Abstract
Understanding the basis of protective immunity is a key requirement for the development of an effective vaccine against infection with Neisseria meningitidis of serogroup B. We have conducted a longitudinal study into the dynamics of meningococcal acquisition and carriage in first-year university students. The detection of carriage of serogroup B meningococci correlated with an increase in detection of serum bactericidal activity (SBA) against both colonizing and heterologous serogroup B strains. Once induced, SBA remained high throughout the study. Although students showed increases in antibodies reactive with capsular polysaccharide and lipopolysaccharide (LPS), these antibody responses were transitory, and their decline was not accompanied by a corresponding decline in SBA. In contrast, there was a significant correlation between the presence of antibodies to the PorA outer membrane protein and SBA against both homologous and heterologous strains. SBA induced by a PorA-negative mutant confirmed the contribution of PorA to heterologous activity. Increases in SBA against a range of serogroup B strains were also observed in students in whom no meningococcal carriage was detected. This heterologous protection could not be associated with the presence of antibodies reacting with capsule, LPS, PorA, PorB, Rmp, Opa, Opc, or pilin, demonstrating that other, as yet unidentified, antigens contribute to the development of immunity to serogroup B meningococci. Identification of such antigens with the ability to induce an effective cross-reactive bactericidal response to a range of strains would be a major step in the production of a universally effective vaccine against infections caused by serogroup B meningococci.
Infection with Neisseria meningitidis (meningococcus) is an important cause of meningitis and septicemia worldwide. Meningococcal infections are of special concern because of their propensity to cause rapidly deteriorating and potentially fatal disease, particularly in children and young adults (19, 24). Humans are the only natural host for meningococci, and healthy carriers are of primary importance in disease transmission (11). During epidemics, contacts of infected individuals tend to carry the epidemic strains (18), and the carriage rate is much higher in close contacts such as family members (10) or among individuals within institutions (24). Nasopharyngeal carriage in closed or semiclosed institutions such as universities may rise to >50% (4), which results in high meningococcal transmission rates (32). The risks of transmission and contracting meningococcal disease are, therefore, increased when many young adults, a group with a high nasopharyngeal meningococcal carriage rate (32), are brought together within the close confines of a university. Students are most at risk in their first year within the university environment when they are likely to be exposed to meningococcal strains not previously encountered (1, 7, 16).
Meningococcal strains are differentiated into serogroups based on the structure of the capsular polysaccharide. In most temperate countries, serogroup B has been the predominant serotype causing disease, followed in frequency by serogroup C. Until recently, our understanding of the relationship between meningococcal carriage and immunity was based largely on the classic studies of Goldschneider and colleagues, who followed an epidemic of serogroup C infection in a military training camp during 1967 and 1968 (8, 9). They found a high prevalence of carriage of the outbreak strain together with high levels of serum bactericidal activity (SBA). They also correlated high levels of SBA with immunity to meningococcal infection and demonstrated that this was due to the presence of antibodies directed against the serogroup C capsular polysaccharide. In contrast, recent studies have reported much lower levels of carriage during outbreaks in universities and other institutions (7, 33). Following a recent serogroup C outbreak at a university in the United Kingdom, we analyzed serum samples taken just before the outbreak and demonstrated only low levels of SBA against serogroup C meningococci (16). The immunization of students with the MenC polysaccharide conjugate vaccine was subsequently introduced into the United Kingdom immunization program, and the number of cases of serogroup C infection has since declined dramatically (2, 26).
A previous study into an outbreak of serogroup C meningococcal disease within a university provided a unique opportunity to investigate immunity to infection in a student population, before and during an outbreak (37). However, given the lack of an effective vaccine against serogroup B strains, there is a continuing need to understand the basis of protective immunity to meningococcal infection. At the time of the outbreak, in contrast to serogroup C, the presence of SBA against serogroup B meningococci in the population was more common and did not correlate with the presence of antibodies directed against capsular polysaccharide but to antibodies directed against the PorA outer membrane protein (37). However, this study was carried out on sera taken at a single time point, 1 month following the outbreak, and could not, therefore, assess the temporal relationship between carriage and development of an immune response. We have, therefore, undertaken a longitudinal study in a new cohort of students during their first year at the same university in order to study the dynamics of meningococcal acquisition and carriage and their influence on the development of both strain-specific and cross-protective immunity to serogroup B meningococcal infection.
MATERIALS AND METHODS
Human volunteers.
This study followed the human experimentation guidelines of the authors' institutions, and informed consent was obtained from participants. Volunteers were sought from first-year undergraduate students living in a single university hall of residence. All had received the meningococcal nonconjugate polysaccharide A/C vaccine prior to entering university at the beginning of the 1999-to-2000 academic year. Samples, which were collected from volunteers 2 weeks after entry to the university (week 0) and on three further occasions during the academic year (weeks 3, 18, and 31), comprised blood, throat swabs, and mouth gargles with phosphate-buffered saline (17).
Detection of meningococcal carriage.
