Abstract
Streptococcus agalactiae is a major cause of bacterial pneumonia, sepsis, and meningitis in human neonates. During the course of infection, S. agalactiae adheres to a variety of epithelial cells but the underlying mechanisms are only poorly understood. The present report demonstrates the importance of the fibrinogen receptor FbsA for the streptococcal adherence and invasion of epithelial cells. Deletion of the fbsA gene in various S. agalactiae strains substantially reduced their binding of soluble fibrinogen and their adherence to and invasion of epithelial cells, indicating a role of FbsA in these different processes. The adherence and invasiveness of an fbsA deletion mutant were partially restored by reintroducing the fbsA gene on an expression vector. Heterologous expression of fbsA in Lactococcus lactis enabled this bacterium to adhere to but not to invade epithelial cells, suggesting that FbsA is a streptococcal adhesin. Flow cytometry experiments revealed a dose-dependent binding of FbsA to the surface of epithelial cells. Furthermore, tissue culture experiments exhibited an intimate contact of FbsA-coated latex beads with the surfaces of human epithelial cells. Finally, host cell adherence and invasion were significantly blocked in competition experiments with either purified FbsA protein or a monoclonal antibody directed against the fibrinogen-binding epitope of FbsA. Taken together, our studies demonstrate that FbsA promotes the adherence of S. agalactiae to epithelial cells but that FbsA does not mediate the bacterial invasion into host cells. Our results also indicate that fibrinogen-binding epitopes within FbsA are involved in the adherence of S. agalactiae to epithelial cells.
Streptococcus agalactiae, also named group B streptococcus, is a frequent colonizer of the gastrointestinal and urogenital tracts of humans (2). However, it is also the cause of substantial pregnancy-related morbidity and has emerged as an increasingly common cause of invasive disease in the elderly and in immunocompromised persons (46). In addition, S. agalactiae is the most common cause of bacterial pneumonia, sepsis, and meningitis in human newborns (2). Neonates acquire S. agalactiae from colonized mothers by aspiration of infected amniotic fluid or vaginal secretions at birth, followed by bacterial adherence to pulmonary epithelial cells (37). Adherence of the bacteria to lung epithelial cells is a prerequisite for the invasion of deeper tissues and the dissemination of the bacteria to the bloodstream. Several studies have demonstrated the adherence of S. agalactiae to epithelial cells both in vitro and in vivo (6, 33, 40, 49). However, the underlying mechanisms of this interaction are only poorly understood. Lipoteichoic acid was initially postulated to mediate the adherence of S. agalactiae to epithelial cells (27, 28), but later studies demonstrated that lipoteichoic acid has cytotoxic rather than adhesive properties against eukaryotic cells (14). As pretreatment of S. agalactiae with protease decreases the bacterial adherence to host cells (26, 40), surface proteins are presently assumed to be important for this process. However, the bacterial determinants that promote adherence of S. agalactiae to epithelial cells have not been elucidated.
Numerous pathogenic bacteria adhere to host cells by surface proteins, termed adhesins, that bind to components of the extracellular matrix (ECM). The ECM of mammalian tissues consists of glycoproteins, including collagen, laminin, fibronectin, and fibrinogen, which form a macromolecular structure underlying epithelial and endothelial cells (20). Several studies have described interactions of S. agalactiae with the ECM proteins laminin, fibronectin, and fibrinogen (22, 38, 42). For each of these binding functions, corresponding bacterial receptors have been identified. In S. agalactiae, the C5a peptidase was shown to play a role in fibronectin binding (4), the Lmb protein mediates binding to human laminin (38), and the proteins FbsA and FbsB are involved in fibrinogen binding (18, 35). On the amino acid level, the fibrinogen-binding proteins FbsA and FbsB are unrelated to each other, but both have a surface-exposed localization in the cell wall of the bacteria. The FbsB protein was shown to bind to human fibrinogen by its N-terminal 388 amino acids (18), whereas the FbsA protein interacts with fibrinogen by repetitive units, each 16 amino acids in length (35). Even a single repeat of FbsA was demonstrated to bind to human fibrinogen (35). Epidemiological studies revealed significant variation in the number of repeats in the FbsA protein between various S. agalactiae strains. Thus, FbsA variants ranging from 3 to 30 repeats have been described for different clinical isolates. The FbsA protein was shown to protect the bacteria from opsonophagocytosis, indicating a role of this protein in the virulence of S. agalactiae.
The present study investigated the importance of FbsA in the adherence and invasion of epithelial cells by S. agalactiae. Defined fbsA deletion mutants were constructed and tested for their interaction with host cells. The effect of plasmid-mediated fbsA expression on bacterial cell adherence and invasion was tested both in S. agalactiae and in Lactococcus lactis. Furthermore, flow cytometry and latex bead experiments were performed to analyze the interaction of FbsA with the surface of epithelial cells. Finally, we tested the influence of the FbsA protein and of FbsA-specific monoclonal antibodies (MAbs) on host cell adherence and invasion by S. agalactiae.
MATERIALS AND METHODS
Bacterial strains, epithelial cells, and growth conditions.
The bacterial strains used in this study are listed in Table 1. Escherichia coli DH5α was used for cloning purposes, and E. coli BL21 served as the host for the production of FbsA fusion protein. L. lactis subsp. cremoris MG1363 was used for heterologous expression of the fbsA gene. S. agalactiae was cultivated at 37°C in Todd-Hewitt yeast broth (THY) containing 1% yeast extract. S. agalactiae strains carrying the plasmid pOri23 or pOrifbsA were grown in the presence of erythromycin (5 μg/ml). E. coli was grown at 37°C in Luria broth, and clones carrying pOri23 or pET28 derivatives (35) or the plasmid pG+ΔfbsA (35) were selected in the presence of erythromycin (300 μg/ml), kanamycin (50 μg/ml), or ampicillin (100 μg/ml). L. lactis was grown at 30°C in M17 medium (Oxoid) supplemented with 0.5% glucose, and strains carrying pOri23 or pOrifbsA were selected with erythromycin (5 μg/ml).
TABLE 1.
