Abstract
We have previously demonstrated that vaccination of mice with plasmid DNA vectors expressing immunodominant mycobacterial genes induced cellular immune responses and significant protection against challenge with Mycobacterium tuberculosis. We demonstrate here, using in vitro-synthesized RNA, that vaccination with DNA or RNA constructs expressing the M. tuberculosis MPT83 antigen are capable of inducing specific humoral and T-cell immune responses and confer modest but significant protection against M. tuberculosis challenge in mice. This is the first report of protective immunity conferred against intracellular bacteria by an RNA vaccine. This novel approach avoids some of the drawbacks of DNA vaccines and illustrates the potential for developing new antimycobacterial immunization strategies.
It is estimated that one-third of the world's population is currently infected with Mycobacterium tuberculosis, and this results in approximately 3 million deaths every year (19). The only tuberculosis vaccine currently available is Mycobacterium bovis bacillus Calmette-Guérin (BCG), but its efficacy varies, particularly against the adult pulmonary form of the disease (14). We have previously shown that vaccination of mice with DNA vectors expressing several mycobacterial genes induced cellular immune responses and significant protection against challenge with M. tuberculosis (10, 22). Mycobacterial MPT83 is a dominant antigen during infection, and immunization of mice with plasmid DNA expressing this antigen induced significant immune responses and protection against challenge with M. bovis (3). Both CD4+- and CD8+-T-cell subpopulations are thought to contribute to the control of mycobacterial infection (4), so a particularly useful aspect of plasmid DNA immunization against tuberculosis is the effective induction of specific CD8+ T cells in addition to CD4+-T-cell responses. However, a major concern with this strategy is the possibility of obtaining homologous-recombination events leading to the activation of cellular oncogenes or the inactivation of tumor suppressor genes (6). An alternative strategy is the administration of RNA-pulsed cells (2) or the injection of naked RNA (9). In fact, evidence indicates that mRNA can be used for in vivo genetic vaccination. Humoral immune responses were elicited following intramuscular injection of in vitro-synthesized RNA encoding human carcinoembryonic antigen (5), and a large amount of antibody was produced after delivery of human α-1-antitrypsin mRNA by gene gun into the epidermis (17). More interestingly, virus-specific cytotoxic T lymphocyte (CTL) responses were induced following immunization with liposome-encapsulated, in vitro-synthesized RNA encoding influenza virus nucleoprotein (12), and in vitro-synthesized, attenuated infectious RNA has been used to induce protection against lethal tick-borne encephalitis virus in mice (11). Here, we used an RNA construct encoding the RNA replicase from the Sindbis virus and the M. tuberculosis MTP83 gene and tested the induction of protective immune responses in a murine model of virulent M. tuberculosis infection.
MATERIALS AND METHODS
Cell culture.
The mouse tumor cell line P815 (European Collection of Cell Cultures, Salisbury, United Kingdom) was grown in RPMI medium (Invitrogen- Life Technologies, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS; Advanced Protein Products, Brierly Hill, United Kingdom). The macrophage-like mouse tumor cell line J774 (European Collection of Cell Cultures) was grown in Dulbecco's modified Eagle's medium (Invitrogen-Life Technologies) supplemented with 10% FCS, 2 mM l-glutamine, 10−5 M 2-mercaptoethanol (Sigma, Dorset, United Kingdom) and antibiotics (100 IU of penicillin/ml and 100 μg of streptomycin/ml [Sigma]). The baby hamster kidney (BHK; European Collection of Cell Cultures) cell line was grown in Dulbecco's modified Eagle's medium supplemented with 5% FCS, 2 mM l-glutamine, 10−5 M 2-mercaptoethanol (Sigma), and antibiotics (100 IU of penicillin/ml and 100 μg of streptomycin/ml).
MPT83 DNA and MPT83 RNA vectors.