The presence of meningococci on throat swabs and in concentrated gargle samples was determined by culture and modified ctrA TaqMan PCR, essentially as described previously (17). For culture, samples were inoculated onto modified New York City Agar (Wessex Media Services, Dorchester, United Kingdom), and the plates were incubated at 37°C for 36 to 48 h in 5% (vol/vol) CO2. Meningococcal DNA was detected by the modified ctrA TaqMan assay developed by the Meningococcal Reference Unit, Manchester, United Kingdom; the assay detects serogroups A, B, C, X, Y, Z, W135, 29E, and some nonserogroupable (NG) strains (5) and has been shown to improve carriage detection over culture alone (17). Reactions contained 1× TaqMan Universal Mix, a 0.3 μM concentration of each primer, 0.2 μM probe (all supplied by Applied Biosystems), and 5 μl of target DNA in a total volume of 25 μl. Dilutions (10-fold) of bacterial DNA covering the range 103 to 107 copies/ml were included with these ctrA assays. PCR amplification conditions consisted of 2 min at 50°C and 10 min at 95°C, followed by 50 cycles of 15 s at 95°C and 1 min at 60°C on a sequence detector system 7700 (Applied Biosystems). Positive controls (containing DNA extracted from serogroup B N. meningitidis and serogroup C N. meningitidis) and negative controls (water only) were included in every experiment. All NG meningococcal cultures or ctrA-positive samples were further characterized by TaqMan siaD PCR for serogroup B- and serogroup C-associated DNA (13, 17).
Typing of N. meningitidis.
Isolates were characterized by serogroup, serotype, and subtype antigens by the Meningococcal Reference Unit (Manchester Public Health Laboratory). The lipopolysaccharide (LPS) immunotype was determined for the serogroup B strains by the Laboratory for Vaccine Research, RIVM, Bilthoven, The Netherlands (31). Further characterization of subtype specificity was obtained by DNA sequence determination of PorA variable regions VR1 and VR2 (3) and comparison with the PorA database (http://neisseria.org/nm/typing/pora/).
ELISA for detection of antibodies to capsular polysaccharide and LPS.
Antibodies directed against the serogroup B capsule were measured as total immunoglobulins by enzyme-linked immunosorbent assay (ELISA) with capsular polysaccharide from an O-acetyl-negative mutant of Escherichia coli K1 (a generous gift from H. Jennings, Ottawa, Canada), which is structurally and immunologically identical to serogroup B polysaccharide, by using goat anti-human immunoglobulins (37). Antibody concentrations were calculated by reference to a positive control serum taken from an individual with high levels of antibody directed against serogroup B polysaccharide, which was assigned a concentration of 100 arbitrary units. Negative controls contained all reagents except serum.
Antibody responses to meningococcal LPS were detected by ELISA (25) with LPS purified from strains MC171 (B:1:P1.19-1,15-11:L1) (this study) and MC58 (B:15:P1.7,16-2:L3) (21) as described previously (37). Concentrations of antibody directed against LPS were calculated by reference to a positive control serum, taken from an individual with high levels of antibody directed against LPS, which was assigned a concentration of 100 arbitrary units.
SDS-PAGE and Western blotting for detection of antibodies to meningococcal outer membranes.
The specificity of the immune response was further investigated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting (37). Control antibodies were used to identify the position of known protein antigens, and the intensity of antibody reactivity was scored semiquantitatively on a scale of 0 to 5 as described elsewhere (37).
SBA directed against N. meningitidis.
The complement-mediated SBA assay was carried out with the isolates described in Table 1 and with strains H44/76 and CE2001, a PorA-negative mutant of strain H44/76 kindly provided by J. Poolman (RIVM). SBA assays were performed essentially as described previously (16) by using human serum at a concentration of 5% (vol/vol) as the exogenous complement source for serogroup B strains (38). Serum bactericidal titers were expressed as the final serum dilution that resulted in a ≥50% reduction in surviving CFU compared with an equivalent negative control containing heat-inactivated complement.
TABLE 1.
Characteristics of carriage strains isolated in this studya
| Serogroup | Serotype | Subtype | LPS type | Phenotype designation | Strain designation | Sourceb
|
||
|---|---|---|---|---|---|---|---|---|
| Student | Week | Site | ||||||
| B | 4 | 5,2 | L3 | A | MC168 | 37 | 3 | T |
| B | NT | 7-2,4 | L3 | B | MC169 | 30 | 3 | T |
| B | 4 | 19,15 | L3 | C | MC170 | 9 | 18 | T |
| B | 1 | 19-1,15-11 | L1 | D | MC171 | 28 | 0 | G |
| B | 1 | 22,14 | L3 | E | MC172 | 15 | 31 | T |
| C | 2b | 5,2 | L3 | F | MC173 | 12 | 3 | G |
| 29E | 4 | 5-1,10-8 | ND | G | MC174 | 5 | 0 | T |
| 29E | NT | 18,25 | ND | H | MC175 | 17 | 18 | T |
| 29E | 14 | 5-1,2-2 | ND | I | MC176 | 39 | 3 | G |
| NG | NT | 17,16-24 | ND | J | MC177 | 33 | 3 | T |
| NG | NT | 5-2,10-25 | ND | K | MC178 | 29 | 3 | T |
Isolates were characterized by serogroup, serotype, and subtype antigens by standard methods at the Meningococcal Reference Unit (Manchester Public Health Laboratory). DNA sequencing of the variable subtype-specific encoding regions of the PorA protein (3) at our laboratory further characterized these strains. ND, not determined; NG, nonserogroupable; NT, nonserotypeable.
