Abstract
This longitudinal prospective study shows that antibodies to the N-terminal block 2 region of the Plasmodium falciparum merozoite surface protein 1 (MSP-1) are associated with protection against clinical malaria in an area of stable but seasonal malaria transmission of Ghana. Antibodies to the block 2 region of MSP-1 were measured in a cohort of 280 children before the beginning of the major malaria transmission season. The cohort was then actively monitored for malaria, clinically and parasitologically, over a period of 17 months. Evidence is presented for an association between antibody responses to block 2 and a significantly reduced risk of subsequent clinical malaria. Furthermore, statistical survival analysis provides new information on the duration of the effect over time. The results support a conclusion that the block 2 region of MSP-1 is a target of protective immunity against P. falciparum and, thus, a promising new candidate for the development of a malaria vaccine.
The asexual erythrocytic stages of Plasmodium falciparum infection are responsible for all clinical manifestations of malaria, and antigens on the asexual merozoite stage are believed to be important in the development of protective immunity to the disease. One such antigen, the P. falciparum merozoite surface protein 1 (Pf MSP-1), is the precursor of the major antigenic complex on the surface of blood merozoites (16, 20, 25). Importantly, since MSP-1 is also expressed in liver schizonts (37), immunity to MSP-1 has the potential to control early liver-derived merozoites before the infection can progress to the blood phase and the disease.
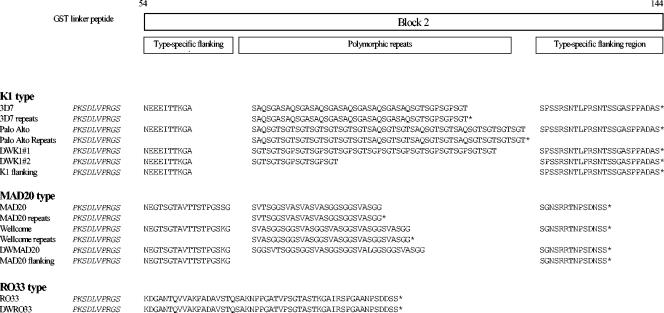
The MSP-1 gene of P. falciparum is divided into 17 distinct blocks that encode conserved, dimorphic or polymorphic regions of the protein (19, 26, 38). MSP-1 alleles belong to one or the other of two families based on the dimorphic sequences and named after the MAD20 and Wellcome isolates (38). The block 2 region, located within the N-terminal half of MSP-1, is more polymorphic, with over 50 different sequence variants identified. However, all block 2 sequences belong to one or another of only three main sequence types represented by prototypic variants originally identified in the K1, MAD20, and RO33 isolates (9, 21, 38). Variants within the K1-like and MAD20-like types of block 2 differ in tri- or hexapeptide repeats (21, 26, 38), whereas block 2 of the RO33 type is a nonrepetitive sequence which varies little between isolates (9, 26) (Fig. 1). Importantly, the main sequence types are recognized as three major block 2 serotypes by human antibodies produced in response to natural P. falciparum infections (6-8).
FIG. 1.
Schematic diagram of MSP-1 block 2 sequences of all recombinant antigens are shown in single amino acid code. Asterisks indicate stop codons.
MSP-1 has been the focus of many epidemiological studies on human immune responses to natural malaria infections (22). Antibodies to the conserved C-terminal MSP-119 are found in the majority of malaria-exposed individuals (14, 31), and the presence of such antibodies has been correlated with protection from clinical symptoms of malaria in some, though not all, studies (12, 15, 31). The roles in human malaria immunity of responses induced by other regions of this large protein remain almost unknown. Early studies suggested that antibodies to the polymorphic and/or dimorphic sequences located in the N-terminal half of MSP-1 are important (10, 17, 27, 41). Gabonese patients who had cleared infections had higher levels of immunoglobulin G (IgG) against an N-terminal fragment of MSP-1 than patients who had persistent infections (10). Furthermore, in another study low and short-lived antibody responses to N-terminal regions of MSP-1 correlated with a higher risk of subsequent reinfection (41). More recently, a new approach combining population genetics with an immunoepidemiological prospective cohort study has identified specifically the block 2 region of Pf MSP-1 as a major target of human immunity against P. falciparum malaria (11). Since block 2 of Pf MSP-1 has no homologue in most animal species of malaria parasites, there is no convenient animal model to validate this new candidate for vaccine development. Thus, to progress further, it is essential to extend immunoepidemiological studies in the natural hosts, i.e., human populations exposed to P. falciparum malaria.
This prospective study is a part of a program to characterize patterns of naturally acquired immune responses to block 2 and other defined proteins of P. falciparum in ethnically varied people exposed to variable levels of malaria infections. Here, block 2-specific antibodies were analyzed in children from an area of seasonal malaria transmission in Dodowa, Ghana, before malaria transmission began. We report significant associations between the presence of IgG (particularly IgG3) to two of the three main types of the block 2 region and subsequent protection from clinical malaria episodes, thus strengthening evidence obtained earlier in The Gambia (11). Furthermore, new information is presented here on the duration of this protection.
MATERIALS AND METHODS
Study area.
The study was conducted in the town of Dodowa (population circa 6,500), 50 km northeast of Accra, Ghana. The area is defined as one of hyperendemicity with seasonal malaria transmission. There are two rainy seasons, a major season from May to August and a minor one between October and November. A relatively dry period follows the second rains and lasts from December to April. Malaria transmission is classified as perennial and stable but is highest during or immediately after the major and minor rainy seasons and lowest during the dry season (12). It is estimated that each individual in Dodowa is exposed to approximately 20 infective bites per year, and 98% of malaria infections are due to P. falciparum (1).
Study population and clinical surveillance.
A cohort of 280 children, typed negative for sickle cell trait, was recruited to the study in April 1994. The children were 3 to 15 years of age; 54% were male and 46% were female. Each age group within the cohort included between 12 and 34 children. The study was approved by the Ghanaian Ministry of Health, and informed parental consent was obtained for each child after an explanation of all procedures involved. The study started in April 1994 and was completed in August 1995. Blood slides were obtained from all children once a month to determine the point prevalence of parasitemia. Throughout this 17-month period, field assistants resident in Dodowa actively monitored the cohort for clinical symptoms and blood parasitemia. During weekly visits to each child, information regarding the child's health status in the previous week was recorded on a standard questionnaire form, and axillary temperature was measured by using a digital thermometer. Blood slides were taken from all children with temperatures of >37.5°C and also from children complaining of symptoms suggestive of malaria, and samples were checked for parasitemia. Parents were instructed to bring sick children to the field assistants also outside the scheduled weekly visits for recording of temperature and blood parasitemia determination by finger prick. Any child with detectable parasitemia and fever was immediately treated with chloroquine. In analyses of the clinical data, individuals were considered to have had clinical malaria if (i) they reported fever and/or had a measured temperature higher than 37.5°C and (ii) they had parasitemia of >5,000 parasites per μl. The parasite cutoff level of 5,000 parasites/ml was based on morbidity and gave a sensitivity and specificity of 90% (40). In children with fever but parasitemia below this threshold, it is uncertain whether the symptoms were due to malaria (36). Data on antibody responses to block 2 and other regions of MSP-1 are based on analyses of plasma samples obtained from 280 children for whom clinical and parasitological data were available for the duration of the 17-month follow-up period.
