Abstract
Interleukin-10 (IL-10) was at first described as a Th2-associated cytokine, although more recent reports have shown that immunosuppression applies to both Th1 and Th2 cell responses, e.g., when produced by T regulatory cells. This concept when applied to human filariasis would argue that high parasite loads are associated with IL-10, while bona fide Th2 responses, mediated by IL-4, IL-5, and IL-13, are associated with parasite containment. To prove this relationship in a causal manner, we investigated the roles of IL-4 and IL-10 in a helminth infection model in which mice genetically deficient for IL-4, IL-10, or IL-4 plus IL-10 were infected with the rodent filaria Litomosoides sigmodontis. Compared to C57BL/6 wild-type and IL-10 knockout (KO) mice, IL-4 KO mice remained susceptible, exhibiting a remarkable number of live adult worms. Interestingly however, when the IL-10 gene was knocked out simultaneously with the IL-4 gene, the susceptibility of IL-4 KO mice was reversed. Although production of IFN-γ was increased in IL-4/IL-10 double-knockout mice, depletion of gamma interferon did not affect worm elimination, so it seems unlikely to be the major factor in mediating resistance in IL-4/IL-10 KO mice. Taken together, the results of this study add proof to the concept that has arisen for human filariasis that IL-10-dependent responses, which are associated with patency, are antagonistic to bona fide Th2 responses, which control parasite loads. The finding that knockout of IL-10 reversed a disease phenotype induced by knockout of IL-4 gives the first causal evidence of an antagonistic activity between IL-4 and IL-10 in an infection in vivo.
Filariasis, which affects more than a 180 million people in the tropics, is a major cause of severe morbidity and considerable socioeconomic problems. Animal models are powerful tools to investigate immune mechanisms induced by different parasite developmental stages. The precise immune mechanisms that govern worm control in mice are only partially understood. The current consensus is that the host elicits a mixed T-helper type 1 (Th1)-Th2 response, which is essential for the containment of different developmental stages. Thus, the Th1 cytokine IFN-γ is needed for adult worm control in murine infection with Brugia malayi (3) and Litomosoides sigmodontis (29), apparently through encapsulation and clearance mediated by neutrophils (1, 29). Furthermore, injection of B. malayi microfilariae (MF) into otherwise uninfected mice stimulated the production of IFN-γ (20, 21, 27, 28). On the other hand, interleukin-5 (IL-5) is essential for vaccine-mediated protection (24) and controls adult worm development and microfilaremia in primary infection (1, 25, 36). Of even more relevance for the present study is that IL-4 mediated pathways are necessary for the control of microfilaremia after full infection with L. sigmodontis L3 organisms that have developed into fertile adult worms (36, 37). In resistant C57BL/6 mice, IL-4 is required to prevent the development of adult worms as well as microfilaremia (22).
The recently developed T regulatory concept (10), when applied to human and animal filariasis, would argue that in filariasis high worm and microfilarial loads are associated with the immunosuppressive cytokine IL-10 (produced by T cells and also a variety of non-T cells), while bona fide Th2 responses, mediated by IL-4, IL-5, and IL-13, are associated with parasite containment (13, 31, 35). However, direct evidence for IL-10 being antagonistic to IL-4 in regard to parasite loads has not been obtained so far for helminth infection. Thus, in murine schistosomiasis, IL-4/IL-10 double-knockout (KO) mice succumbed earlier to infection than their single-KO littermates but showed equivalent parasite loads (15). In the present study, we infected mice deficient for IL-4, IL-10, or both cytokines with the rodent filaria L. sigmodontis. Confirming earlier data (22), adult worms and MF were recovered from IL-4-deficient mice on the resistant C57BL/6 background. However, the additional knockout of IL-10 led to a reversal in worm load. This indicates a counterregulatory role for IL-10 in the susceptibility conferred by IL-4 deficiency.
(This study formed part of a Ph.D. thesis by S. Specht at the Faculty of Biology, University of Hamburg.)
MATERIALS AND METHODS
Animal maintenance and infection of mice with L. sigmodontis.
IL-4/IL-10 KO mice on a C57BL/6 background were obtained from A. Sher and T. Wynn (National Institutes of Health, Bethesda, Md.). IL-10 KO mice on a C57BL/6 background (19) were provided by H. Mossmann (Max Planck Institute for Immunobiology, Freiburg, Germany). IL-4 KO mice and C57BL/6 wild-type (wt) mice (originally from Jackson Laboratories) and the knockout strains mentioned above were housed under specific-pathogen-free conditions in microisolator cages. KO offspring, wild-type C57BL/6 mice, and cotton rats were bred at the animal facilities of the Bernhard Nocht Institute. Natural infections were performed as described previously (2). At least five mice were used for each group.
Parasite recovery.
Adult worms were removed from the pleural cavity and counted at days 42 and 65 postinfection (p.i.). The thoracic cavity was flushed with 2 ml of phosphate-buffered saline (PBS) containing 1% fetal calf serum (FCS), and the worms were allowed to sediment. The microfilaria count was determined from 50 μl of EDTA-treated peripheral blood or 200 μl of pleural cavity flush after staining with Hinkelmann's solution (0.5% [wt/vol] eosin Y, 0.5% [wt/vol] phenol and 0.185 [vol/vol] formaldehyde in distilled water) as described previously (2).
Purification of peripheral blood MF.
MF were purified from the peripheral blood of cotton rats on a Percoll gradient as described previously (6). In brief, isosmotic Percoll was prepared by mixing 9 parts of Percoll (density, 1.130 g/ml) with 1 part of 2.5 M sucrose. Various dilutions of the isosmotic Percoll in 0.25 M sucrose were made to obtain 25, 30, and 35% solutions. The gradient dilutions were layered in 15-ml tubes, and peripheral blood, diluted 1:2, was pipetted onto the Percoll layers. The tubes were then centrifuged at 400 × g for 30 min at room temperature. Recovered MF (between the 25 and 30% layers) were washed with RPMI 1640-1% FCS, and 1.5 × 105 viable MF were injected intravenously into naive mice.