The source of the selected isolate (MC numbers) of each strain phenotype is indicated by the volunteer number followed by time point (weeks 0, 3, 18 or 31) and specimen type (T, throat swab; G, gargle specimen).
Statistical analysis.
For analysis of the correlation between the potential protective effect and presence of specific antibodies, sera were assigned to groups with SBA levels of <1/8 or ≥1/8 (37). The Mann-Whitney U test was used to assess differences between data groups by comparing mean values; probability values of < 0.05 were considered statistically significant.
RESULTS
Carriage of meningococci.
Samples were obtained from a total of 42 students on four occasions during the academic year (weeks 0, 3, 18, and 31). Carriage was determined by culture and PCR of both throat swabs and gargles. Carriers were defined as students detected as positive for meningococci by any one test in any sample and noncarriers were defined as students who tested negative for meningococci by all tests on all samples taken at the four time points.
Meningococcal carriage was detected in 18 students (43%) at some point during the 31-week study period; 10 students acquired meningococci during the course of the study. Twelve students were culture positive on at least one occasion. Characterization of the isolates revealed a total of 11 distinct strains (Table 1) with only two phenotypes (B:4:P1.5,2 and B:NT:P1.7-2,4) detected in more than one student, and each of these phenotypes (designated A and B, respectively) was detected in two students (Table 2). The cumulative carriage rates for the respective meningococcal serogroups were as follows: B, 14% (n = 6); C, 5% (n = 2); 29E, 7% (n = 3); and NG, 17% (n = 7). SiaD PCR was performed on all NG strains to identify meningococci not currently expressing serogroup B capsule but with the genetic potential to do so. All such PCR results were negative.
TABLE 2.
Longitudinal nasopharyngeal carriage of Neisseria meningitidis in students
| Student no. | Meningococcal colonization detected at sampling time pointa
|
|||
|---|---|---|---|---|
| Week 0 | Week 3 | Week 18 | Week 31 | |
| 5 | G | G | Did not attend | Did not attend |
| 9 | − | Did not attend | C | − |
| 10 | + | − | − | − |
| 11 | − | − | + | − |
| 12 | +b | F | − | − |
| 15 | − | − | B | B, E |
| 17 | − | − | H | H |
| 19 | − | + | Did not attend | Did not attend |
| 20 | + | − | − | − |
| 26 | +b | − | − | − |
| 28 | D | − | Did not attend | Did not attend |
| 29 | − | K | K | K |
| 30 | +c | B | B | B |
| 33 | J | J | J | Did not attend |
| 37 | − | A | A | A |
| 38 | − | A | A | − |
| 39 | − | I | I | Did not attend |
| 100 | − | − | + | − |
Nasopharyngeal colonization of meningococci in the student population was determined by culture and PCR of throat swabs and gargle specimens; nine carriers were detected by both culture and PCR, six were detected only by PCR, and three were detected only by culture. The characteristics of the carried strains (A to K) refer to the phenotypes designated in Table 1. +, positive PCR test result but culture negative; −, carriage not detected.
siaD PCR-detected group C-associated DNA.
siaD PCR-detected group B-associated DNA.
Two of the students carrying serogroup B strains remained colonized for at least 28 weeks (volunteers 30 and 37), and one student (volunteer 15) was colonized by a second serogroup B strain (as defined by differences in serotype and subtype) while continuing to carry the first. The carrier of strain MC171 (student 28) received a course of antibiotics as a contact of a sporadic case of meningococcal disease early on in the study and so was excluded from further analysis. One isolate from each of the distinct phenotypes of serogroup B meningococci was selected for further studies (Table 1).
SBA against serogroup B strains.
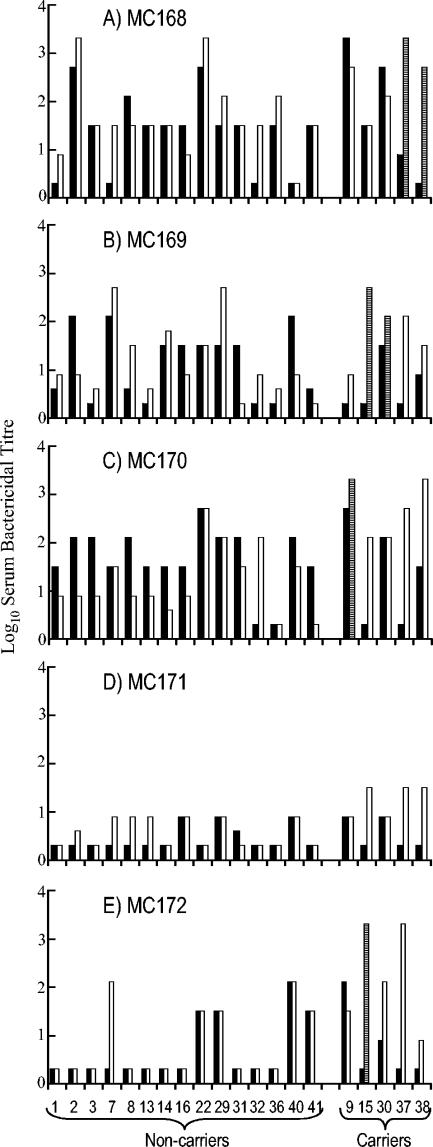
The levels of immunity in the student population to the circulating serogroup B strains were determined at the beginning (week 0) and end points of the 31-week study. Figure 1 shows the SBA against the serogroup B meningococcal strains for the five serogroup B carriers and for the 15 students that attended every session but had no detectable meningococcal carriage. The levels of SBA to the different strains varied considerably, both at the beginning and end of the study. At the beginning of the study, 50 to 80% of students had bactericidal activity (titer of ≥1/8) against strains MC168 (B:4:P1.5,2:L3), MC169 (B:NT:P1.7-2,4:L3), and MC170 (B:4:P1.19,15:L3), but only 30% had bactericidal activity against strains MC171 (B:1:P1.19-1,15-11:L1) and MC172 (B:1:P1.22,14:L3). By the end of the study, these percentages had increased to 75 to 95 and 50%, respectively.
