Abstract
Superantigen peptide antagonists failed to block T-cell activation and cytokine production as well as toxic shock induced by staphylococcal enterotoxin B (SEB) in HLA class II transgenic mice. They also failed to inhibit the binding of SEB to HLA class II molecules as well as activation of human T lymphocytes in vitro.
Bacterial superantigens (SAg) are important clinically (10), as well as being potential agents for bioterrorism and biological warfare (6, 9). Since SAg-mediated immunopathology is T cell dependent, blocking the formation of SAg-major histocompatibility complex class II-T-cell receptor (TCR) complexes would be therapeutically beneficial (see reference 5 for a review). Two groups have shown that peptides derived from the conserved regions of bacterial SAg could mediate such an effect (1-3, 16). While the validity of SAg peptide antagonists has been questioned (8, 14), the major skepticism in these studies was the animal model used. Unlike humans, mice (as well as rabbits) are resistant to SAg-induced toxic shock even at very high doses, and pretreatment with sensitizing agents, such as d-galactosamine or bacterial lipopolysaccharides, is mandatory (10). Therefore, a thorough evaluation of these peptide antagonists in an animal model(s) in which no such sensitizing agents are used would be critical before implementing these interventions in human patients. Since we and others have shown that HLA class II transgenic mice are ideal for recapitulating human immune responses to SAg (4, 11-13, 15, 17, 18), we evaluated the inhibitory potential of the peptide antagonists using this transgenic-mouse model.
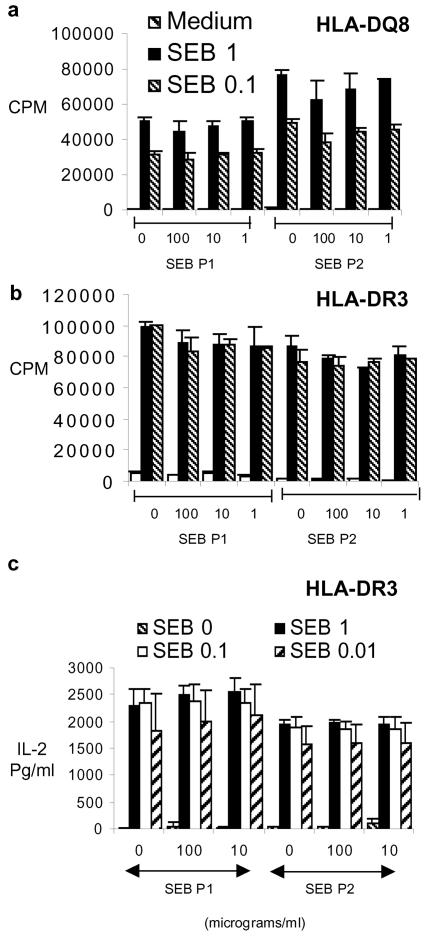
The following peptide antagonists were tested: (i) SEB P1 (SEB 140-151, CMYGGVTEHEGN), described by Visvanathan et al. (16), (ii) SEB P2 (SEB 150-161, TNKKKVTAQELD), (iii) SEB P12 (SEB 150-161, YNKKKATVQELD), and (iv) SEB P12A (d-Ala-YNKKKATVQELD-d-Ala). The last three peptides are described by Arad et al. (3). Peptides were synthesized at the Mayo Clinic Protein Core Facility. In the first set of experiments, we stimulated the splenic mononuclear cells (10 × 106 cells/ml, 100 μl/well) from HLA-DQ8 or HLA-DR3 single transgenic mice with various concentrations (100 μl/well) of highly purified staphylococcal enterotoxin B (SEB), staphylococcal enterotoxin A (SEA), toxic shock syndrome toxin 1 (TSST-1), or streptococcal pyrogenic exotoxin A (SPEA) (Toxin Technologies, Sarasota, Fla.) in the presence or absence of various concentrations of the SEB antagonistic peptides (100 μl/well). The cells were cultured for 48 h in tissue culture medium. Cells were pulsed with tritiated thymidine during the last 18 h of culture, and proliferation was determined by measuring the incorporated radioactivity. While SEB was capable of inducing extensive proliferation in splenocytes from HLA-DQ8 (Fig. 1a and 2) and DR3 transgenic mice (Fig. 1b and 3), addition of the antagonistic peptides at severalfold-higher concentrations did not inhibit this proliferation (Fig. 1 to 3), nor did they inhibit interleukin-2 production, as determined by enzyme-linked immunosorbent assay (Fig. 1c). The antagonistic peptides SEB P12 and SEB P12A also failed to block T-cell activation by other SAg, such as SEA, SPEA, and TSST-1 (Fig. 2 and 3; data with SEB P12 are not shown).