Analysis of developmental stages of MF.
The fertility of the worms was determined by assessing the presence or absence of embryonic stages in squeeze preparations of individual worms. Formalin-fixed worms were placed in a small volume of PBS on a glass slide and cut in the midpoint. Another glass slide was placed on top of the worm, and the two slides were gently pressed together to force the contents of the worms into the phosphate-buffered solution. Uterine tube contents were examined at a magnification of ×40. The stages checked for were morula and fully elongated (stretched) microfilariae. The examiner was blind to the origin of samples.
Cell culture.
The culture of splenocytes was carried out in 96-well culture plates (Greiner, Frickenhausen, Germany) in RPMI 1640 supplemented with 10% FCS, glutamine, and gentamicin at 37°C with 5% CO2. Spleens were teased to single-cell suspension, and erythrocytes were removed by incubation with ammonium chloride. Pleural cavity cells (2 × 105) and whole splenocytes were cultured for 72 h in the presence of medium, 10 μg of L. sigmodontis adult worm antigen per well, or 50 ng of concanavalin A per well. Supernatants were removed and frozen for cytokine determination.
Cytokine assays.
Concentrations of the cytokines IFN-γ, IL-10, and IL-4 in the pleural wash, serum (dilution of 1:10), and culture supernatant were determined by specific two-site enzyme-linked immunosorbent assays (ELISAs), using standard protocols. The antibody pairs for capture and detection (biotinylated) were purchased from BD PharMingen (Heidelberg, Germany) in the combinations recommended. They were used at a concentration of 1 μg/ml. Recombinant cytokines (BD PharMingen) were used as standards. IL-13 ELISA was performed with the Duo ELISA kit (R&D Systems, Wiesbaden, Germany). All ELISAs were developed after incubation with streptavidin-peroxidase complex (1:10,000; Roche-Boehringer, Mannheim, Germany), using 3,5,3′,5′-tetramethylbenzidine (Roth, Karlsruhe, Germany) at 6 mg/ml in dimethyl sulfoxide as the substrate. The sensitivity was 20 pg/ml.
Antibody treatment.
Monoclonal antibodies were purified from hybridoma culture supernatants (XMG 1.2, anti-IFN-γ; TRFK-4, control isotype) by ammonium sulfate precipitation and affinity purification over a protein G column (Pharmacia, Freiburg, Germany) according to standard procedures. Mice were treated at weekly intervals with 1 mg/mouse starting on day 20 until day 41 postinfection.
Antibody isotype analysis.
Microtiter plates were coated overnight with 10 μg of L. sigmodontis adult worm antigen per ml in PBS (pH 9.6). After blocking with 1% bovine serum albumin, sera (1:1,000) were incubated for 5 h at room temperature. After washing, plates were incubated with isotype-specific anti-mouse immunoglobulin antibodies conjugated with biotin (PharMingen) for 1 h. ELISAs were developed after incubation with streptavidin-peroxidase complex (1:10,000; Boehringer), using 3,5,3′,5′-tetramethylbenzidine (Roth) at 6 mg/ml in dimethyl sulfoxide as the substrate.
Statistical analysis.
The nonparametric Mann-Whitney U test, including Bonferroni correction, was performed to assess the statistical differences in parasite loads, microfilarial clearance, and humoral immunoglobulin G (IgG) production. Furthermore, analysis of variance (ANOVA) was used to determine differences attributable to the mouse strains independent of the variance of the single experiments. ANOVA was done for parasite loads, MF counts (which had been log transformed to meet parametric assumptions), and IL-10 and IFN-γ production by splenocytes. A P value of <0.05 was considered to represent a statistically significant difference. Box plots represent medians and percentiles (10, 25, 50, 75, and 90%).
RESULTS
Susceptibility of IL-4 KO mice to L. sigmodontis infection is reversed when IL-10 is knocked out.
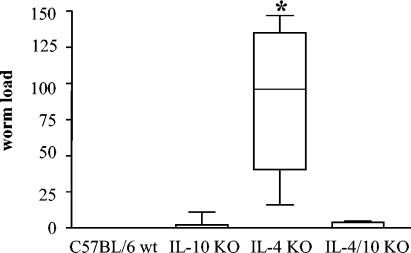
The courses of infection in C57BL/6 wt, IL-4 KO, IL-10 KO, and IL-4/IL-10 double-KO mice were monitored. At day 42 p.i., adult worms were recovered from all mouse strains, with IL-4 KO mice having slightly higher worm loads (data not shown). At day 65 p.i., all worms had been cleared in wild-type mice, whereas IL-4 KO mice still harbored a significant number of worms (Fig. 1). Interestingly, the additional deficiency of IL-10 in IL-4/IL-10 double-knockout mice reversed the susceptibility seen in IL-4 KO mice. Only two out of six double-knockout mice harbored three and five adult worms, respectively, whereas all six IL-4 KO mice contained adult worms (range, 16 to 147). IL-10 KO mice showed worm levels equivalent to those of IL-4/IL-10 KO mice. This finding was reproduced in six additional experiments. ANOVA testing confirmed for all seven experiments that parasite loads were independent from experiment to experiment variation but strictly depended on the single (IL-4 KO) versus double (IL-4/IL-10 KO) deficiency (P < 0.0001). Worms in IL-4 knockout mice also reached sexual maturity and released microfilariae into the thoracic cavity and eventually in the blood. No microfilariae were detected in the pleural cavity or blood of wild-type, IL-10 KO, or IL-4/IL-10 double-knockout mice (Table 1).
