Abstract
Ciliated ependymal cells line the ventricular surfaces and aqueducts of the brain. In ex vivo experiments, pneumolysin caused rapid inhibition of the ependymal ciliary beat frequency and caused ependymal cell disruption. Wild-type pneumococci and pneumococci deficient in pneumolysin caused ciliary slowing, but penicillin lysis of wild-type, not pneumolysin-deficient, pneumococci increased the extent of ciliary inhibition. This effect was abolished by antipneumolysin antibody. Ependymal ciliary stasis by purified pneumolysin was also blocked by the addition of antipneumolysin monoclonal antibodies. These data show that antibiotic lysis of Streptococcus pneumoniae can be detrimental to the ciliated ependyma and that antipneumolysin antibody may have a therapeutic potential.
Ependymal cells lining the ventricular surfaces and aqueducts of the brain form a barrier between the infected cerebrospinal fluid (CSF) of patients with meningitis and their neuronal tissue. Each ependymal cell is covered with around 40 cilia, which beat continuously (1) at a frequency between 35 and 40 Hz. We established an ex vivo model that allowed measurement of the ependymal ciliary beat frequency (CBF), by high-speed video photography, during exposure to bacteria and bacterial toxins. Our initial studies using this model targeted pneumococcal meningitis, for which new, improved therapeutic strategies are urgently required to reduce mortality and neurological damage (11, 12, 13, 16). Currently, only dexamethasone has widely been used as an adjunctive therapy to antibiotic treatment (8). Although its use has shown benefit in a recent European trial (8), no benefit was demonstrated in a clinical trial in Africa (17).
Our recent research has focused on the role of the pneumococcal toxin, pneumolysin, in the pathological process associated with pneumococcal meningitis (12, 13). This work and that of others (5, 24) has led us to believe that blocking the toxic effects of pneumolysin may be advantageous, and the aim of the present study was to determine the ability of antipneumolysin antibodies to reduce damage to the ependymal layer. The rapid release of pneumolysin on antibiotic-induced bacterial lysis (23) is thought to contribute to toxic shock and local tissue damage and may partially be responsible for causing deafness (7). Recently we have shown that pneumococci and pneumolysin cause rapid ependymal ciliary stasis in ependymal brain slices and cultured primary ependymal cells (12), an effect that is mirrored in experimental meningitis in rats (11).
The role of pneumolysin in pneumococcal meningitis has been further elucidated by results from recent in vivo studies (5, 24, 25). The use of β-lactam antibiotics leads to the rapid release of proinflammatory toxic bacterial compounds (18). Therefore, alternative strategies might seek to prevent the release of inflammatory compounds or to block them after they are released. Indeed, a recent study has shown reduced mortality and neuronal injury following treatment of pneumococcal meningitis with inhibitors of bacterial-protein synthesis (19). This reduced mortality and neuronal damage with bacterial-protein synthesis inhibition is likely to be due to a lower level of release of proinflammatory toxic bacterial compounds than of bacteriolytic antibiotics.
Here we show that pneumococci inhibit the ependymal CBF and that inhibitory effects of penicillin-lysed pneumococci and purified pneumolysin on ependymal cilia can be attenuated by antipneumolysin antibodies.
Vibrotome sections (250 μm thick) of ependyma were prepared from the floor of the fourth ventricle of the brains of infant Wistar rats (between 9 and 15 days of age). Each section was submerged under 4 ml of medium 199 as described previously (12). Alternatively, for long-term, low-dose pneumolysin experiments, primary ciliated ependymal cells were cultured as described previously (12).
Beating cilia on ependymal edges were recorded by a high-speed video camera (Kodak EktaPro motion analyzer, model 1012) at a rate of 400 frames per s as previously described (12). At each time point of the study, the CBF was measured at four different areas along each brain slice. Only intact ciliated areas with a thickness in excess of 100 μm were studied at 37°C for 30 min. The CBF measured at this time was used for the baseline reference value. The medium was then exchanged for one of the experimental preparations, and the tissue section was preheated to 37°C, with the observer being blind to its content. The CBF was measured at 30-s intervals for the first 5 min following fluid exchange and then at regular intervals depending on the time course of the experiment. All measurements were taken with the solution temperature between 36.5 and 37.5°C and the pH between 7.35 and 7.45.
