Abstract
A tuberculosis vaccine candidate consisting of a 72-kDa polyprotein or fusion protein based upon the Mtb32 and Mtb39 antigens of Mycobacterium tuberculosis and designated Mtb72F was tested for its protective capacity as a potential adjunct to the Mycobacterium bovis BCG vaccine in the mouse and guinea pig models of this disease. Formulation of recombinant Mtb72F (rMtb72F) in an AS02A adjuvant enhanced the Th1 response to BCG in mice but did not further reduce the bacterial load in the lungs after aerosol challenge infection. In the more stringent guinea pig disease model, rMtb72F delivered by coadministration with BCG vaccination significantly improved the survival of these animals compared to BCG alone, with some animals still alive and healthy in their appearance at >100 weeks post-aerosol challenge. A similar trend was observed with guinea pigs in which BCG vaccination was boosted by DNA vaccination, although this increase was not statistically significant due to excellent protection conferred by BCG alone. Histological examination of the lungs of test animals indicated that while BCG controls eventually died from overwhelming lung consolidation, the majority of guinea pigs receiving BCG mixed with rMtb72F or boosted twice with Mtb72F DNA had mostly clear lungs with minimal granulomatous lesions. Lesions were still prominent in guinea pigs receiving BCG and the Mtb72F DNA boost, but there was considerable evidence of lesion healing and airway remodeling and reestablishment. These data support the hypothesis that the coadministration or boosting of BCG vaccination with Mtb72F may limit the lung consolidation seen with BCG alone and may promote lesion resolution and healing. Collectively, these data suggest that enhancing BCG is a valid vaccination strategy for tuberculosis that is worthy of clinical evaluation.
Disease caused by Mycobacterium tuberculosis continues to result in over 8 million new cases each year, and it remains the highest single cause of mortality from an infectious bacterial pathogen, with an estimated 2 million deaths per year (5, 10, 19, 20). Multiple factors contribute to this epidemic, including the coincidence of AIDS and malnutrition.
The vaccine for tuberculosis, Mycobacterium bovis Bacillus Calmette-Guerin (BCG), has been widely available since the 1950s. It is easy and cheap to produce, and when given to neonates or young children, it is effective in preventing severe manifestations of disease such as meningeal tuberculosis and miliary tuberculosis. However, in terms of the capacity of the vaccine to protect adult humans, it shows a wide range of efficacy, including zero levels of protection in some studies (9, 21). Examination of the substrains of widely distributed BCG has shown evidence of sequential loss of genetic content, leading to the suggestion that the vaccine may have been attenuated to avirulence, thus losing its capacity to confer protection (2-4).
Because of the general realization that BCG has a highly variable protective effect, particularly in terms of preventing adult-onset tuberculosis, a major effort has evolved to try to develop new alternative vaccines. In fact, multiple innovative approaches have been tried, including the development of recombinant vaccines, mutants of existing M. tuberculosis, subunit vaccines, and DNA-based vaccines (12, 15, 17, 18). Many of these candidates have shown some degree of protection in the mouse tuberculosis challenge model, which is the most widely used primary screening model.
One such candidate, Mtb72F, consisting of either a polyprotein vaccine formulated in the AS02A adjuvant or a DNA vaccine, was recently described (22). This polyprotein antigen was derived from two known M. tuberculosis antigens, and when combined with AS02A or delivered as naked DNA, was found to be immunogenic in mice, conferring significant protection against an aerosol challenge infection. In the studies described here, this vaccine was further evaluated in the more stringent guinea pig model (1, 14, 24), a model in which animals develop severe necrosis similar to that seen in untreated tuberculosis in humans. The results of this study show that the Mtb72F protein given as an adjunct to BCG vaccination substantially increased the survival of guinea pigs infected with pulmonary tuberculosis compared to BCG given alone, whereas DNA was less effective but still showed a similar trend. Moreover, whereas animals vaccinated with BCG alone eventually succumbed to increasing lung tissue consolidation, surviving animals given the vaccine combination showed long-term survival and evidence of potential lesion resolution in their lungs. These data support the hypothesis that coadministering or boosting BCG, rather than removing or replacing it, is a valid strategy for improving vaccination against tuberculosis.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free 6- to 8-week-old female C57BL/6 mice and female outbred Hartley guinea pigs (∼500 g in weight) were purchased from Charles River Laboratories (North Wilmington, Mass.) and held under barrier conditions in a biosafety level III animal laboratory.