Plasma samples.
Venous blood samples were obtained from the cohort individuals on two occasions. All 280 children donated samples in April 1994, just before the onset of the major malaria transmission season. Second samples from 266 of the donors were collected in November 1994, after the malaria transmission season. Each sample was drawn aseptically into heparinized Vacutainer tubes (Becton Dickinson, Rutherford, N.J.). Plasmas were separated under sterile conditions and stored at −20°C. Negative control plasma samples were obtained from 34 healthy European adults who were malaria naïve. Positive controls were individual plasmas and a pool of plasmas from adult African donors, selected for high antibody reactivity to MSP-1 antigens (7, 8).
Recombinant antigens.
A panel of recombinant proteins derived from MSP-1, including full-length block 2 sequences of culture-adapted (3D7, Palo Alto, MAD20, Wellcome, and RO33) and field isolates (DWK1#1, DWK1#2, DWMAD20, and DWRO33) and a block 1 (MAD20 variant) protein, were immunologically validated earlier (8). These antigens all induce animal antibodies that recognize parasite-produced MSP-1 with specificities as appropriate for distinct block 2 types expressed by a range of P. falciparum isolates (8). Glutathione S- transferase (GST) fusion proteins containing repeat-only block 2 sequences from the 3D7, Palo Alto, MAD20, and Wellcome isolates and also proteins containing only the K1-type and MAD20-type flanking sequences have been described elsewhere (29). Sequences of the block 2 antigens are shown in Fig. 1. A protein corresponding to the C-terminal MSP-119 was described and immunologically evaluated earlier (5). All antigens were expressed in Escherichia coli as soluble proteins fused to the C terminus of GST of Schistosoma japonicum by using the pGEX-2T vector and purified by published methods (35). Control GST protein was prepared from E. coli harboring pGEX-2T alone.
ELISA.
Human plasma samples were tested by enzyme-linked immunosorbent assay (ELISA) for the presence of IgG antibodies able to recognize the recombinant MSP-1 antigens. Wells of 96-well plates (Immulon 4; Dynatech) were coated with 50 ng of recombinant antigens in 100 μl of coating buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH 9.3]) overnight at 4°C. The wells were washed three times in washing buffer (0.05% Tween 20 in phosphate-buffered saline). Unoccupied protein binding sites were blocked with 200 μl per well of blocking buffer (1% [wt/vol] skim milk powder in washing buffer) for 5 h at room temperature and washed again three times. Human plasma diluted 1:500 in the blocking buffer (100 μl per well) was added to duplicate antigen-coated wells and incubated overnight at 4°C. After three washes, the wells were incubated for 3 h at room temperature with 100 μl per well of horseradish peroxidase-conjugated rabbit anti-human IgG (1:5,000) (Dako Ltd., High Wycombe, United Kingdom). Plates were washed three times before incubating for 15 min at room temperature with 100 μl of substrate (0.1 mg of o-phenylenediamine [Sigma] per ml, 0.012% H2O2) in development buffer (24.5 mM citric acid monohydrate and 52 mM Na2HPO4, pH 5.0). The reaction was stopped by the addition of 25 μl of 2 M H2SO4, and the optical density at 492 nm (OD492) was measured. Corrected OD values for each plasma sample were calculated by subtracting the mean OD value of wells containing the control GST protein alone from the mean OD value obtained with each test MSP-1 antigen. For IgG1 and IgG3 subclass-specific ELISAs, plasma samples were diluted 1:250 in blocking buffer and detected as above by using polyclonal sheep antibodies specific for human IgG1 (1:1,000) or IgG3 (1:1,500) (The Binding Site, Birmingham, United Kingdom).
Cutoff values, at which the binding of antibody from malaria-exposed individuals was regarded as significantly above background, were calculated as the mean plus three standard deviations of OD readings obtained with sera from 34 Scottish blood donors with no history of exposure to malaria. For total IgG, the cutoff OD value for each antigen was as follows: MAD20 block 1, 0.078; 3D7 block 2, 0.206; 3D7 repeats, 0.242; Palo Alto block 2, 0.179; Palo Alto repeats, 0.276; DWK1 block 2 #1, 0.083; DWK1 block 2 #2, 0.064; K1 block 2 flank, 0.267; MAD20 block 2, 0.023; MAD20 repeats, 0.175; Wellcome block 2, 0.208; Wellcome repeats, 0.283; DW MAD20 block 2, 0.069; MAD20 block 2 flank, 0.200; RO33 block 2, 0.063; DW RO33 block 2, 0.061; MSP-119, 0.073. For IgG1-specific ELISA, the cutoff OD value for each antigen was as follows: 3D7 block 2, 0.029; Palo Alto block 2, 0.015; DWK1 block 2 #1, 0.017; DWK1 block 2 #2, 0.015; MAD20 block 2, 0.015; Wellcome block 2, 0.013; DW MAD20 block 2, 0.017; RO33 block 2, 0.017; DW RO33 block 2, 0.020; MSP-119, 0.020. For IgG3-specific ELISA, the cutoff OD value for each antigen was as follows: 3D7 block 2, 0.052; Palo Alto block 2, 0.027; DWK1 block 2 #1, 0.023; DWK1 block 2 #2, 0.023; MAD20 block 2, 0.016; Wellcome block 2, 0.017; DW MAD20 block 2, 0.021; RO33 block 2, 0.020; DW RO33 block 2, 0.019; MSP-119, 0.013.
Statistical analysis.
Statistical analysis was done with the SPSS software package (SPSS Inc., Chicago, Ill.) and the EpiInfo 2000 software package (http://www.cdc.gov/epiinfo/). The χ2 test or Fisher's exact test was used to compare the proportions of children with and without malaria (unprotected and protected) in groups of antibody-positive and antibody-negative individuals and to compare the frequencies of antibody positivity in pre- and postmalaria season samples. To assess the effects of age on associations between antibody positivity (or antibody levels, as measured in absorbance units) and malaria outcome, data were also analyzed by multivariate (logistic regression) analysis, in which age was accounted for as a confounding variable. Survival analysis, according to the Cox proportional hazard model, was used to compare data on first clinical malaria episodes in children for each antigen, with age included in the model as a confounding variable. Differences were considered statistically significant if P was <0.05.
RESULTS
Malaria in the study cohort.
All except two children in the cohort were blood film positive for P. falciparum at one or more of the monthly screenings done to determine parasite point prevalence (12). Interestingly, only some of these infections were symptomatic. The incidence of malaria varied considerably over the year, peaking in August 1994 and again in July 1995 and reaching the lowest levels in April 1994 and February 1995 (12). During the 17-month period of surveillance, 108 of 280 (38.6%) children had at least one clinical episode of malaria, and these children were considered to be susceptible to malaria. A total of 172 of 280 (61.4%) children did not have complaints of fevers or measured febrile temperatures in the presence of asexual parasitemia (>5,000 parasites/ml) at any of the weekly screenings, and these children were considered to be protected from clinical malaria. Susceptible children tended to be younger (median age, 6 years; range, 3 to 15 years) than the protected children (median age, 9 years; range, 3 to 15 years) (P < 0.00001).