To obtain pCMV4.83, we replaced the hsp65 gene with the MPT83 gene in the vector pCMV4.65 (21) by cloning a 0.7-kb BamHI-NotI DNA fragment encoding the MPT83 antigen into BamHI-NotI-digested pCMV4.65. This 0.7-kb DNA insert was generated by PCR using M. tuberculosis H37Rv genomic DNA as the template and the following primers: For83 (5′-ATT GGA TCC GCC ATG ATC AAC GTT CGA GCC-3′) and Rev83 (5′-TAT GCG GCC GCC GAA CGT TAC TGT-3′). Plasmid vectors pCMV3.83 and pMGD20.83 were constructed as described previously (24); plasmid vectors were purified on QIAGEN (Dorking, United Kingdom) columns from Escherichia coli DH5α (Clontech, Cambridge, United Kingdom) and Top10 (Invitrogen, Abington, United Kingdom). The MPT83 RNA construct used in our experiments was synthesized in vitro with the plasmid pSinRep5.83 as the DNA template. This vector was constructed as follows: the MPT83 gene from pCMV4.83 was digested with KpnI-ApaI and cloned into the superlinker plasmid pSL1180 (Amersham-Pharmacia Biotechnologies, Buckinghamshire, United Kingdom) to obtain pSL1180.83. This vector was digested with StuI-ApaI to release the MPT83 gene and cloned into the pSinRep5 vector (Invitrogen). By cloning the green fluorescent protein (GFP) gene into the pSin Rep5 vector as described previously (24), we constructed a control pSinRep5GFP-expressing vector.
Mycobacterial MPT83 expression and purification from E. coli cells.
To express the mycobacterial MPT83 gene in E. coli, we cloned a BamHI-XhoI DNA fragment containing the MPT83 gene, from pCMV4.83, into the pQE30 vector (QIAGEN) to generate pQE30.83, as described previously (24). In this vector, the MPT83 gene is in the same open reading frame as the polyhistidine-tagged sequence, allowing easy purification of the fusion product. Plasmid pQE30.83 was transformed into E. coli M15, and transformants were selected on plates containing ampicillin and kanamycin. The recombinant protein was purified from the bacterial pellets by using nickel-nitrilotriacetic acid resin (QIAGEN); briefly, the pellets were lysed with buffer A (8 M urea, 0.1 M NaH2PO4, 0.01 M Tris base [pH 8.0]) on ice for 30 min, and then the solution was centrifuged at 12,000 × g. Supernatants were loaded onto 8 ml of resin. The protein was eluted with a different buffer (8 M urea, 0.1 M NaH2PO4, 0.01 M Tris-HCl [pH 5.9 to 4.3]). The resulting fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The semipurified MPT83 protein was dialyzed and detoxified using a Slide-A-Lyzer dialysis cassette (Pierce & Warriner, Cheshire, United Kingdom).
Transient transfection of MPT83 DNA and MPT83 RNA vectors.
CV1 or BHK cells (1 × 105 to 2 × 105) in 2 ml of the appropriate growth medium supplemented with serum were plated in six-well culture plates. Transfections were performed with Lipofectin (Life Technologies) and 4 to 6 μg of DNA or RNA in accordance with the manufacturer's instructions.
Generation of stably transfected cells expressing MPT83.
To generate stably transfected cells expressing MPT83, P815, and J774, cells were transfected with Lipofectin (Life Technologies) as described previously (22), with linearized pCMV4.83. Cells were selected with Geneticin (G418, 1 mg/ml; Life Technologies) for 1 month. Clonal isolates of G418-selected cells were prepared by limiting dilution and were analyzed for MPT83 expression by reverse transcription (RT)-PCR and Western blotting.
In vitro transcription of pSinRep583 or pSinRep5GFP.
In vitro transcription reactions with the SP6 transcription kit (Invitrogen), in accordance with the manufacturer's instructions, were used to synthesize the recombinant RNA used for the transfection or vaccination experiments. The purified RNA was capped, DNase digested, phenol-chloroform extracted, and finally dissolved in RNase-free saline solution to a final concentration of 1 mg/ml and stored at −70°C until use.
RNA isolation and RT-PCR.