Experimental vaccines.
In mouse experiments, BCG (5 × 106 CFU) was given subcutaneously. Negative control mice were given saline or DNA empty vector only. Adjuvants consisted of formulations based upon mixtures of MPL and QS-21 adjuvants combined with an oil-in-water emulsion (referred to as AS02A) (GlaxoSmithKline Biologicals, Rixensart, Belgium) (6, 23). In BCG-boosted mice, animals were first vaccinated with BCG (5 × 106 CFU) subcutaneously, rested for 3 weeks, and then boosted twice at 3-week intervals with a dose of 8 μg of recombinant Mrb72F (rMtb72F) formulated in AS02A or 100 μg of Mtb72F DNA via the intramuscular route. In guinea pig experiments, BCG was given either alone (intradermally at a dose of 103 CFU) or coadministered with 20 μg of rMtb72F. Alternatively, guinea pigs were first vaccinated with BCG, rested for 3 weeks, and then boosted with two subsequent intramuscular immunizations of a 400-μg dose of Mtb72F DNA, again at 3-week intervals.
Experimental infections.
M. tuberculosis H37Rv and M. bovis BCG Pasteur were grown from low-passage seed lots in Proskauer-Beck liquid medium containing 0.05% Tween 80 to early mid-log phase. Cultures were aliquoted into 1-ml tubes and stored at −70°C until used. Thawed aliquots were diluted in double-distilled sterile water to the desired inoculum concentrations. An aerosol generation device (Glas-Col, Terre Haute, Ind.) was used to expose the animals to an aerosol of M. tuberculosis and was calibrated to deliver approximately 20 bacilli into each guinea pig lung. Mice were infected in a similar manner with approximately 100 bacilli.
To assess bacterial loads, individual whole-organ homogenates were plated on nutrient Middlebrook 7H11 Bacto agar (Becton Dickinson Microbiology Systems, Cockeysville, Md.). Organs from the BCG-vaccinated mice were grown on medium supplemented with 2 μg of 2-thiophene-carboxylic acid hydrazide/ml to selectively inhibit the growth of the residual BCG bacteria in the test organs. Bacterial colonies were counted after 2 to 3 weeks of incubation at 37°C. The protective effects of vaccination were expressed as log10 reductions in bacterial counts in immunized mice compared with bacterial counts in the controls.
Survival assays.
Survival times for infected guinea pigs were determined by observing animals on a daily basis for changes in food consumption, evidence of labored breathing, and behavioral changes. In addition, animals were weighed on a weekly basis until a sustained drop in weight was observed over several days, indicating illness. At this point, the animal was euthanized and the lung and spleen were removed aseptically. The lower cranial lung lobe was used for inspection of pathology, and the right cranial lung lobe and the spleen were cultured to determine numbers of CFU of M. tuberculosis.
IFN-γ enzyme-linked immunosorbent assay.
Spleen cells from mice were cultured in duplicate 96-well tissue culture plates at 2.5 × 105 cells/well with or without Mtb72F. After 72 h, supernatants were harvested and analyzed for gamma interferon (IFN-γ) by a double sandwich enzyme-linked immunosorbent assay with specific antibodies (BD Pharmingen).
IFN-γ Elispot assay.
The number of IFN-γ-expressing cells in single cell suspensions was determined by Elispot. A MultiScreen 96-well plate (Millipore) was coated with 10 μg of rat anti-mouse IFN-γ capture antibody (BD Pharmingen)/ml and incubated overnight at 4°C. Plates were washed with phosphate-buffered saline (PBS) and blocked with RPMI containing 10% fetal calf serum for 1 h. Spleen cells were plated in duplicate at 105 cells per well in 100 μl and stimulated with 10 μg of Mtb72F/ml plus 0.2 ng of interleukin-2/ml for 48 h at 37°C. The plates were then washed with PBS containing 0.1% Tween and incubated overnight at 4°C with a biotin-conjugated rat anti-mouse IFN-γ secondary antibody at 5 μg/ml in PBS containing 0.1% Tween and 0.5% bovine serum albumin. The filters were developed by using the Vectastain ABC avidin peroxidase conjugate and Vectastain AEC substrate kits (Vector Laboratories). The reaction was stopped by washing with deionized water. Plates were then dried, and spots were counted.