Antibody responses to block 2 and other regions of MSP-1 depend on exposure and age.
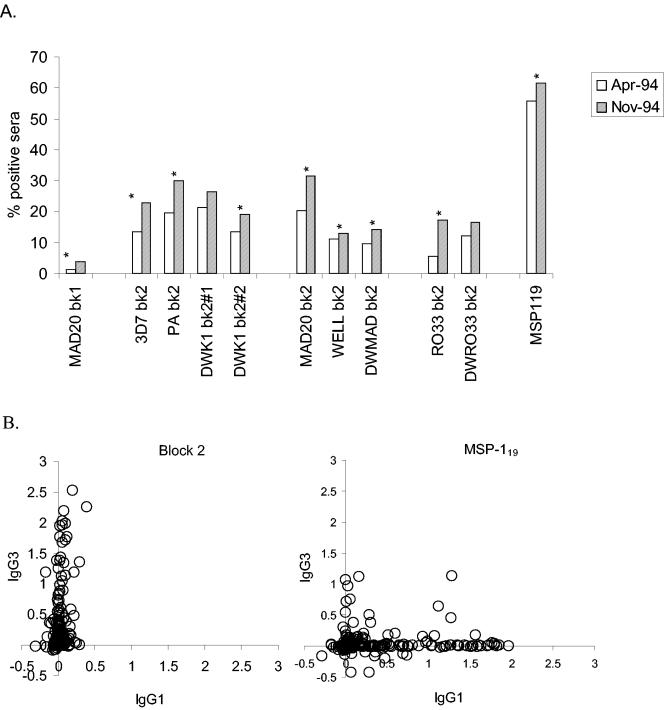
Seropositivity frequencies of children with antibodies to 11 antigens derived from MSP-1 were measured by using plasma samples taken in April before the start of seasonal malaria transmission and after the season in November 1994. In the preseason samples from 280 children, the most commonly recognized antigen was MSP-119 (56%), followed by various block 2 constructs (between 2.5 to 22%), while only 1.4% of the samples contained detectable levels of antibodies to the block 1 antigen (Fig. 2). After the transmission season, the frequencies of seropositivity moderately increased, with 62% of children having detectable antibodies to MSP-119, followed by 13 to 32% with antibodies to block 2 antigens, and 3.8% antibody positive for block 1. These seasonal increases were statistically significant for all antigens tested, except for block 1 and two block 2 variants (DWK1#1 and DWRO33) (Fig. 2A).
FIG. 2.
Frequencies and isotype biases of IgG antibodies to different regions of MSP-1. (A) Frequencies of seropositive individuals in the cohort before malaria transmission season (April 1994; n = 280) and after the season (November 1994; n = 266). Percentages of antibody-positive individuals for each full-length Block 2 antigen, Block 1, and MSP-119 are shown as bars. Asterisks indicate pairs of frequencies that significantly increased between the two samplings (P < 0.05). (B) Comparisons between IgG1 and IgG3 subclasses to Block 2 and MSP-119. Each point represents levels of antigen-specific subclasses in the April sample from one individual.
The overall prevalence of antibody positivity to any block 2 antigen tested was age dependent, increasing from 19.4% in the group of 3- to 5-year-olds, 45% in the group of 6- to 9-year-olds, to 58.3% in the group of 10- to 15-year-olds. In comparison, little increase in antibody prevalence with age was seen to MSP-119, (51.1, 56, and 59.4% in the three age groups, respectively).
Antibodies to block 2 and protection from malaria.
The relative risks of clinical malaria were compared over a 17-month period between groups of children with or without antibodies to different regions of MSP-1 at the start of the period. Lower frequencies of clinical malaria were seen among children who had IgG antibodies to block 2 (Table 1, group A). For most block 2 antigens, there were statistically significant associations between the presence of detectable IgG and a lowered relative risk of clinical malaria episodes during the follow-up period (Table 1, group A, and Table 2). Antibodies to most full-length antigens from the two common block 2 types, namely the K1- and MAD20-like types, were significantly associated with protection in this cohort, as shown by the relative risk values for IgG-positive children, specific for each antigen, which were significantly less than 1.0 (Table 1). Antibodies to two antigens (3D7 and DWK1#2), for which there was no statistically significant association, still showed the same trend. This association was also statistically significant for antibodies to the repeat-only antigens of the MAD20 block 2 type. For antibodies to the repeat-only antigens of the K1 type, a trend toward reduced risk was apparent, although this was not statistically significant (Table 1). The association of antibodies to block 2 with a reduced risk of clinical malaria was also statistically highly significant when the data were analyzed for children who were antibody positive for any block 2 variant, versus those without antibodies to any variant tested (P = 0.009) (Table 1). Children with antibodies to both the MAD20-like and K1-like types also had a significantly lower incidence of clinical malaria episodes (P = 0.0038) (Table 1). All significant associations between antibody positivity to any block 2 antigen that had a P value of < 0.01 remained statistically significant (P < 0.05), when age was accounted for as a confounding variable by multivariate logistic regression analysis (data not shown). When antibody levels (i.e., corrected absorbance values) against the block 2 antigens (rather than positive-negative scoring of antibody reactivity) were analyzed by multinomial logistic regression analysis for associations with reduced incidence of malaria, highly significant P values (0.01 or less) were obtained for all flock 2 antigens tested. This finding indicates that differences in the absolute levels of antibody against flock 2, as measured by absorbance values in ELISAs, are important in terms of protective effect.
TABLE 1.
