster regimen (pRECFA + HG3). Cell cultures were stimulated with 25 μg of purified heat-denatured CFA/I fimbriae/ml. IgG subclass (two determinations) and cytokine (one determination) detection values are represented by end-point reverse titers ± SE and mean nanograms per milliliter ± SE, respectively. *, statistically different values (P < 0.05) compared to results obtained with pRECFA-vaccinated mouse groups.
Recognition of native CFA/I fimbriae by antibodies raised in mice subjected to the primer-booster immunization regimen.
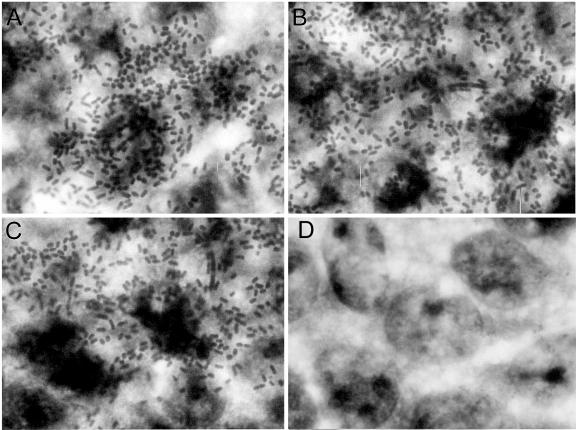
To efficiently inhibit the binding of CFA/I+ ETEC strains, anti-CfaB antibodies raised in vaccinated mice must recognize epitopes located on the surfaces of intact CFA/I fimbriae and must block binding to host cell glycolipid receptors (35). To evaluate the protective potential of the anti-CfaB serum pools collected from mice who were subjected to different immunization regimens, we used human type A red blood cells in IHA tests and measured the ability of sera to block the binding of CFA/I+ ETEC bacteria to in vitro-cultured Caco-2 cells. As shown in Table 2 and Fig. 6, serum pools from mice who were immunized only with pRECFA or with the HG3 strain did not inhibit hemagglutination or binding of the CFA/I+ ETEC 258909-3 strain to Caco-2 cells. On the other hand, serum samples harvested from mice who were subjected to the combined DNA primer-Salmonella booster regimen promoted complete inhibition of the binding properties of the CFA/I fimbriae expressed by live ETEC strains, even after dilution to titers similar to those derived from pRECFA-vaccinated mice. Similarly, only anti-CfaB antibodies present in fecal extracts of mice subjected to the primer-booster regimen were able to inhibit the adhesive properties of the CFA/I fimbriae (Table 2). These results indicate that the primer-booster regimen also enhanced the specificity of antibodies to epitopes involved in the binding properties of the CFA/I fimbriae.
TABLE 2.
Antiadhesive properties of serum pools harvested from mice subjected to different regimens on live CFA/I+ ETEC bacteria, as evaluated by the ability to inhibit CFA/I-mediated binding to human erythrocytes (IHA) and Caco-2 cells
| Vaccination regimena | IHA resultb
|
Caco-2 cells with bound bacteria (%)c | |
|---|---|---|---|
| Serum | Fecal extract | ||
| Nonimmunized | — | — | 92 |
| pRECFA | — | — | 90 |
| HG3 | 1:4 | 1:4 | 88 |
| pRE4 + HG3 | 1:4 | 1:4 | 87 |
| pRECFA + HG3 | 1:512 | 1:16 | 4* |
Serum pools were from mice subjected to the following immunization protocols: nonimmunized; pRECFA, mice immunized with two i.m. doses (100 μg/dose) of pRECFA (titer of 4,268); HG3, mice immunized with two p.o. doses of the HG3 strain (1010 CFU/dose) (titer of 305); pRE4 + HG3, mice immunized with two doses of pRE4 followed 2 weeks later by two booster doses with the HG3 strain (titer of 420); pRECFA + HG3, mice subjected to the primer-booster regimen, with a 2-week interval between the priming and boosting immunizations (diluted in PBS to a final titer of 4,500).
CFA/I-specific antiadhesive potencies were measured by IHA with human group A red blood cells and the ETEC 258909-3 strain. Results are maximal dilutions of serum or fecal extract samples that yielded visible inhibition of the hemagglutination reactions. —, no visible reaction.