FIG. 1.
Single-cell suspensions of splenocytes from HLA-DQ8 (a) and HLA-DR3 (b and c) transgenic mice were cultured for 48 h in the presence of medium alone or various concentrations of SEB. SEB antagonistic peptides were also added at the indicated concentrations. (a and b) Splenocyte proliferation, as determined by measuring tritiated-thymidine uptake. (c) Interleukin-2 (IL-2) production in vitro by splenocytes from HLA-DR3 transgenic mice, as measured by sandwich enzyme-linked immunosorbent assay. Each bar represents the mean ± standard error from at least four independent experiments.
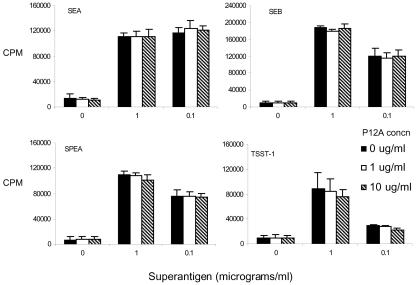
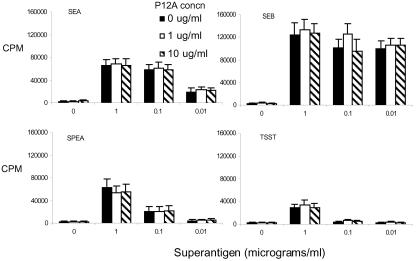
FIG. 2.
Single-cell suspensions of splenocytes from HLA-DQ8 transgenic mice were cultured for 48 h in the presence of medium alone or various concentrations of the indicated SAg. SEB P12A was added at the indicated concentrations. Cell proliferation was determined by measuring tritiated-thymidine uptake. Each bar represents the mean ± standard error from at least four independent experiments.
FIG. 3.
Single-cell suspensions of splenocytes from HLA-DR3 transgenic mice were cultured for 48 h in the presence of medium alone or various concentrations of the indicated SAg. SEB P12A was added at the indicated concentrations. Cell proliferation was determined by measuring tritiated-thymidine uptake. Each bar represents the mean ± standard error. Representative data from two of the four independent but similar experiments are given.
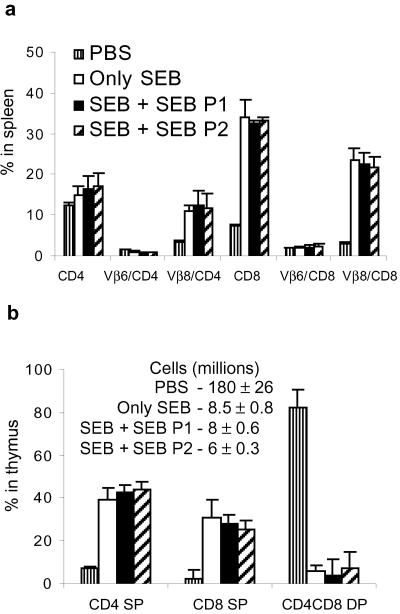
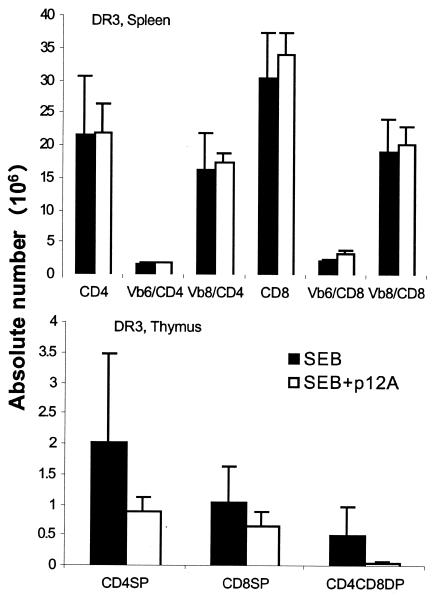
To determine the in vivo inhibitory potential of the peptide antagonists, HLA-DR3 transgenic mice were challenged with 10 μg of SEB and simultaneously received a 10-fold-higher concentration of SEB P1, SEB P2, or phosphate-buffered saline (PBS). Mice were sacrificed 3 days later, and the expansion of the TCR Vβ8+ (SEB-reactive) CD4+ and CD8+ T cells was enumerated along with TCR Vβ6+ (SEB-nonreactive) T cells by flow cytometry. While there was a 5- to 10-fold increase in the TCR Vβ8+ CD4+ and CD8+ T cells in SEB-treated mice over the number in PBS-treated mice, coinjection of the antagonistic peptides did not block SEB-induced T-cell expansion in the spleens (Fig. 4a). Administration of SAg also causes massive