Incidence of clinical malaria in antibody-positive and -negative groups of children to particular recombinant antigenic fragments of MSP-1
| Antibody group and antigena | Proportion (%) of clinical malaria (no. of positive samples/total no. of samples) among:b
|
RR (95% CI)c | P valued | |
|---|---|---|---|---|
| IgG-positive children | IgG-negative children | |||
| Group A | ||||
| K1 Block 2 type | ||||
| 3D7 full | 39.5 (15/38) | 38.4 (93/242) | 1.03 (0.67-1.57) | 0.902 |
| 3D7 rpts | 27.8 (10/36) | 40.2 (98/244) | 0.69 (0.40-1.20) | 0.154 |
| Palo Alto full | 27.1 (19/70) | 42.4 (89/210) | 0.64 (0.42-0.97) | 0.023* |
| Palo alto rpts | 15.4 (2/13) | 39.7 (106/267) | 0.39 (0.11-1.40) | 0.079 |
| DWK1#1 full | 26.7 (16/60) | 41.8 (92/220) | 0.64 (0.41-1.00) | 0.033* |
| DWK1#2 full | 28.9 (11/38) | 40.1 (97/242) | 0.72 (0.43-1.22) | 0.19 |
| K1 flanking | 28.6 (6/21) | 39.4 (102/259) | 0.73 (0.36-1.45) | 0.328 |
| MAD20 Block 2 type | ||||
| MAD20 full | 21.1 (12/57) | 43.0 (96/223) | 0.49 (0.29-0.83) | 0.002** |
| MAD20 rpts | 14.3 (3/21) | 40.5 (105/259) | 0.35 (0.12-1.02) | 0.017* |
| Wellcome full | 12.9 (4/31) | 41.8 (104/249) | 0.31 (0.12-0.78) | 0.002** |
| Wellcome rpts | 13.3 (2/15) | 40.0 (106/265) | 0.33 (0.09-1.22) | 0.039* |
| DWMAD20 full | 11.1 (3/27) | 41.5 (105/253) | 0.27 (0.09-0.79) | 0.002** |
| MAD20 flanking | 14.3 (1/7) | 39.2 (107/273) | 0.36 (0.06-2.25) | 0.181 |
| RO33 Block 2 type | ||||
| RO33 full | 31.3 (5/16) | 39.0 (103/264) | 0.80 (0.38-1.68) | 0.536 |
| DWRO33 full | 38.2 (13/34) | 38.6 (95/246) | 0.99 (0.63-1.56) | 0.966 |
| K1 plus MAD20 type | 16.7 (6/36) | 41.8 (102/244) | 0.40 (0.19-0.84) | 0.0038** |
| Any Block 2 | 29.6 (34/115) | 44.8 (74/165) | 0.66 (0.47-0.92) | 0.009** |
| MSP119 | 37.8 (59/156) | 39.5 (49/124) | 0.96 (0.71-1.29) | 0.772 |
| MAD20 Block 1 | 25 (1/4) | 38.8 (107/276) | 0.64 (0.12-3.54) | 0.574 |
| Group B | ||||
| K1 Block 2 type | ||||
| 3D7 full | 6.25 (1/16) | 40.5 (107/264) | 0.15 (0.02-1.03) | 0.006** |
| Palo Alto full | 30.0 (15/50) | 40.4 (93/230) | 0.74 (0.47-1.17) | 0.169 |
| DWK1#1 full | 30.0 (6/26) | 40.1 (102/254) | 0.57 (0.28-1.18) | 0.088 |
| DWK1#2 full | 21.4 (3/11) | 39.5 (105/266) | 0.54 (0.20-1.50) | 0.176 |
| MAD20 Block 2 type | ||||
| MAD20 full | 25.0 (8/32) | 40.3 (100/248) | 0.62 (0.33-1.15) | 0.094 |
| Wellcome full | 26.7 (12/45) | 40.9 (96/235) | 0.65 (0.39-1.09) | 0.073 |
| DWMAD20 full | 38.5 (5/13) | 38.6 (103/267) | 1.00 (0.49-2.02) | 0.993 |
| RO33 Block 2 type | ||||
| RO33 full | 35.3 (6/17) | 38.8 (102/161) | 0.91 (0.47-1.76) | 0.775 |
| DWRO33 full | 38.2 (13/34) | 38.6 (95/246) | 0.99 (0.63-1.56) | 0.966 |
| Any Block 2 | 32.2 (38/118) | 43.2 (70/162) | 0.75 (0.54-1.02) | 0.06 |
| MSP119 | 39 (69/108) | 37.9 (39/103) | 1.03 (0.76-1.40) | 0.853 |
| Group C | ||||
| K1 Block 2 type | ||||
| 3D7 full | 30.2 (13/43) | 40.1 (95/237) | 0.75 (0.47-1.22) | 0.223 |
| Palo Alto full | 31.3 (21/67) | 40.8 (87/213) | 0.77 (0.52-1.13) | 0.163 |
| DWK1#1 full | 26.8 (19/71) | 42.6 (89/209) | 0.63 (0.41-0.95) | 0.018* |
| DWK1#2 full | 32.7 (17/52) | 39.9 (91/228) | 0.82 (0.54-1.25) | 0.334 |
| MAD20 Block 2 type | ||||
| MAD20 full | 20.0 (13/65) | 44.2 (95/215) | 0.45 (0.27-0.75) | 0.0004*** |
| Wellcome full | 28.8 (21/73) | 42.0 (87/207) | 0.68 (0.46-1.02) | 0.045* |
| DWMAD20 full | 22.2 (8/36) | 41 (100/244) | 0.54 (0.29-1.02) | 0.031* |
| RO33 Block 2 type | ||||
| RO33 full | 41.7 (20/48) | 37.9 (88/232) | 1.11 (0.76-1.61) | 0.598 |
| DWRO33 full | 36.4 (16/44) | 38.9 (92/236) | 0.93 (0.61-1.42) | 0.744 |
| Any Block 2 | 29.5 (41/139) | 47.5 (67/141) | 0.62 (0.46-0.85) | 0.002** |
| MSP119 | 30.6 (33/108) | 39.3 (75/191) | 0.78 (0.56-1.09) | 0.133 |
Group A, IgG-positive and IgG-negative individuals; Group B, IgG1-positive and IgG1-negative individuals; and Group C, IgG3-positive and IgG3-negative individuals.
Percentages apply to children with antibodies to regions of MSP-1 at the start of the study period who subsequently contracted malaria in the follow-up period.
The calculated relative risk (RR) of malaria in the antibody positive groups is shown with 95% confidence intervals (CI).
*, P < 0.05; **, P < 0.01; ***, P < 0.001. All protective associations with P values below 0.01 remained statistically significant when analyzed by multiple logistic regression, after adjusting for age as a confounding variable in the analysis.
TABLE 2.
Survival analysis of time to clinical malariaa
| Antigen | Regression coefficient (B) | Hazard ratio | 95% CI | P value |
|---|---|---|---|---|
| K1 Block 2 type | ||||
| 3D7 Block 2 | −1.035 | 0.355 | (0.117-1.075) | 0.067 |
| 3D7 repeats | −0.448 | 0.639 | (0.246-1.659) | 0.358 |
| Palo Alto Block 2 | −1.223 | 0.294 | (0.088-0.982) | 0.047* |
| Palo Alto repeats | −0.939 | 0.391 | (0.055-2.801) | 0.350 |
| DWK1#1 Block 2 | −1.060 | 0.347 | (0.121-0.989) | 0.048* |
| DWK1#2 Block 2 | −1.580 | 0.206 | (0.033-1.284) | 0.091 |
| K1 flanking | −0.844 | 0.430 | (0.091-2.031) | 0.287 |
| MAD20 Block 2 type | ||||
| MAD20 Block 2 | −1.847 | 0.158 | (0.032-0.779) | 0.023* |
| MAD20 repeats | −0.858 | 0.424 | (0.091-1.985) | 0.276 |
| Wellcome Block 2 | −2.209 | 0.110 | (0.014-0.878) | 0.037* |
| Wellcome repeats | −1.210 | 0.298 | (0.047-1.873) | 0.197 |
| DWMAD20 Block 2 | −1.364 | 0.256 | (0.064-1.023) | 0.054 |
| MAD20 type flanking | −1.770 | 0.170 | (0.010-2.927) | 0.223 |
| RO33 Block 2 type | ||||
| RO33 Block 2 | −0.432 | 0.649 | (0.184-2.283) | 0.500 |
| DWRO33 Block 2 | −0.153 | 0.858 | (0.284-2.590) | 0.786 |
| Any Block 2 | −0.453 | 0.636 | (0.351-1.152) | 0.135 |
| Block 1 | −0.419 | 0.658 | (0.107-4.047) | 0.651 |
| MSP-119 | 0.049 | 1.050 | (0.809-1.362) | 0.715 |
Survival analysis calculated by using Cox proportional hazards model of the times to clinical malaria episodes in the cohort, for each antigen tested. Age was included in each model as a confounding variable as follows: coefficient (B), −0.703; hazard ratio, 0.495; 95% confidence interval (CI), (0.354-0.693); P value, < 0.0001***.