FIG. 1.
SBA of students against each serogroup B meningococcal carriage strain. Shown are the SBA titers of students (as numbered on the x axis of panel E) against each serogroup B strain detected in this study. Filled bars indicate the SBA titer at the start of the study (week 0), and open bars show bactericidal antibody levels at the end (week 31) of the study. Noncarriers and carriers are grouped by parentheses (as shown in panel E) on the x axis. Striped bars indicate bactericidal activity of carriers at week 31 against their homologous colonizing strain(s).
There were distinct differences in the ability of sera from the noncarriers to kill the different serogroup B strains (Fig. 1). For example, volunteer 32 was the only individual who showed no SBA against any of the serogroup B carriage strains at week 0. By week 31, this individual had developed bactericidal activity against strains MC170, MC168, and MC169. Volunteer 36 demonstrated SBA at week 0 only to strain MC168; by week 31, this student had developed some activity against strain MC169 but not to the other three serogroup B carriage strains. In contrast, student 29 demonstrated SBA against all of the serogroup B carriage strains throughout the study.
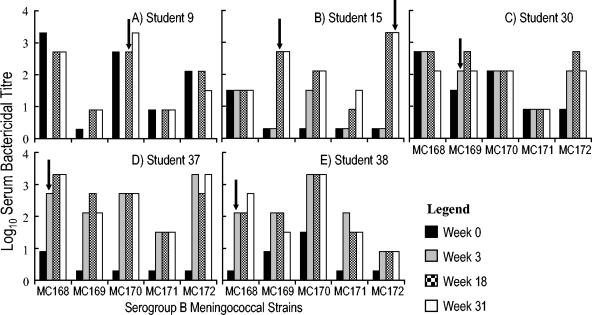
In order to study the effect of carriage on immunity, all sera taken at any session from serogroup carriers were tested for SBA against the serogroup B strains. Student 30, who was colonized at the beginning of the study, was found to have bactericidal activity throughout the study to the entire panel of serogroup B strains (Fig. 2). Sera from the other carriers revealed increases in SBA both to their homologous and heterologous strains during the course of the study. Subjects 9, 15, 37, and 38 became colonized by serogroup B meningococci during the course of the study. Two of these students (15 and 38) had nonprotective levels of bactericidal antibody (titer of <1/8) to their homologous strain in sera taken prior to colonization, with a significant rise in bactericidal activity detected in sera taken at the time point that colonization was first detected (Fig. 2B and E). In each case the rise in titer against the homologous strain was accompanied by an increase in titer against the other serogroup B strains. Thus, at week 0, student 38 had preexisting bactericidal activity to strains MC169 and MC170; colonization with strain MC168 was detected at week 3, and at the same time increases in SBA were detected against the homologous and all four heterologous strains. Similarly, student 15 had preexisting bactericidal activity only against strain MC168 at week 0, became colonized with strain MC169 at week 18, and demonstrated activity not only against the homologous strain but also against the other carriage strains, to which he had previously lacked activity. These included a second serogroup B isolate from the student, strain MC172, which was only detected at week 31. Student 37 had low but potentially protective antibodies (titer of 1/8) only against strain MC168. Colonization with MC168 at week 3 was accompanied by an increase in bactericidal activity, not only to the carriage strain but also to the four heterologous strains. In contrast to the other colonized students, volunteer 9 showed high levels of preexisting SBA to the colonizing strain (MC170) and lacked bactericidal activity against only one strain (MC169) at week 0. Following colonization with MC170, significant activity against strain MC169 also developed. Thus, despite the different patterns of SBA before and after carriage, all carriers had developed bactericidal activity to all serogroup B carriage strains by the end of the study.
FIG. 2.
SBA of serogroup B carriers against all serogroup B meningococcal carriage strains. The SBA titers of individual students colonized by serogroup B meningococci are shown. The MC numbers (MC168 to MC172) are the designations of the serogroup B carriage strains detected in the present survey (Table 2). The arrows indicate the time point(s) at which serogroup B colonization was first detected. The four time points are shown in chronological order as indicated on the figure. Student 9 did not attend the second session; therefore, there is no data for this individual at that time point.
Immune response to serogroup B capsular polysaccharide.
