Abstract
Tuberculosis remains the leading cause of death among infectious diseases, accounting for more than two million deaths annually. The incidence of the disease is increasing globally, partially because of the resurgence of drug-resistant strains of Mycobacterium tuberculosis. Calixarenes are macrocyclic oligomers, some of which are able to modify the growth of M. tuberculosis in infected cells. Most experimental work has been carried out with Macrocyclon, also known as HOC 12.5EO. In this study, we demonstrate that Macrocyclon is effective in controlling M. tuberculosis infections, and we provide evidence that its effect is partially mediated by an l-arginine-dependent mechanism of macrophage activation that involves the activity of the inducible nitric oxide synthase. We also show that Macrocyclon is effective in athymic and major histocompatibility complex class II−/− mice and synthesized a number of structurally related calixarenes expressing significant antimycobacterial activity.
Mycobacterium tuberculosis infects one-third of the world's population, and it accounts for more deaths each year than any other infectious bacterium (13). The problem, associated with multiple-drug resistance (12), has prompted a great interest in understanding new alternatives in host-mediated mechanisms of disease intervention. A new therapeutic agent, with activity mediated through a host-derived effector mechanism, would be particularly attractive, since it could be less susceptible to selection for drug resistance; if the balance between the pathogenic mycobacteria and the macrophage can be manipulated in favor of the host macrophage, it may be possible to develop novel adjunctive therapies for tuberculosis control.
Calixarenes have been used as building blocks for host molecules with numerous applications in supramolecular chemistry (5); some were identified as having antimycobacterial activity (3, 7). Most experimental work has been carried out with the compound Macrocyclon, also known as HOC 12.5EO, which was prepared by reacting the macrocycle HOC under basic conditions with ethylene oxide to give a heterogeneous compound with an average polyethylene glycol (PEG) chain of 12.5 U (3). The compound HOC was prepared from t-octylphenol and formaldehyde by a modified Zinke-Ziegler procedure; for many years, it was believed to be a cyclic tetrameric compound (3). Although the antibacterial mechanism of action of HOC compounds is not known, we have excluded extracellular inhibition of mycobacterial growth by Macrocyclon treatment (3, 7, 8). Therefore, it is believed that they work through a host-mediated mechanism (7), a view supported by reports showing activity in a wide range of in vivo models of infection in addition to tuberculosis (10). In this study, we have extended observations on the parent preparation, Macrocyclon, to show that it significantly affects mycobacterial growth in murine macrophages by a mechanism requiring inducible nitric oxide synthase (iNOS) activity. In addition, we show that Macrocyclon is effective in athymic and major histocompatibility complex class II−/− (MHC-II−/−) mice, and we have synthesized new structurally related calixarene compounds which show significant antimycobacterial activity.
MATERIALS AND METHODS
Mice.
MHC-II−/− mice were obtained as a breeding nucleus (kindly provided by D. Gray, Hammersmith Hospital, London, United Kingdom, with permission from D. Mathis, Institut National de la Santé et de la Recherche Medicale) and were bred at the National Institute for Medical Research (NIMR). Female C57BL/6 or BALB/c mice, 6 to 8 weeks old, were obtained from the Specific Pathogen Free Unit at NIMR. Athymic BALB/cnu/nu (nude) mice were obtained from a breeding colony at NIMR. Experiments were carried out in the United Kingdom according to the Home Office Animal Scientific Act of 1986.
Calixarene synthesis.
Macrocyclon (compound 1) was obtained from original stock produced in 1960 (synthesized by J. Cornforth); p-tert-butylcalix[8]arene (compound 2) and p-tert-butylcalix[6]arene (compound 3) were synthesized as described by Gutsche et al. (6) and Munch and Gutsche (11). Compounds 4, 5, and 6 were prepared by the recently reported two-step procedure (9) to access hydrophilically substituted calixarenes. Synthesis of compound 5 was as follows: potassium carbonate was added to calixarene (compound 2) in dry acetonitrile at 40°C, and the reaction mixture was stirred for 1 h. 17-(2H-Tetrahydropyran-2-yloxy)-3,6,9,12,15-pentaoxaheptadecyl bromide, dissolved in acetonitrile, was then added dropwise, and the contents were heated at 80°C for 4 days. Purification was carried out with a neutral alumina column. To this material, sodium hydride (60% dispersion in mineral oil; 2 equivalents per OH) was added in dry tetrahydrofuran. After 20 min, 17-(2H-tetrahydropyran-2-yloxy)-3,6,9,12,15-pentaoxaheptadecyl bromide was added in dry tetrahydrofuran. The reaction mixture was heated at reflux for 4 days; water was added, and the solvent was removed in vacuo, to give an oil which was purified by neutral alumina chromatography (ethyl acetate/methanol). Deprotection was achieved by stirring in dichloromethane/methanol (50:50) containing a 10% concentration of HCl to give 5 m/z (matrix-assisted laser desorption ionization-time of flight) 3432.6 [MNa-H]+. All calixarenes used demonstrated less than 0.2 endotoxin unit/mg of endotoxin and did not induce detectable levels of cytotoxicity or affect apoptosis in cultured macrophages, as detected by the lactate dehydrogenase assay and the cell death detection (apoptosis) assay (Roche Diagnostics, East Sussex, United Kingdom).