The encapsulated Streptococcus pneumoniae strain D39 (2) and an isogenic, pneumolysin-negative mutant (PLN-A) (3, 22) were used. Bacteria were grown and standard inocula were prepared as previously described (6). For use, bacteria were sedimented (4,000 × g for 10 min) and resuspended in medium 199 to a concentration of 108 CFU/ml (12). Heat killing was done by incubation of 1-ml samples at 60°C for 1 h. The addition of penicillin G (1 mg/ml for 3 h) achieved complete lysis of the bacteria. Culture and microscopy were undertaken to confirm the death and lysis of pneumococci. Recombinant pneumolysin was purified from Escherichia coli as previously described (15).
Three pneumolysin-neutralizing monoclonal antibodies were used: PLY-4, PLY-5, and PLY-7 (9). These monoclonal antibodies are specific for pneumolysin and fall into two groups. Antibody PLY-4 prevents toxin pore formation, whereas PLY-5 and PLY-7 inhibit toxin binding to cells (9). The minimum amount of antibody needed to neutralize the lytic activity of toxin in a sample was determined in a standard neutralization assay (9), and 10 times that amount was added to the preparation to be used in the ependyma experiments. Brain sections and antibodies were incubated together for 30 min at 37°C prior to use.
All data presented are means ± standard deviations or standard error means of results of four to nine independent experiments. Statistical analysis was done by analysis of variance, followed by the paired or unpaired Student t test, with Bonferroni's correction for repeated measures.
The results were of interest and as follows. One hundred fifty hemolytic units (HU) of pneumolysin per ml caused ciliary stasis within 2 min. In control samples, the CBF was unchanged over 3 h (36.4 ± 2.9 Hz at 0 h, 40 ± 4.2 Hz at 3 h; P > 0.05). Sloughing of cilia and cytoplasmic extrusion were seen following exposure to pneumolysin, and this effect was not observed in the control group or in any of the groups preincubated with any of the antipneumolysin antibodies. Ependymal cilia preincubated with any of the three monoclonal antipneumolysin antibodies were protected against the rapid ciliary stasis caused by purified pneumolysin (Table 1). The CBF did not change (P > 0.05) over a 3-h period (Table 1), and no cellular damage was observed. The antibody alone had no effect (P > 0.05) on the ependymal CBF (Table 1).
TABLE 1.
Ependymal ciliary beat frequency during incubation of brain ependymal cilia with antipneumolysin antibodies, pneumolysin plus antipneumolysin antibodies, or pneumolysin alone
| Treatment (no. of expts) | CBF (Hz) (SD)
|
|||
|---|---|---|---|---|
| Baseline reading | 60 min posttreatment | 120 min posttreatment | 180 min posttreatment | |
| Control (6) | 42.2 (3.4) | 44.6 (2) | 36.3 (4.6) | 40.65 (2.9) |
| Pneumolysin at 1 μg/ml (4) | 36.1 (3.5) | 0a | 0a | 0a |
| Antibody PLY-4 (4) | 36.2 (2.8) | 40 (2.3) | 36 (6) | 33.6 (3.7) |
| Antibody PLY-5 (4) | 37.9 (3.5) | 37.5 (4.5) | 38.5 (4) | 39.8 (6.2) |
| Antibody PLY-7 (4) | 39.5 (6.1) | 43 (3) | 41.2 (4) | 42.8 (3.9) |
| Pneumolysin at 1 μg/ml + antibody PLY-4 (6) | 36.3 (3.8) | 40 (3.6) | 36 (3.5) | 33.1 (10.0) |
| Pneumolysin at 1 μg/ml + antibody PLY-5 (6) | 36.0 (4.8) | 36 (4.5) | 35 (5) | 34.9 (7.4) |
| Pneumolysin at 1 μg/ml + antibody PLY-7 (6) | 38.0 (3.5) | 38 (2.6) | 39.8 (2.9) | 40.1 (1.8) |
There was total ciliary stasis within 2 min.