deletion of CD4-CD8 double-positive (DP) lymphocytes in the thymus (7). While there was a profound loss of CD4-CD8 DP thymocytes in mice receiving SEB alone, injection of the antagonistic peptides did not prevent this deletion (Fig. 4b). SEBP12A also failed to inhibit SEB-induced peripheral T-cell activation (Fig. 5, top) or thymocyte deletion (Fig. 5, bottom) in vivo in DR3 mice.
FIG. 4.
HLA-DR3 transgenic mice were challenged with a single (10-μg) dose of SEB. Simultaneously, they also received 100 μg of either SEB P1 or SEB P2 or PBS alone. Mice were sacrificed 3 days later, and the distributions of various T-cell subsets in spleen (a) and thymus (b) were determined by flow cytometry. Numbers indicate the absolute cell counts in thymus. Each bar represents the mean ± standard deviation from at least three mice.
FIG. 5.
HLA-DR3 transgenic mice were challenged with a single (10-μg) dose of SEB alone or SEB with 100 μg of SEB P12A. Mice were sacrificed 3 days later, and the distributions of various T-cell subsets in spleen (top) and thymus (bottom) were determined by flow cytometry. Each bar represents the mean ± standard deviation from at least four or five mice. SP, single positive.
We have shown previously that HLA transgenic mice are highly susceptible to SAg-induced shock even without presensitization with lipopolysaccharide or d-galactosamine (11). Two consecutive injections of 10 μg of SEB at 48-h intervals induced toxic shock, which resulted in 100% mortality (Table 1). Coadministration of SEB antagonistic peptides (100-μg doses at 0 and 48 h) at these two time points did not confer any protection from toxic shock (Table 1), indicating the failure of SAg antagonistic peptides. We have observed that DQ8 transgenic mice with a disrupted IL-10 gene (DQ8.IL-10−/−) are highly sensitive to SEB-induced toxic shock (G. Rajagopalan and C. S. David, unpublished observation). While a single dose of SEB (10 μg) caused 100% mortality in DQ8.IL-10−/− mice, the peptide antagonist SEB P12A offered no protection (Table 1). However, SEB-induced mortality in DR3 mice could be completely prevented when T-cell costimulation through CD28 was blocked by the CTLA-4Fc fusion protein (100 μg; Chimerigen, Allston, Mass.) administered at the time of SEB challenge (both at 0 and 48 h) (Table 1).
TABLE 1.