M. tuberculosis culture.
A total of 250 ml of Dubos medium containing 10 ml of Dubos albumin supplement (Difco Laboratories, Surrey, United Kingdom) was inoculated with M. tuberculosis H37Rv and incubated in a 37°C rotating incubator. The bacterial cells were resuspended in 20 ml of Dulbecco's modified Eagles medium (DMEM; Flow Laboratories, High Wycombe, United Kingdom) supplemented with 50% fetal calf serum (FCS; Advanced Protein Products, Brierly Hills, United Kingdom).
Isolation and culture of macrophages.
Peritoneal cells were pelleted, washed, and cultured in six-well plates (Nunc, Roskilde, Denmark) at 1 × 104 to 5 × 104 cells/ml in DMEM containing 10% FCS. After 3 to 4 days, the nonadhering cells were removed, and the medium was replaced with prewarmed DMEM medium containing 10% FCS and Macrocyclon at a final concentration of 2.5 mg/ml. The cells were infected 24 to 48 h later. Murine bone marrow-derived macrophages were isolated from the hind legs. The cells were resuspended into Iscove's modified Dulbecco's medium and cultured in six-well plates at 1 × 104 to 5 × 104 cells/ml in Iscove's modified Dulbecco's medium (Flow Laboratories) complemented with 5% FCS, 10 ng of either recombinant granulocyte-macrophage colony-stimulating factor (Sigma, Dorset, United Kingdom) or macrophage colony-stimulating factor (a kind gift of A. O'Garra, NIMR)/ml, 2 mM l-glutamine, and 2-mercaptoethanol (1 × 10−5 M) (Sigma); the adherent cells were used after 5 to 6 days of culture.
M. tuberculosis growth in murine macrophages.
Peritoneum- or bone marrow-derived macrophages were infected for 6 h with viable M. tuberculosis H37Rv at a low dose (1 bacilli/2 cells). CFU bacterial counts were determined 6 h postinfection and then 4, 7, and 11 days postinfection by lysing the cells with 0.2% saponin in phosphate-buffered saline (Sigma) for 1 h and then preparing 10-fold dilutions in saline. Dilutions were plated onto 7H11 solid medium, and CFU were counted 20 days after incubation at 37°C. All the calixarene compounds were used in vitro at a final concentration of 2.5 mg/ml. Either inhibitor L-NAME (l-N6-nitro-arginine-methyl-ester) or D-NAME (d-N6-nitro-arginine-methyl ester) (Sigma) at a 2 mM concentration was added to the infected cells immediately after infection. The RPMI 1640 select amine kit culture medium (Invitrogen-Life Technologies, Paisley, United Kingdom) without l-arginine was used in some of the experiments or was supplemented with 120.5 mg of l-arginine/ml (50% l-arginine) or 241 mg of l-arginine/ml (normal l-arginine levels).
M. tuberculosis infection of mice.
Each mouse received 2 × 105 mycobacterial cells intravenously. The infection was monitored by removal of the lungs and spleens of infected mice at various intervals; the baseline level of infection of each tissue was estimated by harvesting organs from the mice 18 h after infection and determining viable counts. The tissues were weighed and homogenized by shaking the tissues with 2-mm-diameter glass beads in chilled saline with a Mini-Bead Beater (Biospec Products, Bartlesville, Okla.), and 10-fold dilutions of the suspension were plated in duplicate onto Dubos 7H11 agar supplemented with Dubos oleic albumin complex supplement (Difco Laboratories). Numbers of CFU were determined after the plates had been incubated at 37°C for approximately 20 days. In the experiments testing the new calixarenes, CFU were determined 24 to 35 days after the infection.