Bacterial strains used in this study
| Species and strain | Serotype | Derivation and properties | Reference |
|---|---|---|---|
| S. agalactiae | |||
| 706 S2 | la | Clinical isolate | 35 |
| 706 S2 ΔfbsA | la | fbsA deletion mutant of 706 S2 | This study |
| O176 H4A | II | Clinical isolate | 35 |
| O176 H4A ΔfbsA | II | fbsA deletion mutant of O176 H4A | This study |
| 6313 | III | Clinical isolate | 35 |
| 6313 ΔfbsA | III | fbsA deletion mutant of 6313 | 35 |
| SS1169 | V | Clinical isolate | 35 |
| SS1169 ΔfbsA | V | fbsA deletion mutant of SS1169 | This study |
| O90R | Undefined capsule mutant of serotype la typing strain O90 | ATCC 12386 (23) | |
| L. lactis subsp. cremoris MG1363 | Plasmid-free derivative of strain NCDO 712 | 13 | |
| E. coli | |||
| DH5α | F−gyrA96 (Nalr) recA1 relA1 endA1 thi-1 hsdR17 (rK− mK+), glnV44 Δ(lacZYA-argF)U169 deoR (φ80dΔ(lacZ)M15) | 19 | |
| BL21(DE3) | F−ompT gal [dcm][lon] hsdSB (rB− mB−) λ prophage carrying the T7 polymerase gene | 11 |
The cell line A549 (ATCC CCL-185) was obtained from the American Type Culture Collection. A549 is a human lung carcinoma cell line which has many characteristics of type II alveolar pneumocytes. A549 cells were propagated in RPMI tissue culture medium (Gibco BRL) with 10% fetal calf serum in a humid atmosphere at 37°C with 5% CO2.
Construction of fbsA deletion mutants of S. agalactiae.
The fbsA gene was deleted in S. agalactiae strains O90R, 706 S2, O176 H4A, and SS1169 according to the procedure described by Schubert et al. (35). Briefly, the thermosensitive plasmid pG+ΔfbsA was transformed into the S. agalactiae strains by electroporation, and transformants were selected by growth on erythromycin agar at 30°C. Cells in which pG+ΔfbsA had integrated into the chromosome were selected by growth of the transformants at 37°C with erythromycin selection as described previously (24). Integrant strains were serially passaged for 5 days in liquid medium at 30°C without erythromycin selection to facilitate the excision of plasmid pG+ΔfbsA, leaving the desired fbsA deletion in the chromosome. Dilutions of the serially passaged cultures were plated onto agar, and single colonies were tested for erythromycin sensitivity to identify pG+ΔfbsA excisants. Chromosomal DNA of erythromycin-sensitive S. agalactiae excisants was tested by Southern blotting after HindIII digestion by using a digoxigenin-labeled fbsA flanking fragment as described previously (35).
Plasmid-mediated expression of fbsA in S. agalactiae and L. lactis.
The fbsA structural gene, including its ribosomal binding site, was amplified from chromosomal S. agalactiae 6313 DNA by PCR with primers 5′GTTTAGTGGATCCGAAGTAAGGAGAAAATTAATTGTTC and 5′ATCCCATATAATGACCTC, and the PCR product was directly ligated into the TA cloning vector pDrive (Qiagen). The fbsA gene was subsequently isolated by BamHI digestion and ligated into the BamHI-digested E. coli-Streptococcus expression vector pOri23 (31). Plasmid pOri23 possesses the strong promoter P23 from L. lactis (45), which is silent in E. coli but allows a constitutive, high-level expression of heterologously expressed genes in different gram-positive bacteria (31). After cloning of the fbsA gene in pOri23, its orientation was determined by HindIII digestion, and the resulting plasmid was termed pOrifbsA. Vector pOri23 and plasmid pOrifbsA were transformed by electroporation into S. agalactiae and L. lactis with subsequent erythromycin selection. L. lactis cells were made competent and transformed as described elsewhere (47). Sequencing of the insert of pOrifbsA revealed in the fbsA gene two A→G transitions, which result in amino acid exchanges in the FbsA protein. A mutation at bp 328 in the structural fbsA gene results in an exchange of N110 to S110 which does not affect the fibrinogen binding of the respective repeat unit (35). However, a mutation at bp 881 leads to an exchange of D310 to G310, which significantly reduces the fibrinogen-binding capability of the respective repeat unit (35). Thus, pOrifbsA-mediated fbsA expression results in the synthesis of an FbsA protein with 18 instead of 19 functional fibrinogen-binding repeats.
Antibodies and human proteins.
Affinity-purified polyclonal rabbit antifibrinogen antibodies were obtained from Dako Biochemicals. Fibrinogen, fibronectin, and polyclonal rabbit antifibronectin antibodies were purchased from Sigma-Aldrich. Fibrinogen was passed through a gelatin-Sepharose column to remove residual contaminating fibronectin in the preparation. The purity of the fibrinogen preparation was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining and by Western blotting with antifibronectin antibodies. The generation and characterization of the anti-FbsA MAbs 5H2 and 2B1 are described elsewhere (30).
Binding of soluble 125I-labeled fibrinogen to S. agalactiae.
Purified human fibrinogen was radiolabeled with 125I, using the chloramine T method (21). Binding of labeled fibrinogen to S. agalactiae was performed as described previously (35). Statistical analysis was performed with a Student's paired t test, and differences were considered significant at a P value of ≤0.05.
Preparation of hexahistidyl-tagged fusion proteins.
The FbsA fusion protein originates from S. agalactiae 6313 and possesses 19 repeats, each 16 amino acids in length (35). The Bsp protein is a surface protein from S. agalactiae that plays a role in the morphogenesis of the bacteria (32) and served as a control in the present study. The fusion proteins were synthesized in recombinant E. coli BL21 by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) after the culture had reached an optical density at 600 nm of 1.0. The cells were disrupted with a French pressure cell, and purification of the fusion protein was performed according to the instructions of Qiagen by Ni2+ affinity chromatography.
Adherence and invasion assays.
Adherence of S. agalactiae or L. lactis to epithelial cells and internalization into epithelial cells were assayed as described previously (17). Briefly, A549 cells were transferred to 24-well tissue culture plates at approximately 4 × 105 cells per well and cultivated overnight in RPMI tissue culture medium supplemented with 10% fetal calf serum. After replacement of the medium with 1 ml of fresh medium, the cells were infected with S. agalactiae or L. lactis at a multiplicity of infection of 10:1 and incubated at 37°C for 2 h. The infected cells were subsequently washed three times with phosphate-buffered saline (PBS). The number of cell-adherent bacteria was determined by lysis of the eukaryotic cells with distilled water and subsequent determination of CFU by plating appropriate dilutions of the lysates on THY agar. Intracellular bacteria were determined after a further incubation of the infected cells for 2 h with RPMI medium containing penicillin G (10 U) and streptomycin (0.01 mg) to kill extracellular bacteria. After three washes with PBS, the epithelial cells were lysed in distilled water and the amount of intracellular bacteria was quantitated by plating serial dilutions of the lysate onto THY agar plates. All samples were tested in triplicate, and experiments were repeated at least three times.