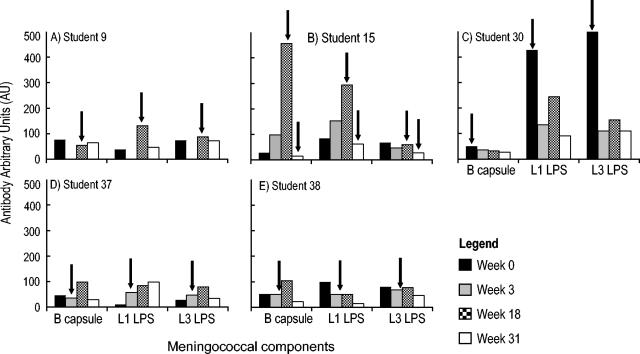
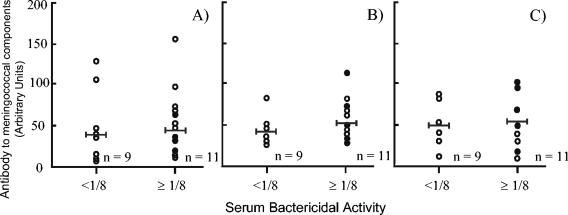
The sera from serogroup B carriers and noncarriers were analyzed by ELISA for the presence of antibodies directed against the serogroup B capsular polysaccharide (Fig. 3). Student 30, who was colonized at the beginning of the study, showed low levels of capsular polysaccharide antibody that declined during the study. Student 15 showed a large but transitory rise in serogroup B antibodies at the time of colonization. Students 37 and 38 showed similar, but lower, transitory rises in antibodies. In each case the decline in anticapsular antibodies was not accompanied by a concomitant decline in SBA against any of the serogroup B strains. The potential bactericidal effect of antibodies directed against capsular polysaccharide was further analyzed by a comparison between the antibody concentration and bactericidal activity in samples taken at week 31 (Fig. 4A). There was no significant difference (P > 0.05) between the mean serogroup B capsular antibody concentration for the students with SBA of <1/8 and those with SBA of ≥1/8.
FIG. 3.
Serum antibodies directed against specific meningococcal components in serogroup B carriers. Antibodies directed against the serogroup B capsular polysaccharide (B cap) and LPS immunotypes L1 and L3 were determined by ELISA, and results are shown for individual students. The data are presented as antibody concentration levels obtained at each sampling time point as detailed in the legend of Fig. 2, with arrows indicating the time point(s) at which serogroup B colonization was first detected.
FIG. 4.
Antibody directed against meningococcal components in relation to SBA. Serum samples of carriers and noncarriers were reacted in ELISAs with serogroup B capsular polysaccharide (A), LPS immunotype L3 (B), and LPS immunotype L1 (C). Antibody levels are shown in relation to SBA against the serogroup B carriage strain MC171 at the same time point (week 31), and the bar indicates the geometric mean of the data collected. Similar results were obtained for the other strains. The immune data of serogroup B carriers are indicated by filled circles.
Immune response to LPS.
In the present study, four of the five serogroup B strains possessed the LPS immunotype L3, whereas only strain MC171 was characterized as L1. The students' serum samples were tested for the presence of antibodies directed against both LPS immunotypes by using ELISA. All sera demonstrated the presence of antibodies that reacted against both LPS immunotypes. As with antibodies directed against the capsular polysaccharide, some students showed increases in antibody levels following colonization, but these increases were transitory (Fig. 3B and C) and not accompanied by a decline in SBA. Similarly, there was no significant difference between the mean anti-LPS antibody concentration for the students with SBA of <1/8 and those with SBA of ≥1/8 (Fig. 4B and C).
SDS-PAGE and Western blotting for detection of antibodies to outer membranes of serogroup B meningococci.
The sera from serogroup B carriers were subjected to SDS-PAGE and Western blotting against both the homologous and heterologous carriage strains. In general, colonization and the development of bactericidal activity were accompanied by the presence of antibodies directed against the homologous PorA protein. Analysis of all serum samples taken from carriers revealed that there was a significant correlation (P < 0.01) between SBA and the presence of antibodies to the homologous PorA protein. No significant correlation (P > 0.05) could be detected between SBA and the presence of the other protein antigens, PorB, Opc, Opa, Rmp, and pilin. In addition, there was also a significant correlation (P < 0.01) between the bactericidal activity of sera from carriers and the presence of antibodies to the heterologous PorA proteins.
By the end of the survey an increasing percentage of noncarriers had bactericidal activity against the serogroup B carriage strains, which varied from 33% for strain MC172 up to 93% for strain MC168. However, there was no association between SBA and the presence of antibodies directed against PorA or any of the other protein antigens tested (P > 0.05).
Bactericidal activity of sera against PorA-negative mutant.
In order to confirm the contribution of antibodies directed against PorA to the SBA of carrier sera against heterologous strains, serum samples from the five serogroup B carriers were tested against meningococcal strain H44/76 (B:15:P1.7,16) and a corresponding mutant lacking PorA (Table 3). Two of the five serum samples showed a significant decrease in titer against the PorA-negative mutant compared with wild type; a third serum sample showed an equivocal result, while the two remaining samples showed no significant differences.
TABLE 3.