Administration of calixarenes.
A total of 25 mg of Macrocyclon was diluted in 200 μl of endotoxin-free saline and was injected intraperitoneally into each mouse 48 to 72 h before infection and 48 to 72 h after infection. The doses of the new calixarenes used in the in vivo experiments were extrapolated from the antimycobacterial activities first tested in cultured macrophages when compared to Macrocyclon; mice were injected similarly as follows: compound 4, 25 mg; compound 5, 10 mg; and compound 6, 15 mg.
RESULTS
l-Arginine metabolism and iNOS activity are required for Macrocyclon-induced antimycobacterial activity in murine macrophages.
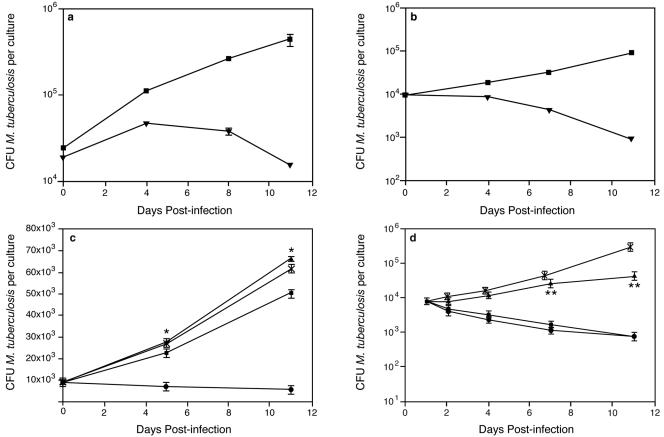
To test for Macrocyclon-induced antimycobacterial activity, we isolated murine bone marrow- or peritoneum-derived macrophages and treated them with Macrocyclon; the cells were infected for 6 to 8 h, and M. tuberculosis growth was determined 6 h postinfection and then 4, 7, and 11 days postinfection. Although we observed some variability in the rate of mycobacterial growth in macrophages between experiments, we consistently found that Macrocyclon treatment significantly affected mycobacterial growth in murine macrophages (Fig. 1a and b); importantly, we confirmed that Macrocyclon treatment did not induce apoptosis of murine macrophages and did not protect M. tuberculosis-infected macrophages from apoptosis (data not shown). A well-characterized cell-mediated antimycobacterial mechanism is effected in macrophages via the iNOS production of toxic reactive nitrogen intermediates. This l-arginine-dependent antimicrobial mechanism of murine macrophages has been established to be effective against M. tuberculosis both in vitro (1, 14) and in vivo (2). Because macrophages use l-arginine to synthesize nitric oxide and polyamines, we determined if l-arginine was necessary for the observed antimycobacterial effect. Macrocyclon-induced antimycobacterial activity required the presence of l-arginine; macrophages cultured in the absence of l-arginine did not exhibit an enhanced ability to control mycobacterial growth (Fig. 1c). We then tested if the antimycobacterial activity observed after Macrocyclon treatment could be modified by the use of pharmacological inhibitors of iNOS. Using L-NAME, a specific inhibitor of iNOS, we showed that inhibition of iNOS activity in treated, infected macrophages significantly inhibited antimycobacterial activity induced by Macrocyclon, while the inactive enantiomer (D-NAME), had no significant effect on Macrocyclon activity (Fig. 1d), demonstrating that iNOS activity is required for Macrocyclon-induced antimycobacterial control in cultured macrophages.
FIG. 1.
M. tuberculosis H37Rv growth in murine macrophages treated with Macrocyclon. (a) Bone marrow or (b) peritoneal macrophages were treated with Macrocyclon (▾) or saline (▪) as described in Materials and Methods and were infected with M. tuberculosis H37Rv. (c) M. tuberculosis H37Rv growth in Macrocyclon-treated bone marrow-derived macrophages in the presence of different levels of l-arginine. No l-arginine (▴), 50% l-arginine levels (▪), and normal l-arginine levels (•) are shown. (d) M. tuberculosis H37Rv growth in Macrocyclon-treated bone marrow-derived macrophages in the absence (▪) or presence of inhibitor L-NAME (▴) or D-NAME (•). Control cultures (X) received saline only. Error bars represent the standard errors of the mean of three culture wells. Results shown are one representative experiment of three separate experiments. *, P < 0.001; Student's t test for no l-arginine versus normal l-arginine. **, P < 0.001; Student's t test for Macrocyclon versus Macrocyclon L-NAME.
