Abstract
Mast cells are important for protective immunity to intestinal helminth infections and as mediators of allergic disease. Their role in protozoan infections is less well described. We have therefore analyzed mast cell responses and parasite control in mice infected with the protozoan Giardia lamblia. We also measured immunoglobulin A (IgA) responses to the parasite, as IgA can have a protective role in this model. c-kitw/wv mice failed to make parasite-specific IgA, mount a mast cell response, or eliminate the infection. Anti-c-kit-treated C57BL/6 mice had normal IgA responses, lacked mast cell responses, had reduced interleukin-6 (IL-6) mRNA in the small intestine, and failed to control the infection within 10 days. IL-9-deficient mice had a significant but reduced mast cell response and still controlled the infection within 2 weeks. Interestingly, IL-6-deficient mice had enhanced mast cell responses yet failed to rapidly control the infection. However, prevention of mast cell responses in IL-6-deficient mice by anti-c-kit treatment did not lead to parasite elimination. Both IL-6- and IL-9-deficient mice had normal IgA production. IL-6-deficient mice had significant serum levels of mast cell mediators, histamine and mast cell protease 1, following infection. Together, these results show that mast cells are important for the rapid control of Giardia infections in mice. Furthermore, they show that IL-6 is not necessary for these mast cell responses. Instead, they suggest that mast cell production of IL-6 appears to be important for control of this infection.
Giardia lamblia (syn. Giardia duodenalis, Giardia intestinalis) is a protozoan parasite which replicates exclusively in the lumen of the small intestine of a wide variety of mammalian hosts (reviewed in reference 11). Giardia infections are practically universal in the developing world, and the Centers for Disease Control and Prevention estimates that two million infections occur in the United States each year. Infections in young children are particularly common. In some cases, these infections result in acute or chronic symptoms, including malabsorptive diarrhea, cramps, and nausea, although asymptomatic infections are common. Most individuals effectively control these infections within a few weeks, although the precise immune mechanisms responsible for eliminating parasites are unknown. It also remains unclear whether symptomatic disease is due to immune-mediated pathology or parasite-derived factors or both.
Immunity to G. lamblia has been shown to occur in at least two distinct phases in mice. Normally mice eliminate this infection within 2 weeks. T cells and interleukin-6 (IL-6) are important in this early phase, since SCID mice, anti-CD4-treated mice, Tcrβ gene-targeted mice, and IL-6-deficient mice all fail to control these infections within this time frame (2, 4, 29, 36). In contrast, antibodies are not necessary in this phase, as B-cell-deficient mice eliminate the majority of parasites within 2 weeks (29). If infections persist beyond this initial phase, a second phase of the immune response can occur. This is best seen in IL-6-deficient mice that eliminate the parasites between 4 and 8 weeks postinfection (36). This correlates with the appearance of antiparasite immunoglobulin A (IgA) antibodies in the intestinal fluid that react with all parasites present in a population expressing diverse surface antigens. The longer time required for antibody-dependent control of these infections is consistent with the ability of the parasite to undergo antigenic variation (23). B-cell-deficient mice lack this second phase of immunity and have extended low-level infections, further supporting a role for antibodies in the control of chronic infections (19, 32). Thus, an IL-6-dependent pathway can control the infection early on while a B-cell-dependent pathway plays a role later on and may be important for preventing chronic infections. Similarly, human patients with X-linked agammaglobulinemia or common variable immunodeficiency are at risk for chronic giardiasis.
Immunity to the related parasite Giardia muris is similar, although B cells appear to play a more important role earlier. Anti-IgM-treated mice and xid mutant mice have defects in controlling this infection, and Langford et al. and Snider et al. recently showed that B-cell-deficient mice and IgA-deficient mice have significant defects in controlling G. muris infection early on (19, 30, 31). Nevertheless, significant decreases in parasite numbers were observed between 1 and 3 weeks postinfection in the absence of antibodies, consistent with the existence of an antibody-independent mechanism for controlling this infection as well. T cells are also important in this model, since nude mice and anti-CD4-treated mice have defects controlling G. muris infection (16, 27).