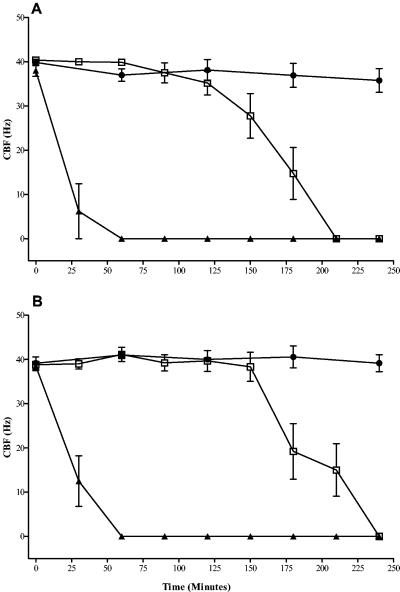
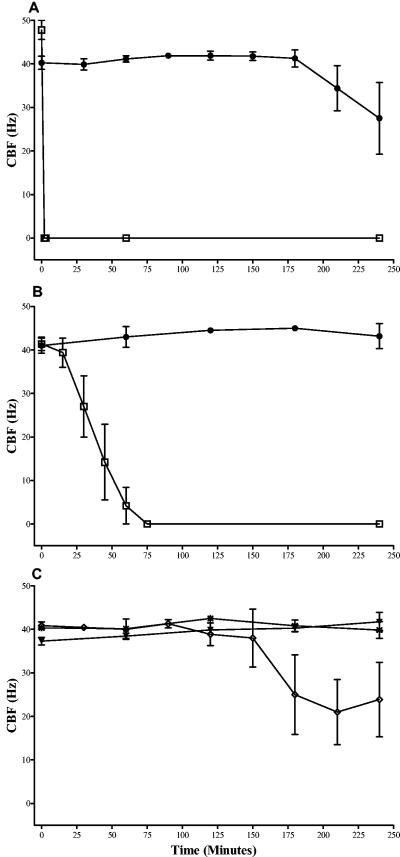
There was no significant difference (P > 0.05) in the times required for ciliary stasis following incubation for the two strains (wild-type pneumococci [Fig. 1A ] and pneumolysin-negative pneumococci [Fig. 1B]) at 108 CFU/ml. In the presence of 107 CFU of the wild type per ml (Fig. 1A) or 107 CFU of pneumolysin-negative pneumococci per ml (Fig. 1B), similar patterns were seen, with ciliary stasis occurring between 90 and 240 min after exposure to either strain. No ciliary stasis was observed in the presence of 106 CFU of wild-type pneumococci (Fig. 1A) or PLN-A (Fig. 1B) per ml. When cilia were exposed to heat-killed wild-type pneumococci (108 CFU/ml), there was no change in either the baseline CBF (over 240 min; P > 0.05) or the integrity of the ependymal edges. The application of 108 (Fig. 2A) or 107 (Fig. 2B) CFU of penicillin-lysed wild-type pneumococci per ml caused ciliary stasis within 3 or 75 min, respectively. There was significant (P < 0.05) inhibition of the CBF with 108 CFU of penicillin-lysed PLN-A per ml at times after 150 min (Fig. 2C) but no inhibition with 107 CFU of penicillin-lysed PLN-A per ml.
FIG. 1.
Inhibition of the ependymal CBF by intact wild-type pneumococci (A) or intact pneumolysin-negative pneumococci (B). Ependyma were given 108 CFU/ml (▴), 107 CFU/ml (□), or 106 CFU/ml (•). Each point is the mean ± the standard error of the mean of results from between five and nine independent experiments.
FIG. 2.
Effects of penicillin-lysed wild-type (D39) pneumococci at 108 CFU/ml (A) and at 107 CFU/ml (B) on the ependymal CBF in the absence (□) and presence (•) of antipneumolysin antibody (PLY-4). (C) Effects of penicillin-lysed PLN-A at 107 CFU/ml (✠) and 108 CFU/ml (◊) and of D39 at 106 CFU/ml (▾) on the ependymal CBF. Each point represents the mean ± the standard error of the mean of results from between five and seven independent experiments.