Histological analysis.
Tissues were fixed in 10% neutral buffered formalin for a minimum of 2 weeks. In each case, the left caudal lung lobe was sectioned through the middle of the lobe. The lobe was the submerged in formalin for 2 weeks or more. The tissues were embedded in paraffin wax. Sections (3 μm) were cut and stained with hematoxylin and eosin. The tissues were coded and evaluated by a veterinary pathologist without prior knowledge of time or treatment group.
Statistical method.
The difference in bacterial numbers between control mice and vaccinated mice were compared by a one-way analysis of variance of the log10 CFU. Statistical differences in guinea pig survival times were calculated by using the log rank test.
RESULTS
Boosting BCG with Mtb72F-AS02A in mice increases Th1 response but does not result in increased protection against challenge with M. tuberculosis.
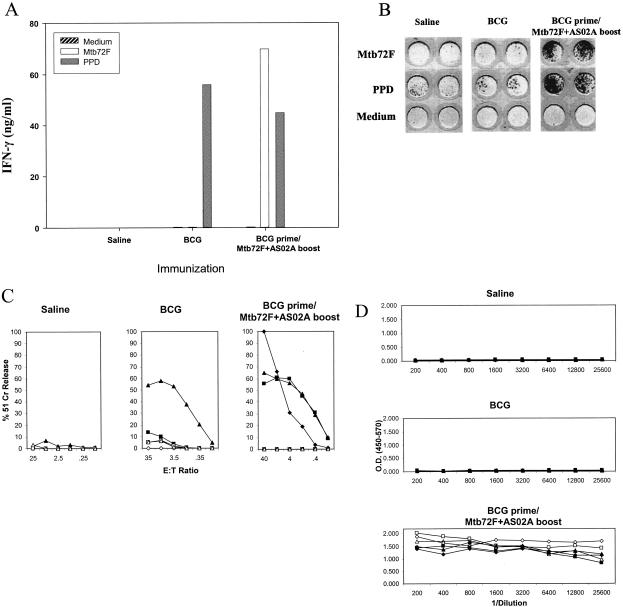
As demonstrated recently (22), mice immunized with the polyprotein Mtb72F formulated in AS02A adjuvant or the corresponding DNA vaccine construct resulted in the induction of a strong Mtb72F-specific Th1 response. In addition, a strong cytotoxic T lymphocyte (CTL) response was observed in the DNA-immunized group. To assess this material further, and to determine whether immune responses to the BCG vaccine could be improved by boosting, spleen cells from mice vaccinated with BCG and boosted with Mtb72F-AS02A were analyzed in vitro. Mice immunized with BCG alone responded weakly following stimulation in vitro with Mtb72F, but a strong IFN-γ response was seen in mice given BCG followed by the Mtb72F-AS02A boost (Fig. 1A). This was further confirmed by IFN-γ Elispot assay (Fig. 1B).
FIG. 1.
(A) Spleen cell secretion of IFN-γ following stimulation with Mtb72F. Mice were immunized with BCG or BCG boosted with Mtb72F in an AS02A formulation. Data shown are from pooled cells from three mice. PPD, purified protein derivative. (B) Elispot analysis of immunized mice, vaccinated as described above. (C) CTL responses to Mtb32c. Harvested spleen cells were tested for cytolytic activity against EL4 cells expressing Mtb32c. Data shown are the individual responses of three mice against EL4 expressing Mtb32c (filled symbols) or EL4 cell controls (open symbols). E:T ratio, effector-to-target cell ratio. (D) Serum antibody responses to Mtb72F. Open symbols show immunoglobulin G1 antibody titers, and closed symbols show immunoglobulin G2a responses. O.D., optical density.
In further experiments, CTL responses were determined in these mice to the CD8+-T-cell epitope expressed by the Mtb32c component of the Mtb72F polyprotein. One animal given BCG showed evidence of responding, whereas all animals covaccinated with Mtb72F generated strong CTL responses (Fig. 1C). Finally, antibody responses to Mtb72F were also determined and found to be highly elevated in mice receiving the Mtb72F boost (Fig. 1D).