FIG. 1.
L. sigmodontis-infected IL-4 KO mice contain significantly (Mann-Whitney U test and Bonferroni correction, P < 0.0024) more adult parasites than L. sigmodontis-infected C57BL/6, IL-10 KO, or IL-4/IL-10 KO mice at day 65 postinfection. Viable parasites were obtained by pleural lavage and counted. Results from one of six consistent experiments comprising six to eight animals per group are shown.
TABLE 1.
Development of patent infection in IL-4 KO mice but not in IL-10 KO strainsa
| Strain | No. of MF inb:
|
|
|---|---|---|
| Pleural lavage fluid (200 μl) | Blood (50 μl) | |
| C57BL/6 | 0 | 0 |
| IL-4 KO | 31 (1-1,982)c | 0 (0-113)d |
| IL-10 KO | 0 | 0 |
| IL-4/IL-10 KO | 0 | 0 |
Blood and pleural cavity MF statuses in L. sigmodontis-infected C57BL/6 and IL-10-, IL-4, and IL-4/IL-10-deficient mice were assessed at day 65 p.i.
Medians (10 to 90% percentiles) are given.
P < 0.0001 by ANOVA of log-transformed pleural lavage fluid MF counts.
P = 0.0198 by ANOVA of log-transformed blood MF counts.
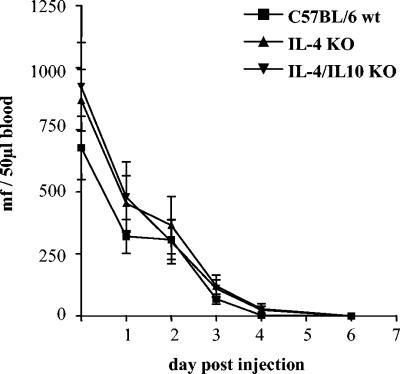
Equivalent clearance of intravenously injected microfilariae in all mouse strains.
In order to monitor clearance of MF without interference of adult worms, 100,000 MF of L. sigmodontis were injected intravenously. IL-10 KO mice were not available for this experiment but are known to show declines of MF equivalent to those in wild-type C57BL/6 mice (17). All mouse strains cleared the MF with similar kinetics (Fig. 2). A 50% drop in microfilaremia was seen on day 1 following injection in all strains. No microfilaremia was detected past day 6. This indicates that there is no direct effect of cytokine deficiency on MF containment.
FIG. 2.
There is no difference in the clearance of microfilariae (mean values ± standard errors of the means) following intravenous injection of 100,000 MF in C57BL/6, IL-4 KO, and IL-4/IL-10 KO mice (n = 8 in each group). Results from one of two consistent experiments are shown.
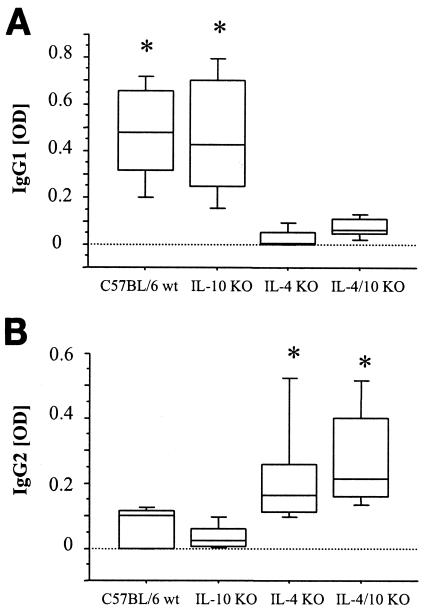
Filaria-specific antibody responses do not correlate with the parasite load.
L. sigmodontis antigen-specific antibodies in the sera on day 65 p.i. were investigated. Consistent with the fact that IL-4 is a major factor in class switching to IgG1, the two strains deficient for IL-4 produced little to no filaria-specific IgG1 (Fig. 3A). Conversely, these strains displayed elevated levels of IgG2a (Fig. 3B). Τhe fact that immunoglobulin subclass production followed the pattern of IL-4 expression but not that of parasite loads suggests that antifilarial antibodies of these subclasses do not correlate with parasite containment in these mouse strains.
FIG. 3.
Humoral analysis of C57BL/6, IL-4 KO, IL-4/IL-10 KO, and IL-10 KO mice following infection. Results for filarial specific IgG1 (A) and IgG2a (B) are shown, representing one of two consistent experiments. For each isotype, four or five mice were bled at day 65 p.i. by retro-orbital venipuncture. Sandwich ELISA was performed to determine the filaria-specific concentration of the relevant isotype in serum. *, significant difference in IgG concentration (Mann-Whitney U test and Bonferroni correction, P = 0.0044).
Anti-IFN-γ antibody does not alter parasite loads in IL-4/IL-10 KO mice.
Given that in BALB/c mice an impact of IFN-γ on the control of adult worms was shown earlier (29), it was of interest to monitor IFN-γ production in the different mouse strains after L. sigmodontis infection. At day 65 p.i., splenocytes of KO strains in comparison to wild-type mice displayed increased levels of IFN-γ in vitro upon stimulation with mitogen or L. sigmodontis antigen (Fig. 4), with much larger amounts being produced by the IL-4/IL-10 double-knockout mice than by IL-4 single-knockout mice. In IL-4/IL-10 KO and IL-10 KO mice, elevated basal secretion of IFN-γ was observed.
FIG. 4.