No statistically significant associations were found between antibodies to the flanking sequences of either the K1 or the MAD20 block 2 types and reduced risk of malaria (Table 1). This is in agreement with results from another cohort from The Gambia (29). Antibodies to the conserved block 1 and MSP-119 regions, or to the nonpolymorphic RO33 block 2 type, were not associated with a reduced risk of subsequent malaria episodes in this cohort (Table 1).
The P values shown in Table 1 were calculated without corrective adjustments for multiple comparisons, mainly because the multiple facets of the analyses introduce considerable uncertainty about the number of independent tests performed or implied. Here the testing is not of independent null hypotheses but related ones; block 2 antigens within each block 2 type share epitopes and amino acid sequence homologies, and some tested block 2 antigens (e.g., block 2 flanking antigens) are, in fact, subsections of other, longer antigens in the same assays. Following the suggestions of Rothman (32) and Perneger (28), we prefer to show the data in their unadjusted form to allow value judgments to be made of the significance of the reduction in malaria incidence for each antibody to each antigen in terms of its biological plausibility.
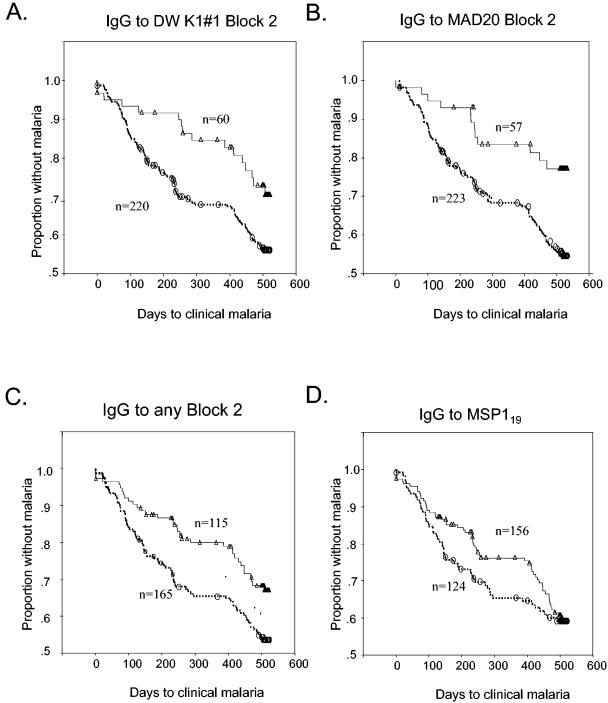
Survival analysis of time to clinical malaria.
The data were subjected to survival analysis by using Cox's proportional hazards regression model, including age as a covariate in the analysis to estimate, as another index of protection, the relative risk of malaria episodes subsequent to the determination of antibodies to specific antigens. This analysis revealed clear associations between the presence of IgG to the block 2 region and the time free from clinical malaria (Table 2 and Fig. 3). Differences in malaria-free survival times between antibody-positive versus antibody-negative groups were particularly clear during the first transmission season following the initial testing for antibodies in April 1994 (Fig. 3A and B, day 0 to day 250). The protective association of antibodies detected in these individuals appeared to be relatively short-lived, as differences in survival rates were negligible beyond the end of the first transmission season (Fig. 3A to C, day 400 onwards). However, this still indicates protective associations between possession of antibodies to block 2 and a reduced risk of malaria for at least 250 days. There was no significant association between antibodies to either the RO33 block 2 type or MSP-119 and this index of protection, although there was a somewhat lower incidence of malaria in the group with antibodies to MSP-119 in the first transmission season (Fig. 3D).
FIG. 3.
Kaplan-Meier plots of the cumulative incidence of first malaria episodes between antibody-positive and antibody-negative groups of children over the 17-month follow-up period. Proportions of children who remained malaria free in the antibody-positive and antibody-negative groups are shown as solid lines and dashed lines, respectively. Censored cases in the follow-up are shown by open symbols. The effects of IgG antibodies to different antigens are analyzed in separate panels.
Children with antibodies to any block 2 antigen had a significantly lowered risk of clinical malaria over time (Fig. 3C). Recategorization of the children as positive or negative for clinical malaria episodes during shorter periods of surveillance (between 1 and 11 months following the first blood sampling) did not change the associations between anti-block 2 antibody positivity and clinical protection (data not shown). Depending on the block 2 antigen, the relative risk (expressed as a hazard ratio in Cox proportional hazards model) of a malaria episode varied from 0.11 to 0.858 (Table 2). For the K1 and MAD20 block 2 types, this association was statistically significant for four of the antigens tested (Table 2, Palo Alto, DWK1#1, MAD20, and Wellcome), and the weaker associations in both these two types still showed the same trend (Table 2, all other K1- and MAD20-type antigens). No such association was seen with antibodies to the conserved antigens (block 1 and MSP-119) or to the RO33 block 2 type (Table 2). In summary, these data support the hypothesis that antibodies to the K1 and MAD20 types of block 2 contribute significantly to protection against malaria in this cohort.
IgG subclass specificities of anti-MSP-1 antibody responses.
Seeking an explanation for the relatively fast decline of the protective effect of anti-block 2 antibodies, IgG subclasses of antibodies to various MSP-1 antigens were determined in the cohort by using IgG1 and IgG3 subclass-specific reagents (6). All 280 plasma samples from April 1994 were tested against all full-length block 2 antigens and against MSP-119. Other block 2 antigens contained within the sequences of the full-length antigens (i.e., repeat-only and flanking antigens) were not analyzed due to the very limited volumes of serum available. Limited serum volumes precluded subclass analysis of the November samples from the same cohort. The responses to MSP-119 were predominantly of the IgG1 subclass, with a few sera containing IgG3 alone or both IgG1 and IgG3 (Fig. 2B). In marked contrast, antibodies to all block 2 antigens were restricted predominantly to the IgG3 subclass, with only some sera also showing weak IgG1 reactivities (Fig. 2B). Consequently, the associations between antibodies to block 2 and reduced risks of malaria were statistically significant for most antigens when the IgG3 subclass was measured but not when IgG1 was assessed (Table 1, groups B and C).