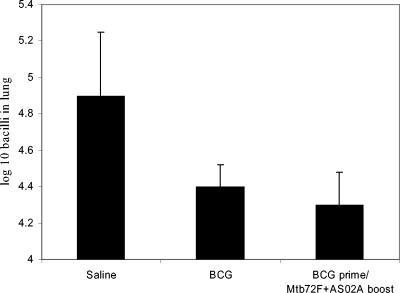
To further determine whether these strong responses to Mtb72F translated into improved protection, the two groups of vaccinated mice were exposed to a low-dose aerosol challenge infection. However, as shown in Fig. 2, the levels of protection measured were not significantly different between the two groups. Similarly, boosting of the BCG vaccine with Mtb72F DNA resulted in a strong enhancement of Mtb72F-specific Th1 responses (IFN-γ, CTL, and antibody) but without an improvement in the protection seen with BCG vaccination alone (data not shown).
FIG. 2.
Expression of protective immunity in the lung conferred by vaccination. Both BCG and BCG boosted with Mtb72F-AS02A significantly reduced bacterial loads in the lung (P < 0.01), but the vaccine groups were not themselves significantly different. Data shown are means ± standard errors of the means (n = 5 mice) for animals sacrificed 30 days after aerosol challenge.
Survival studies with Mtb72F polyprotein in the guinea pig model.
In the studies described above, boosting of BCG with rMtb72F-AS02A did not enhance protection in mice above that of BCG alone. In our experience, however, such data have to be regarded with caution, given that the mouse aerosol model has a very limited window of protection. Moreover, as demonstrated elsewhere (1), differences that are not obvious in terms of a short-term protection assay can in fact be discerned if guinea pig survival assays are used as an additional readout. These assays can potentially reveal pathological changes that may be beneficial to the animal that may or may not be associated with a drop in the lung bacterial load per se.
In a first such study, groups of guinea pigs were covaccinated with rMtb72F plus BCG prior to low-dose aerosol challenge. In this study (Fig. 3), animals given saline died in approximately 20 to 25 weeks, with similar results seen for guinea pigs given the adjuvant formulation alone (data not shown). As anticipated, BCG controls lived for approximately 1 year, although a single animal survived for the length of the experiment. An additional control group of BCG plus adjuvant only was not included for economic reasons; we have not to date seen any effect of the AS adjuvants (or DNA vectors for that matter; see below) on BCG-induced survival in several other studies (data not shown). In the group receiving the two vaccines together, one animal died at 52 weeks and another died at 75 weeks, but the other three remained healthy and retained their body weight through to week 113, when the study was curtailed. This extended protection was statistically significant (P = 0.03, one-sided log rank test).
FIG. 3.
Kaplan-Meier survival curves (n = 5) showing that covaccination of guinea pigs with BCG mixed with Mtb72F protein substantially increased survival after low-dose aerosol challenge with M. tuberculosis.
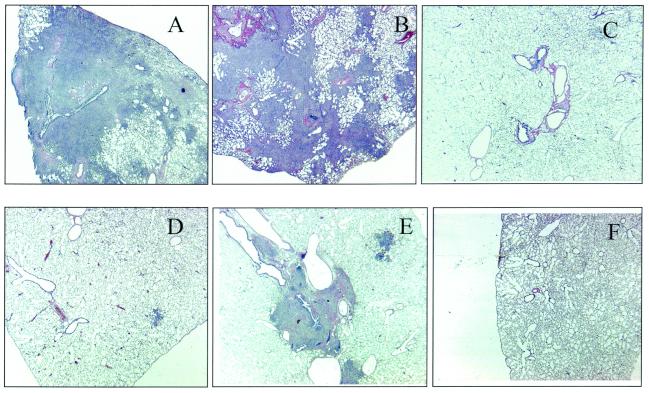
Each of the three surviving guinea pigs that had received BCG plus Mtb72F retained 200 to 800 viable bacilli in their lungs, indicating that they had been infected with M. tuberculosis. The histopathology seen in these animals is shown in Fig. 4. In the animals, most of the lung tissues had been destroyed by extensive necrosis and mineralization, as anticipated. However, in animals given BCG, multiple coalescing granulomas were seen in animals dying after about 1 year. These granulomas were lymphocytic in nature but had slowly grown to the extent that they eventually consolidated much of the lung tissues, resulting in animal death. In the single BCG-vaccinated guinea pig and in the three guinea pigs vaccinated with BCG plus rMtb72F surviving to week 113 of the experiment, granulomatous tissues could be seen in the lungs, but they were mostly very small and invariably associated with large airways or blood vessels.