Total RNA from mouse tissue was isolated by using RNazol B solution (AMS Biotechnology, Abingdon, United Kingdom) in accordance with the manufacturer's instructions. Tissue samples were first homogenized in RNazol B (2 ml per 100 mg of tissue) with a few strokes in a glass-Teflon homogenizer. Cells were lysed by the addition of 0.1 ml of RNazol B per 106 cells. A DNase treatment was included to avoid DNA contamination; the reaction mix consisted of RNA samples in a solution of 60 μl of diethyl pyrocarbonate (Sigma) water, 3.75 μl of 0.1 M MgSO4, 7.5 μl of 1 M sodium acetate, 1 μl of RNase inhibitor (Promega, Southampton, United Kingdom), and 10 U of DNase I (Roche Diagnostic, East Sussex, United Kingdom). Diethyl pyrocarbonate water (1.75 μl) was incubated at 37°C for 15 min, followed by a phenol-chloroform extraction and RNA precipitation with ethanol. RT was carried out as described previously (24). The MPT83 gene was amplified using the following primers: the forward primer ATT GGA TCC GCC ATG ATC AAC GTT CAG and the reverse primer TAT GCG GCC GCC GAA CGT TAC TGT. The PCR program was 94°C for 45 s, 60°C for 45 s, and 72°C for 90 s for 30 cycles. The final extension temperature was 72°C for 7 min. The resulting PCR product was visualized by electrophoresis on 2% agarose gels.
Anti-MPT83 antibody levels.
The specific anti-MPT83 antibody levels were measured by enzyme-linked immunosorbent assay (ELISA), as described previously (24). Briefly, microplates (Maxisorp Nunc immunoplates; Nunc, Roskilde, United Kingdom) were adsorbed with 1 μg of recombinant MPT83 protein in 100 μl of carbonate-bicarbonate buffer (pH 9.6). Plates were incubated at 37°C for 1 h and then kept at 4°C overnight. They were then rinsed once with PBST (20 mM NaH2PO4 and 150 mM NaCl [pH 9.2] containing 0.05% Tween 20) and blocked with 5% powdered milk in PBST for 2 h at room temperature. The plates were then washed, and 100 μl of diluted sera from vaccinated mice was added and incubated for 1 h at room temperature. The plates were then extensively washed with PBST and incubated for 2 h at 37°C with 100 μl of anti-mouse immunoglobulin G conjugated to horseradish peroxidase (Dako, Cambridgeshire, United Kingdom). The plates were again washed with PBST and incubated with the substrate TMB (Life Technologies) for 30 min at room temperature, and the reaction was stopped with 20 μl of 4 M sulfuric acid. Absorbance was measured at 450 nm in an ELISA reader (Bio-Tek Instruments, Hertfordshire, United Kingdom).
Cytokine assays.
Pooled spleen cells or column (R&D Systems, Abingdon, United Kingdom)-purified T cells from three to five mice were obtained after four MPT83 DNA or MPT83 RNA injections. Cells were adjusted to a concentration of 4 × 106 cells/ml and were cultured with or without syngeneic irradiated spleen cells in round-bottom microwell plates (Nunc) by using RPMI medium supplemented with 2 mM glutamine, HEPES, 50 μM 2-mercaptoethanol, antibiotics (100 IU of penicillin/ml and 100 μg of streptomycin/ml), and 10% FCS. The cells were cultured with the recombinant MPT83 protein or with MPT83 peptides at a final concentration of 10 μg/ml, and supernatants were harvested after 24 h for interleukin-2 (IL-2) and after 72 h for gamma interferon (IFN-γ). Peptides were synthesized by solid-phase peptide synthesis. Supernatants from three separate wells were pooled and stored at −20°C until assayed. IL-2 and IFN-γ levels were detected by using mouse cytokine ELISA kits (Amersham International, Amersham, United Kingdom) in accordance with the manufacturer's instructions.
CTL assay.