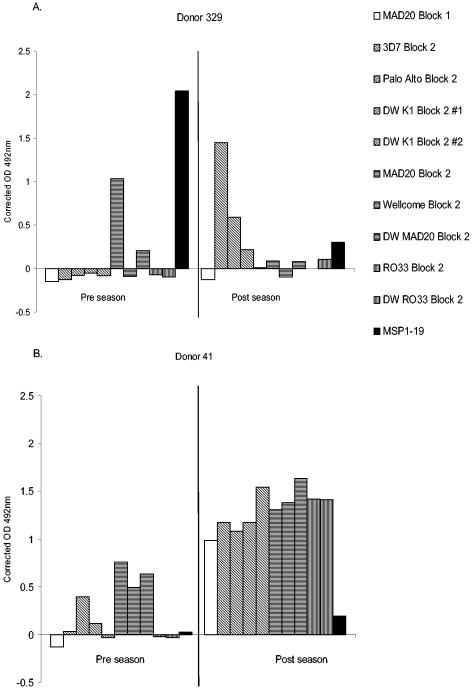
Specificities of antibody responses to MSP-1 block 2 are not fixed.
Paired plasma samples collected before and after malaria transmission (April and November 1994) from 236 individuals were tested for the presence of antibodies to block 2 antigens, and the results were then analyzed for changes or similarities in antibody specificities for different variants and/or types of block 2 antigens. Among initially antibody-negative children (210 to 265 individuals, depending on the antigen), 18 to 43 became antibody positive for one or another block 2 antigen between April and November. Among those children who were initially block 2 antibody positive (between 16 and 70, depending on the antigen), between 3 to 16 became antibody negative for any one block 2 antigen by November. Some antibody-positive individuals were seen to acquire additional block 2 antibody specificities that had not been present in the earlier sample (Fig. 4A and B), and some individuals who acquired new block 2 antibody specificities between April and November also became antibody negative for the block 2 type for which they had been positive in April (Fig. 4A). The changing patterns of acquisition and/or loss of block 2 antibody specificities by a significant number of children indicates that the responses seen are possibly a result of new infections and generally do not remain fixed in this cohort.
FIG. 4.
Changes in the specificity of Block 2 antibodies. Typical patterns of changes in Block 2 specificity with time are illustrated by antibody reactivities of pairs of serum samples taken from donors 329 and 41. Antibody reactivity is expressed as the OD492, comparing preseason (April 1994) and postseason (November 1994) samples from each donor side by side. MSP-1 antigens are shaded by type, as shown in the key. Panel A shows a changeover from a narrow MAD20-type specificity to K1-type specificities. Panel B shows boosts of antibody responses to K1 and MAD20 types and also the acquisition of antibodies against the RO33 type.
DISCUSSION
One aim of this study was to compare the apparent immunogenicity of the polymorphic block 2 and two conserved regions of MSP-1 in children living under conditions of stable but seasonal malaria transmission in Dodowa. Between 5 and 32% of the children had antibodies to one or another block 2 antigen, with a total of 41% of the cohort positive for at least one block 2 antigen before malaria transmission season in April, increasing to 53% positive after the season. These frequencies are in broad agreement with results of earlier studies carried in other populations from areas of different malaria endemicity (41). Anti-block 2 antibody frequencies tend to be lower than the frequency of antibodies to the conserved MSP-119 (55 to 60%). However, exposure to any one type of block 2 antigen is less frequent than to conserved domains like MSP-119, and, thus, the probability of a sustained primary or memory antibody response to a parasite expressing the same type of block 2 antigen is likely to be lower. Exposure and, thus, responses to internal polymorphic repetitive sequences from individual block 2 variants are likely to be lower still, as illustrated here by the low seroprevalence of antibodies to the repeat-only block 2 antigens (5 to 13%). An age-dependent increase in antibody prevalence to block 2, similar to the age-dependent prevalence of antibodies to several other merozoite antigens, probably reflects the number of past malaria infections experienced by each child (2, 34). This contrasts with the small age-related rise in antibody prevalence to MSP-119. These results are in agreement with earlier assays performed on this same cohort (12) and probably reflect the fact that MSP-119 is a nonpolymorphic portion of MSP-1 to which all children in the cohort had been previously exposed. However, at 56%, the prevalence of antibodies to MSP-119 detected in this study is higher than the frequency of 31% reported with the same set of plasma samples and the same recombinant antigen (5, 14). The sensitivity of the assay used here may be higher than in the earlier study that used a higher plasma dilution (1:1,000 compared to 1:500 here). The frequency of MSP-119 antibodies seen in this work is closer to that observed in The Gambia (11), in Sudan (7), and in Kenya (4). Antibody to MSP-1 block 1 antigen was detected in only a few individuals (four in April and nine in November), thus extending earlier observations of low antibody prevalence to block 1 in other cohorts in The Gambia and Sudan (7, 11) and of others in Senegal (where a synthetic peptide containing part of the block 1 sequence was used [24]). These findings indicate that although block 1 is conserved, it may be a poor immunogen and that antibody responses to block 1 antigen are not boosted by natural P. falciparum infections.
Antibodies to the full-length block 2 antigens were associated with a statistically significant reduced risk of clinical malaria episodes over a 17-month follow-up period. Survival analysis of this effect over time shows that the final outcome reflects a significant prolongation of time without malaria over the first malaria season (some 250 days or longer). This indicates a relatively short persistence of effective antibodies, their production, or both. The interpretation is in keeping with the finding that serum IgG to block 2 antigens in these Ghanaian children is predominantly of the IgG3 subclass. This further extends recent findings in three other East African populations (6), showing a strong IgG3 bias to be a general feature of human response to block 2 (in contrast to IgG1-biased responses to MSP-119).
The association between IgG (especially IgG3) responses to block 2 and a significantly reduced risk of clinical disease is important evidence that the block 2 region of MSP-1 is a significant target of protective immune responses to P. falciparum. Biases to either IgG1 or IgG3 have been observed in human responses to several defined protein antigens of P. falciparum. Similar to the IgG3 bias of responses to block 2, the main response to another merozoite surface protein (MSP-2) of P. falciparum is of the IgG3 subclass (33, 39). IgG3 antibodies are short-lived (half-life, 7 days), and thus require continued production to persist at levels that would have immediate protective effects. The presence of IgG3 block 2-specific antibody before malaria transmission indicates either that antigen (or parasitemia) persists in antibody-positive individuals or that, in the absence of antigenic stimulation, block 2-specific B cells persist at sufficiently high frequency to confer a protective effect for prolonged periods. The general association between the presence of cytophilic IgG subclasses, namely IgG1 and IgG3, and protection against malaria has been shown by others (3, 13). Such antibodies are proposed to play a role in protection through several mechanisms, including inhibition of parasite invasion by agglutination and/or opsonization of merozoites, agglutination of parasites within erythrocytes, and monocyte-mediated cellular inhibition. If there is sufficient preexisting antibody to clear or inactivate the parasites rapidly, it is possible that no further anti-block 2 response would ensue. It might be difficult to envisage how low (but significantly above background) levels of antibody could protect children from clinical malaria episodes up to 17 months later. We suggest, however, that even low levels of preexisting antibody (where parasite antigen is in excess) would enhance antigen presentation, leading to rapid secondary responses able to control liver and/or blood merozoites after new P. falciparum infections. From the presented survival analysis, the association between anti-block 2 antibodies and protection from malaria over this long survey period remains statistically significant, even though antibodies to block 2 may decay to levels not immediately functionally protective. That naturally acquired protection is relatively short-lived does not preclude the possibility that any vaccine based on block 2 could induce antibody responses that would have longer-lasting effects. Persistence of antigen in depots (in the context of vaccination) might well provide the stimulus for more efficient immune responses to MSP-1 and/or the parasite itself.