The inhibition of adhesion of the ETEC 258909-3 strain to in vitro-cultivated Caco-2 cell monolayers was also measured. The results are expressed as percentages of Caco-2 cells with at least one attached bacterium visible under an optical microscope. All serum pools were used at a final dilution of 1:3 in d-mannose-containing PBS. Data were based on the total number of cells counted in two independent determinations. *, statistically different value (P < 0.05) compared to results obtained with serum samples from other vaccinated mouse groups.
FIG. 6.
Inhibition of binding properties of CFA/I fimbriae by serum samples collected from mice subjected to different immunization regimens. The CFA/I+ ETEC 258990-3 strain was incubated with different serum samples for 1 h at 37°C, at a final dilution of 1:3, before being added to Caco-2 cell cultures. The inhibition of bacterial binding was evaluated by assays using serum pools from nonimmunized mice (A), serum pools from mice who were immunized with two i.m. pRECFA doses (titer of 4,268) (B), serum pools from mice who were immunized with two p.o. doses of the HG3 strain (reverse titer of 305) (C), or PBS-diluted serum pools from mice subjected to the pRECFA priming-HG3 strain boosting immunization regimen with a two-week interval between the priming and boosting doses (titer of 4,500) (D). Magnification, ×2,000. The experiment was repeated twice with the same observed results.
Antibodies raised in mice subjected to the primer-booster immunization regimen confer protection to newborn mice challenged with a CFA/I+ ETEC strain.
As a step toward an evaluation of the protective role of antibodies raised in mice who were subjected to the primer-booster immunization regimen, we adapted a previously described newborn mouse challenge assay (10) for use with the human-derived ETEC 258909-3 strain and the DBA2 mouse lineage. Initial experiments allowed us to show that intrastomach inoculation of 107 CFU of the ETEC strain resulted in 100% mortality of DBA2 neonate mice within 6 days after the challenge. Moreover, a previous incubation of the bacteria with antibodies raised in mice who were immunized with purified CFA/I fimbriae reduced the observed neonate mortality to values similar to those attained with a nonadherent and nontoxigenic isogenic ETEC strain (data not shown). As shown in Table 3, the mortality rate of DBA2 neonate mice inoculated with the ETEC 258909-3 strain which had previously been incubated with a serum pool from mice who were subjected to the vaccine regimen was reduced to 18% of that for mice inoculated with bacteria that were incubated with nonimmune sera. Serum samples harvested from mice who were immunized with two doses of pRECFA or two doses of the HG3 strain were not able to confer any protection to the challenged neonates (Table 3). As a positive control, a pooled serum sample harvested from mice who were immunized with purified CFA/I fimbria preparations reduced the mortality frequency to 25% of that caused by an ETEC strain that was treated with nonimmune sera. These results further demonstrate that antibodies raised in mice subjected to the combined vaccine regimen can recognize and neutralize the adhesive properties of the CFA/I fimbriae.
TABLE 3.
Mortality rates of DBA2 neonate mice challenged with the ETEC 258909 strain, which had been incubated with serum pools from mice subjected to different immunization regimens
| Serum samplea | Anti-CfaB titerb | No. of deaths/no. of inoculated mice (%)c |
|---|---|---|
| Nonimmunized | 8/8 (100) | |
| pRECFA | 4,268 | 12/12 (100) |
| pRE4 + HG3 | 420 | 10/10 (100) |
| pRECFA + HG3 4w | 10,663 | 2/11 (18)* |
| CFA/I fimbriae | 14,753 | 2/8 (25)* |
Tested serum samples were as follows: nonimmunized mice; pRECFA, mice immunized with two i.m. pRECFA doses; HG3, mice vaccinated with two p.o. doses of the HG3 strain; pRE4 + HG3, mice immunized with two doses of pRE4 followed 2 weeks later by two booster doses with the HG3 strain; pRECFA + HG3 4w, mice subjected to the primer-booster regimen, with a 4-week interval between the last DNA dose and the first Salmonella booster; CFA/I, mice vaccinated with three i.p. doses (10 μg each) of purified CFA/I fimbriae.
Titers of the tested serum samples, as determined for IgG ELISA plates coated with denatured CFA/I fimbriae.