Macrocyclon activity in vivo.
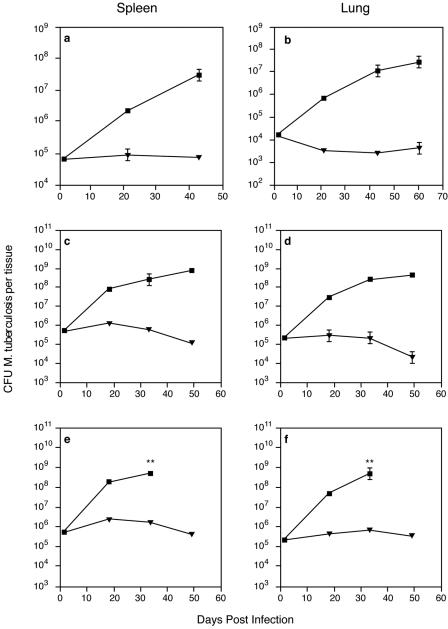
We next sought to examine Macrocyclon activity in vivo: groups of BALB/c mice were infected with 5 × 106 to 7 × 106 viable M. tuberculosis H37Rv cells and injected with Macrocyclon; control mice received saline. Macrocyclon-treated mice had significantly lower numbers of viable M. tuberculosis cells in the spleens and lungs (Fig. 2a and b), demonstrating that this compound is active in vivo. To test if the mechanism of action in vivo required conventional CD4+ and CD8+ T-cell-mediated immunity, we carried out experiments using MHC-II−/− mice (Fig. 2c and d), which lack conventional CD4+ T cells, and athymic nu/nu mice (Fig. 2e and f), which lack both conventional CD4+ and CD8+ T cells. In both cases, Macrocyclon was effective in reducing the viable counts of M. tuberculosis in the spleens and lungs of the infected mice, suggesting that Macrocyclon effect in vivo is independent of the action of conventional CD4+ and CD8+ T cells.
FIG. 2.
M. tuberculosis H37Rv growth in spleens and lungs of mice treated with Macrocyclon or saline. (a and b) Groups of three to five BALB/c mice were infected with M. tuberculosis H37Rv and treated with Macrocyclon as described in Materials and Methods. (c and d) Groups of three to five MHC-II−/− mice were infected and treated as described in Materials and Methods. (e and f) Groups of three to five BALB/c nu/nu mice were infected and treated as described in Materials and Methods. Results for saline-treated mice (▪) and Macrocyclon-treated mice (▾) are shown. Results shown are one representative experiment of two or three separate experiments. **, experiments were terminated at day 35 due to the severity of infection.
New calixarenes are effective against M. tuberculosis infection in mice.
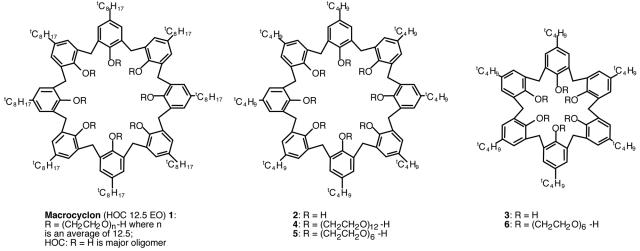
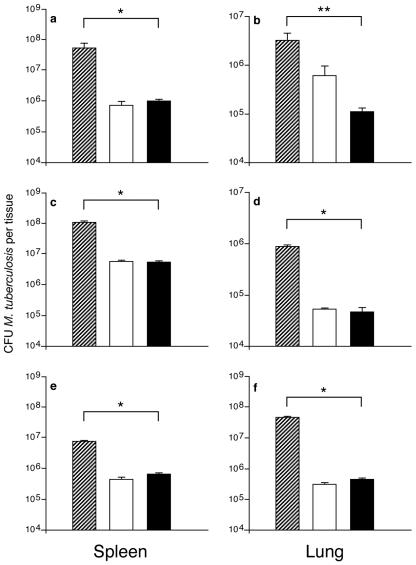
Macrocyclon was prepared to give a heterogeneous compound with an average polyethylene glycol chain of 12.5 U; for many years, it was believed to be a cyclic tetrameric compound (3). More recent investigations revealed that HOC was a mixture of oligomers in which the cyclic octamer was the major compound (15); we therefore wished to determine if homogeneous calixarenes (Fig. 3, compounds 2 to 6) were effective against M. tuberculosis infection. Athymic mice were given one dose of each calixarene 2 days before intravenous infection, followed by a second identical dose given 2 to 3 days after infection. Our results clearly demonstrated that the three calixarenes—compound 4 (Fig. 4a and b), compound 5 (Fig. 4c and d), and compound 6 (Fig. 4e and f)—induced significant antimycobacterial activities in vivo, which were comparable to those conferred by Macrocyclon.