It has been suggested that, in some individuals, responses to block 2 antigens may become fixed to a few specific epitopes, regardless of the parasite type infecting the host, and that this may be due to “clonal imprinting” or “original antigenic sin” (24). This immunoevasion hypothesis suggests that an individual's B cell repertoire against block 2 might become selected and fixed by a first or early exposure to a particular parasite antigenic variant, thereby preventing the recognition of other variants in subsequent infections (18, 30). Here, 18% of children in the cohort produced IgG to more than one block 2 type, and numerous examples of changing specificities were seen between the start and the end of the malaria transmission season (Fig. 4). Thus, in this cohort at least, responses of individuals have not been fixed to any particular MSP-1 block 2 type, and these data give no support to the hypothesis of original antigenic sin for block 2. The more likely explanation for the relatively low frequencies of antibodies to block 2 lies in the combination of antigenic polymorphism of this region with the short-lived production and short half-life of specific IgG3.
The recombinant antigens used here mimic the antigenic structure of the block 2 region of Pf MSP-1 (7), and the panel of antigens used contains sufficient diversity in the two block 2 types that contain repetitive regions to detect the majority of antibody responses directed to this region of MSP-1. While it is possible that linear peptides may represent epitopes present in the block 2 region of MSP-1, no immunogenicity studies with these peptides have been reported which might determine their usefulness in assessing immune responses in naturally exposed human populations (24). It is not excluded that in some individuals, variant-specific (rather than type-specific) antibodies were present which were not detectable by the variants in our panel. Single-variant-specific responses do occur, albeit at low frequencies. However, the high correlation of “within type” recognition of block 2 variants (data not shown) argues against a high frequency of narrowly specific antibody recognition of single block 2 variants, rather than recognition of most variants within a type (7). Antibodies to block 2 are directed predominantly against epitopes that are shared within a type rather than against variant-specific epitopes. The lower frequency of block 2 repeat-specific IgG versus type-specific IgG seen in our cohort (Table 1) argues that repetitive sequences in block 2 are not immunodominant in this antigen. However, there are important epitopes within the repetitive sequences that cross-react between variants within a type (29). The sequence polymorphism in the K1 and MAD20 types of block 2 looks initially quite extensive (26) but is created by no more than four to five tripeptide motifs, present in variable numbers and in a limited number of combinations (23).
This prospective cohort study in an area of stable but seasonal malaria transmission, which has combined accurate monitoring of clinical status with sensitive assays of antibodies to block 2, has confirmed and extended the seroepidemiological evidence in support of the protective role for antibodies to MSP-1 block 2 in naturally exposed populations (11). IgG3 responses to block 2 are associated with protection from subsequent clinical malaria episodes in Ghanaian children, as shown by using our panel of immunologically well characterized recombinant antigens. The protective potential of immunity to block 2 should now be verified directly through the experimental immunization of primates.
Acknowledgments
We thank all the children of Dodowa who participated in this study and the Ghanaian health workers who conducted the vital clinical follow-up of the cohort.
This work was funded by a grant from the Wellcome Trust (grant no. 057270) and by Danish Development Assistance (Danida), grant 14.Dan.8.L.306 for the Enhancement of Research Capacity (ENRECA) program in countries of endemicity.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Afari, E. A., M. Appawu, S. Dunyo, A. Baffoe-Wilmot, and F. K. Nkrumah. 1995. Malaria infection, morbidity and transmission in two ecological zones in southern Ghana. Afr. J. Health Sci. 2:312-316. [PubMed] [Google Scholar]
- 2.Beck, H. P., I. Felger, B. Genton, N. Alexander, F. al-Yaman, R. F. Anders, and M. Alpers. 1995. Humoral and cell-mediated immunity to the Plasmodium falciparum ring-infected erythrocyte surface antigen in an adult population exposed to highly endemic malaria. Infect. Immun. 63:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branch, O. H., V. Udhayakumar, A. W. Hightower, A. J. Oloo, W. Hawley, B. L. Nahlen, P. B. Bloland, D. C. Kaslow, and A. A. Lal. 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kilodalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am. J. Trop. Med. Hyg. 58:211-219. [DOI] [PubMed] [Google Scholar]
- 5.Burghaus, P. A., and A. A. Holder. 1994. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein Mol. Biochem. Parasitol. 64:165-169. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh, D. R., C. Dobano, I. M. Elhassan, K. Marsh, A. Elhassan, L. Hviid, E. A. T. G. Khalil, T. G. Theander, D. E. Arnot, and J. S. McBride. 2001. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect. Immun. 69:1207-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanagh, D. R., I. M. Elhassan, C. Roper, V. J. Robinson, H. Giha, A. A. Holder, L. Hviid, T. G. Theander, D. E. Arnot, and J. S. McBride. 1998. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J. Immunol. 161:347-359. [PubMed] [Google Scholar]
- 8.Cavanagh, D. R., and J. S. McBride. 1997. Antigenicity of recombinant proteins derived from Plasmodium falciparum merozoite surface protein 1. Mol. Biochem. Parasitol. 85:197-211. [DOI] [PubMed] [Google Scholar]
- 9.Certa, U., D. Rotmann, H. Matile, and R. Reber-Liske. 1987. A naturally occurring gene encoding the major surface antigen precursor p190 of Plasmodium falciparum lacks tripeptide repeats. EMBO J. 6:4137-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chizzolini, C., A. Dupont, J. P. Akue, M. H. Kaufmann, A. Verdini, A. Pessi, and G. Del Giudice. 1988. Natural antibodies against three distinct and defined antigens of Plasmodium falciparum in residents of a mesoendemic area in Gabon. Am. J. Trop. Med. Hyg. 39:150-156. [DOI] [PubMed] [Google Scholar]
- 11.Conway, D. J., D. R. Cavanagh, K. Tanabe, C. Roper, Z. S. Mikes, N. Sakihama, K. A. Bojang, A. M. J. Oduola, P. G. Kremsner, D. E. Arnot, B. M. Greenwood, and J. S. McBride. 2000. A principal target of immunity to malaria identified by molecular population genetic and immunological analysis. Nat. Med. 6:689-692. [DOI] [PubMed] [Google Scholar]