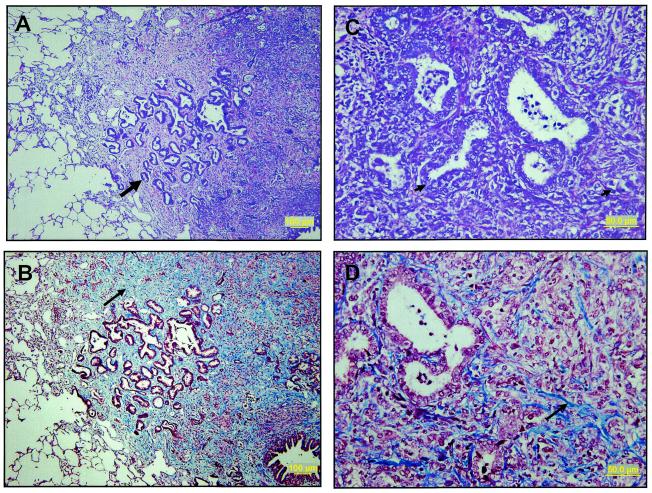
FIG. 4.
Histologic appearance of lung tissues harvested from guinea pigs vaccinated with either BCG alone or with BCG plus Mtb72F. (A) Saline control animal showing extensive tissue destruction and necrosis (week 20). (B) Typical appearance of BCG control animals showing extensive consolidation of the lung by lymphocytic granulomatous tissue formation (week 42). (C to F) Representative sections from surviving animals vaccinated with BCG plus Mtb72F. There are occasional small areas of granulomatous tissue and fibrosis; most of the lung fields are clear.
We also examined the histologic appearance of the two guinea pigs that died despite being boosted with Mtb72F. In these animals, we found consolidated lesions consisting of extensive multifocal to coalescing granulomas effacing the majority of the pulmonary parenchyma (Fig. 5), suggesting that, similar to the BCG control group, these animals also died from lung consolidation.
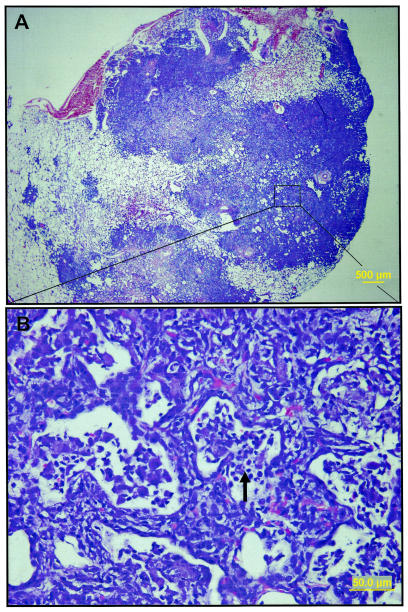
FIG. 5.
Representative light photomicrographs of lungs from animals dying despite Mtb72F boosting. Extensive multifocal to coalescing granulomatous inflammation effaces the majority of pulmonary parenchyma (A). A higher magnification of these affected areas (rectangle in panel A) shows marked thickening of the interalveolar septa with infiltrates of lymphocytes and macrophages, fewer neutrophils, and cellular debris (arrow) that fill remnant alveoli (B). The sections were stained with hematoxylin and eosin.
Studies with Mtb72F delivered as a DNA vaccine.
Given the very encouraging results of the above studies, parallel experiments were performed with an alternative vaccination strategy comprising a BCG prime and an Mtb72F boost. Mtb72F DNA was used instead of the rMtb72F-AS02A formulation to boost the BCG vaccine because in several previous experiments, on its own, the DNA vaccine has consistently provided protection in the guinea pig model. In this study, protection by BCG alone was more extended, with three of five animals dying by about 80 weeks and the other two animals surviving to the end of the study at 108 weeks (Fig. 6). In the group receiving the two vaccines together, four of five animals survived to 104 weeks, with one dying just before the experiment was ended. Because of the longer than anticipated protection seen in the BCG only group, the two groups were not statistically significant (P = 0.27) in terms of survival.
FIG. 6.
Kaplan-Meier survival curves (n = 5) comparing the survival of BCG controls versus guinea pigs vaccinated with BCG and boosted twice with DNA encoding Mtb72F.