Mortality rates of DBA2 neonate mice after challenge with 2 × 107 CFU of the ETEC 258909 strain, which was previously incubated for 1 h with the tested serum samples. Serum samples were tested at a final dilution of 1:25 (HG3, nonimmune, and pRECFA + HG3 4w) or 1:50 (CFA/I). *, statistically different value (P < 0.05) compared with the results obtained with serum samples from mice immunized with pRECFA.
DISCUSSION
Vaccine strategies based on priming with DNA followed by boosting with recombinant viruses have been shown to induce unprecedented levels of specific immunity and, in same cases, protection from diseases that are not yet preventable by vaccines (2, 33, 34, 37, 40). Most of these reports were based on the parenteral administration of both priming and boosting immunization, leading to the activation of systemic immune responses, such as cytotoxic CD8+ T cells (37, 40). In this work, we reported the synergic induction of both serum and intestinal antibody responses in mice subjected to a primer-booster regimen based on parenteral priming with a DNA vaccine and oral boosting with a recombinant Salmonella vaccine strain encoding the same heterologous antigen derived from an enteric bacterial pathogen, the structural subunit of the ETEC CFA/I fimbriae. A positive impact of the primer-booster vaccine strategy was also noted on the ability of antibodies to neutralize the binding properties of the CFA/I fimbriae. Although a previous report has described a DNA priming-Salmonella boosting immunization regimen based on parenteral administration of the recombinant Salmonella strain (31), as far as we know, the present report is the first one to describe that oral administration of a Salmonella vaccine strain can both quantitatively and qualitatively improve the induced antibody responses targeting the encoded heterologous antigen. Based on this evidence, the proposed primer-booster regimen can be considered an alternative for the development of vaccines against noninvasive enteric bacteria, such as ETEC, as well as nonenteric pathogens.
The sequential parenteral priming with a DNA vaccine and oral boosting with a recombinant Salmonella strain were shown to comprise the most adequate regimen for enhancing antibody responses in both sera and intestinal mucosa of vaccinated mice. Inverting the vaccine administration order (oral priming with Salmonella followed by i.m. boosting with DNA) resulted in an apparent antagonistic effect on the induced antibody responses, causing a reduction in the specific antibody levels among vaccinated animals. The reduced immunogenicity of the inverted vaccine regimen may be associated with the induction of oral tolerance in vaccinated animals due to repeated exposure to the antigen via the oral route, resulting in subsequent suppression of the parenterally expressed antigen. Opposite results were reported for a similar primer-booster immunization regimen based on a recombinant vaccinia virus vector, which elicited enhanced immune responses in mice primed via the nasal route and boosted later with a DNA encoding the same heterologous antigen (11). The distinct nature of the vectors and the diverse administration sites most probably explain the contrasting behaviors of the bacterial and virus vectors used for priming immune responses in mice who are subsequently boosted with DNA vaccines. Recombinant Salmonella strains can more efficiently target the afferent sites of the GALT, while recombinant poxviruses are clearly more appropriate for priming immune responses when delivered via the respiratory tract. Moreover, the intranasal environment represents a more sensitive and less tolerance-prone mucosal site than the intestinal lumen (32).
The nature of the oral booster antigen had a significant impact on the induced systemic and, more relevantly, secreted antibody responses among DNA-primed Salmonella-boosted mice. Although enhanced serum anti-CfaB IgG responses were detected in mice who received boosters with inactivated HG3 or live CFA/I+ ETEC bacteria, live recombinant Salmonella reproducibly activated better secreted antibody responses in mice subjected to the primer-booster regimen, which probably reflects the ability of live Salmonella to efficiently target gut mucosa afferent sites, invade, and multiply into deeper tissues and organs (20). As expected, the ages of the animals at the beginning of the immunization regimen had a profound effect on the magnitude of the elicited secreted and systemic antibody responses. Mice who were immunized at earlier ages systematically showed stronger antibody responses to the encoded antigen than older individuals. Interestingly, while young animals could mount enhanced serum IgG responses when they were primed 7 days after birth, the synergic effect of the primer-booster regimen on the mucosal immune responses was better detected among animals who were 4 weeks old, which suggested a delay in GALT maturation. An obvious point was the fact that, irrespective of the ages of the mice and the magnitudes of the antibody responses, all animals subjected to the primer-booster regimen developed enhanced antigen-specific antibody responses compared to age-matched mice who were vaccinated only with the DNA vaccine. These results indicate that the primer-booster vaccine regimen can be active on individuals at early and late ages, which is a particularly relevant feature for vaccines designed for ETEC as well as for other enteric pathogens, which inflict higher morbidity and mortality rates among individuals at extreme ages (4).