FIG. 3.
Schematic structure of the new calixarene compounds used in this study.
FIG. 4.
M. tuberculosis H37Rv growth in spleens and lungs of BALB/c nu/nu mice treated with Macrocyclon or new calixarene compounds. Results show one representative experiment. Groups of three to five mice were treated with Macrocyclon or the new calixarene compounds, as described in Materials and Methods. Hatched bars represent data for saline-treated mice, white bars represent data for Macrocyclon-treated mice, and black bars represent data for mice treated with the new calixarenes: compound 4 (a and b), compound 5 (c and d), or compound 6 (e and f). *, P < 0.001; Student's t test. **, P < 0.01; Student's t test.
DISCUSSION
In this work, we have shown that Macrocyclon significantly affected mycobacterial growth in murine macrophages by a mechanism involving l-arginine metabolism and iNOS activity. The beneficial effect of Macrocyclon in vivo seems to be independent of the action of conventional CD4+ and CD8+ T cells, as demonstrated by the clear antimycobacterial effect of Macrocyclon in reducing the viable counts of M. tuberculosis in the spleens and lungs of athymic and MHC-II knockout mice. Importantly, however, the relevance of CD1-dependent T cells in Macrocyclon function in vivo was not properly addressed in our experiments; therefore, additional experiments using Rag or T-cell receptor α/β and γ/δ double-knockout mice are needed to clarify this issue. Our data are in line with previous reports on the action of Macrocyclon in murine macrophages (7) and confirm the notion that calixarenes principally enhance nonspecific innate immune defense mechanisms in murine macrophages. Previous work demonstrated that lipid metabolism is affected in Macrocyclon-treated cells and suggested that Macrocyclon antimycobacterial activity correlated with inhibition of triglyceride lipase and phospholipases (8); our results provide evidence that l-arginine metabolism and iNOS activity are required for Macrocyclon-induced antimycobacterial activity in murine macrophages. Importantly, however, because specific inhibition of iNOS affected the antimycobacterial activity induced by Macrocyclon in infected macrophages by only 50 to 70%, it is likely that iNOS-independent mechanisms of action also exist. Consistent with this hypothesis, macrophage lipase activity is inhibited by Macrocyclon (8), and ex vivo-isolated macrophages from Macrocyclon-treated mice demonstrated significant up-regulation of arginase I (data not shown); further studies with iNOS knockout mice and enzyme specific inhibitors will help to clarify this issue.
Importantly, we describe in this work new calixarene compounds with clear in vivo antimycobacterial activities. The three compounds tested here (compounds 4, 5, and 6) possess t-butyl groups at the upper rim, together with defined-length PEG chains: PEG-12-OH, PEG-6-OH, and PEG-6-OH, respectively. Compounds 4 and 5 are also octamers, while compound 6 is the hexamer ring. Macrocyclon, by comparison, has a t-octyl group at the upper rim with polymeric PEG chain lengths at the lower rim of 12.5 repeat units (on average), and the macrocycle is mainly the octamer. Our results demonstrate that a PEG chain of six repeat units is sufficient to produce calixarenes with high antimycobacterial activities and that a chain extension to PEG-12 offers no significant advantages.
Cornforth et al. (4) also carried out synthetic studies involving the preparation of HOC 6EO and HOC 10EO, using HOC and presynthesized PEG of 6 and 10 repeat units, respectively. These compounds were reported to possess slightly lower chemotherapeutic activities than Macrocyclon, suggesting that the upper-rim moiety together with the PEG chain length may be important. Since the synthesis and purification of long PEG chains (more than six repeat units) is nontrivial and since t-butyl calixarenes are accessible at higher yields than most other calixarenes, our results are important for future structure activity studies. Interestingly, both the hexamer and octamer had similar activities, suggesting that ring size is not crucial. However, when the unsubstituted rings of compounds 2 and 3 were tested in vivo, compound 2 had a slight antimycobacterial effect, while compound 3 was inactive (data not shown). Thus, we suggest that ring cavity size may be important when there is no functionalization at the lower rim. However, this is less critical, particularly for t-butylcalix[8]arenes and t-butylcalix[6]arenes when PEG is substituted.