Exposure to 108 and 107 CFU of lysed wild-type pneumococci per ml was associated with marked cellular extrusion and ciliary sloughing along most of the ependyma. The sloughing of cilia and cytoplasmic extrusion from many ependymal cells were similar in extent to those seen following exposure to 150 HU of pneumolysin per ml. Lysis of 108 CFU of wild-type pneumococci released 1,266 HU of pneumolysin. Only minor damage of the ependyma was observed in the presence of penicillin-lysed PLN-A (108 CFU/ml).
Mixing the antipneumolysin monoclonal antibody PLY-4 with 107 CFU of penicillin-lysed wild-type pneumococci per ml prevented (P < 0.05) ciliary slowing over a 240-min period (Fig. 2B). PLY-4 also had a statistically significant (P < 0.05) protective effect against 108 CFU penicillin-lysed pneumococci per ml (Fig. 2A). Ependymal cellular damage by penicillin-lysed wild-type pneumococci also was prevented by two other antipneumolysin antibodies tested (PLY-5 and PLY-7 [data not shown]). Each antibody also had the effect of halting the extrusion of the cellular contents.
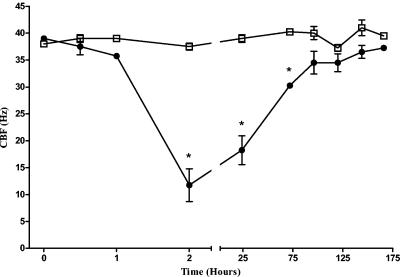
Using purified pneumolysin at very low levels (1 HU), we showed that at times after 1 h, there was a significant (P < 0.05) decrease in the CBF of ependymal cells in culture compared to that of the control (Fig. 3). At these levels, the cilia did not stop beating, and following medium exchange at 2 h and after continued culture, the pneumolysin-treated CBF recovered to the speeds of the control cells. At 72 h, there was no significant difference in the CBFs of control and pneumolysin-treated cells.
FIG. 3.
Effect of 1 HU of pneumolysin (•) on the cultured ependymal CBF compared to that of no pneumolysin (control) (□). The graph shows a significant (*, P < 0.05) inhibition compared with values for the control at 2, 24, and 72 h after exposure to pneumolysin followed by a recovery in the CBF to control levels. Each point is the mean ± the standard error of the mean of results from four independent experiments.
Morbidity and mortality rates of patients suffering from pneumococcal meningitis are increased when numbers of bacteria in the CSF exceed 107 CFU/ml (4). At concentrations of 107 CFU/ml or more, intact wild-type or pneumolysin-deficient pneumococci caused ependymal ciliary stasis, whereas after lysis, only wild-type bacteria were inhibitory. These observations are consistent with the results of our previous study (13), in which we showed that both bacterial pneumolysin and hydrogen peroxide could cause stasis.
Penicillin-induced lysates of wild-type pneumococci caused rapid inhibition of the CBF and caused structural damage to the ependymal layer that was more severe than that of intact pneumococci. This result is not surprising, as antibiotic lysis of D39 pneumococci causes a dramatic rise in extracellular pneumolysin (13). This effect was blocked by the addition of antipneumolysin antibody. Pneumolysin seems to have a dual action on the ependymal CBF: at low levels (1 HU), it is able to reduce the CBF without causing cell death (Fig. 3). Recovery of the CBF is possible over longer times (Fig. 3), but at high levels (150 HU), pneumolysin is cytolytic and irreversibly inhibits the ependymal cilia (16). The mechanism of the low-dose action of pneumolysin-induced ciliary slowing remains to be elucidated. Preparations of penicillin-lysed pneumolysin-negative pneumococci (PLN-A) had minimal effect on the CBF or on the integrity of the ependyma. Not only do these findings support the hypothesis that pneumolysin release is largely responsible for the rapid ciliary stasis and structural damage of the ependyma seen following antibiotic lysis, they strongly suggest that antipneumolysin agents have a therapeutic potential.
Penicillin-induced lysis of PLN-A at 108 CFU/ml caused some ciliary stasis after 150 min. This effect may represent a direct toxic effect of the pneumococcal cell wall on the cilia. The mechanism of action of pneumococcal cell wall toxicity to ependymal cilia requires further study.