Mast cell responses have been suggested in protection against both G. lamblia, and G. muris infections. Infections in gerbils with G. lamblia or in mice with G. muris have shown that mast cells accumulate in the small intestine following infection (15, 20, 34). In addition, mast cell-deficient (c-kitw/wf) mice were unable to control G. muris infections (8), suggesting that mast cell responses may be involved in controlling these infections. However, c-kit mutant animals exhibit multiple defects including anemia, abnormal γδ T cell development, and an absence of intestinal pacemaking activity (13, 26, 28). Cyproheptadine (an antagonist of histamine H1 receptors) treatment prolonged infections with G. muris, suggesting that histamine release from mast cells may be involved in Giardia immunity (34). Importantly, attempts to reconstitute the mast cell response in the c-kitw/wf mice by adoptive transfer of bone marrow mast cells did not restore resistance to infection (8), and cyproheptadine has numerous effects unrelated to its antihistaminic properties, making it difficult to precisely implicate mast cells in resistance to Giardia infection based on these data.
We have therefore analyzed G. lamblia infections and mast cell and IgA responses in c-kit mutant as well as anti-c-kit-treated mice to determine whether mast cells have a role in T-cell-dependent immunity to Giardia. We have also examined infections and measured mast cell and IgA responses in IL-6 and IL-9 mutant mice to determine whether these cytokines are important for mast cell responses in the intestine during Giardia infections.
MATERIALS AND METHODS
Mice.
BALB/c, C57BL/6J, B6.129S2-Il6tm1Kopf, WBB6 F1/J c-kit+/+, and WBB6/F1J c-kitw/wv mice were obtained from Jackson Laboratories (Bar Harbor, Maine). IL-9 gene-targeted mice (33) were backcrossed onto the BALB/c background for six generations and then maintained as an inbred line. IL-9 mutant mice were obtained from Andrew McKenzie (Cambridge University) and bred at Georgetown University under specific-pathogen-free conditions, although the colony carried infections with Helicobacter hepaticus. All experiments were performed with the approval of the Georgetown University Animal Care and Use Committee.
Parasites and infections.
G. lamblia (strain GS/M/H7) was cultured and used for infections as previously described (36). Mice were infected by gavage with 5 × 105 to 10 × 105 parasites in phosphate-buffered saline (PBS), and parasite numbers in the small intestines were determined at different days postinfection. To count parasites, 10-cm sections of small intestine were minced in 4 ml of PBS, kept on ice for >30 min to release parasites, and counted on a hemocytometer. Because Giardia requires bile for growth, we routinely analyzed parasite numbers in the duodenum, beginning just distal to the junction of the small intestine with the common bile duct.
Mast cell blockade in vivo.
Anti-c-kit rat IgG2b (ACK-2) (25) and control rat IgG2b (J1.2) were provided by Fred Finkelman (University of Cincinnati) as ammonium sulfate-purified ascites fluid. Mice were treated with 0.5 mg of antibody in 200 μl of PBS by intraperitoneal injection on days 2, 4, 6, and 8 postinfection. Mice were analyzed on day 10 postinfection.
Intestinal antibody responses.
Intestinal washes were collected from the 5-cm segment of jejunum distal to that used for counting parasites by flushing the lumen with 0.5 ml of PBS/mouse. The presence of antiparasite antibodies was determined as previously described (36). Briefly, parasite cultures were allowed to attach to glass slides and fixed with cold methanol-acetone (1:1). Slides were blocked with 5% goat serum in PBS and then incubated with intestinal washes diluted 1:5 in PBS plus 5% goat serum, followed by goat anti-mouse IgA, goat anti-mouse IgG, or goat anti-mouse IgM conjugated to fluorescein isothiocyanate (Southern Biotechnology Associates, Birmingham, Ala.). Giardia-specific monoclonal antibodies of each isotype were used as controls for the specificity and sensitivity of each secondary reagent. Slides were mounted with Vectashield plus propidium iodide (Vector Laboratories, Burlingame, Calif.) and viewed with a Zeiss Axiophot microscope. Images were collected with a CoolSnap fx camera (Roper Scientific, Trenton, N.J.) by using OpenLab software (Improvision, Cambridge, Mass.). Images were processed with Photoshop (Adobe Systems, San Jose, Calif.).
Mast cell staining.