Bactericidal activity of carrier sera against a meningococcal PorA-negative straina
| Student no. | Titer against strain H44/76
|
|
|---|---|---|
| PorA+ | PorA− | |
| 9 | 32,000 | 8,000 |
| 15 | 8,000 | 8,000 |
| 30 | 5,600 | 4,000 |
| 37 | 132,000 | 32,000 |
| 38 | 8,000 | 8,000 |
Sera from serogroup B carriers taken at the end of the study (week 31) were tested for bactericidal activity against strain H44/76 (B:15:P1:7,16) and the corresponding PorA-negative mutant. Titres are expressed as the final serum dilution that resulted in a ≥50% reduction in surviving CFU and are the mean of two independent experiments.
DISCUSSION
Despite the importance of the carrier state in the natural history of meningococcal disease, there is limited information available on the temporal relationship between the acquisition of carriage and the development of immunity to infection. The classic studies of Goldschneider and colleagues in the 1960s revealed the importance of antibodies directed against capsular polysaccharide in the development of immunity against serogroup C infection in military recruits (8, 9). More recently, colonization of military recruits with serogroups X and W135 has been shown to be accompanied by an increase in bactericidal activity against the colonizing strain, which was associated with presence of antibodies to the PorA outer membrane protein (15). Given the lack of an effective vaccine, the effect of carriage on the development of immunity to serogroup B infection is of particular importance. One study of long-term carriage in students revealed that carriage of serogroup B strains may persist for many months (1) and that carriage was associated with an increase in serum immunoglobulin G reactive with the colonizing strain, but this study did not examine responses to individual antigens (27). Previous studies on immunity and carriage in a university population were carried out at a single time point and showed an association between bactericidal activity to serogroup B and the presence of antibodies to the PorA outer membrane protein (37). In the present study we were able to take advantage of a heightened awareness of meningococcal infection following the earlier institutional outbreak to undertake a longitudinal study of meningococcal carriage in a cohort of new students at the same university. In particular, we were able to follow students over a 7-month period and to examine the effect of carriage on individual immune responses and the development of SBA against serogroup B meningococci, the generally accepted “gold standard” correlate of protective immunity (35).
The levels of meningococcal carriage were high, with at least 43% of students carrying meningococci at some point during the study. Although the numbers are small, the level of carriage of serogroup C meningococci (5%; n = 2) was comparable to the equivalent population at the time of the outbreak (7). These results are consistent with observations that immunization with capsular polysaccharide alone may not eliminate carriage of the corresponding meningococcal serogroup (12, 6).
Carriage of serogroup B meningococci was significantly higher than carriage of serogroup C, with colonization detected in 14% (n = 6) of individuals at some point in the study. Although such carriers represent a potential reservoir of infection for nonimmune individuals, it is interesting that with most of the serogroup B carriage strains being different, strain acquisition was not from among the students followed. The detection of carriage correlated with an increase in the detection of bactericidal activity against the colonizing strain; this included one student (volunteer 15) who was colonized by two distinct strains and who developed bactericidal activity to both. Once induced, bactericidal activity remained high throughout the study. In contrast, although students showed increases in antibodies reactive with capsular polysaccharide and LPS, these antibody responses were transitory, and their decline was not accompanied by a corresponding decline in SBA. The lack of correlation between antibodies to these antigens and the presence of bactericidal activity is consistent with previous studies of patients recovering from meningococcal infection and recipients of experimental outer membrane vaccines, where the major antigen associated with production of a bactericidal immune response was the PorA protein (36, 20, 14). A similar correlation between the presence, at a single time point, of bactericidal activity against serogroup B meningococci and antibodies to PorA reacting with the test strain was also noted in a previous study of the student population (37). Major findings, however, of the present study were the development of bactericidal activity not only against the serogroup B colonizing strain but also against the other serogroup B strains found in the population. The association between this bactericidal activity and the presence of antibodies to PorA detected by Western blotting suggests that PorA antibodies contribute to the heterologous activity, and this was confirmed by the observation that some serum samples showed significantly reduced bactericidal activity against a PorA-negative mutant.
It is not possible to strictly identify noncarriers, since colonization may occur transiently between sampling sessions. Indeed, the development of bactericidal activity against a range of strains was also reflected in increased SBA among students in whom no carriage was detected. This heterologous protection could not be associated with the presence of antibodies reacting with PorA or any of the other major protein antigens studied. Nevertheless, by the end of their first year, all students had bactericidal activity against some, but not necessarily all, of the serogroup B strains isolated from the cohort. It is possible that such serological data from individuals in whom colonization is only transient may provide useful information on recent exposure, particularly with regard to the strains circulating within a population.
These results suggest that at least three different mechanisms are responsible for generating immunity to serogroup B meningococci over the course of the study. First, carriage of serogroup B meningococci elicits antibodies to the PorA protein, resulting in a subtype-specific response. Second, this colonization also results in a cross-reactive response, for which PorA is at least partially responsible, that generates bactericidal activity against other serogroup B strains. Third, transient colonization with serogroup B or other meningococcal serotypes generates a cross-reacting response against other antigens that could not be identified in this study. A similar effect has been reported from a study of serum samples from Brazilian children immunized with an outer membrane-based vaccine; this study reported a complex pattern of recognition of bactericidal antibodies, some with subtype specificity directed against PorA and others with wider reactivity that recognized an unidentified target antigen (22).