SEB-induced mortality in HLA class II transgenic mice
| Mouse group | Treatmenta | Mortality |
|---|---|---|
| DR3 | No peptide | 6/6 |
| DR3 | SEB P1 | 3/3 |
| DR3 | SEB P2 | 3/3 |
| DR3 | SEB P12A | 4/4 |
| DQ8.IL-10−/− | No peptide | 3/3 |
| DQ8.IL-10−/− | SEB P12A | 3/3 |
| DR3 | CTLA-4Fc | 0/4 |
All DR3 mice received 10 μg of SEB at 0 and 48 h. Where indicated, 100 μg of peptides or CTLA-4Fc was injected intraperitoneally just before each injection of SEB. DQ8.IL-10−/− mice received only one injection of SEB (10 μg) and one injection of the peptide (200 μg).
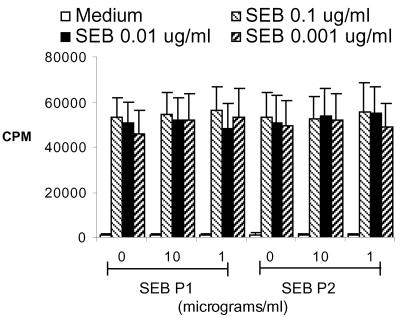
The ability of antagonistic peptides to block SEB-induced proliferation of human peripheral blood mononuclear cells (PBMC) in vitro was also evaluated. For this, PBMC from healthy donors were obtained from a blood bank and were cultured (5 × 106/ml, 100 μl/well) for a total of 48 h with different concentrations of SEB (100 μl/well) in the presence of various concentrations of the peptide antagonists (100 μl/well) in accordance with a standard protocol. Cell proliferation was determined by measuring thymidine incorporation. No inhibition in SEB-induced T-cell proliferation by the antagonists was observed (Fig. 6). Finally, the ability of peptide antagonists to block the binding of SEB to HLA class II molecules was studied. For this human PBMC (0.5 × 106 cells) were incubated with 10 μg of biotinylated SEB (Toxin Technologies) alone or with various concentrations of SAg peptide antagonists or with nonbiotinylated SEB as competitors. After incubation and washing, cell-bound biotinylated SEB was detected by phycoerythrin-labeled streptavidin by flow cytometry. While the nonbiotinylated SEB could significantly block the binding of biotinylated SEB in a dose-dependent manner, the peptide antagonists did not show any inhibition (Fig. 7). Incubation of the peptide antagonists with PBMC prior to addition of biotinylated SEB also did not have any inhibitory effect (data not shown). Overall, SAg peptide antagonists failed to inhibit SAg-induced T-cell activation in vitro as well as in vivo and hence warrant further evaluation before their clinical use. The applicability of HLA class II transgenic mice in such studies is underscored.
FIG. 6.
PBMC from healthy individuals were cultured in vitro with the indicated concentrations of SEB along with different concentrations of SEB antagonistic peptides for 48 h. Cell proliferation was determined by measuring thymidine incorporation. Each bar represents the mean ± standard error from at least five different individuals.
FIG. 7.
PBMC from healthy individuals were incubated with 10 μg of nonbiotinylated SEB (A) or 10 μg of biotinylated SEB (B to F) along with the indicated concentrations of SEB P1 (C) or SEB P2 (D) or nonbiotinylated SEB (E and F). The extent of SEB binding to cells was determined by using avidin-labeled phycoerythrin for flow cytometry. Representative data from two similar experiments are given.
Acknowledgments
This study was supported by NIH grant AI14764.
We thank Julie Hanson and her crew for excellent mice husbandry and Michelle Smart for characterizing the transgenic mice.