To measure CTL activities, 2 weeks after completion of the immunization procedures, single-cell suspensions of spleen cells from vaccinated animals were obtained and depleted of erythrocytes in red blood cell lysis buffer (Life Technologies). Cells were plated in 24-well plates, with 1× 106 to 5 × 106 cells/ml in RPMI complete medium and with 1 × 105 to 5 × 105 J774-83 cells irradiated (120 Gy) in the presence of recombinant IL-2 (50 IU/ml; Roche Diagnostic) for 6 days. CD8+ T cells harvested from the stimulated cultures were tested for cytotoxic activity by JAM CTL assays as described previously (13). Cell-specific lysis was calculated by the following formula: percent specific lysis = (counts per minute for S − counts per minute for E/counts per minute for S) × 100, where E is retained DNA in the presence of killers and S is retained DNA in the absence of killers. Thymidine-labeled P815.83 cells were used as targets.
Infection and immunization procedures.
Female BALB/c mice from 6 to 8 weeks old were obtained from in-house animal facilities (National Institute for Medical Research, London, United Kingdom). For DNA and RNA vaccinations, mice were injected intramuscularly with 50 μg of plasmid DNA or RNA in 50 μl of saline into each quadriceps muscle on four occasions at 3-week intervals. As controls, mice received the corresponding constructs without inserts or plasmids expressing GFP or live BCG. Infection was induced by injecting 5 × 105 viable CFU of M. tuberculosis H37Rv into a lateral tail vein. Five weeks later, the bacterial loads in the lungs and spleens were evaluated. Briefly, the organs were weighed and homogenized in phosphate-buffered saline with a mini-bead beater (Biospec Products, Bathesville, Okla.). Serial 10-fold dilutions of the homogenates were plated on Middlebrook 7H11 Bacto agar (Difco Laboratories, Surrey, United Kingdom). Colonies were counted 3 to 4 weeks later, and results are expressed as numbers of CFU per gram of tissue.
Experiments were carried out in the United Kingdom according to the Home Office Animal Scientific Act of 1986.
RESULTS
MPT83 expression following MPT83 DNA or MPT83 RNA transfection or injection.
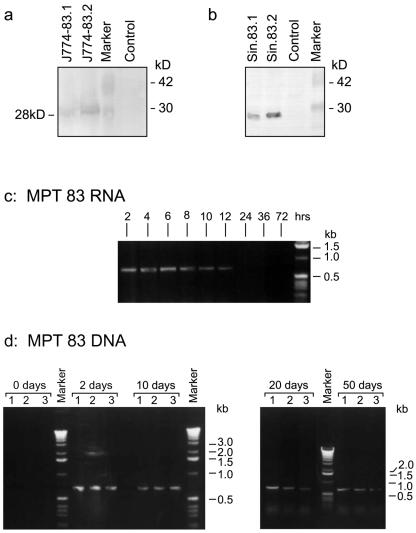
We initially confirmed the expression of MPT83 antigen by a Western blot analysis with specific monoclonal antibodies following DNA transfection of P815 or J774 cells (Fig. 1a) and following transfection of BHK cells with MPT83 RNA (Fig. 1b), synthesized using a Sindbis virus-derived expression vector system (23). We next compared the kinetics of MPT83 gene expression in vivo following intramuscular gene injection of mice by RT-PCR. We found that while specific MPT83 expression was detectable in DNA-immunized mice for at least 50 days following injection, immunization with RNA led to transient MPT83 gene expression, which was detectable for up to 12 h but was undetectable 24 h after injection (Fig. 1c and d).
FIG. 1.
MPT83 antigen expression following MPT83 DNA or MPT83 RNA transfection. Western blot analysis of MPT83 expression by J774 (a)- or BHK (b)-transfected cells. Whole-cell extracts were prepared from two independent J774 cell lines stably transfected with CMV4.83 or BHK cells transiently transfected with MPT83 RNA prepared according to the protocol described in Materials and Methods. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted onto nitrocellulose membranes, and incubated with the specific anti-MPT83 monoclonal antibody 12/6/1. Also shown are kinetics of MPT83 expression, in vivo, following intramuscular injection with MPT83 RNA (c) or with MPT83 DNA (d). Total RNA was purified from the injected muscles and reverse transcribed, and the cDNA was amplified by PCR using MPT83-specific primers. The plasmids used were pCMV4.83 (lanes 1), pCMV3.83 (lanes 2), and pMGD20.83 (lanes 3) (24). The MPT83 RNA was synthesized in vitro using the plasmid pSinRep5.83 as the DNA template and the SP6 transcription kit as described in Materials and Methods.