Splenocytes of infected mice with IL-10 deficiency show higher IFN-γ production at day 65 p.i. after restimulation with filarial antigen (B) or concanavalin A (ConA) (C), as do unstimulated (A) cells. Sandwich ELISA was performed to determine the murine IFN-γ concentration. Results from two consistent experiments, comprising four or five animals per group, were combined by using ANOVA, which showed that this difference is not due to variations from infections. The asterisks indicate significantly higher IFN-γ levels in IL-10 and IL-4/IL-10 KO strains compared to wild-type and IL-4 KO mice (P < 0.0001) (A, B, and C) and in IL-4/IL-10 KO mice compared to IL-10 KO mice (P < 0.05) (A and B).
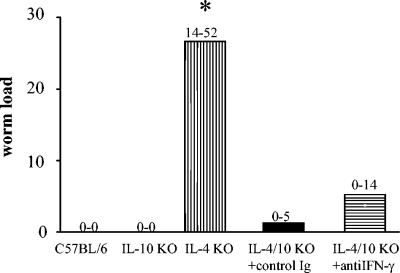
To find out whether the increase in IFN-γ production in IL-4/IL-10 double-knockout mice was responsible for their resistance to a patent infection, anti-IFN-γ antibody was injected into the peritoneal cavities of these mice at weekly intervals from day 20 to day 41 p.i. in a separate experiment. Mice were sacrificed and analyzed for their worm containment at 65 days p.i. Consistently, the number of worms found in the thoracic cavities of IL-4 KO mice was significantly higher than that in all other strains. However, in two separate experiments no difference was observed between anti-IFN-γ-treated and control Ig-treated IL-4/IL-10 KO mice (Fig. 5). There was also no significant difference (determined by ANOVA) when data from the two experiments were combined. An insufficient efficacy of the anti-IFN-γ antibody treatment is not likely, since rectal prolapse development in the IL-4/IL-10 KO strain (observed in approximately one-third of cases) was reversed each time after the repeated injections. In addition, analysis of IFN-γ levels at day 65 p.i., i.e., 23 days after the last antibody administration, showed that IFN-γ levels were highly elevated in IL-4/IL-10 KO mice (420 ± 253 pg/ml [mean ± standard error) but were reduced to the level of that of IL-4 KO mice (11 ± 9 pg/ml) in anti-IFN-γ-treated IL-4/IL-10 KO mice (7 ± 6 pg/ml). As a further control experiment, IFN-γ KO mice on a C57BL/6 background were infected with L. sigmodontis. The course of parasite infection appeared to be equivalent to that in C57BL/6 wt mice in that at day 65 p.i. (i.e., at the time point when IL-4/IL-10 KO mice treated with anti-IFN-γ were assessed), neither strain contained adult worms (data not shown). This further suggests that in mice on the C57BL/6 background, IFN-γ is not a major mediator of parasite control.
FIG. 5.
Treatment of infected IL-4/IL-10 KO mice with anti-IFN-γ antibodies (horizontal stripes) has no effect on worm load compared to that in IL-4/IL-10 mice treated with control Ig. C57BL/6, IL-10 KO, IL-4 KO, and IL-4/IL-10 KO mice were naturally infected with L. sigmodontis, and the worm load was analyzed 65 days later. *, significant increase in IL-4 KO mice compared to all other genotypes of mice (P < 0.05), with four to seven mice per group (four in the antibody-treated groups).
IL-4 KO mice permit worm embryogenesis, while IL-4/IL-10 KO mice do not.
In order to monitor the effects of IL-4 and IL-10 on worm fertility, adult female worms recovered from the thoracic cavity were subjected to an analysis of embryogenesis, using the squeeze preparation technique. Development from oocytes to stretched and motile micofilariae was accomplished only in IL-4 KO mice. However, embryos prepared from worms recovered from IL-4/IL-10 double-KO mice failed to develop past the morula stage (Table 2). These data suggest that IL-4 and IL-10 exert contrasting effects on embryogenesis in murine filariasis.
TABLE 2.
Fertility of Litomosoides worms as determined by squeeze preparations
| Strain | No. of mice | No. of worms | Embryo development in female worms at day 65 p.i.
|
|
|---|---|---|---|---|
| No. of worms positive for morula stage | No. of worms positive for stretched MF | |||
| C57BL/6 | 7 | 2 | 0 | 0 |
| IL-4 KO | 8 | 31 | 23 | 17 |
| IL-10 KO | 8 | 7 | 0 | 0 |
| IL-4/IL-10 KO | 8 | 9 | 9 | 2 |
IL-10 levels in sera are elevated in IL-4 KO mice.
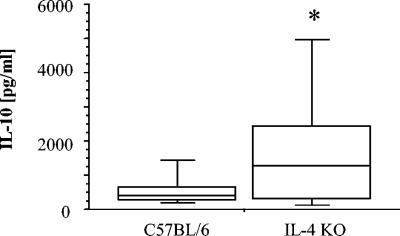
The data described above suggested that IL-10 might be involved, probably indirectly, in the embryogenesis and thus in permissivity towards filarial infection in IL-4 KO mice. In support of this, blood was drawn from the animals from three experiments and then analyzed collectively by Student's t test. Sera from IL-4 KO mice showed elevated levels of IL-10 at day 65 p.i. compared to those from C57BL/6 wt mice (Fig. 6).
FIG. 6.
IL-10 levels in sera of mice infected naturally with L. sigmodontis. Significantly (P = 0.0347) elevated levels were observed in IL-4 KO mice. The data represent the combined analysis of sera from animals of three experiments analyzed with Student's t test. ANOVA confirmed that the significant difference did not arise from variation in the natural infection model.
IL-13 is not elevated in IL-4/IL-10 KO mice.