Rapid ciliary beating causing continual movement of CSF close to the ventricular surface may prevent initial margination of pneumococci to the protective ependymal layer during infection and play a role in host defense. Therefore, rapid cessation of ciliary movement and ependymal damage would increase the exposure of the brain surface to other toxic factors from the bacteria and the host immune cells. Long-term effects of ependymal damage are also likely. The precise role of the ependyma in relation to the host immune response to pneumococcal meningitis in vivo cannot be determined from this in vitro study alone. Defective ependymal ciliary movement alone has been linked to the development of hydrocephalus (14). Rats with generalized primary ciliary dyskinesia have a very high incidence of hydrocephalus (21). However, ciliary dysfunction alone cannot be the cause of hydrocephalus, because not all patients with defective cilia develop hydrocephalus (14).
The role of pneumolysin in the pathogenesis of pneumococcal meningitis has been studied by Friedland and colleagues (10), who reported that a pneumolysin-deficient strain of S. pneumoniae caused meningeal inflammation in rabbits that was indistinguishable from that induced by the parent pneumolysin-producing strain. Since that report, however, others have shown a direct link between the severity of meningitis and pneumolysin in experimental meningitis (5, 24, 25). The introduction of antibiotics and, more recently, the addition of steroid therapy in the form of dexamethasone have improved the survival of patients with pneumococcal meningitis (8). However, additional therapeutic strategies are needed to reduce the high levels of mortality and morbidity. It is known that pneumococcal meningitis causes a breach of the blood-brain barrier (20); therefore, antibodies may be given intravenously to patients with severe meningitis symptoms. We believe that the use of antipneumolysin antibodies as an adjunctive therapy for severe pneumococcal meningitis merits investigation.
Editor: J. N. Weiser
REFERENCES
- 1.Afzelius, B. A. 1995. Role of cilia in human health. Cell Motil. Cytoskelet. 32:95-97. [DOI] [PubMed] [Google Scholar]
- 2.Avery, O. T., C. M. MacLeod, and M. McCarty. 1979. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Inductions of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 149:297-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohr, V., O. B. Paulson, and N. Rasmussen. 1984. Pneumococcal meningitis. Late neurologic sequelae and features of prognostic impact. Arch. Neurol. 41:1045-1049. [DOI] [PubMed] [Google Scholar]
- 5.Braun, J. S., J. E. Sublett, D. Freyer, T. J. Mitchell, J. L. Cleveland, E. I. Tuomanen, and J. R. Weber. 2002. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J. Clin. Investig. 109:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canvin, J. R., A. P. Marvin, M. Sivakumaran, J. C. Paton, G. J. Boulnois, P. W. Andrew, and T. J. Mitchell. 1995. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J. Infect. Dis. 172:119-123. [DOI] [PubMed] [Google Scholar]
- 7.Comis, S. D., M. P. Osborne, J. Stephen, M. J. Tarlow, T. L. Hayward, T. J. Mitchell, P. W. Andrew, and G. J. Boulnois. 1993. Cytotoxic effects on hair cells of guinea pig cochlea produced by pneumolysin, the thiol activated toxin of Streptococcus pneumoniae. Acta Oto-Laryngol. 113:152-159. [DOI] [PubMed] [Google Scholar]
- 8.de Gans, J., and D. van de Beek. 2002. Dexamethasone in adults with bacterial meningitis. N. Engl. J. Med. 347:1549-1556. [DOI] [PubMed] [Google Scholar]
- 9.de los Toyos, J. R., F. J. Méndez, J. F. Aparicio, F. Vázquez, M. del Mar Garcia Suárez, A. Fleites, C. Hardisson, P. J. Morgan, P. W. Andrew, and T. J. Mitchell. 1996. Functional analysis of pneumolysin by use of monoclonal antibodies. Infect. Immun. 64:480-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedland, I. R., M. M. Paris, S. Hickey, S. Shelton, K. Olsen, J. C. Paton, and G. H. McCracken. 1995. The limited role of pneumolysin in the pathogenesis of pneumococcal meningitis. J. Infect. Dis. 172:805-809. [DOI] [PubMed] [Google Scholar]