To assess the effect of FbsA, Bsp, polyclonal antifibrinogen, or polyclonal antifibronectin antibodies on the adherence and invasion of S. agalactiae, the adherence and invasion assays were performed as described above, with the following modifications. A549 cells in tissue culture wells were incubated for 15 min in 100 μl of PBS with different amounts of purified proteins or antibodies as described elsewhere (25). Bacterial cells were then added in tissue culture medium, and the wells were incubated at 37°C for 2 h. To analyze the effect of fibrinogen, fibronectin, or anti-FbsA MAbs on the bacterial adherence and invasion, S. agalactiae 6313 was incubated for 15 min in 500 μl of RPMI medium containing different amounts of fibrinogen, fibronectin, or the MAbs. Subsequently, the bacteria were used to infect A549 cells, and the remainder of the experiment was carried out as described above.
Fluorescence-activated cell sorter analysis.
Binding of purified FbsA protein to A549 cells was performed essentially as described by Taschner et al. (43). In brief, 5 × 106 A549 cells were pelleted by centrifugation at 4°C and washed with 10% bovine serum albumin (BSA) in PBS. Subsequently, the cells were incubated for 45 min on ice with 5 μg of Fc fragments (Dianova) and washed two times with 10% BSA in PBS. The cells were incubated on ice for 1 h with different concentrations of FbsA fusion protein or with 25 μg of a His-tagged S. pyogenes surface protein (Spy0416) as a negative control. Subsequently, the cells were washed two times with 10% BSA in PBS and incubated for 1 h on ice with an anti-His tag MAb (1:100) (Qiagen). After two washings with 10% BSA in PBS, fluorescein isothiocyanate (FITC)-labeled anti-mouse immunoglobulin G (1:500) (Dako) was added and the suspension was incubated for 1 h on ice. The cells were again washed two times with 10% BSA in PBS and fixed for 30 min with 1% paraformaldehyde in PBS. The fluorescence of 104 cells was quantitated in a FACSCalibur flow cytometer (Becton Dickinson), using a laser at 488 nm and the FL1 PMT (530/30 nm). The data were analyzed with the WinMDI software, displaying the fluorescence intensity on the x axis and the number of events on the y axis. The geometric mean value was calculated with the MinMDI software as n root(a1 × a2 × a3… .an). The geometric mean takes into account the weighting of the data distribution.
Scanning electron microscopy of FbsA-coated latex beads.
Approximately 109 latex beads (3-μm diameter) (Sigma) were washed three times in PBS. One half was resuspended in 1.0 ml of PBS containing FbsA fusion protein (500 μg/ml), and the remaining half was resuspended in 1.0 ml of PBS with BSA (10 mg/ml). The beads were incubated overnight at 4°C with end-over-end rotation. After pelleting of the beads by centrifugation, the amount of remaining protein in the supernatant was determined with a Bradford protein assay kit (Bio-Rad). The beads were washed once with PBS and blocked for 2 h with BSA (10 mg/ml) in PBS at room temperature. The beads were washed twice with PBS and once with RPMI plus 10% FCS and resuspended in RPMI plus 10% FCS. Confluent A549 cells in 24-well plates were inoculated with 2 × 108 beads per well in a total volume of 1.0 ml for 2 h at 37°C in a 5% CO2 atmosphere. The cells were washed five times with PBS and fixed with 3% paraformaldehyde and 4% glutaraldehyde in 100 mM phosphate buffer (pH 7.4) for scanning electron microscopy. Scanning electron microscopy was performed with a Zeiss DSM 962 microscope.
RESULTS
Various S. agalactiae strains require the fbsA gene for fibrinogen binding.
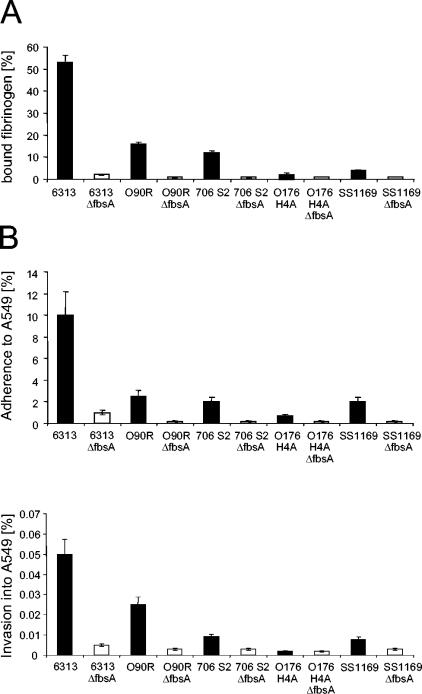
In the clinical S. agalactiae isolate 6313 (serotype III), the FbsA protein is essential for the attachment of the bacteria to human fibrinogen (35). To analyze the importance of FbsA for the fibrinogen binding of various clinical S. agalactiae isolates, the fbsA gene was deleted in the genomes of S. agalactiae 706 S2 (serotype Ia), O176 H4A (serotype II), and SS1169 (serotype V). The fbsA gene was also deleted in the chromosome of the S. agalactiae grouping strain O90R, a capsule mutant of the serotype Ia strain O90. By Southern blot analysis, the successful deletion of fbsA in the genomes of the respective strains was confirmed (data not shown). The different S. agalactiae strains and their fbsA mutants were subsequently tested for their binding of 125I-labeled fibrinogen (Fig. 1A). S. agalactiae 6313 showed significant binding of radiolabeled fibrinogen, whereas strains O90R and 706 S2 exhibited moderate binding and strains SS1169 and O176 H4A showed only little binding of soluble fibrinogen. However, in all of the tested strains, the deletion of the fbsA gene resulted in a loss of their fibrinogen-binding activity, indicating that fbsA is essential for the fibrinogen binding of various S. agalactiae strains.
FIG. 1.
Binding of radiolabeled fibrinogen (A) and host cell adherence and invasion (B) by different S. agalactiae strains and their fbsA deletion mutants. Binding of 125I-labeled fibrinogen was quantitated by incubating a defined number of bacteria with a defined amount of radiolabeled fibrinogen and relating the amount of fibrinogen bound to bacteria to the total amount of fibrinogen added. To determine the adherence and invasiveness of the different strains with the lung epithelial cell line A549, equal numbers of each streptococcal strain were used to infect A549 cells, and the numbers of cell-adherent and internalized bacteria were related to the number of input bacteria. The values represent the means ± standard errors of the means from three independent experiments, each performed in triplicate. The values for the mutant strains are statistically significant compared to those for the parental strains, with a P value of ≤0.05.