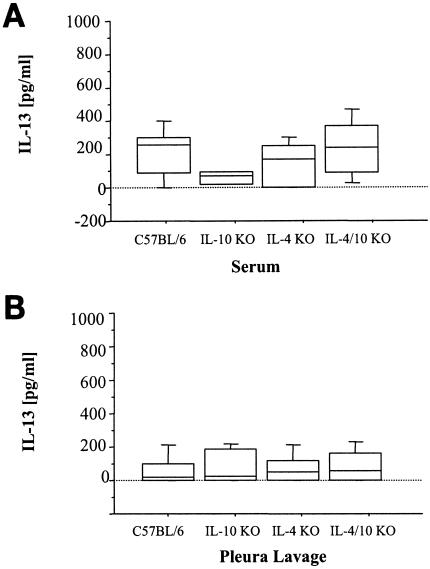
In order to look for other possible mechanisms of parasite control in IL-4/IL-10 KO mice, IL-13 levels in serum and pleural lavage fluid were analyzed at day 65 p.i. No significant difference between mouse strains was observed when four independent experiments with sera (Fig. 7A) (ANOVA, P > 0.05) and eight independent experiments with pleural lavage fluid (Fig. 7B) (ANOVA, P > 0.05) were analyzed. This argues against the hypothesis that elevated IL-13 mediates resistance in IL-4/IL-10 KO mice compared to full susceptibility in IL-4 KO mice.
FIG. 7.
IL-13 levels in sera (A) and pleural lavage fluid (B) of mice naturally infected with L. sigmodontis. No significant differences could be observed between C57BL/6, IL-4 KO, IL-10 KO, and IL-4/IL-10 KO mice when analyses of four (A) and eight (B) experiments were combined and analyzed with Student's t test. ANOVA confirmed that the significant difference did not arise from variation in the natural infection model.
DISCUSSION
Infections with filarial worms in humans and mice typically induce a Th2 response as do other helminth infections, with the classic hallmarks being elevated production of IL-4, IL-5, and IL-13 as well as elevated levels of serum IgE and eosinophilia. For quite some time after the establishment of the Th1-Th2 dichotomy, IL-10 was also considered a Th2 marker, at least in mice, due to its profound antagonistic effects on Th1 responses, while inhibitory effects on IL-5 production by human T cells have been known for a longer time (32, 41, 42). Increasing evidence from animal models of allergic diseases suggests that IL-10 acts as an inhibitor of Th2 responses in vitro and in vivo (8).
However, a direct antagonism between IL-4 and IL-10 has not been shown so far. The novel finding in this study is that causal evidence for such an antagonism can be directly demonstrated for parasite loads in a helminth infection. Thus, while deficiency of IL-4 permitted persistence of filarial worms and microfilarial production, this permissivity was reversed in mice deficient in both IL-4 and IL-10 (Fig. 1). The presence of IL-10 and absence of IL-4 in this system were associated with embryo development (Table 2).
Our results on the role of IL-10 in compromising Th2 cytokine-mediated protection differ from earlier data for murine T. muris infection, where deficiency of IL-10 and IL-4 led to a failure to eliminate Trichuris muris larvae (33). In this model, IL-4/IL-10 double-KO mice showed neither an increased nor a reduced larval burden in comparison to single-knockout mice. The differences from murine filariasis may be due to (i) the fact that IL-10 per se is essential for worm control in T. muris and (ii) the fact that Th1 responses promote susceptibility to T. muris infection and are similarly induced in mice deficient for IL-10, IL-4, or both cytokines, while in murine filariasis Th1 responses either do not influence parasite loads (C57BL/6 background) or are associated with protection (29).
One may conclude from this comparison that different mechanisms of parasite control are effective in different helminth infections. Accordingly, in experimental murine schistosomiasis, mice deficient for either IL-10 or IL-4 or for both cytokines displayed parasite loads that were not different from those of wild-type mice (15, 16, 40).
Differences from schistosomiasis were also found in regard to infection-induced IFN-γ production in IL-4/IL-10 KO mice. While IL-4/IL-10 KO mice had an excessive Th1 response to schistosomiasis and died significantly earlier than did single-KO mice, in our study IL-4/IL-10 KO mice did not succumb to infection, although IFN-γ levels were strongly elevated compared to those in wt and IL-4 KO mice and were elevated to a lesser extent compared to those in IL-10 KO mice (Fig. 4). In addition, the role of IFN-γ in parasite control seems to differ between schistosomiasis and filariasis: in BALB/c mice the complete absence of IFN-γ resulted in increased loads of adult filariae, whereas only small amounts of IFN-γ, as observed in IL-12 KO mice, were sufficient to limit adult worm loads to the level in BALB/c control mice (29). This is different on the C57BL/6 background, where IFN-γ deficiency does not result in elevated worm loads (data not shown).
Still, the control experiment with C57BL/6 IFN-γ KO mice does not exclude a protective role for IFN-γ in IL-4/IL-10 KO mice, all the more since elevated IFN-γ levels were observed in these mice. Therefore, we administered neutralizing anti-IFN-γ monoclonal antibody at weekly intervals from day 20 to 41, i.e., at time points when maturation of worms and embryogenesis take place. While the injections did reverse rectal prolapse development, thus demonstrating in vivo efficacy, they did not influence L. sigmodontis infection. Although we cannot fully exclude the possibility that the antibody might have reached the colonic tissue and prevented rectal prolapse but would not affect the local inflammatory environment that controls the worms, our data rather argue against IFN-γ being the major factor of resistance in IL-4/IL-10 KO mice.
Surprisingly, the ratio of IFN-γ induction by mitogen or specific antigen over medium in splenic cell cultures was rather low (Fig. 4). This correlated with high baseline secretion by cells from IL-10 and IL-4/IL-10 KO strains. This may be due to the existence of T cells, which are already stimulated in vivo by the L. sigmodontis infection, or may reflect the fact the sources other than T cells contribute to IFN-γ production in these mice.