Two- to three-centimeter segments of small intestine distal to the segment used for collecting parasites (after collection of intestinal washes) were fixed in 10% formalin, embedded in paraffin, and sectioned. Tissue sections were stained for chloroacetate esterase activity as described previously (12) and counterstained with hematoxylin. Slides were viewed as for the fluorescence microscopy, except that the microscope was also equipped with a CRI filter wheel (Cambridge Research, Cambridge, Mass.). Mast cell numbers were then determined by an observer who was unaware of the identities of the samples.
Serum histamine and mast cell protease measurements.
Venous blood was collected from mice immediately prior to sacrifice and allowed to clot overnight at 4°C. Sera were collected after centrifugation and stored in aliquots at −20°C until tested. Histamine levels were measured in sera diluted 1:20 with PBS by using a competitive enzyme-linked immunosorbent assay system (Immunotech; Beckman Coulter, Fullerton, Calif.). Mouse mast cell protease levels were determined by capture enzyme-linked immunosorbent assay (Moredun Scientific, Midlothian, Scotland, United Kingdom).
RT-PCR.
Small intestinal RNA was prepared from a 1- to 2-cm segment of the small intestine distal to that used for enumerating parasites. RNA was purified by using RNA-STAT-60 (Teltest, Inc., Midland, Tex.). Reverse transcription (RT) and amplification of IL-6 and hypoxanthine phosphoribosyltransferase cDNA were performed as described previously (36). Briefly, 5 μg of RNA was reverse transcribed with SuperScript II reverse transcriptase (Invitrogen, Carlsbad, Calif.). cDNA was then amplified with Taq polymerase and primers for hypoxanthine phosphoribosyltransferase to determine whether samples contained similar amounts of cDNA. IL-6 cDNA was then amplified and analyzed on 1.5% agarose gels in 0.5× Tris-borate-EDTA buffer.
Statistics.
Data were analyzed by either the Student t test or the Mann-Whitney test. The nonparametric Mann-Whitney test was used when analyzing parasite numbers, since data were not normally distributed, and t tests were used for comparisons of mast cell numbers, histamine, and mast cell protease levels. Analyses were performed with GraphPad Prism (version 3.0cx; GraphPad Software, Inc., San Diego, Calif.). P values of <0.05 were considered significant.
RESULTS
Mast cell-deficient mice fail to control infections.
Our laboratory has recently shown that antibodies are not required for control of G. lamblia infections in mice (29). In this phase of the infection, parasite control requires IL-6 and normally occurs within the first 2 weeks (2, 36). Since mast cells have been suggested to control G. muris infections (8, 34), we wanted to determine whether mast cells were involved in the early control of G. lamblia infections and how mast cell responses were related to IL-6. We therefore began by infecting genetically mast cell-deficient c-kitw/wv mice with G. lamblia and determining whether they were able to control the infection. Whereas wild-type mice controlled G. lamblia infections within 1 to 2 weeks, the c-kit mutant mice failed to control the infections even after 5 months (Table 1). The small decrease in parasite numbers seen late in the infection of the c-kit mutant mice was not statistically significant. As expected, no mast cells were seen in the intestines of c-kit mutant mice even after infection with G. lamblia (data not shown). Thus, the c-kitw/wv mice exhibit a profound inability to control infections with G. lamblia during the early phase of infection as well as a defect in the later phase.
TABLE 1.
G. lamblia infections in mast cell-deficient mice
| Groupa | Day postinfection | 104 Parasites (avg ± SD)b |
|---|---|---|
| WBB6F1/J c-kit+/+ | 4 | 5.8 ± 3.3 |
| 10 | 0.3 ± 0.6 | |
| 29 | 0.4 ± 0.7 | |
| 172 | ND | |
| WBB6F1/J c-kitw/wv | 4 | 51.7 ± 26.2c |
| 10 | 98.8 ± 56.7c | |
| 29 | 16.7 ± 18.5c | |
| 172 | 27.9 ± 14.5c |
Groups of 4 to 6-week-old female mice were infected with 106 G. lamblia on day 0.
Average and standard deviation of the number of parasites found in the small intestines on the indicated day postinfection. Each group consisted of 3 mice per time point. ND, none detected. Data are representative of the results from three separate experiments.