S. agalactiae host cell adherence and invasion is FbsA dependent.
To investigate the importance of FbsA for host cell adherence and invasion of S. agalactiae, strains 6313, O90R, 706 S2, O176 H4A, and SS1169, and their isogenic fbsA mutants were used in tissue culture experiments with the human lung epithelial cell line A549. As shown in Fig. 1B, strain 6313 efficiently adhered to and invaded A549 cells, whereas strains O90R, 706 S2, and SS1169 showed moderate, and strain O176 H4A, showed low adherence to and invasion of A549 cells. Interestingly, the capabilities of the various strains for host cell adherence and invasion correlated with their capabilities for fibrinogen binding, indicating a putative connection between fibrinogen binding and host cell interaction in S. agalactiae. In line with this, the deletion of the fbsA gene substantially reduced the host cell adherence to and invasion of the different S. agalactiae strains. Only the invasiveness of strain O176 H4A, which already was very low, was not further reduced upon deletion of the fbsA gene. Our findings therefore indicate a prominent role of the fibrinogen-binding protein FbsA in the interaction of S. agalactiae with epithelial cells. To study the importance of FbsA for host cell adherence and invasion of S. agalactiae in more detail, we focused on the highly adherent and invasive S. agalactiae strain 6313.
Plasmid-mediated expression of fbsA partially restores host cell adherence to and invasion of S. agalactiae 6313 ΔfbsA.
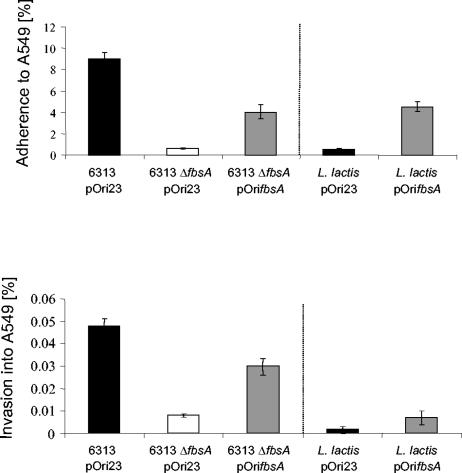
To complement the fbsA deficiency of mutant 6313 ΔfbsA, we attempted to clone from strain 6313 the entire fbsA gene, including its promoter region, into the E. coli-Streptococcus shuttle vector pAT28. Despite several attempts, we repeatedly failed to clone the fbsA gene into this vector. As the promoter of the fbsA gene is very active in both E. coli and S. agalactiae (reference 17 and unpublished results), we hypothesized that overexpression of fbsA by its own promoter might be toxic to E. coli and S. agalactiae. We therefore cloned the fbsA gene, devoid of its promoter region, into the E. coli-Streptococcus expression vector pOri23 (31), resulting in plasmid pOrifbsA. After transformation of the plasmids pOri23 and pOrifbsA into S. agalactiae 6313 and 6313 ΔfbsA, respectively, strain 6313 ΔfbsA(pOrifbsA) showed about 80% fibrinogen binding compared to strain 6313(pOri23) (30). The recombinant strains were subsequently examined for their capacities for adhesion to and invasion of A549 cells (Fig. 2). S. agalactiae strains 6313(pOri23) and 6313 ΔfbsA(pOri23) showed adherence and invasion rates comparable to those of their plasmid-free parental strains, demonstrating that the vector pOri23 does not influence the adherence and invasion properties of these strains. In contrast, plasmid-mediated expression of fbsA in strain 6313 ΔfbsA(pOrifbsA) significantly increased its adherence to and invasion of A549 cells compared to strain 6313 ΔfbsA(pOri23). Our findings therefore demonstrate that the reduced adherence to and invasion of A549 cells by mutant 6313 ΔfbsA are due to its fbsA deficiency and not to unrelated mutations in its chromosome. However, the adhesive and invasive efficiencies of 6313 ΔfbsA(pOrifbsA) were significantly lower than those of 6313(pOri23), indicating that pOri23-driven expression of fbsA does not fully complement the fbsA deficiency of mutant 6313 ΔfbsA.
FIG. 2.
Adherence to and invasion of the lung epithelial cell line A549 by S. agalactiae strains 6313(pOri23), 6313 ΔfbsA(pOri23), and 6313 ΔfbsA(pOrifbsA) and by L. lactis(pOri23) and L. lactis(pOrifbsA), respectively. The epithelial cell line A549 was infected with equal amounts of bacteria of each strain, and the numbers of cell-adherent and internalized bacteria were related to the number of input bacteria. The dotted line separates the results obtained with S. agalactiae and L. lactis from each other. Each experiment was performed at least three times in triplicate. Error bars indicate standard errors of the means.
Heterologous expression of fbsA in Lactococcus lactis confers the ability to adhere to host cells.
To investigate whether S. agalactiae factors other than FbsA are required for the bacterial adherence and invasion of host cells, plasmids pOri23 and pOrifbsA were introduced in L. lactis, a gram-positive bacterium that naturally does not adhere to epithelial cells. Recombinant L. lactis(pOri23) revealed no binding to human fibrinogen whereas L. lactis(pOrifbsA) exhibited significant fibrinogen binding, equal to that of S. agalactiae 6313 ΔfbsA(pOrifbsA) (30). Both L. lactis strains were tested in tissue culture experiments for their adhesive and invasive capacities with A549 cells (Fig. 2). L. lactis(pOri23) exhibited no adherence to and invasion of A549 cells, whereas L. lactis(pOrifbsA) showed significant adherence to A549 cells but only little invasion into this cell line. Of note is that host cell adherence of L. lactis(pOrifbsA) was of the same order of magnitude as that of the complemented S. agalactiae strain 6313 ΔfbsA(pOrifbsA) (Fig. 2). These findings demonstrate that FbsA does not require an S. agalactiae coreceptor for host cell adherence. Our results also suggest that FbsA promotes bacterial adherence to but not invasion into host cells.
The FbsA protein binds directly to A549 cells.