IL-13 is yet another cytokine that could be responsible for the reversion of susceptibility in IL-4/IL-10 compared to IL-4 KO mice. Therefore, the levels of this cytokine in the sera and thoracic cavity fluids of the mice were determined (Fig. 7), but no significant differences were found. This result, however, still does not fully exclude a biological role for IL-13, because its functional efficiency is controlled by expression of an IL-13 decoy receptor (sIL-13Rα2-Fc). Since IFN-γ was found (via IL-12) to downregulate decoy receptor expression and thus to increase the functional effect of IL-13 (7), it is possible that elevated IFN-γ levels in IL-4/IL-10 KO mice might have led to increased function of IL-13. An elegant way to prove such a compensatory effect of IL-13 would have been the treatment with recombinant IL-13 decoy receptor (formerly from Wyeth, Cambridge, Mass.), which unfortunately is no longer available.
Deficiency of IL-4 drastically increases susceptibility to filariasis, and in particular to L. sigmodontis infection, in mice of both susceptible BALB/c and resistant C57BL/6 backgrounds. Microfilaremia is enhanced throughout (22, 36, 37), while in IL-4 KO mice on a C57BL/6 background the life span of adult worms also appears to be prolonged (22). The precise mechanism of IL-4 action, however, remains unclear. Antibodies do not play a major role, given that μMT KO mice, which have no functional B cells, do not display an altered course of parasite infection (26, 37). A direct effect of IL-4 on worm embryogenesis could be excluded in our earlier work, given that IL-4Rα KO mice displayed grossly the same phenotype as IL-4 KO mice (36). The finding of elevated levels of IL-10 in sera of IL-4 KO mice in the present study may indicate that IL-10 promotes patency in these mice. This would be in line with our finding of patency reversal by additional deficiency of IL-10 as well as with an earlier study of L. sigmodontis infection in which IL-10 was found to be necessary for microfilariaremia persistence upon transfer of adult fertile female filariae (17).
Evidence for antagonistic effects of IL-4 and IL-10 can also be found in human filarial infection. In onchocerciasis, patients with generalized disease and high microfilarial skin loads have peripheral blood mononuclear cells (PBMC) that show low proliferation to onchocercal antigen but produce large amounts of IL-10. This is in contrast to the hyperreactive Sowda form of onchocerciasis, in which microfilariae are effectively reduced by Th2-mediated mechanisms and in which PBMC proliferate vigorously and produce highly elevated IL-5 but only little IL-10 upon antigen-specific stimulation (4, 9, 12). In another large survey, IL-5 production in whole blood of patients was inversely related to MF skin loads (5). At least part of the IL-10 response is driven by regulatory T cells (Tr-1 cells), which could be cloned from PBMC as well as from onchocercomas. These Tr-1 cells were shown to inhibit Th1 and Th2 clones in vitro (31). In PBMC from patients with lymphatic filariasis, high MF loads also were positively associated with IL-10 and correlated negatively with IL-5 (18, 23, 30). While a direct interventional study with humans to prove these associations is ethically not possible, our results using the well defined murine filariasis model of L. sigmodontis infection provide the first causal evidence to date for an antagonism between IL-4 and IL-10 in filariasis control. There are several potential pathways of IL-10 downregulation of parasite control mechanisms. While direct action of IL-10 on worm fertility can in principle not be excluded, there exist several other proven avenues of IL-10 downregulating the effector function of effector cells such as macrophages, eosinophils, and/or neutrophils (34, 38, 39, 42).
Increasing evidence for an antagonism between IL-4 and IL-10 comes from the field of allergy. Thus, transfer of Tr-1 cells inhibited antigen-specific serum IgE responses in OVA-immunized mice (8). In humans, antigen-specific desensitization was associated with an increase of antigen-specific production of IL-10, suggesting that antigen-specific IL-10 induction is key to a novel concept of treatment of allergy and asthma.
Given that protective immune mechanisms against helminths and allergic responses have a common evolutionary basis, it seems logical that the antagonistic downregulatory mechanisms also are similar. Thus, the genetic predisposition to allergy can be interpreted as an evolutionary advantage in the defense against helminths. Rather, the same genetic variants that are associated with atopy (11) are also linked to hyperreactivity against onchocerciasis, which is harmful to the host (14). The elucidation of antagonistic effects between IL-4 and IL-10 therefore improves our understanding of both helminth infection and allergy. Inasmuch as IL-10 induction may prevent allergy, neutralization of IL-10 may improve vaccination against helminths, as has been shown for schistosomiasis (16). The present study has given the first causal evidence of an antagonism of IL-4 and IL-10 in regard to parasite loads in a helminth infection.
Acknowledgments
We thank K. Fischer for expert technical assistance and B. Richter, Y. Richter, and A. Ali for maintaining the colonies of KO mice. We also thank H. Mossmann (MPI for Immunobiology, Freiburg, Germany) for the IL-10 KO strain.