Despite the lack of statistical difference, however, there were obvious differences when the two groups were compared in terms of pathology (Fig. 7). Guinea pigs vaccinated with BCG alone had extensive lymphocytic granulomatous lesions, as expected, but as these animals began to die, it was apparent that many lesions had developed prominent central areas of coagulative necrosis. However, unlike necrotic lesions in saline controls, there was no evidence of any process of mineralization starting in the BCG-vaccinated animals.
FIG. 7.
Histologic appearance of lung tissues harvested from guinea pigs vaccinated with BCG alone or vaccinated with BCG and boosted twice with Mtb72F DNA. (A) Saline control guinea pig euthanized at week 17 showing extensive coalescing granulomatous inflammation with a central focus of necrosis that is undergoing mineralization (arrow). (B) DNA vector control animal (week 30) with region (arrow) of central coagulative necrosis. (C) BCG-vaccinated guinea pig euthanized at week 79. A large granulomatous structure has degenerated with central necrosis (arrow) but with no apparent mineralization. (D) Covaccinated guinea pig surviving to curtailment of the study. Lesions in animals from this group had less-extensive mixed inflammation than animals from other groups, with extensive fibrous connective tissue deposition and small airway reestablishment (arrow), indicating lesion resolution. Bars, 100 μm.
In contrast to lung tissues showing severe granulomatous inflammation in the BCG controls, guinea pigs receiving BCG followed by boosting with Mtb72F DNA showed signs of healing, in that the necrosis and inflammation had been replaced by fibrous connective tissue (Fig. 7D). Moreover, in these areas, small airways were clearly being reestablished. Additional examples are shown in Fig. 8.
FIG. 8.
Further illustration of airway reestablishment. Light photomicrographs from guinea pigs surviving to the end of the experiment are shown. There are extensive foci of resolving granulomatous inflammation in which highly branched and regenerating bronchioles appear (thick arrow) (A). These airways are supported by fibrous connective tissue (thin arrow) that effaces the pulmonary parenchyma (B). Higher magnifications of the affected area (C and D) show regenerating bronchioles and alveoli lined by a hyperplastic epithelium (arrowheads) and increased fibrous connective tissue (D), with minimal residual inflammation. The sections shown in panels A and C were stained with hematoxylin and eosin, and the sections shown in panels B and D were stained with Masson's trichrome.
DISCUSSION
In a recent study (22), it was shown that vaccines based upon the Mtb72F polyprotein construct can prolong the survival of guinea pigs exposed to a low-dose aerosol infection with M. tuberculosis H37Rv to an extent that was within the range (40 to 70 weeks) consistently seen in animals protected with the Pasteur substrain of BCG. We extend these observations in the present study to show that when the two vaccines were combined, the survival times observed were consistently longer than that seen in BCG controls. In the case of BCG mixed with rMtb72F, three of five animals were still alive and healthy 113 weeks after aerosol exposure, and a similar percentage were protected at 108 weeks by BCG boosted with Mtb72F DNA. In the experiment with a mixture of BCG and rMtb72F, this amounted to essentially a doubling of the survival time seen in BCG controls. In the study with the Mtb72F DNA boost, survival was not significantly increased at a statistical level, but important differences were observed at the histopathological level.
In this regard, examination of the lungs of animals receiving both BCG and Mtb72F surprisingly showed mostly clear lungs with scattered small pockets of granulomatous inflammation observed mainly around large vessels, an observation in spectacular contrast to that of animals given BCG alone, in which much of the lung tissues had been consolidated prior to the death of these animals. Interestingly, in the two animals that died despite boosting by Mtb72F, the same type of lymphocytic lung consolidation was seen as in the BCG control animals, but it was less pronounced. In further studies, replacing the Mtb72F-AS02A protein-based vaccine with a DNA vaccine was also tried, and whereas evidence for better survival was less convincing, lesions in the BCG controls and in the Mtb72F DNA-boosted animals were clearly different. These data suggested that the BCG group had been strongly protected but had reached a point after about 60 to 70 weeks where lesions were breaking down due to necrosis. This in itself is novel information, since previous work on this model suggested that lung consolidation leading to asphyxiation was the primary cause of death (15). In the group receiving both vaccines, however, a clearly different pathological process must have been taking place, since the tissues showed considerable evidence of tissue healing, including the establishment of small airways.