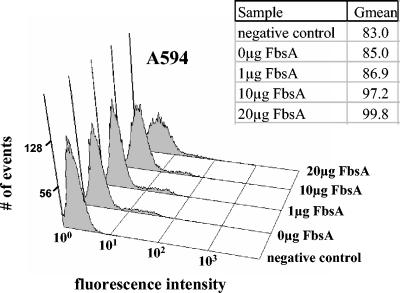
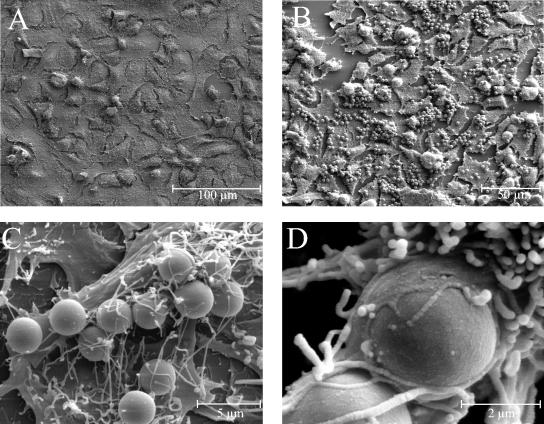
Flow cytometry and latex bead experiments were performed to investigate the interaction of FbsA with A549 cells in more detail. In flow cytometry experiments, a dose-dependent binding of the FbsA fusion protein to A549 cells was observed (Fig. 3), suggesting that FbsA binds directly to host cells. To further investigate the interaction of FbsA with epithelial cells, latex beads were coated with the FbsA fusion protein and tested for their interaction with human A549 cells. As a control, BSA-coated latex beads were analyzed for their binding to A549 cells. By scanning electron microscopy, BSA-coated latex beads were rarely found associated with A549 cells (Fig. 4A), while FbsA-coated beads bound in high numbers to A549 cells (Fig. 4B). Attachment of the FbsA-coated beads to the plasma membrane of A549 cells was characterized by contact with microvilli and structures that resembled early pseudopods (Fig. 4C). In a few cases, the pseudopod appeared to surround the surface of the bead, indicating that the bead was finally internalized (Fig. 4D). Taken together, the results from our flow cytometry and latex bead experiments indicate a direct interaction of FbsA with structures on the surface of A549 cells.
FIG. 3.
Detection of FbsA binding to the surface of A549 cells by flow cytometry. A549 cells were incubated with different amounts of purified FbsA fusion protein as described in Materials and Methods and tested with mouse anti-His tag antibodies and FITC-coupled anti-mouse antibodies for the interaction of FbsA with the host cell surface. The fluorescence intensity is given on the x axis, and the number of events (cells) is given on the y axis. The graphs show an increased fluorescence intensity of the cells upon binding of the FITC-coupled antibody-FbsA protein complex, demonstrating a concentration-dependent binding of FbsA to A549 cells. Gmean represents the geometric mean, which takes into account the weighting of the data distribution.
FIG. 4.
Binding of FbsA-coated latex beads to human A549 cells. Latex beads were coated with either BSA (A) or FbsA fusion protein (B to D), and the interaction of the coated beads with the lung epithelial cell line A549 was analyzed by scanning electron microscopy.
The FbsA protein blocks the bacterial adherence to and invasion of A549 cells.
As the previous experiments had demonstrated direct binding of FbsA to the surface of epithelial cells, we investigated the effect of externally added FbsA fusion protein on the adherence to and invasion of A549 cells by S. agalactiae. Pretreatment of A549 cells with 50 μg of FbsA fusion protein per ml reduced the adherence of S. agalactiae 6313 by 51% ± 6% and reduced its invasion by 46% ± 7%. Similarly, preincubation of A549 cells with 100 μg of FbsA per ml inhibited the adherence of strain 6313 by 71% ± 5% and its invasion by 73% ± 7%. Pretreatment of A549 cells with a 100-μg/ml concentration of the S. agalactiae protein Bsp, which plays a role in the morphogenesis of the bacteria (32), did not influence the bacterial adherence to and invasion of A549 cells (data not shown). These results demonstrate that externally added FbsA protein can specifically block host cell adherence to and invasion of S. agalactiae.
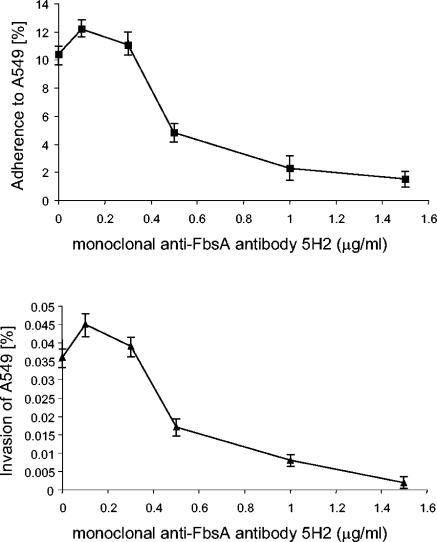
A MAb against the fibrinogen-binding site of FbsA blocks the bacterial adherence.
To better understand the interaction of FbsA with the host cell surface at the molecular level, we used MAbs directed against different epitopes of the FbsA protein (30). MAb 5H2 binds to the repeat region of FbsA, thereby blocking the fibrinogen binding of the FbsA protein. In contrast, MAb 2B1 binds to the repeat region of FbsA without interfering with the binding of FbsA to human fibrinogen. After preincubation of S. agalactiae 6313 with either of the two MAbs, the streptococcal host cell adherence and invasion were quantitated in tissue culture experiments. As depicted in Fig. 5, increasing concentrations of MAb 5H2 caused a dose-dependent inhibition of the bacterial adherence and invasiveness. Preincubation of strain 6313 with 1.5 μg of MAb 5H2 per ml almost completely blocked the streptococcal adherence to and invasion of A549 cells. In contrast, preincubation of strain 6313 with up to 10 μg of MAb 2B1 per ml did not influence its host cell adherence or invasion (data not shown).
FIG. 5.
Competitive inhibition of streptococcal adherence and invasion by MAb 5H2, which specifically blocks the binding of FbsA to human fibrinogen. Tissue culture experiments were performed after pretreatment of S. agalactiae 6313 with different amounts of MAb 5H2. Each experiment was performed at least three times in triplicate. Error bars indicate standard errors of the means.
Tissue culture experiments were performed to analyze the importance of host cell fibrinogen for the streptococcal adherence to and invasion of epithelial cells. Pretreatment of strain 6313 with human fibrinogen caused a dose-dependent inhibition of the adherence to and invasion of epithelial cells (data not shown), but it also resulted in the previously described clumping of the bacteria (18). We therefore tested the effect of polyclonal antifibrinogen antibodies on the adherence to and invasion of A549 cells by S. agalactiae. Pretreatment of A549 cells with up to 200 μg of polyclonal antifibrinogen antibodies per ml did not influence the adherence and invasiveness of strain 6313 (data not shown). However, S. agalactiae 6313, preincubated with antifibrinogen antibodies, revealed the same fibrinogen binding (52% ± 5%) as the untreated strain (Fig. 1), demonstrating that the antifibrinogen antibodies do not interfere with the binding of S. agalactiae to human fibrinogen. Thus, the role of surface-exposed fibrinogen in FbsA-mediated host cell adherence remains to be determined.