Financial support from the German Research Foundation (grant Ho/2009/1-1/3 and 1/4) is gratefully acknowledged.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Al-Qaoud, K. M., E. Pearlman, J. Klukowski, T. Hartung, B. Fleischer, and A. Hoerauf. 2000. A new mechanism for IL-5 dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int. Immunol. 12:899-908. [DOI] [PubMed] [Google Scholar]
- 2.Al-Qaoud, K. M., A. Taubert, H. Zahner, B. Fleischer, and A. Hoerauf. 1997. Infection of BALB/c mice with the filarial nematode Litomosoides sigmodontis: role of CD4+ T cells in controlling larval development. Infect. Immun. 65:2457-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babu, S., L. M. Ganley, T. R. Klei, L. D. Shultz, and T. V. Rajan. 2000. Role of gamma interferon and interleukin-4 in host defense against the human filarial parasite Brugia malayi. Infect. Immun. 68:3034-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brattig, N., C. Nietz, S. Hounkpatin, R. Lucius, F. Seeber, U. Pichlmeier, and T. Pogonka. 1997. Differences in cytokine responses to Onchocerca volvulus extract and recombinant Ov33 and OvL3-1 proteins in exposed subjects with various parasitologic and clinical states. J. Infect. Dis. 176:838-842. [DOI] [PubMed] [Google Scholar]
- 5.Brattig, N. W., B. Lepping, C. Timmann, D. W. Büttner, Y. Marfo, C. Hamelmann, and R. D. Horstmann. 2002. Onchocerca volvulus-exposed persons fail to produce interferon-gamma in response to O. volvulus antigen but mount proliferative responses with interleukin-5 and IL-13 production that decrease with increasing microfilarial density. J. Infect. Dis. 185:1148-1154. [DOI] [PubMed] [Google Scholar]
- 6.Chandrashekar, R., U. R. Rao, P. B. Parab, and D. Subrahmanyam. 1986. Brugia malayi: rat cell interactions with infective larvae mediated by complement. Exp. Parasitol. 62:362-369. [DOI] [PubMed] [Google Scholar]
- 7.Chiaramonte, M. G., M. Mentink-Kane, B. A. Jacobson, A. W. Cheever, M. J. Whitters, M. E. Goad, A. Wong, M. Collins, D. D. Donaldson, M. J. Grusby, and T. A. Wynn. 2003. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J. Exp. Med. 197:687-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cottrez, F., S. D. Hurst, R. L. Coffman, and H. Groux. 2000. T regulatory cells 1 inhibit a Th2-specific response in vivo. J. Immunol. 165:4848-4853. [DOI] [PubMed] [Google Scholar]
- 9.Doetze, A., J. Satoguina, G. Burchard, T. Rau, C. Löliger, B. Fleischer, and A. Hoerauf. 2000. Antigen-specific cellular hyporesponsiveness in generalized onchocerciasis is mediated by Th3/Tr1-type cytokines IL-10 and TGF-beta but not by a Th1 to Th2 shift. Int. Immunol. 12:623-630. [DOI] [PubMed] [Google Scholar]
- 10.Groux, H. 2001. An overview of regulatory T cells. Microbes Infect. 3:883-889. [DOI] [PubMed] [Google Scholar]
- 11.Heinzmann, A., X. Q. Mao, M. Akaiwa, R. T. Kreomer, P. S. Gao, K. Ohshima, R. Umeshita, Y. Abe, S. Braun, T. Yamashita, M. H. Roberts, R. Sugimoto, K. Arima, Y. Arinobu, B. Yu, S. Kruse, T. Enomoto, Y. Dake, M. Kawai, S. Shimazu, S. Sasaki, C. N. Adra, M. Kitaichi, H. Inoue, K. Yamauchi, N. Tomichi, F. Kurimoto, N. Hamasaki, J. M. Hopkin, K. Izuhara, T. Shirakawa, and K. A. Deichmann. 2000. Genetic variants of IL-13 signalling and human asthma and atopy. Hum. Mol. Genet. 9:549-559. [DOI] [PubMed] [Google Scholar]
- 12.Hoerauf, A. 2002. Immune effectors important in protective resistance, p. 109-125. In T. Klei and T. V. Rajan (ed.), World class parasites: the filariae. Kluwer Academic Press, New York, N.Y.
- 13.Hoerauf, A., and N. Brattig. 2002. Resistance and susceptibility in human onchocerciasis—-beyond Th1 vs. Th2. Trends Parasitol. 18:25-31. [DOI] [PubMed] [Google Scholar]
- 14.Hoerauf, A., S. Kruse, N. W. Brattig, A. Heinzmann, B. Mueller-Myhsok, and K. A. Deichmann. 2002. The variant Arg110Gln of human IL-13 is associated with an immunologically hyper-reactive form of onchocerciasis (sowda). Microbes Infect. 4:37-42. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann, K. F., A. W. Cheever, and T. A. Wynn. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164:6406-6416. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann, K. F., S. L. James, A. W. Cheever, and T. A. Wynn. 1999. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J. Immunol. 163:927-938. [PubMed] [Google Scholar]
- 17.Hoffmann, W. H., A. W. Pfaff, H. Schulz-Key, and P. T. Soboslay. 2001. Determinants for resistance and susceptibility to microfilaraemia in Litomosoides sigmodontis filariasis. Parasitology 122:641-649. [DOI] [PubMed] [Google Scholar]
- 18.King, C. L., S. Mahanty, V. Kumaraswami, J. S. Abrams, J. Regunathan, K. Jayaraman, E. A. Ottesen, and T. B. Nutman. 1993. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Investig. 92:1667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263-274. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence, R. A., J. E. Allen, and C. A. Gray. 2000. Requirements for in vivo IFN-gamma induction by live microfilariae of the parasitic nematode, Brugia malayi. Parasitology 120:631-640. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence, R. A., J. E. Allen, J. Osborne, and R. A. Maizels. 1994. Adult and microfilarial stages of the filarial parasite Brugia malayi stimulate contrasting cytokine and Ig isotype responses in BALB/c mice. J. Immunol. 153:1216-1224. [PubMed] [Google Scholar]