Our results indicated that the administration of 50 μg of DNA/dose was enough to efficiently prime both systemic and mucosal antibody responses to the commonly encoded antigen among mice subjected to the primer-booster regimen, representing a significant economy of the amount of DNA required for optimal immune responses. The interval between the two priming DNA doses and the first oral booster dose with the recombinant Salmonella strain was the most relevant parameter affecting the antibody-inducing properties of the priming-boosting immunization regimen. Mice who received the booster 8 weeks after the last DNA dose expressed maximal anti-CfaB serum IgG titers which were at least 1 order of magnitude higher than those detected in animals subjected to the standard regimen based on an interval of 2 weeks between priming and boosting. Similar results were observed for the CfaB-specific fecal IgA responses, but with an optimal interval of 4 weeks between the priming and boosting immunizations. The different optimal periods for the development of maximized antibody responses in both the systemic and intestinal environments point to the differences in the kinetics of antigen processing and stimulation of afferent cells in the two compartments. Moreover, such results suggest that the optimal vaccine regimen should take into account the type of antibody response to be preferentially activated for an ideal protective immunological state. Although they had different magnitudes, the enhancements of the serum IgG responses promoted by the primer-booster regimen could be detected for all tested mice groups compared to control groups that were immunized only with the DNA vaccine. Such evidence suggests that pRECFA-primed animals develop long-term memory responses that are able to trigger specific antibody responses once boosted with live recombinant Salmonella strains. In principle, for natives living in zones of endemicity, parenteral priming with an anti-ETEC DNA vaccine would elicit immunological memory, leading to enhanced antifimbria responses after later exposures with the locally prevailing ETEC strains, while tourists could have the alternative to improve their protective immunological status after the administration of an oral vaccine before traveling to areas of endemicity.
An analysis of the cellular basis of the responses elicited in mice subjected to the primer-booster regimen emphasized the role of CD4+ Th1 cells on the induced immune responses. No significant change on the Th cell stimulation pattern could be detected between animals subjected to the primer-booster regimen and those immunized with only one vaccine type, as demonstrated both by the IgG1/IgG2a ratios and by the cytokines produced by CFA/I-stimulated splenocytes. The activation of secreted IgA responses with the concomitant triggering of a prevailing Th1 response, characterized by the secretion of IFN-γ and a lack of detectable IL-4 production by activated lymphocytes, may be explained by the presence of alternate IgA-inducing pathways mediated by cytokines such as IL-10 which are stimulated upon oral immunization with Salmonella vaccine strains (39). These observations also indicated that changes in the expression levels of anti-CfaB antibodies raised in mice subjected to the primer-booster regimen did not reflect the differential activation of T helper lymphocyte subsets.
The ability to bind and block the adhesive properties of bacterial fimbriae is an essential feature of a protective antibody response aiming for the control of an enteroadhesive pathogen such as ETEC. In contrast to the case for serum samples collected from mice who were vaccinated only with pRECFA or with two doses of the HG3 strain, serum and fecal antibodies raised in mice subjected to the primer-booster regimen could bind and neutralize the adhesive properties of a CFA/I+-expressing ETEC strain, as demonstrated by their abilities to prevent adhesion to in vitro-cultured Caco-2 cells and to inhibit hemagglutination. The expression of the CfaB subunit genetically fused to the HSV-1 gD protein by pRECFA-transfected cells resulted in the loss of conformational epitopes on the surfaces of intact CFA/I fimbriae, which are important for the generation of neutralizing antibodies (1). Thus, besides the inability to actively secrete IgA responses, antibodies raised in mice who were immunized with parenterally delivered DNA vaccines are usually affected by a loss of the epitope specificity required for the adequate recognition of native bacterial antigens. The recovery of the neutralizing properties of the induced antibodies indicates that the primer-booster regimen both quantitatively and qualitatively improved the antibody responses elicited by the DNA vaccine and suggests that the recombinant Salmonella strain played a major role in the affinity maturation of established systemic B-cell responses. Such an effect may reflect, at least in part, the affinity maturation and selective expansion of regulatory T cells during transient infection with the recombinant Salmonella strain (6).