Immune responses induced after MPT83 DNA or RNA injection.
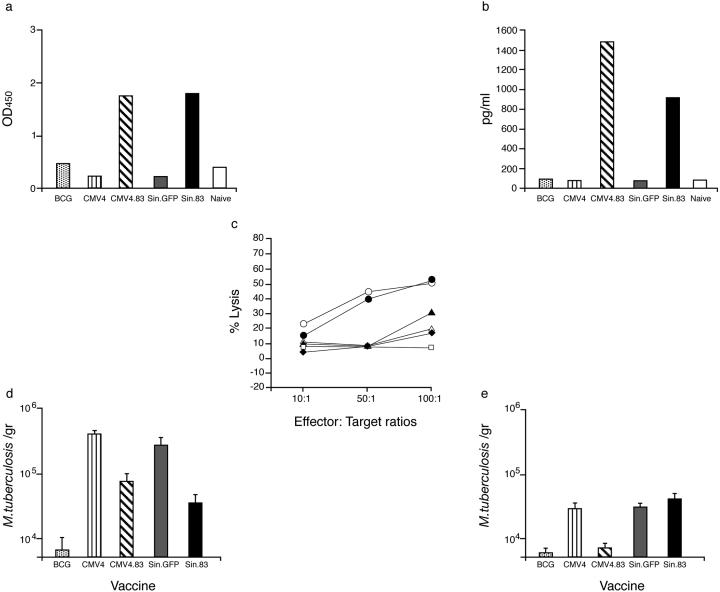
Consistent with the results of gene expression in vivo, antigen-specific immunoglobulin G antibody responses were detected after a series of intramuscular MPT83 DNA or MPT83 RNA injections (Fig. 2a). In addition, spleen T cells from either MPT83 DNA- or MPT83 RNA-injected mice specifically secreted IFN-γ in vitro in response to MPT83 protein stimulation (Fig. 2b). These responses, however, were not obtained after injection of DNA or RNA vectors that did not contain the mycobacterial MPT83 gene. We also tested the ability of immune CD8+ T cells to lyse MPT83-transfected cells in standard CTL assays. Cells from mice that were vaccinated with either MPT83 DNA or MPT83 RNA induced significant lysis of MPT83-transfected cells, whereas cells from mice that received control DNA or RNA vectors did not show any cytotoxic activity (Fig. 2c), demonstrating that immunization with MPT83 RNA or DNA resulted in specific priming of humoral and cellular immune responses.
FIG. 2.
Immune responses induced after MPT83 DNA or RNA injection. Groups of three to five BALB/c mice received MPT83 DNA (CMV4.83) or MPT83 RNA (Sin.83) intramuscularly on four occasions at 3- to 4-week intervals or were immunized similarly with a DNA control (CMV4) or RNA control (Sin.GFP). Humoral and cellular responses were determined 4 weeks after four DNA or RNA injections. A group of mice received one injection of BCG vaccine intradermally, and an additional group of mice (Naive) was not vaccinated. (a) Antibody levels from pooled sera (diluted 1:1,000) from three to five immunized mice tested against the recombinant MPT83 antigen. The mean absorbance readings of triplicate determinations are shown. Standard deviations were less than 20%. OD450, optical density at 450 nm. (b) IFN-γ produced by immune T cells from vaccinated mice in response to MPT83 antigen stimulation. (c) Induction of MPT83-specific CTL activity after MPT83 DNA or MPT83 RNA injection. Specific CTL activities were determined in triplicate against P815.83 cells as described in Materials and Methods. No significant differences from values for P815 control cells were observed. Also shown are percentages of lysis of effector cells from MPT83 DNA (•), MPT 83 RNA (○), a control RNA vector (⧫), a control DNA vector (⋄), and BCG-vaccinated (▴) or Naive (□) mice. (d and e) Protection against M. tuberculosis infection by immunization with MPT83 DNA or MPT83 RNA. Groups of five BALB/c mice were injected with MPT83 DNA (CMV4.83) or MPT83 RNA (Sin.83) as described in Materials and Methods and 4 weeks (d) or 6 months (e) later were infected with virulent M. tuberculosis H37Rv. Controls were BCG-CMV4 (DNA)-, or Sin.GFP (RNA)-vaccinated mice; 6 weeks later, the number of live bacteria (mean ± standard deviation) in the lungs was determined. Results shown are from one representative experiment of two or three separate experiments.