- 22.Le Goff, L., T. J. Lamb, A. L. Graham, Y. Harcus, and J. E. Allen. 2002. IL-4 is required to prevent filarial nematode development in resistant but not susceptible strains of mice. Int. J. Parasitol. 32:1277-1284. [DOI] [PubMed] [Google Scholar]
- 23.Mahanty, S., H. E. Luke, V. Kumaraswami, P. R. Narayanan, V. Vijayshekaran, and T. B. Nutman. 1996. Stage-specific induction of cytokines regulates the immune response in lymphatic filariasis. Exp. Parasitol. 84:282-290. [DOI] [PubMed] [Google Scholar]
- 24.Martin, C., K. M. Al-Qaoud, O. Bain, K. Paehle, B. Fleischer, and A. Hoerauf. 2000. IL-5 is essential for protection after immunization against murine filariasis but not during primary infection. Med. Microbiol. Immunol. 189:67-74. [DOI] [PubMed] [Google Scholar]
- 25.Martin, C., L. Le Goff, M. N. Ungeheuer, P. N. Vuong, and O. Bain. 2000. Drastic reduction of a filarial infection in eosinophilic interleukin-5 transgenic mice. Infect. Immun. 68:3651-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin, C., M. Saeftel, P. N. Vuong, S. Babayan, K. Fischer, O. Bain, and A. Hoerauf. 2001. B-cell deficiency suppresses vaccine-induced protection against murine filariasis but does not increase the recovery rate for primary infection. Infect. Immun. 69:7067-7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osborne, J., S. J. Hunter, and E. Devaney. 1996. Anti-interleukin-4 modulation of the Th2 polarized response to the parasitic nematode Brugia pahangi. Infect. Immun. 64:3461-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearlman, E., F. E. Hazlett, W. H. Boom, and J. W. Kazura. 1993. Induction of murine T helper cell responses to the filarial nematode Brugia malayi. Infect. Immun. 61:1105-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saeftel, M., L. Volkmann, S. Korten, N. Brattig, K. M. Al-Qaoud, B. Fleischer, and A. Hoerauf. 2001. Lack of IFN-γ confers impaired neutrophil granulocyte function and imparts prolonged survival of adult filarial worms in murine filariasis. Microbes Infect. 3:203-213. [DOI] [PubMed] [Google Scholar]
- 30.Sartono, E., Y. C. Kruize, A. Kurniawan, R. M. Maizels, and M. Yazdanbakhsh. 1997. Depression of antigen-specific interleukin-5 and interferon-gamma responses in human lymphatic filariasis as a function of clinical status and age. J. Infect. Dis. 175:1276-1280. [DOI] [PubMed] [Google Scholar]
- 31.Satoguina, J., M. Mempel, J. Larbi, M. Badusche, C. Loliger, O. Adjei, G. Gachelin, B. Fleischer, and A. Hoerauf. 2002. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect. 4:1291-1300. [DOI] [PubMed] [Google Scholar]
- 32.Schandene, L., C. Alonso-Vega, F. Willems, C. Gerard, A. Delvaux, T. Velu, R. Devos, M. de Boer, and M. Goldman. 1994. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J. Immunol. 152:4368-4374. [PubMed] [Google Scholar]
- 33.Schopf, L. R., K. F. Hoffmann, A. W. Cheever, J. F. Urban, Jr., and T. A. Wynn. 2002. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 168:2383-2392. [DOI] [PubMed] [Google Scholar]
- 34.Stampfli, M. R., M. Cwiartka, B. U. Gajewska, D. Alvarez, S. A. Ritz, M. D. Inman, Z. Xing, and M. Jordana. 1999. Interleukin-10 gene transfer to the airway regulates allergic mucosal sensitization in mice. Am. J. Respir. Cell Mol. Biol. 21:586-596. [DOI] [PubMed] [Google Scholar]
- 35.Steel, C., and T. B. Nutman. 2003. Ctla-4 in filarial infections: implications for a role in diminished T cell reactivity. J. Immunol. 170:1930-1938. [DOI] [PubMed] [Google Scholar]
- 36.Volkmann, L., O. Bain, M. Saeftel, S. Specht, K. Fischer, F. Brombacher, K. I. Matthaei, and A. Hoerauf. 2003. Murine filariasis: interleukin 4 and interleukin 5 lead to containment of different worm developmental stages. Med. Microbiol. Immunol. 192:23-31. [DOI] [PubMed] [Google Scholar]
- 37.Volkmann, L., M. Saeftel, B. Fleischer, and A. Hoerauf. 2001. Interleukin-4 is essential for the control of microfilariae in murine infection with the filaria Litomosoides sigmodontis. Infect. Immun. 69:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, P., P. Wu, M. I. Siegel, R. W. Egan, and M. M. Billah. 1995. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J. Biol. Chem. 270:9558-9563. [DOI] [PubMed] [Google Scholar]
- 39.Williams, L., L. Bradley, A. Smith, and B. Foxwell. 2004. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J. Immunol. 172:567-576. [DOI] [PubMed] [Google Scholar]
- 40.Wynn, T. A., and K. F. Hoffmann. 2000. Defining a schistosomiasis vaccination strategy—is it really Th1 versus Th2? Parasitol. Today 16:497-501. [DOI] [PubMed] [Google Scholar]
- 41.Zuany-Amorim, C., C. Creminon, M. C. Nevers, M. A. Nahori, B. B. Vargaftig, and M. Pretolani. 1996. Modulation by IL-10 of antigen-induced IL-5 generation, and CD4+ T lymphocyte and eosinophil infiltration into the mouse peritoneal cavity. J. Immunol. 157:377-384. [PubMed] [Google Scholar]
- 42.Zuany-Amorim, C., S. Haile, D. Leduc, C. Dumarey, M. Huerre, B. B. Vargaftig, and M. Pretolani. 1995. Interleukin-10 inhibits antigen-induced cellular recruitment into the airways of sensitized mice. J. Clin. Investig. 95:2644-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]