The lack of a relevant ETEC animal model has restricted efficacy studies of potential vaccines prior to volunteer administration, a step that is not feasible for most vaccine research laboratories in the world. In an attempt to evaluate the protective efficacy of the proposed ETEC vaccine regimen, we adapted a previously described neonate challenge model (10) for a human-derived ETEC strain. Based on that model, we demonstrated that serum antibodies raised in mice subjected to the DNA primer-Salmonella booster vaccine regimen conferred enhanced protection levels (82%) to newborn DBA2 mice who were lethally challenged with a CFA/I+ ETEC strain. As expected, sera from mice who were immunized only with the DNA vaccine did not confer any protection to the inoculated mice, due to the generation of antibodies that were unable to bind and neutralize surface-exposed epitopes required for the binding of the CFA/I fimbriae to host cell receptors (1). Such observations further suggest that antibodies raised in mice subjected to the combined vaccine regimen both quantitatively and qualitatively improve the elicited antibody responses. Nonetheless, a definite demonstration of the protective potential of the proposed vaccine regimen will require additional testing with appropriate animal models, such as the removable intestinal tie-adult rabbit diarrhea model (38), or the enrollment of human volunteers in a vaccine trial.
So far, vaccines against ETEC-associated diarrhea have been mainly restricted to inactivated ETEC strains producing different colonization factors and to attenuated Salmonella or Shigella strains that have been genetically modified to express ETEC CFAs (3, 16). The present results indicate that a vaccine regimen based on priming with a DNA vaccine followed by boosting with a recombinant Salmonella strain expressing the same ETEC fimbrial protein can be an alternative for the development of ETEC vaccines based on the primer-booster concept, a vaccine approach which has been successfully used against several virus- and parasite-associated diseases. Constructions based on multiepitope DNA vaccines or the combination of several plasmids encoding different proteins is feasible and may help us to develop multitarget vaccines aiming for the control of ETEC colonization as well as infections with pathogens with heterogenous antigen compositions.
Acknowledgments
We acknowledge the invaluable technical assistance of M. N. Oliveira and M. Normandia. We also thank J. M. A. Mosig, M. R. D'Imperio Lima, L. R. Sardinha, and K. R. Bastos for their important contributions to the ELISPOT assays as well as I. A. Abrahamsohn and T. Mosca for cytokine measurement experiments.
This work was supported by CNPq and FAPESP grants.
Editor: J. T. Barbieri
REFERENCES
- 1.Alves, A. M. B., M. O. Lasaro, D. F. Almeida, and L. C. S. Ferreira. 1998. Epitope specificity of antibodies raised against enterotoxigenic Escherichia coli CFA/I fimbriae in mice immunized with naked DNA. Vaccine 16:9-15. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, R. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Barry, E. M., Z. Altboum, G. Losonsky, and M. M. Levine. 2003. Immune responses elicited against multiple enterotoxigenic Escherichia coli fimbriae and mutant LT expressed in attenuated Shigella vaccine strains. Vaccine 21:333-340. [DOI] [PubMed] [Google Scholar]
- 4.Black, R. E. 1990. Epidemiology of traveller's diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12:S73-S79. [DOI] [PubMed] [Google Scholar]
- 5.Bühler, T., H. Hoschützky, and K. Jann. 1991. Analysis of colonization factor antigen I, an adhesin of enterotoxigenic Escherichia coli O78:H11: fimbrial morphology and location of the receptor-binding site. Infect. Immun. 59:3876-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busch, D. H., and E. G. Pamer. 1999. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 189:701-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, G. H., W. C. Wilcox, D. L. Sodora, D. Long, J. Z. Levin, and R. J. Eisenberg. 1988. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J. Virol. 62:1932-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darji, A., C. A. Guyman, and B. Gerstel. 1997. Oral somatic transgene vaccination using attenuated S. Typhimurium. Cell 91:765-775. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 10.Duchet-Suchaux, M., C. Le Maitre, and A. Bertin. 1990. Differences in susceptibility of inbred and outbred infant mice to enterotoxigenic Escherichia coli of bovine, porcine and human origin. J. Med. Microbiol. 56:185-190. [DOI] [PubMed] [Google Scholar]