Protection against M. tuberculosis infection by immunization with MPT83 DNA or MPT83 RNA.
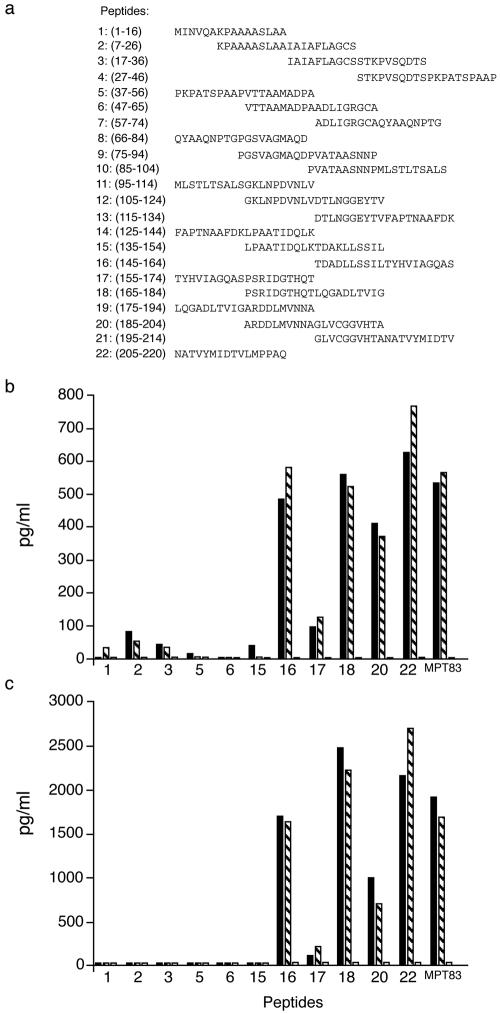
We then compared the abilities of MPT83 RNA and DNA injections to confer protection against tuberculosis after challenging vaccinated animals with M. tuberculosis H37Rv, 4 weeks after the last polynucleotide injection. We demonstrated that MPT83 DNA or MPT83 RNA vaccination induced a modest but significant protective effect in mice after challenge infection (Fig. 2d) (P < 0.05, Student's t test). However, while both MPT83 DNA and MPT83 RNA induced significant short-term protective responses, only MPT83 DNA-vaccinated mice exhibited significant protection when they were tested 6 months after vaccination (Fig. 2e) (P < 0.05, Student's t test). CMV4 and Sin.GFP vector control-vaccinated mice showed no significant protection when they were compared to nonvaccinated mice (data not shown). In addition, we analyzed in greater detail antigen-specific immune responses from MPT83 DNA- or MPT83 RNA-vaccinated mice by measuring IL-2 and IFN-γ responses from immune T cells, restimulated in vitro with synthetic 20-mer peptides spanning the complete sequence of the mature MPT83 mycobacterial antigen (Fig. 3a). We observed that the patterns of reactivity were consistently very similar, with significant levels of IL-2 (Fig. 3b) and IFN-γ (Fig. 3c) being produced by immune T cells in response to peptides spanning the C-terminal region of the molecule.
FIG. 3.
Specific T-cell responses induced after MPT83 DNA or MPT83 RNA injection. (a) A series of overlapping peptides spanning the MPT83 sequence was used to characterize the fine T-cell reactivity of MPT83 RNA- or MPT83 DNA-vaccinated mice. (b and c) IL-2 (b) and IFN-γ (c) production by immune T-cell cultures from MPT83 RNA (black bars)- or MPT83 DNA (hatched bars)- vaccinated mice, in response to 10 μg of each peptide/ml. Results shown are for peptides that demonstrated some reactivity. Cytokine responses were determined 2 weeks after the last MPT83 DNA (CMV4.83) or MPT83 RNA (Sin.83) injection. Results shown are from one representative experiment of two separate experiments. ELISA readings were assayed in duplicate, with standard deviations being <20%.