DISCUSSION
The adherence of streptococci to epithelial cells is a key event in the infection process that allows the colonization of host epithelial surfaces (37). Following colonization, the bacteria may eventually penetrate the epithelial barrier and disseminate to the bloodstream and deeper tissues. Adherence is frequently mediated by specific interactions between streptococcal cell wall proteins and components of the host ECM. The ECM is a three-dimensional structure of glycoproteins and contains the proteins collagen, laminin, fibronectin, and fibrinogen (15, 16, 20). S. agalactiae, a frequent cause of neonatal pneumonia, was recently shown to synthesize the fibrinogen-binding proteins FbsA and FbsB (18, 35). The present study aimed to investigate the importance of FbsA for the binding of S. agalactiae to human fibrinogen and for the bacterial adherence to and invasion of epithelial cells.
Previously, the fbsA gene was found to be widely distributed in different S. agalactiae strains and to be essential for the fibrinogen binding of S. agalactiae 6313 (35). However, the relevance of FbsA for the fibrinogen binding of other clinical S. agalactiae isolates remained unclear. Here, we provide evidence that various S. agalactiae strains, belonging to different serotypes, require FbsA for an efficient interaction with human fibrinogen. Interestingly, the fbsB gene, encoding the second fibrinogen-binding protein in S. agalactiae, was found not to influence the fibrinogen binding of the bacteria (18). Our results therefore suggest that FbsA is of general importance for the fibrinogen binding of S. agalactiae.
It is noteworthy that the S. agalactiae strains investigated in the present study showed significant differences in their ability to interact with human fibrinogen. Recently, the internal repeats of the highly repetitive FbsA protein were shown to mediate fibrinogen binding, and even a single repeat of FbsA was demonstrated to interact with fibrinogen (35). The FbsA proteins of S. agalactiae 6313, O90R, 706 S2, 176 H4A, and SS1169 differ from each other in that they possess 19, 10, 17, 3, and 30 internal repeats, respectively. Interestingly, strain SS1169 showed only weak fibrinogen binding, although its FbsA protein carries 30 internal repeats. Similarly, strain O90R, possessing an FbsA protein with 10 internal repeats, bound larger amounts of fibrinogen than strain 706 S2, whose FbsA protein carries 17 repetitive units. However, the capacity of FbsA for fibrinogen binding was previously shown to correlate with its number of repeats (35). It can thus be speculated that the fibrinogen binding of a given strain is to only some extent controlled by the repeat numbers of its FbsA protein. Possibly the analyzed strains differ with respect to their fbsA expression, the transport of the FbsA protein across the cytoplasmic membrane, or the anchoring of FbsA to the cell wall. Alternatively, the capsules of the different strains may influence their fibrinogen-binding properties. In a report by Chhatwal et al. (8), the capsule of S. agalactiae was demonstrated to interfere with the bacterial binding to fibrinogen. Studies are therefore under way to investigate the expression of the fbsA gene in the various strains and the importance of the capsule for fibrinogen binding.
Plasmid-mediated expression of fbsA only partially restored the adherence and invasion capabilities of the complemented strain 6313 ΔfbsA(pOrifbsA). However, this strain exhibits about 80% fibrinogen binding compared to the parental strain 6313 (30). Furthermore, enzyme-linked immunosorbent assay experiments with whole bacteria and MAb 5H2 revealed equal amounts of FbsA on the surfaces of S. agalactiae 6313 and 6313 ΔfbsA(pOrifbsA) (data not shown). Our findings therefore indicate similar expression levels of the fbsA promoter (PfbsA) and of promoter P23 in plasmid pOri23 during growth in complex media. However, P23 expression is constitutive in gram-positive bacteria (31), while that of PfbsA is highly regulated (17, 34). The differences in bacterial adherence and invasion between 6313 and 6313 ΔfbsA(pOrifbsA) may thus be explained by different fbsA expression levels in tissue culture experiments. Although it is highly speculative, one could hypothesize that tissue culture conditions might significantly upregulate PfbsA-driven fbsA expression in strain 6313. This would result in increased synthesis of FbsA protein, thereby allowing enhanced host cell adherence of S. agalactiae 6313 compared to strain 6313 ΔfbsA(pOrifbsA).
The adherence of S. agalactiae to epithelial surfaces is a process that requires specific interactions between bacterial adhesins and host receptors. In various in vitro models, S. agalactiae was shown to adhere to different epithelial cells (7, 40, 41, 44, 48), but the molecular basis of this interaction is currently only poorly understood. The laminin-binding protein Lmb has been speculated to play a role in the colonization of epithelial surfaces (37), but this hypothesis has not been experimentally tested. The transcriptional regulator RogB and the oligopeptide permease Opp from S. agalactiae were recently shown to control both the expression of the fbsA gene and the bacterial adherence to epithelial cells (17, 34). These findings indicated a link between the fibrinogen receptor FbsA and the adherence of S. agalactiae to epithelial cells. In the present study, different experimental approaches unambiguously demonstrate that the FbsA protein alone is sufficient to promote the adherence of S. agalactiae to epithelial cells. Thus, FbsA represents the first adhesin identified in these bacteria. As the adherence of S. agalactiae to epithelial surfaces is the initial event in the colonization of host surfaces, FbsA may thus play an important role in the development of a vaccine against these bacteria.
Competition experiments and the analysis of fbsA deletion mutants indicated that FbsA might also play a role in the invasion of epithelial cells by S. agalactiae. However, adherence is frequently a prerequisite for the successful invasion of host cells (12). In line with this, the adherence of the fbsA deletion mutants was reduced by the same order of magnitude as was their host cell invasion. Similar results were obtained in the competition experiments with purified FbsA protein or MAb 5H2. Furthermore, FbsA-coated latex beads bound in high number to epithelial cells but were only rarely seen in the process of internalization by host cells. Finally, plasmid-mediated fbsA expression allowed L. lactis to adhere to but not to enter epithelial cells. Thus, our findings suggest that FbsA does not promote the invasion of S. agalactiae into epithelial cells. Interestingly, the fibrinogen-binding protein FbsB was recently shown to mediate the invasion of S. agalactiae into epithelial cells (18). Thus, fibrinogen-binding proteins obviously play a prominent role in both host cell adherence and invasion by S. agalactiae. The FbsB protein, however, is not the only invasin in S. agalactiae. The C5a peptidase (7), the hemolysin CylE (10), the alpha C protein (3, 5), and protein Spb1 (1), which is unique to serotype III-3, also have been shown to play a role in the entry of S. agalactiae into host cells. This indicates that after FbsA-mediated adherence, different proteins are involved in the entry of S. agalactiae into host cells.