Our working hypothesis, being determined in ongoing studies, is that infected animals initially develop lesions, but whereas these lesions continue to develop in the BCG controls, they are limited in covaccinated animals. Whereas we cannot yet assume that the pathological processes we observed in the two studies reflect the same basic mechanisms, it seems evident that a process leading to substantial lesion healing and resolution had taken place in the majority of guinea pigs given BCG plus Mtb72F. In animals boosted with the DNA vaccine, the pathology we observed may reflect a slower process and perhaps an earlier stage, with wound healing and airway reestablishment that potentially may precede the lesion absorption seen in the animals receiving the protein vaccine. The driving force behind this may reflect the degree of destruction of the bacterial inoculum; whereas BCG reduces the load in the lungs fairly effectively, many remain in the form of a persistent disease. In the dually vaccinated animals, the bacterial load may be further reduced to a point where the granulomatous response is not further triggered, allowing lesion resolution (in the surviving animals given BCG plus Mtb72F, the bacterial counts were all <1,000). In work to be reported elsewhere, vaccination of primates with BCG boosted with Mtb72F-AS02A seems to follow a similar pattern, with evidence of lesions early (on X-ray plates) followed by some degree of apparent resolution; in contrast, these lesions progress in primates receiving BCG only and these animals die (S. Reed, personal communication).
These studies also demonstrate the additional and more detailed information provided by the guinea pig model of tuberculosis compared to the mouse model. As shown above, vaccination of mice with the dual vaccines clearly increased the Th1 response, measured in terms of IFN-γ production, and gave strong CTL responses. However, when exposed to aerosol challenge infection with M. tuberculosis, both groups of vaccinated animals had similar levels of protection. Whether this is a true measure of protective capacity is unclear, however, for the simple reason that the mouse model has a relatively narrow window (about 1 log) in which the bacterial load in the lungs is contained in vaccinated animals. As discussed before (16), these limitations to the otherwise useful mouse model can be addressed by further evaluations of the guinea pig model. Here again, changes in bacterial load in the lungs may not always be evident, but other factors, notably the nature of the pathological response in the lungs, be it necrotic or lymphocytic, may directly influence the ability of the animal to control the infection, thus resulting in prolonged survival. Hence, by monitoring the survival of infected guinea pigs, one can (i) determine whether survival is statistically different from that of saline, adjuvant, or BCG controls, (ii) determine whether survival is associated with a more beneficial pathology in the lungs, and (iii) by monitoring weight loss indicating eventual euthanasia, harvest lung tissue for bacterial count determination.
Although the first wave of innovation in the recent drive to develop a new tuberculosis vaccine involved candidates designed to replace BCG, this strategy may be neither ethical nor practical. A viewpoint, voiced before (15), has been that if BCG has a protective effect to some degree in children but not so in adults, then this implies that something has been lost (presumably the capacity to express immunological memory) which can potentially be replaced if truly lost or boosted if waning. Studies with mice (8), in which we were able to boost immunity in BCG-vaccinated animals in the latter stages of their lifespan by mid-life inoculation with a subunit vaccine, seem to support the latter theory. Studies from others (7, 11, 13) showing that BCG vaccination in mice can be boosted by DNA vaccines encoding individual antigens, such as Ag85, similarly support this concept.
In this regard, therefore, the results of the present study clearly support the hypothesis that immunity engendered by BCG in the stringent guinea pig model can be improved by combination with Mtb72F. However, it remains to be determined whether Mtb72F needs to be given at the same time or soon after BCG to be effective or whether it can be given some time later (a true booster in the classical sense). Either way, this form of prime-boost strategy clearly warrants further investigation.
In conclusion, therefore, these data indicate that Mtb72F-AS02A is a valid candidate for clinical trial evaluations. Moreover, it shows that boosting or augmenting BCG, rather than trying to replace it, is a valid strategy for future tuberculosis control.
Acknowledgments
This work was supported by U.S. Public Health Service grants AI-75320, AI-44373, AI-49505, and AI-45707 from the National Institutes of Health.