DISCUSSION
Recent evidence indicates that several mycobacterial antigens can generate significant levels of protective immunity when they are administered with adjuvants to guinea pigs (1, 16) or when they are given as DNA vaccines to mice (7, 8, 10, 22, 25). In this study, we demonstrated that DNA or RNA gene constructs expressing MPT83 were able to induce protective immunity against virulent M. tuberculosis challenge in mice; it should be stressed, however, that the protective response obtained was modest compared to those of other mycobacterial antigens and was not better than that of the BCG vaccine (7, 8, 22, 25). In addition, an interesting aspect of our study is that while both MPT83 DNA and MPT83 RNA induced significant short-term protective responses, only MPT83 DNA-vaccinated mice exhibited significant protection when they were tested 6 months after vaccination. It is tempting to speculate that the longer persistence of antigen expression in MPT83 DNA-vaccinated mice, 40 to 50 days after DNA injection in contrast to 10 to 12 h in the case of MPT83 RNA, is necessary for the maintenance of protection in our model. This notion is consistent with the work of Ochsenbein et al. (15), who demonstrated, using lymphocytic choriomeningitis virus infection in mice, that while the frequency of memory CD8+ T cells was largely independent of antigen persistence, the protective ability of these cells was correlated with in vivo persistence of the antigen.
It is clear, however, from our results that MPT83 DNA- and MPT83 RNA-vaccinated mice generated antigen-specific, cell-mediated, and humoral immune responses. Similar antibody levels were generated, and the IL-2 and IFN-γ responses secreted by immune T cells in response to recombinant antigen stimulation were also comparable when the mice were tested 4 weeks after three or four polynucleotide injections. We concluded, therefore, that RNA vaccination achieved priming of specific immune responses; the transient nature of the expression seen with RNA immunization is likely to minimize many of the safety issues which have been raised for DNA vaccination. However, immunization with RNA appears to result in short-lasting protective immunity. This approach, therefore, combined with efficient strategies specific for in vivo boosting of the immune responses using different vectors (18, 20), could be an important tool for the development of safer and more-effective vaccines against tuberculosis.
Acknowledgments
We are grateful to the World Health Organization for giving a studentship to Tian Xue.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A. Frank, M. A. Lui, J. B. Ulmer, K. Huygen, D. M. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boczkowski, D., S. K. Nair, J. K. Nam, H. K. Lyerly, and E. Gilboa. 2000. Induction of tumor immunity and cytotoxic T lymphocytes responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 60:1028-1034. [PubMed] [Google Scholar]
- 3.Chambers, M. A., H.-M. Vordermeier, A. Whelan, N. Commander, R. Tascon, D. Lowrie, and R. G. Hewinson. 2000. Vaccination of mice and cattle with plasmid DNA encoding the Mycobacterium bovis antigen MPB83. Clin. Infect. Dis. 30:283-287. [DOI] [PubMed] [Google Scholar]
- 4.Chan, J., and S. H. E. Kaufmann. 1994. Immune mechanisms of protection, p. 389-415. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, D.C.