Although the present study and previous studies convincingly demonstrate the binding of FbsA to human fibrinogen (35), the eukaryotic molecules that allow FbsA-mediated adherence to host cells remain to be determined. Externally added fibrinogen significantly inhibited the adherence of S. agalactiae to epithelial cells; however, it also caused a dose-dependent clumping of the bacteria (18). Thus, the inhibition of streptococcal adherence may be caused by the clumping of the bacteria. Host cell adherence was also unaffected by the addition of polyclonal antifibrinogen antibodies. However, these antibodies also did not block the binding of the bacteria to fibrinogen, suggesting that FbsA binds to a highly conserved region within human fibrinogen, which does not allow the production of antibodies. In an approach to define those sites in FbsA that are involved in host cell adherence, we made use of MAbs 5H2 and 2B1. Both antibodies bind to FbsA in its repeat region; however, MAb 5H2 blocks the binding of FbsA to fibrinogen, while MAb 2B1 does not interfere with this interaction (30). Interestingly, we found that MAb 5H2 competitively blocked the adherence of S. agalactiae to epithelial cells, while MAb 2B1 had no effect on the streptococcal adherence. This result indicates that fibrinogen-binding epitopes in the repeat region of FbsA are involved in the adherence of S. agalactiae to epithelial cells. As fibrinogen was already detected on the surface of A549 cells (16), fibrinogen might thus play a role in FbsA-mediated adherence of S. agalactiae. Alternatively, FbsA may bind to a different ligand on A549 cells. Interestingly, the fibrinogen-binding protein ClfB from Staphylococcus aureus was recently shown to interact with cytokeratin 10 on the surface of eukaryotic cells (29). Moreover, the fibrinogen-binding protein ClfA from S. aureus was found to interact with a platelet membrane protein that is distinct from fibrinogen (36). These findings demonstrate that bacterial fibrinogen-binding proteins may interact with distinct ligands on the host cell surface.
Several pathogenic bacteria possess fibrinogen-binding proteins that also interact with human fibronectin, and it is the fibronectin-binding activity which mediates the bacterial adherence and invasion of host cells (9, 25, 39). Moreover, commercially available fibrinogen preparations are frequently contaminated with fibronectin. Thus, one might argue that FbsA-mediated cell adherence is brought about by binding of FbsA to human fibronectin on the surface of A549 cells. However, FbsA was previously demonstrated not to interact with human fibronectin (35). In addition, neither preincubation of S. agalactiae 6313 with fibronectin nor pretreatment of A549 cells with antifibronectin antibodies had an effect of the bacterial adherence and invasion (data not shown). These results demonstrate that fibronectin is obviously not involved in FbsA-mediated adherence of S. agalactiae to host cells. Currently, studies to identify the nature of the ligand(s) to which FbsA binds during the course of host cell adherence by S. agalactiae are under way.
Editor: J. N. Weiser
REFERENCES
- 1.Adderson, E. E., S. Takahashi, Y. Wang, J. Armstrong, D. V. Miller, and J. F. Bohnsack. 2003. Subtractive hybridization identifies a novel predicted protein mediating epithelial cell invasion by virulent serotype III group B Streptococcus agalactiae. Infect. Immun. 71:6857-6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, C. J., and M. S. Edwards. 1995. Group B streptococcal infections, p. 980-1054. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant. W. B. Saunders, Philadelphia, Pa.
- 3.Baron, M. J., G. R. Bolduc, M. B. Goldberg, T. C. Auperin, and L. C. Madoff. 2004. Alpha C protein of group B Streptococcus binds host cell surface glycosaminoglycan and enters cells by an actin-dependent mechanism. J. Biol. Chem. 279:24714-24723. [DOI] [PubMed] [Google Scholar]
- 4.Beckmann, C., J. D. Waggoner, T. O. Harris, G. S. Tamura, and C. E. Rubens. 2002. Identification of novel adhesins from group B streptococci by use of phage display reveals that C5a peptidase mediates fibronectin binding. Infect. Immun. 70:2869-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolduc, G. R., M. J. Baron, C. Gravekamp, C. S. Lachenauer, and L. C. Madoff. 2002. The alpha C protein mediates internalization of group B Streptococcus within human cervical epithelial cells. Cell Microbiol. 4:751-758. [DOI] [PubMed] [Google Scholar]
- 6.Broughton, R. A., and C. J. Baker. 1983. Role of adherence in the pathogenesis of neonatal group B streptococcal infection. Infect. Immun. 39:837-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, Q., D. Stafslien, S. S. Purushothaman, and P. Cleary. 2002. The group B streptococcal C5a peptidase is both a specific protease and an invasin. Infect. Immun. 70:2408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chhatwal, G. S., C. Lämmler, and H. Blöbel. 1984. Guanidine extraction enhances the binding of human fibrinogen to group-B streptococci. Med. Microbiol. Immunol. 173:19-27. [DOI] [PubMed] [Google Scholar]
- 9.Cue, D., H. Lam, and P. P. Cleary. 2001. Genetic dissection of the Streptococcus pyogenes M1 protein: regions involved in fibronectin binding and intracellular invasion. Microb. Pathog. 31:231-242. [DOI] [PubMed] [Google Scholar]
- 10.Doran, K. S., J. C. Chang, V. M. Benoit, L. Eckmann, and V. Nizet. 2002. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J. Infect. Dis. 185:196-203. [DOI] [PubMed] [Google Scholar]
- 11.Dubendorff, J. W., and F. W. Studier. 1991. Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J. Mol. Biol. 219:45-59. [DOI] [PubMed] [Google Scholar]
- 12.Elsinghorst, E. A. 1994. Measurement of invasion by gentamycin resistance, p. 405-419. In J. N. Abelson and M. I. Simon (ed.), Bacterial pathogenesis, part B. Academic Press, New York, N.Y.
- 13.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldschmidt, J. C., Jr., and C. Panos. 1984. Teichoic acids of Streptococcus agalactiae: chemistry, cytotoxicity, and effect on bacterial adherence to human cells in tissue culture. Infect. Immun. 43:670-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guadiz, G., L. A. Sporn, R. A. Goss, S. O. Lawrence, V. J. Marder, and P. J. Simpson-Haidaris. 1997. Polarized secretion of fibrinogen by lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 17:60-69. [DOI] [PubMed] [Google Scholar]
- 16.Guadiz, G., L. A. Sporn, and P. J. Simpson-Haidaris. 1997. Thrombin cleavage-independent deposition of fibrinogen in extracellular matrices. Blood 90:2644-2653. [PubMed] [Google Scholar]
- 17.Gutekunst, H., B. E. Eikmanns, and D. J. Reinscheid. 2003. Analysis of RogB-controlled virulence mechanisms and gene expression in Streptococcus agalactiae. Infect. Immun. 71:5056-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutekunst, H., B. E. Eikmanns, and D. J. Reinscheid. 2004. The novel fibrinogen-binding protein FbsB promotes the invasion of Streptococcus agalactiae into epithelial cells. Infect. Immun. 72:3495-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan, D. 1985. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 20.Hay, E. D. 1991. Cell biology of extracellular matrix. Plenum Press, New York, N.Y.