We thank JoLynn Trout, Robert Christensen, Pamela Ovendale, and Jeff Guderian for excellent technical assistance.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A. Frank, M. A. Lui, J. B. Ulmer, K. Huygen, D. M. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behr, M. A., and P. M. Small. 1997. Has BCG attenuated to impotence? Nature 389:133-134. [DOI] [PubMed] [Google Scholar]
- 3.Behr, M. A., and P. M. Small. 1999. A historical and molecular phylogeny of BCG strains. Vaccine 17:915-922. [DOI] [PubMed] [Google Scholar]
- 4.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 5.Bloom, B. R., and C. J. Murray. 1992. Tuberculosis: commentary on a reemergent killer. Science 257:1055-1064. [DOI] [PubMed] [Google Scholar]
- 6.Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358:1927-1934. [DOI] [PubMed] [Google Scholar]
- 7.Britton, W. J., and U. Palendira. 2003. Improving vaccines against tuberculosis. Immunol. Cell Biol. 81:34-45. [DOI] [PubMed] [Google Scholar]
- 8.Brooks, J. V., A. A. Frank, M. A. Keen, J. T. Bellisle, and I. M. Orme. 2001. Boosting vaccine for tuberculosis. Infect. Immun. 69:2714-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 10.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. W.H.O. Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 11.Feng, C. G., U. Palendira, C. Demangel, J. M. Spratt, A. S. Malin, and W. J. Britton. 2001. Priming by DNA immunization augments protective efficacy of Mycobacterium bovis Bacille Calmette-Guerin against tuberculosis. Infect. Immun. 69:4174-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann, S. H. 2000. Is the development of a new tuberculosis vaccine possible? Nat. Med. 6:955-960. [DOI] [PubMed] [Google Scholar]
- 13.Kirman, J. R., T. Turon, H. Su, A. Li, C. Kraus, J. M. Polo, J. Belisle, S. Morris, and R. A. Seder. 2003. Enhanced immunogenicity to Mycobacterium tuberculosis by vaccination with an alphavirus plasmid replicon expressing antigen 85A. Infect. Immun. 71:575-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMurray, D. N. 2003. Hematogenous reseeding of the lung in low-dose, aerosol-infected guinea pigs: unique features of the host-pathogen interface in secondary tubercles. Tuberculosis (Edinburgh) 83:131-134. [DOI] [PubMed] [Google Scholar]
- 15.Orme, I. M. 1999. Beyond BCG: the potential for a more effective TB vaccine. Mol. Med. Today 5:487-492. [DOI] [PubMed] [Google Scholar]
- 16.Orme, I. M. 2003. The mouse as a useful model of tuberculosis. Tuberculosis (Edinburgh) 83:112-115. [DOI] [PubMed] [Google Scholar]
- 17.Orme, I. M. 1999. New vaccines against tuberculosis. The status of current research. Infect. Dis. Clin. North Am. 13:169-185, vii-viii. [DOI] [PubMed] [Google Scholar]
- 18.Orme, I. M., D. N. McMurray, and J. T. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 9:115-118. [DOI] [PubMed] [Google Scholar]
- 19.Raviglione, M. C. 2003. The TB epidemic from 1992 to 2002. Tuberculosis (Edinburgh) 83:4-14. [DOI] [PubMed] [Google Scholar]
- 20.Raviglione, M. C., and A. Pio. 2002. Evolution of W.H.O. policies for tuberculosis control, 1948-2001. Lancet 359:775-780. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues, L. C., and P. G. Smith. 1990. Tuberculosis in developing countries and methods for its control. Trans. R. Soc. Trop. Med. Hyg. 84:739-744. [DOI] [PubMed] [Google Scholar]
- 22.Skeiky, Y. A. W., M. R. Alderson, J. A. Guderian, P. A. Ovendale, L. Brandt, A. Campos-Neto, Y. Lobet, W. Dalemans, I. M. Orme, and S. G. Reed. 2004. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72f, delivered as naked DNA or as recombinant protein. J. Immunol. 172:7618-7628. [DOI] [PubMed] [Google Scholar]
- 23.Stoute, J. A., M. Slaoui, D. G. Heppner, P. Momin, K. E. Kester, P. Desmons, B. T. Wellde, N. Garcon, U. Krzych, and M. Marchand. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N. Engl. J. Med. 336:86-91. [DOI] [PubMed] [Google Scholar]
- 24.Turner, O. C., R. J. Basaraba, and I. M. Orme. 2003. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infect. Immun. 71:864-871. [DOI] [PMC free article] [PubMed] [Google Scholar]