- 5.Conry, R. M., A. F. LoBuglio, M. Wright, L. Sumerel, M. J. Pike, F. Johanning, R. Benjamin, D. Lu, and D. T. Curiel. 1995. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 55:1397-1400. [PubMed] [Google Scholar]
- 6.Donnelly, J., K. Berry, and J. B. Ulmer. 2003. Technical and regulatory hurdles for DNA vaccines. Int. J. Parasitol. 33:457-467. [DOI] [PubMed] [Google Scholar]
- 7.Huygen, K. 2003. On the use of DNA vaccines for the prophylaxis of mycobacterial diseases. Infect. Immun. 71:1613-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J. P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 9.Leitner, W. W., H. Ying, and N. P. Restifo. 1999. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine 18:765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowrie, D. B., C. L. Silva, M. J. Colston, S. Ragno, and R. Tascon. 1997. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine 15:45-49. [DOI] [PubMed] [Google Scholar]
- 11.Mandl, C. W., J. H. Aberle, H. Holzmann, S. L. Allison, and F. H. Heinz. 1998. In vitro-synthesized infectious RNA as an attenuated live vaccine in a flavivirus model. Nat. Med. 4:1438-1440. [DOI] [PubMed] [Google Scholar]
- 12.Martinon, F., S. Krishnan, G. Lenzen, R. Magne, E. Gomardi, J. G. Guillet, J. P. Levy, and P. Meulien. 1993. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 23:1719-1722. [DOI] [PubMed] [Google Scholar]
- 13.Matzinger, P. 1991. The JAM test. J. Immunol. Methods 145:185-192. [DOI] [PubMed] [Google Scholar]
- 14.McKinney, J. D., W. R. Jacobs, Jr., and B. Bloom. 1998. Persisting problems in tuberculosis, p. 51-146. In R. M. Krause (ed.), Emerging infections. Academic Press, San Diego, Calif.
- 15.Ochsenbein, A. F., U. Karrer, P. Klenerman, A. Althage, A. Ciurea, H. Shen, J. F. Miller, J. L. Whitton, H. Hengartner, and R. M. Zinkernagel. 1999. A comparison of T cell memory against the same antigen induced by virus versus intracellular bacteria. Proc. Natl. Acad. Sci. USA 96:9293-9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pal, P. G., and M. A. Horwitz. 1992. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect. Immun. 60:4781-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu, P., P. Ziegelhoffer, J. Sun, and N. S. Yang. 1996. Gene gun delivery of mRNA in situ results in efficient transgene expression and genetic immunisation. Gene Ther. 3:262-268. [PubMed] [Google Scholar]
- 18.Ramshaw, I. A., and A. J. Ramsay. 2000. The prime-boost strategy: exciting prospects for improved vaccination. Immunol. Today 21:163-165. [DOI] [PubMed] [Google Scholar]
- 19.Raviglione, M. C. 2003. The TB epidemic from 1992 to 2002. Tuberculosis 83:4-14. [DOI] [PubMed] [Google Scholar]
- 20.Skinner, M. A., B. M. Buddle, D. N. Wedlock, D. Keen, G. W. de Lisle, R. E. Tascon, J. C. Ferraz, D. B. Lowrie, P. J. Cockle, H. M. Vordermeier, and R. G. Hewinson. 2003. A DNA prime-Mycobacterium bovis BCG boost vaccination strategy for cattle induces protection against bovine tuberculosis. Infect. Immun. 71:4901-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tascon, R. E., M. J. Colston, E. Stavropoulos, S. Ragno, D. Gregory, and D. B. Lowrie. 1997. Protection against tuberculosis by plasmid DNA, p. 181-185. In G. Gregoriadis, B. McCormack, and A. C. Allison (ed.), Vaccine design: the role of cytokine networks. Plenum Press, New York, N.Y.
- 22.Tascon, R. E., M. J. Colston, S. Ragno, E. Stavropoulos, D. Gregory, and D. B. Lowrie. 1996. Vaccination against tuberculosis by DNA injection. Nat. Med. 2:888-892. [DOI] [PubMed] [Google Scholar]
- 23.Xiong, C., R. Levis, P. Shen, S. Schlesinger, C. M. Rice, and H. V. Huang. 1989. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science 243:1188-1191. [DOI] [PubMed] [Google Scholar]
- 24.Xue, T. 1999. Investigation of antigen MPT83 for genetic vaccination against Mycobacterium tuberculosis. Ph.D. thesis. University of London, London, United Kingdom.
- 25.Zhu, X., N. Venkataprasad, H. S. Thangaraj, M. Hill, M. Singh, J. Ivanyi, and H. M. Vordermeier. 1997. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. DNA. J. Immunol. 158:5921-5926. [PubMed] [Google Scholar]