- 21.Hunter, W. H., and F. C. Greenwood. 1962. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature 194:495-496. [DOI] [PubMed] [Google Scholar]
- 22.Lämmler, C., G. S. Chhatwal, and H. Blöbel. 1983. Binding of human fibrinogen and its polypeptide chains to group B streptococci. Med. Microbiol. Immunol. 172:149-153. [DOI] [PubMed] [Google Scholar]
- 23.Lancefield, R. C., M. McCarty, and W. N. Everly. 1975. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J. Exp. Med. 142:165-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maguin, E., H. Prevost, S. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massey, R. C., M. N. Kantzanou, T. Fowler, N. P. Day, K. Schofield, E. R. Wann, A. R. Berendt, M. Höök, and S. J. Peacock. 2001. Fibronectin-binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell Microbiol. 3:839-851. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki, S., O. Leon, and C. Panos. 1988. Adherence of Streptococcus agalactiae to synchronously growing human cell monolayers without lipoteichoic acid involvement. Infect. Immun. 56:505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nealon, T. J., and S. J. Mattingly. 1984. Role of cellular lipoteichoic acids in mediating adherence of serotype III strains of group B streptococci to human embryonic, fetal, and adult epithelial cells. Infect. Immun. 43:523-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nealon, T. J., and S. J. Mattingly. 1985. Kinetic and chemical analyses of the biologic significance of lipoteichoic acids in mediating adherence of serotype III group B streptococci. Infect. Immun. 50:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Brien, L. M., E. J. Walsh, R. C. Massey, S. J. Peacock, and T. J. Foster. 2002. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell Microbiol. 4:759-770. [DOI] [PubMed] [Google Scholar]
- 30.Pietrocola, G., A. Schubert, L. Lisai, M. Torti, J. R. Fitzgerald, T. J. Foster, D. J. Reinscheid, and P. Speziale. FbsA, a fibrinogen-binding protein from Streptococcus agalactiae, in press. [DOI] [PubMed]
- 31.Que, Y. A., J. A. Haefliger, P. Francioli, and P. Moreillon. 2000. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect. Immun. 68:3516-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinscheid, D. J., C. Stoesser, K. Moeller, K. Ehlert, R. W. Jack, B. E. Eikmanns, and G. S. Chhatwal. 2002. Influence of proteins Bsp and FemH on cell shape and peptidoglycan composition in group B streptococcus. Microbiology 148:3245-3254. [DOI] [PubMed] [Google Scholar]
- 33.Rubens, C. E., H. V. Raff, J. C. Jackson, E. Y. Chi, J. T. Bielitzki, and S. L. Hillier. 1991. Pathophysiology and histopathology of group B streptococcal sepsis in Macaca nemestrina primates induced after intraamniotic inoculation: evidence for bacterial cellular invasion. J. Infect. Dis. 164:320-330. [DOI] [PubMed] [Google Scholar]
- 34.Samen, U., B. Gottschalk, B. J. Eikmanns, and D. J. Reinscheid. 2004. Relevance of peptide uptake systems to the physiology and virulence of Streptococcus agalactiae. J. Bacteriol. 186:1398-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schubert, A., K. Zakikhany, M. Schreiner, R. Frank, B. Spellerberg, B. E. Eikmanns, and D. J. Reinscheid. 2002. A fibrinogen receptor from group B streptococcus interacts with fibrinogen by repetitive units with novel ligand binding sites. Mol. Microbiol. 46:557-569. [DOI] [PubMed] [Google Scholar]
- 36.Siboo, I. R., A. L. Cheung, A. S. Bayer, and P. M. Sullam. 2001. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect. Immun. 69:3120-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spellerberg, B. 2000. Pathogenesis of neonatal Streptococcus agalactiae infections. Microbes Infect. 2:1733-1742. [DOI] [PubMed] [Google Scholar]
- 38.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, N. Schnitzler, R. Lütticken, and A. Podbielski. 1999. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67:871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talay, S. R., A. Zock, M. Rohde, G. Molinari, M. Oggioni, G. Pozzi, C. A. Guzman, and G. S. Chhatwal. 2000. Co-operative binding of human fibronectin to Sfbl protein triggers streptococcal invasion into respiratory epithelial cells. Cell Microbiol. 2:521-535. [DOI] [PubMed] [Google Scholar]
- 40.Tamura, G. S., J. M. Kuypers, S. Smith, H. Raff, and C. E. Rubens. 1994. Adherence of group B streptococci to cultured epithelial cells: roles of environmental factors and bacterial surface components. Infect. Immun. 62:2450-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura, G. S., and A. Nittayajarn. 2000. Group B streptococci and other gram-positive cocci bind to cytokeratin 8. Infect. Immun. 68:2129-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura, G. S., and C. E. Rubens. 1995. Group B streptococci adhere to a variant of fibronectin attached to a solid phase. Mol. Microbiol. 15:581-589. [DOI] [PubMed] [Google Scholar]
- 43.Taschner, S., A. Meinke, A. von Gabain, and A. P. Boyd. 2002. Selection of peptide entry motifs by bacterial surface display. Biochem. J. 367:393-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teti, G., F. Tomasello, M. S. Chiofalo, G. Orefici, and P. Mastroeni. 1987. Adherence of group B streptococci to adult and neonatal epithelial cells mediated by lipoteichoic acid. Infect. Immun. 55:3057-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Vossen, J. M., D. van der Lelie, and G. Venema. 1987. Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl. Environ. Microbiol. 53:2452-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waite, D. C., E. J. Alper, and B. J. Mady. 1996. Adult group B streptococcal disease. Ann. Intern. Med. 125:152-153. [DOI] [PubMed] [Google Scholar]
- 47.Wells, J. M., P. W. Wilson, and R. W. Le Page. 1993. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Bac-teriol. 74:629-636. [DOI] [PubMed] [Google Scholar]
- 48.Winram, S. B., M. Jonas, E. Chi, and C. E. Rubens. 1998. Characterization of group B streptococcal invasion of human chorion and amnion epithelial cells in vitro. Infect. Immun. 66:4932-4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zawaneh, S. M., E. M. Ayoub, H. Baer, A. C. Cruz, and W. N. Spellacy. 1979. Factors influencing adherence of group B streptococci to human vaginal epithelial cells. Infect. Immun. 26:441-447. [DOI] [PMC free article] [PubMed] [Google Scholar]