Editor: A. D. O'Brien
REFERENCES
- 1.Arad, G., D. Hillman, R. Levy, and R. Kaempfer. 2004. Broad-spectrum immunity against superantigens is elicited in mice protected from lethal shock by a superantigen antagonist peptide. Immunol. Lett. 91:141-145. [DOI] [PubMed] [Google Scholar]
- 2.Arad, G., D. Hillman, R. Levy, and R. Kaempfer. 2001. Superantigen antagonist blocks Th1 cytokine gene induction and lethal shock. J. Leukoc. Biol. 69:921-927. [PubMed] [Google Scholar]
- 3.Arad, G., R. Levy, D. Hillman, and R. Kaempfer. 2000. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat. Med. 6:414-421. [DOI] [PubMed] [Google Scholar]
- 4.DaSilva, L., B. C. Welcher, R. G. Ulrich, M. J. Aman, C. S. David, and S. Bavari. 2002. Humanlike immune response of human leukocyte antigen-DR3 transgenic mice to staphylococcal enterotoxins: a novel model for superantigen vaccines. J. Infect. Dis. 185:1754-1760. [DOI] [PubMed] [Google Scholar]
- 5.Hong-Geller, E., and G. Gupta. 2003. Therapeutic approaches to superantigen-based diseases: a review. J. Mol Recognit. 16:91-101. [DOI] [PubMed] [Google Scholar]
- 6.Kaempfer, R., G. Arad, R. Levy, and D. Hillman. 2002. Defense against biologic warfare with superantigen toxins. Isr. Med. Assoc. J. 4:520-523. [PubMed] [Google Scholar]
- 7.Kishimoto, H., and J. Sprent. 2001. A defect in central tolerance in NOD mice. Nat. Immunol. 2:1025-1031. [DOI] [PubMed] [Google Scholar]
- 8.Llewelyn, M., and J. Cohen. 2001. Superantigen antagonist peptides. Crit. Care 5:53-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madsen, J. M. 2001. Toxins as weapons of mass destruction. A comparison and contrast with biological-warfare and chemical-warfare agents. Clin. Lab. Med. 21:593-605. [PubMed] [Google Scholar]
- 10.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 11.Rajagopalan, G., M. K. Smart, S. Cheng, C. J. Krco, K. L. Johnson, and C. S. David. 2003. Expression and function of HLA-DR3 and DQ8 in transgenic mice lacking functional H2-M. Tissue Antigens 62:149-161. [DOI] [PubMed] [Google Scholar]
- 12.Rajagopalan, G., M. K. Smart, C. J. Krco, and C. S. David. 2002. Expression and function of transgenic HLA-DQ molecules and lymphocyte development in mice lacking invariant chain. J. Immunol. 169:1774-1783. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopalan, G., M. K. Smart, E. V. Marietta, and C. S. David. 2002. Staphylococcal enterotoxin B-induced activation and concomitant resistance to cell death in CD28-deficient HLA-DQ8 transgenic mice. Int. Immunol. 14:801-812. [DOI] [PubMed] [Google Scholar]
- 14.Roggiani, M., J. A. Stoehr, S. B. Olmsted, Y. V. Matsuka, S. Pillai, D. H. Ohlendorf, and P. M. Schlievert. 2000. Toxoids of streptococcal pyrogenic exotoxin A are protective in rabbit models of streptococcal toxic shock syndrome. Infect. Immun. 68:5011-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sriskandan, S., M. Unnikrishnan, T. Krausz, H. Dewchand, S. Van Noorden, J. Cohen, and D. M. Altmann. 2001. Enhanced susceptibility to superantigen-associated streptococcal sepsis in human leukocyte antigen-DQ transgenic mice. J. Infect. Dis. 184:166-173. [DOI] [PubMed] [Google Scholar]
- 16.Visvanathan, K., A. Charles, J. Bannan, P. Pugach, K. Kashfi, and J. B. Zabriskie. 2001. Inhibition of bacterial superantigens by peptides and antibodies. Infect. Immun. 69:875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welcher, B. C., J. H. Carra, L. DaSilva, J. Hanson, C. S. David, M. J. Aman, and S. Bavari. 2002. Lethal shock induced by streptococcal pyrogenic exotoxin A in mice transgenic for human leukocyte antigen-DQ8 and human CD4 receptors: implications for development of vaccines and therapeutics. J. Infect. Dis. 186:501-510. [DOI] [PubMed] [Google Scholar]
- 18.Yeung, R. S., J. M. Penninger, T. Kundig, W. Khoo, P. S. Ohashi, G. Kroemer, and T. W. Mak. 1996. Human CD4 and human major histocompatibility complex class II (DQ6) transgenic mice: supersensitivity to superantigen-induced septic shock. Eur. J. Immunol. 26:1074-1082. [DOI] [PubMed] [Google Scholar]