Present experimental vaccines based largely on meningococcal outer membranes have been shown to be effective in inducing bactericidal activity (23, 28, 29). The antigens that have been identified as contributing to bactericidal activity are restricted to the PorA and Opc proteins. The Opc protein is not a viable vaccine candidate since it shows variable expression and is absent from many disease isolates. Previous studies have demonstrated subtype-specific immunity in accordance with the immunodominance of variable epitopes located on loops 1 and 4 of the predicted secondary structure of the PorA protein (34, 30). The present studies show that other, as yet unidentified, epitopes both on PorA and other meningococcal proteins contribute to the development of immunity to serogroup B meningococci. The identification of such epitopes with the ability to induce a more effective cross-reactive bactericidal response to a range of meningococcal strains would be a major step in the production of a universally effective vaccine against infections caused by serogroup B meningococci.
Acknowledgments
We thank S. Wootton, University of Southampton, for university liaison, J. Argent for blood sampling, R. Athersuch (HPA, Southampton, United Kingdom) for help with sample processing and A. Tuck (HPA, Southampton, United Kingdom) for bacteriological advice. We are grateful to H. Jennings (Ottawa, Canada) for the gift of E. coli K1 capsular polysaccharide, B. Kuipers (RIVM) for immunotyping of LPS, and J. Goddard (University of Southampton) for advice on statistical analysis. We are also grateful for the cooperation of the first-year students who participated in this study.
This work was supported by grants from Hope (The Wessex Medical Trust) and The University of Southampton Strategic Development Fund.
Editor: J. N. Weiser
REFERENCES
- 1.Ala'Aldeen, D. A., K. R. Neal, K. Ait-Tahar, J. S. Nguyen-van-Tam, A. English, T. J. Falla, P. M. Hawkey, and R. C. Slack. 2000. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J. Clin. Microbiol. 38:2311-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balmer, P., R. Borrow, and E. Miller. 2002. Impact of meningococcal C conjugate vaccine in the UK. J. Med. Microbiol. 51:717-722. [DOI] [PubMed] [Google Scholar]
- 3.Brooks, J. L., R. J. Fallon, and J. E. Heckels. 1995. Sequence variation in class 1 outer membrane protein in Neisseria meningitidis isolated from patients with meningococcal infection and close household contacts. FEMS Microbiol. Lett. 128:145-150. [DOI] [PubMed] [Google Scholar]
- 4.Cartwright, K. A. V. 1995. Meningococcal carriage and disease, p. 115-146. In K. A. V. Cartwright (ed.) Meningococcal disease. John Wiley & Sons, Chichester, United Kingdom.
- 5.Corless, C. E., M. Guiver, R. Borrow, V. Edwards-Jones, A. J. Fox, and E. B. Kaczmarski. 2001. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J. Clin. Microbiol. 39:1553-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez, S., I. Arreaza, I. Santiago, A. Malvar, S. Berron, J. A. Vazquez, and X. Hervada. 2003. Impact of meningococcal vaccination with combined serogroups A and C polysaccharide vaccine on carriage of Neisseria meningitidis C. J. Med. Microbiol. 52:75-77. [DOI] [PubMed] [Google Scholar]
- 7.Gilmore, A., G. Jones, M. Barker, N. Soltanpoor, and J. M. Stuart. 1999. Meningococcal disease at the University of Southampton: outbreak investigation. Epidemiol. Infect. 123:185-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenfield, S., and H. A. Feldman. 1967. Familial carriers and meningococcal meningitis. N. Engl. J. Med. 277:497-502. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield, S., P. R. Sheehe, and H. A. Feldman. 1971. Meningococcal carriage in a population of normal families. J. Infect. Dis. 123:67-73. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood, B. M., M. Hassan-King, and H. C. Whittle. 2003. Prevention of secondary cases of meningococcal disease in household contacts by vaccination. Br. Med. J. 1:1317-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guiver, M., R. Borrow, J. Marsh, S. J. Gray, E. B. Kaczmarski, D. Howells, P. Boseley, and A. J. Fox. 2000. Evaluation of the Applied Biosystems automated TaqMan polymerase chain reaction system for the detection of meningococcal DNA. FEMS Immunol. Med. Microbiol. 28:173-179. [DOI] [PubMed] [Google Scholar]
- 14.Guttormsen, H. K., L. M. Wetzler, and C. O. Solberg. 1994. Humoral immune response to class 1 outer membrane protein during the course of meningococcal disease. Infect. Immun. 62:1437-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, G. R., M. Christodoulides, J. L. Brooks, A. R. O. Miller, K. A. V. Cartwright, and J. E. Heckels. 1998. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: subtype stability and specificity of the immune response following colonisation. J. Infect. Dis. 178:451-459. [DOI] [PubMed] [Google Scholar]
- 16.Jones, G. R., J. N. Williams, M. Christodoulides, K. Jolley, and J. E. Heckels. 2000. Lack of immunity in university students prior to an outbreak of serogroup C meningococcal infection. J. Infect. Dis. 181:1172-1175. [DOI] [PubMed] [Google Scholar]