- 11.Hirst, R. A., B. Gosai, A. Rutman, P. W. Andrew, and C. O'Callaghan. 2003. Streptococcus pneumoniae damages the ciliated ependyma of the brain during meningitis. Infect. Immun. 71:6095-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirst, R. A., A. Rutman, K. Sikand, P. W. Andrew, T. J. Mitchell, and C. O'Callaghan. 2000. Effect of pneumolysin on rat brain ciliary function: comparison of brain slices with cultured ependymal cells. Pediatr. Res. 47:381-384. [DOI] [PubMed] [Google Scholar]
- 13.Hirst, R. A., K. S. Sikand, A. Rutman, T. J. Mitchell, P. W. Andrew, and C. O'Callaghan. 2000. Relative roles of pneumolysin and hydrogen peroxide from Streptococcus pneumoniae in inhibition of ependymal ciliary beat frequency. Infect. Immun. 68:1557-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiefer, M., R. Eymann, S. von Tiling, A. Muller, W. I. Steudel, and K. H. Booz. 1998. The ependyma in chronic hydrocephalus. Child's Nerv. Syst. 14:263-270. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell, T. J., J. A. Walker, F. K. Saunders, P. W. Andrew, and G. J. Boulnois. 1989. Expression of the pneumolysin gene in Escherichia coli: rapid purification and biological properties. Biochim. Biophys. Acta 1007:67-72. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed, B. J., T. J. Mitchell, P. W. Andrew, R. A. Hirst, and C. O'Callaghan. 1999. The effect of the pneumococcal toxin, pneumolysin on brain ependymal cilia. Microb. Pathog. 27:303-309. [DOI] [PubMed] [Google Scholar]
- 17.Molyneux, E. M., A. L. Walsh, H. Forsyth, M. Tembo, J. Mwenechanya, K. Kayira, L. Bwanaisa, A. Njobvu, S. Rogerson, and G. Malenga. 2002. Dexamethasone treatment in childhood bacterial meningitis in Malawi: a randomised controlled trial. Lancet 360:211-218. [DOI] [PubMed] [Google Scholar]
- 18.Nau, R., and W. Bruck. 2002. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci. 25:38-45. [DOI] [PubMed] [Google Scholar]
- 19.Nau, R., and H. Eiffert. 2002. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin. Microbiol. Rev. 15:95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quagliarello, V. J., W. J. Long, and W. M. Scheld. 1986. Morphologic alterations of the blood-brain barrier with experimental meningitis in the rat. Temporal sequence and role of encapsulation. J. Clin. Investig. 77:1084-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu, A., and M. Koto. 1992. Ultrastructure and movement of the ependymal and tracheal cilia in congenitally hydrocephalic WIC-Hyd rats. Child's Nerv. Syst. 8:25-32. [DOI] [PubMed] [Google Scholar]
- 22.Shoemaker, N. B., and W. R. Guild. 1974. Destruction of low efficiency markers is a slow process occurring at a heteroduplex stage of transformation. Mol. Gen. Genet. 128:283-290. [DOI] [PubMed] [Google Scholar]
- 23.Spreer, A., H. Kerstan, T. Böttcher, J. Gerber, A. Siemer, G. Zysk, T. J. Mitchell, H. Eiffert, and R. Nau. 2003. Reduced release of pneumolysin by Streptococcus pneumoniae in vitro and in vivo after treatment with nonbacteriolytic antibiotics in comparison to ceftriaxone. Antimicrob. Agents Chemother. 47:2649-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wellmer, A., G. Zysk, J. Gerber, T. Kunst, M. von Mering, S. Bunkowski, H. Eiffert, and R. Nau. 2002. Decreased virulence of a pneumolysin-deficient strain of Streptococcus pneumoniae in murine meningitis. Infect. Immun. 70:6504-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winter, A. J., S. D. Comis, M. P. Osborne, M. J. Tarlow, J. Stephen, P. W. Andrew, J. Hill, and T. J. Mitchell. 1997. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect. Immun. 65:4411-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]