- 12.Dodoo, D., T. G. Theander, J. A. Kurtzhals, K. Koram, E. Riley, B. D. Akanmori, F. K. Nkrumah, and L. Hviid. 1999. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect. Immun. 67:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Druilhe, P., and J.-L. Perignon. 1997. A hypothesis about the chronicity of malaria infection. Parasitol. Today 13:353-357. [DOI] [PubMed] [Google Scholar]
- 14.Egan, A. F., J. A. Chappel, P. A. Burghaus, J. S. Morris, J. McBride, A. A. Holder, D. C. Kaslow, and E. M. Riley. 1995. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP1(19), the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect. Immun. 63:456-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan, A. F., J. Morris, G. Barnish, S. Allen, B. M. Greenwood, D. C. Kaslow, A. A. Holder, and E. M. Riley. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect. Dis. 173:765-769. [DOI] [PubMed] [Google Scholar]
- 16.Freeman, R. R., and A. A. Holder. 1983. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J. Exp. Med. 158:1647-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fruh, K., O. Doumbo, H. M. Muller, O. Koita, J. McBride, A. Crisanti, Y. Toure, and H. Bujard. 1991. Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect. Immun. 59:1319-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Good, M. F., Y. Zevering, J. Currier, and J. Bilsborough. 1993. “Original antigenic sin,” T cell memory, and malaria sporozoite immunity: an hypothesis for immune evasion. Parasite Immunol. 15:187-193. [DOI] [PubMed] [Google Scholar]
- 19.Holder, A. A., M. J. Blackman, P. A. Burghaus, J. A. Chappel, I. T. Ling, N. McCallum-Deighton, and S. Shai. 1992. A malaria merozoite surface protein (MSP1)-structure, processing and function. Mem. Inst. Oswaldo Cruz 87(Suppl. 3):37-42. [DOI] [PubMed] [Google Scholar]
- 20.Holder, A. A., and R. R. Freeman. 1984. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J. Exp. Med. 160:624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holder, A. A., M. J. Lockyer, K. G. Odink, J. S. Sandhu, V. Riveros-Moreno, S. C. Nicholls, Y. Hillman, L. S. Davey, M. L. Tizard, R. Schwarz, et al. 1985. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature 317:270-273. [DOI] [PubMed] [Google Scholar]
- 22.Holder, A. A., and E. M. Riley. 1996. Human immune response to MSP-1. Parasitol. Today 12:173. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, G. F., C. Daubenberger, W. Huber, H. Matile, M. Tanner, and G. Pluschke. 2000. Sequence diversity of the merozoite surface protein 1 of Plasmodium falciparum in clinical isolates from the Kilombero District, Tanzania. Acta Trop. 74:51-61. [DOI] [PubMed] [Google Scholar]
- 24.Jouin, H., C. Rogier, J. F. Trape, and O. Mercereau-Puijalon. 2001. Fixed, epitope-specific, cytophilic antibody response to the polymorphic block 2 domain of the Plasmodium falciparum merozoite surface antigen MSP-1 in humans living in a malaria-endemic area. Eur. J. Immunol. 31:539-550. [DOI] [PubMed] [Google Scholar]
- 25.McBride, J. S., and H. G. Heidrich. 1987. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 23:71-84. [DOI] [PubMed] [Google Scholar]
- 26.Miller, L. H., T. Roberts, M. Shahabuddin, and T. F. McCutchan. 1993. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1). Mol. Biochem. Parasitol. 59:1-14. [DOI] [PubMed] [Google Scholar]
- 27.Muller, H. M., K. Fruh, A. von Brunn, F. Esposito, S. Lombardi, A. Crisanti, and H. Bujard. 1989. Development of the human immune response against the major surface protein (gp190) of Plasmodium falciparum. Infect. Immun. 57:3765-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perneger, T. V. 1998. What's wrong with Bonferroni adjustments. BMJ 316:1236-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polley, S. D., K. K. Tetteh, D. R. Cavanagh, R. J. Pearce, J. M. Lloyd, K. A. Bojang, D. M. Okenu, B. M. Greenwood, J. S. McBride, and D. J. Conway. 2003. Repeat sequences in block 2 of Plasmodium falciparum merozoite surface protein 1 are targets of antibodies associated with protection from malaria. Infect. Immun. 71:1833-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley, E. M. 1996. The role of MHC- and non-MHC-associated genes in determining the human immune response to malaria antigens. Parasitology 112:S39-S51. [PubMed] [Google Scholar]
- 31.Riley, E. M., S. Allen, M. Troye-Blomberg, S. Bennett, H. Perlmann, G. Andersson, L. Smedman, P. Perlmann, and B. M. Greenwood. 1991. Association between immune recognition of the malaria vaccine candidate antigen Pf155 RESA and resistance to clinical disease: a prospective study in a malaria-endemic region of West Africa. Trans. R. Soc. Trop. Med. Hyg. 85:436-443. [DOI] [PubMed] [Google Scholar]
- 32.Rothman, K. J. 1990. No adjustments are needed for multiple comparisons. Epidemiology 1:43-46. [PubMed] [Google Scholar]
- 33.Rzepczyk, C. M., K. Hale, N. Woodroffe, A. Bobogare, P. Csurhes, A. Ishii, and A. Ferrante. 1997. Humoral immune responses of Solomon Islanders to the merozoite surface antigen 2 of Plasmodium falciparum show pronounced skewing towards antibodies of the immunoglobulin G3 subclass. Infect. Immun. 65:1098-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi, Y. P., U. Sayed, S. H. Qari, J. M. Roberts, V. Udhayakumar, A. J. Oloo, W. A. Hawley, D. C. Kaslow, B. L. Nahlen, and A. A. Lal. 1996. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect. Immun. 64:2716-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 36.Smith, T., J. A. Schellenberg, and R. Hayes. 1994. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat. Med. 13:2345-2358. [DOI] [PubMed] [Google Scholar]
- 37.Szarfman, A., D. Walliker, J. S. McBride, J. A. Lyon, I. A. Quakyi, and R. Carter. 1988. Allelic forms of gp195, a major blood-stage antigen of Plasmodium falciparum, are expressed in liver stages. J. Exp. Med. 167:231-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanabe, K., M. Mackay, M. Goman, and J. G. Scaife. 1987. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 195:273-287. [DOI] [PubMed] [Google Scholar]
- 39.Taylor, R. R., D. B. Smith, V. J. Robinson, J. S. McBride, and E. M. Riley. 1995. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect. Immun. 63:4382-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theisen, M., D. Dodoo, A. Toure-Balde, S. Soe, G. Corradin, K. K. Koram, J. A. Kurtzhals, L. Hviid, T. Theander, B. Akanmori, M. Ndiaye, and P. Druilhe. 2001. Selection of glutamate-rich protein long synthetic peptides for vaccine development: antigenicity and relationship with clinical protection and immunogenicity. Infect. Immun. 69:5223-5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tolle, R., K. Fruh, O. Doumbo, O. Koita, M. N′Diaye, A. Fischer, K. Dietz, and H. Bujard. 1993. A prospective study of the association between the human humoral immune response to Plasmodium falciparum blood stage antigen gp190 and control of malarial infections. Infect. Immun. 61:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]