P = 0.05 compared to wild-type controls by the Mann-Whitney test.
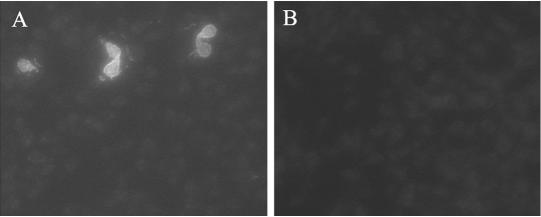
IgA has long been thought to be involved in the control of giardiasis (19). Furthermore, results in the IL-6-deficient mice suggested that while IgA cannot initially control infections with G. lamblia due to the ability of the parasite to undergo antigenic variation, IgA was effective later in the infection (36). Since the c-kitw/wv mice failed to control the infection after 5 months, we also examined the production of parasite-specific IgA in c-kitw/wv mice and wild-type controls. As expected, IgA produced 14 days postinfection from wild-type mice reacted with only a subset of parasites present in a diverse in vitro population (Fig. 1A). Antiparasite IgG and IgM were not detectable in the intestinal washes of these mice, consistent with IgA being the major antibody isotype secreted into the small intestine (data not shown). Moreover, c-kit mutant mice did not have antiparasite IgA in their intestinal fluid at this time (Fig. 1B). This suggests that a defect in IgA production in c-kitw/wv mice may be involved in their inability to control Giardia infections.
FIG. 1.
Intestinal IgA Responses in G. lamblia-infected mice. Intestinal fluid was collected from the jejuna of 14-day-old infected c-kit+/+ (A) or c-kitw/wv (B) mice and used to stain trophozoites from in vitro cultures of G. lamblia. Fluorescein isothiocyanate-conjugated anti-IgA was used to demonstrate the presence or absence of IgA in the intestinal fluid, and propidium iodide (data not shown) was used to identify the parasites.
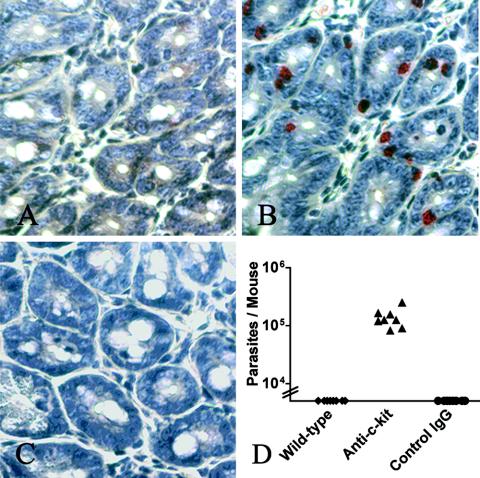
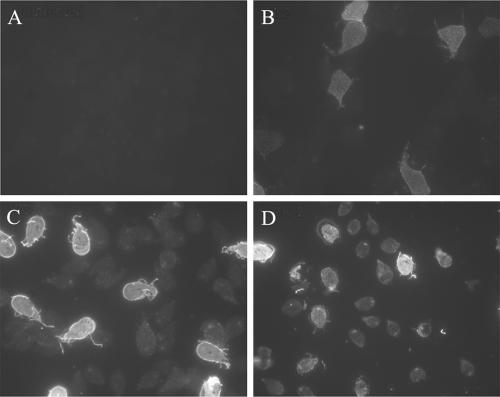
The inability of c-kitw/wv mice to produce antiparasite IgA makes it impossible to conclude that the absence of mast cells in these mice is responsible for the failure to control Giardia infections. The lack of IgA itself may contribute to the failure to control infections or may reflect a defect in T-cell-dependent immune responses in general. We therefore used a second approach to study mast cell function in vivo, a blockade of mast cell responses with noncytolytic anti-c-kit antibodies. The anti-c-kit antibody ACK-2 has been used to specifically inhibit mast cell responses during murine infections with Trichinella spiralis (14) and during mast cell-dependent diarrhea (3). Furthermore, while ACK-2 treatment of neonatal mice can affect the development of numerous cell types, including melanocytes and interstitial cells of Cajal in the intestinal tract, these effects require treatment within the first few days postpartum (21, 24). Short-term treatment of adult mice with this antibody does not affect these other cells. We therefore analyzed mast cell responses in wild-type mice infected with Giardia with and without injection of anti-c-kit antibodies during the infections. Whereas uninfected mice had few mast cells in the small intestine, mice infected with G. lamblia accumulated large numbers of mast cells in the small intestine (Fig. 2A and B). As expected, injection of anti-c-kit antibodies completely prevented the accumulation of mast cells in the small intestines of the infected mice (Fig. 2C). It also inhibited the ability of these mice to eliminate their infections (Fig. 2D). Since treatment with ACK-2 may have interfered with hematopoietic cell development, we also examined IgA production in