- 11.Eo, S. K., M. Gierynska, A. A. Kamar, and B. T. Rouse. 2001. Prime/boost immunization with DNA vaccine: mucosal route of administration changes the rules. J. Immunol. 166:5473-5479. [DOI] [PubMed] [Google Scholar]
- 12.Eo, S. K., S. Lee, S. Chun, and B. T. Rose. 2001. Modulation of immunity against herpes simplex virus infection via mucosal genetic transfer of plasmid DNA encoding chemokines. J. Virol. 75:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fennelly, G. J., S. A. Khan, M. A. Abadi, T. F. Wild, and B. R. Bloom. 1999. Mucosal DNA vaccine immunization against measles with a highly attenuated Shigella flexneri vector. J. Immunol. 162:1603-1610. [PubMed] [Google Scholar]
- 14.Gaastra, W., and A. M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli. Trends Microbiol. 4:444-452. [DOI] [PubMed] [Google Scholar]
- 15.Gastão da Silva, A. P., J. F. Jacysyn, and I. A. Abrahamsohn. 2003. Resistant mice lacking interleukin-12 become susceptible to Trypanosoma cruzi infection but fail to mount a T helper type 2 response. Immunology 108:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giron, J. A., J. G. Su, C. R. Gonzalez, D. Hone, J. B. Kaper, and M. M. Levine. 1995. Simultaneous expression of CFA/I and CS3 colonization factor antigens of enterotoxigenic Escherichia coli by ΔaroC, ΔaroD Salmonella typhi vaccine strain CVD-908. Vaccine 13:939-946. [DOI] [PubMed] [Google Scholar]
- 17.Guillobel, H. C. R., J. I. Carinhanha, L. Cardenas, J. D. Clements, D. F. de Almeida, and L. C. S. Ferreira. 2000. Adjuvant activity of a nontoxic mutant of Escherichia coli heat-labile enterotoxin on systemic and mucosal immune responses elicited against a heterologous antigen carried by a live Salmonella enterica serovar Typhimurium vaccine strain. Infect. Immun. 68:4349-4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillobel, H. C. R., M. G. Luna, E. F. Camacho, D. F. Almeida, and L. C. S. Ferreira. 1998. Immunization against the colonization factor antigen I of enterotoxigenic Escherichia coli by administration of a bivalent Salmonella typhimurium aroA strain. Braz. J. Med. Biol. Res. 31:545-554. [DOI] [PubMed] [Google Scholar]
- 19.Han, S. W., J. Z. de Moraes, C. L. Silva, R. Camas, and M. M. Rodríguez. 2003. DNA vaccines, p. 329-361. In Y. E. Khudyakov and H. A. Fields (ed.), Artificial DNA. Methods and applications. CRC Press, Boca Raton, Fla.
- 20.Hormaeche, C. E., H. S. Joysey, L. DeSilva, M. Ighar, and B. A. D. Stocker. 1990. Immunity induced by live attenuated Salmonella vaccines. Res. Microbiol. 141:757-764. [DOI] [PubMed] [Google Scholar]
- 21.Jertborn, M., C. Ahren, J. Holmgren, and A. M. Svennerholm. 1998. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine 16:255-260. [DOI] [PubMed] [Google Scholar]
- 22.Jones, D. H., S. Corris, S. McDonald, J. C. Clegg, and G. H. Farrar. 1997. Poly(dl-lactide-co.glycolide)-encapsulated plasmid DNA elicits systemic and mucosal antibody responses to encoded protein after oral administration. Vaccine 15:814-817. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko, H., I. Bednarek, A. Wierybicki, I. Kiska, M. Dmochowski, T. J. Wasik, Y. Kaneko, and D. Kozbor. 2000. Oral DNA vaccination promotes mucosal and systemic immune responses to HIV envelope glycoprotein. Virology 267:8-16. [DOI] [PubMed] [Google Scholar]
- 24.Kent, S. J., A. Yhao, S. J. Best, J. D. Chandler, D. B. Boyle, and I. A. Ramshaw. 1998. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant cowpox virus. J. Virol. 72:10180-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klavinskis, L. S., L. Gao, C. Barnfield, T. Lehner, and D. Burman. 1997. Mucosal immunization with DNA-liposome complexes. Vaccine 15:818-820. [DOI] [PubMed] [Google Scholar]