- 17.Jordens, J. Z., J. N. Williams, G. R. Jones, and J. E. Heckels. 2002. Detection of meningococcal carriage by culture and PCR of throat swabs and mouth gargles. J. Clin. Microbiol. 40:75-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristiansen, B. E., Y. Tveten, and A. Jenkins. 1998. Which contacts of patients with meningococcal disease carry the pathogenic strain of Neisseria meningitidis? A population based study. Br. Med. J. 317:621-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kvalsvig, A. J., and D. J. Unsworth. 2002. The immunopathogenesis of meningococcal disease. J. Clin. Pathol. 56:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandrell, R. E., and W. D. Zollinger. 1989. Human immune response to meningococcal outer membrane protein epitopes after natural infection or vaccination. Infect. Immun. 57:1590-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuinness, B. T., I. N. Clarke, P. R. Lambden, A. K. Barlow, J. T. Poolman, D. M. Jones, and J. E. Heckels. 1991. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet 337:514-517. [DOI] [PubMed] [Google Scholar]
- 22.Milagres, L. G., M. C. Gorla, C. T. Sacchi, and M. A. Rodrigues. 1998. Specificity of bactericidal antibody response to serogroup B meningococcal strains in Brazilian children after immunization with an outer membrane vaccine. Infect. Immun. 66:4755-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. A. Melles, V. S. D. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 62:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peltola, H. 1983. Meningococcal disease: still with us. Rev. Infect. Dis. 5:71-91. [DOI] [PubMed] [Google Scholar]
- 25.Quakyi, E. K., C. E. Frasch, N. Buller, and T. Chao-Ming. 1999. Immunization with meningococcal outer-membrane protein vesicles containing lipooligosaccharide protects mice against lethal experimental Group B Neisseria meningitidis infection and septic shock. J. Infect. Dis. 180:747-754. [DOI] [PubMed] [Google Scholar]
- 26.Ramsay, M. E., N. J. Andrews, C. L. Trotter, E. B. Kaczmarski, and E. Miller. 2003. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. Br. Med. J. 326:365-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson, K., K. R. Neal, C. Howard, J. Stockton, K. Atkinson, E. Scarth, J. Moran, A. Robins, I. Todd, E. Kaczmarski, S. Gray, I. Muscat, R. Slack, and D. A. A. Ala'aldeen. 2002. Characterization of humoral and cellular immune responses elicited by meningococcal carriage. Infect. Immun. 70:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenqvist, E., E. A. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouppe van der Voort, E. M., M. Schuller, J. Holst, P. de Vries, P. van der Ley, G. van den Dobbelsteen, and J. Poolman. 2000. Immunogenicity studies with a genetically engineered hexavalent PorA and a wild-type meningococcal group B outer membrane vesicle vaccine in infant cynomolgus monkeys. Vaccine 18:1334-1343. [DOI] [PubMed] [Google Scholar]
- 30.Rouppe van der Voort, E. M., H. van Dijken, B. Kuipers, J. van der Biezen, P. van der Ley, J. Meylis, I. Claassen, and J. Poolman. 1998. Human B- and T-cell responses after immunization with a hexavalent PorA meningococcal outer membrane vesicle vaccine. Infect. Immun. 65:5184-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholten, R. J. P. M., B. Kuipers, H. A. Valkenburg, J. Dankert, W. D. Zollinger, and J. T. Poolman. 1994. Lipo-oligosaccharide immunotyping of Neisseria meningitidis by a whole-cell ELISA with monoclonal antibodies. J. Med. Microbiol. 41:236-243. [DOI] [PubMed] [Google Scholar]
- 32.Stephens, D. S. 1999. Uncloaking the meningococcus: dynamics of carriage and disease. Lancet 353:941-942. [DOI] [PubMed] [Google Scholar]
- 33.Tappero, J. W., R. Reporter, J. D. Wenger, B. A. Ward, M. W. Reeves, T. S. Missbach, B. D. Plikaytis, L. Mascola, and A. Schuchat. 1996. Meningococcal disease in Los Angeles County, California, and among men in the county jails. N. Engl. J. Med. 335:833-840. [DOI] [PubMed] [Google Scholar]
- 34.van der Ley, P., J. E. Heckels, M. Virji, P. Hoogerhout, and J. T. Poolman. 1991. Topology of outer membrane porins in pathogenic Neisseria spp. Infect. Immun. 59:2963-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermont, C., and G. van den Dobbelsteen. 2002. Neisseria meningitidis serogroup B: laboratory correlates of protection. FEMS Immunol. Med. Microbiol. 34:89-96. [DOI] [PubMed] [Google Scholar]
- 36.Wedege, E., E. A. Hoiby, E. Rosenqvist, and G. Bjune. 1998. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccinees and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect. Immun. 66:3223-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams, J. N., G. R. Jones, M. Christodoulides, and J. E, Heckels. 2003. Serological correlates of protection against meningococci in a cohort of university students, before and during an outbreak of serogroup C infection. J. Infect. Dis. 187:1433-1441. [DOI] [PubMed] [Google Scholar]
- 38.Zollinger, W. D., and R. E. Mandrell. 1983. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect. Immun. 40:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]