Understanding the final mechanisms involved in the control of bacterial infections by activated macrophages is of paramount importance for the treatment and control of infectious diseases. At present, it is not known whether Macrocyclon has any effects on other bacterial species; similarly, a fundamental priority will be to investigate whether Macrocyclon is also effective in controlling mycobacterial infections by human macrophages.
Acknowledgments
We are grateful to J. W. Cornforth for providing Macrocyclon.
We are grateful to the MRC and to the BBSRC for support (grant 31/B10963 to G.H. and A.C.H.) and to Glaxo SmithKline (Action TB program) for a studentship (to K.J.G.).
We also thank J. Skehel for encouragement and critical reading of the manuscript.
We declare that we have no competing financial interest.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1993. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan, J., K. Tanaka, D. Carroll, J. Flynn, and B. R. Bloom. 1995. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 63:736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornforth, J. W., P. D. Hart, G. A. Nicholls, R. J. Rees, and J. A. Stock. 1955. Antituberculous effects of certain surface-active polyoxyethylene ethers. Br. J. Pharmacol. 10:73-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornforth, J. W., E. D. Morgan, K. T. Potts, and R. J. Rees. 1973. Preparation of antituberculosis polyoxyethylene ethers of homogeneous structures. Tetrahedron 29:1659-1667. [Google Scholar]
- 5.Gutsche, C. D. 1998. Calixarenes revisited. Royal Society of Chemistry, London, England.
- 6.Gutsche, C. D., B. Dhawan, K. H. No, and R. Muthukrishnan. 1981. Calixarenes. 4. The synthesis, characterization, and properties of the calixarenes from p-tert-butylphenol. J. Am. Chem. Soc. 103:3782-3792. [Google Scholar]
- 7.Hart, P. D. 1968. Mycobacterium tuberculosis in macrophages: effect of certain surfactants and other membrane-active compounds. Science 162:686-689. [DOI] [PubMed] [Google Scholar]
- 8.Hart, P. D., J. A. Armstrong, and E. Brodaty. 1996. Calixarenes with host-mediated potency in experimental tuberculosis: further evidence that macrophage lipids are involved in their mechanism of action. Infect. Immun. 64:1491-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hervé, G., D. U. Hahn, A. C. Hervé, K. J. Goodworth, A. M. Hill, and H. C. Hailes. 2003. The selective functionalisation and difunctionalisation of p-substituted calix[6]arene and calix[8]arenes using hydrophilic moieties. Org. Biomol. Chem. 1:427-435. [DOI] [PubMed] [Google Scholar]
- 10.Jacques, P. J., J. Delville, G. M. Dom, and J. M. Gillet. 1983. Effects of anti-infectious immunomodulators in leprosy and malaria in the mouse. Bull. Soc. Pathol. Exot. Filiales 76:584-587. [In French.] [PubMed] [Google Scholar]
- 11.Munch, J. H., and C. D. Gutsche. 1989. p-tert-Butylcalix[8]arene. Org. Synth. 68:243-245. [Google Scholar]
- 12.Pablos-Mendez, A., M. C. Raviglione, A. Laszlo, N. Binkin, H. L. Rieder, F. Bustreo, D. L. Cohn, C. S. Lambregts-van Weezenbeek, S. J. Kim, P. Chaul, and P. Nunn. 1998. Global surveillance for antituberculosis—drug resistance, 1994-1997. N. Engl. J. Med. 338:1641-1649. [DOI] [PubMed] [Google Scholar]
- 13.Raviglione, M. C. 2003. The TB epidemic from 1992 to 2002. Tuberculosis 83:4-14. [DOI] [PubMed] [Google Scholar]
- 14.Schable, U. E., H. L. Collins, and S. H. Kaufmann. 1998. Confrontation between intracellular bacteria and the immune system. Adv. Immunol. 66:1277-1281. [DOI] [PubMed] [Google Scholar]
- 15.Ungaro, R., V. Bocchi, D. Foina, A. Pochinia, and G. D. Andretti. 1982. Synthesis, 1H NMR, 13C NMR spectra and conformational preference of open chain ligands on lipophilic macrocycles. Tetrahedron 38:373-378. [Google Scholar]