- 26.Kuklin, N., M. Daheshia, K. Karem, E. Manickan, and B. T. Rose. 1997. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J. Virol. 71:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasaro, M. O., A. M. Alves, H. C. Guillobel, D. F. Almeida, and L. C. Ferreira. 1999. New vaccine strategies against enterotoxigenic Escherichia coli. II. Enhanced systemic and secreted antibody responses against the CFA/I fimbriae by priming with DNA and boosting with a live recombinant Salmonella vaccine. Braz. J. Med. Biol. Res. 32:241-246. [DOI] [PubMed] [Google Scholar]
- 28.Letvin, N. L., D. C. Monteriori, Y. Yasutomi, H. C. Perry, M. E. Davies, C. Lekutis, M. Alroy, D. C. Freed, C. I. Lord, L. K. Handt, M. A. Liu, and J. W. Shiver. 1997. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc. Natl. Acad. Sci. USA 94:9378-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine, M. M., J. Galen, E. Barry, F. Noriega, S. Chatfield, M. Sztein, G. Dougan, and C. Tacket. 1996. Attenuated Salmonella as live oral vaccines against typhoid fever and as live vectors. J. Biotechnol. 44:193-196. [DOI] [PubMed] [Google Scholar]
- 30.Lundholm, P., Y. Asukara, J. Hinkula, E. Lucht, and B. Wahren. 1999. Induction of mucosal IgA by a novel jet delivery technique for HIV-1 DNA. Vaccine 17:2036-2042. [DOI] [PubMed] [Google Scholar]
- 31.Mollenkopf, H. J., D. G. Triebkorn, P. Andersen, J. Hess, and S. H. E. Kaufmann. 2001. Protective efficacy against tuberculosis of ESAT-6 secreted by live Salmonella typhimurium vaccine carrier strain and expressed by naked DNA. Vaccine 19:4028-4035. [DOI] [PubMed] [Google Scholar]
- 32.Pickett, T. E., M. F. Pasetti, J. E. Galen, M. B. Sztein, and M. M. Levine. 2000. In vivo characterization of the murine intranasal model for assessing the immunogenicity of attenuated Salmonella enterica serovar Typhi strains as live mucosal vaccines and as live vectors. Infect. Immun. 68:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramshaw, I. A., and A. J. Ramsay. 2000. The prime/boost strategy: exciting prospects for improved vaccination. Immunol. Today 2:163-165. [DOI] [PubMed] [Google Scholar]
- 34.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S.-L. Hu, G. P. Mazzara, D. L. Panicali, J. G. Herndon, R. Glicknam, M. A. Candido, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenge by DNA priming and recombinant poxvirus booster immunizations. Nat. Med. 5:526-534. [DOI] [PubMed] [Google Scholar]
- 35.Rudin, A., and A. M. Svennerholm. 1996. Identification of a cross-reactive continuous B-cell epitope in enterotoxigenic Escherichia coli colonization factor antigen I. Infect. Immun. 64:4508-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sansonetti, P. J. 1998. Slaying the Hydra all at once or head by head? New candidates show promise in protecting against the diverse pathogens that cause diarrheal diseases. Nat. Med. 4:499-500. [DOI] [PubMed] [Google Scholar]
- 37.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. S. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 38.Spira, W. M., R. B. Sack, and J. L. Froehlich. 1981. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect. Immun. 32:739-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanCott, J. L., H. F. Staats, D. W. Pascual, M. Roberts, S. N. Chatfield, M. Yamamoto, M. Coste, P. B. Cartes, H. Kiyono, and J. R. MacGhee. 1996. Regulation of mucosal and systemic antibody responses by T helper cell subset, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J. Immunol. 156:1504-1514. [PubMed] [Google Scholar]
- 40.Woodberry, T., J. Gardner, S. L. Elliott, S. Leyer, D. M. Purdie, P. Chaplin, and A. Suhrbier. 2003. Prime boost vaccination strategies: CD8 T cell numbers, protection, and Th1 bias. J. Immunol. 170:2599-2604. [DOI] [PubMed] [Google Scholar]
- 41.Xiang, Y. Q., S. Paquini, and H. C. J. Ertl. 1999. Induction of genital immunity by DNA priming and intranasal booster immunization with a replication-defective adenoviral recombinant. J. Immunol. 162:6716-6723. [PubMed] [Google Scholar]
- 42.Xiang, Z. Q., and H. C. J. Ertl. 1995. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity 2:129-135. [DOI] [PubMed] [Google Scholar]