anti-c-kit-treated mice to determine whether T-cell-dependent antibody responses were affected. While intestinal fluid from uninfected mice contained no detectable anti-Giardia IgA (Fig. 3A), fluid from infected mice had IgA which reacted with a subset of the parasites present in our in vitro populations (Fig. 3B to D). This pattern of IgA reactivity is consistent with antibodies specific for the variant-specific surface proteins of the parasite. No differences in the pattern of IgA reactivity were seen among infected mice that were not treated with IgG or that received either control IgG or anti-c-kit IgG (Fig. 3B to D). It remains possible that ACK-2 antibody treatment may have led to differences in the quantity of IgA secreted into the intestine. Importantly, these data show that the lack of IgA in c-kit mutant mice is not due to the absence of mast cells but represents an additional defect in these mice. Furthermore, together, these data strongly argue that mast cells are required during the early phase of control of Giardia infections in mice.
FIG. 2.
Mast cell responses and infections in anti-c-kit-treated mice. C57BL/6J mice were not infected (A) or infected (B and C) with 106 G. lamblia trophozoites and treated on days 2, 4, 6, and 8 with either control IgG (B) or anti-c-kit antibody (C). On day 10 postinfection, small intestines were fixed in formalin and paraffin sections were stained for chloroacetate esterase activity (red) to visualize mast cells and counterstained with hematoxylin. Similar results were seen with 8 mice/group. (D) Parasite numbers were determined in C57BL/6J mice infected with Giardia for 10 days and untreated (diamonds), treated with anti-c-kit (triangles), or treated with control IgG (circles). Each symbol represents a single mouse (n = 8 mice/group). *, P < 0.05 compared to wild-type controls. Data are representative of the results from three independent experiments.
FIG. 3.
IgA responses in anti-c-kit-treated mice infected with G. lamblia. Intestinal washes from uninfected C57BL/6J mice (A), 10-day-old infected C57BL6/J mice treated with control antibody J1.2 (B), untreated (C), or treated with anti-c-kit antibody ACK-2 (D) were used to stain in vitro cultures of G. lamblia with an IgA-specific secondary antibody. IgA detection was performed on at least 8 mice/group with similar results. Data are representative of the results from three independent experiments.
IL-9 is not necessary for mast cell accumulation.
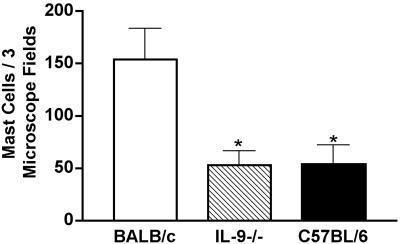
IL-9 has been implicated in inducing mast cell responses during helminth infections. Townsend et al. showed that IL-9-deficient mice had markedly reduced mast cell accumulation in the lungs of mice instilled with Schistosoma mansoni eggs (33). To determine whether IL-9 was also needed for intestinal mast cell responses and protection against Giardia infections, IL-9-deficient mice were infected with G. lamblia. Since the IL-9 deletion was present on the BALB/c background, we infected both wild-type BALB/c and C57BL/6 mice as controls to facilitate comparisons with our earlier analyses with C57BL/6 mice. At 5 days postinfection, the number of parasites in the IL-9-deficient mice was similar to that seen in C57BL/6 mice and significantly lower than that seen in BALB/c controls (Table 2). By 2 weeks postinfection, the IL-9-deficient mice completely eliminated detectable Giardia parasites, the same as both sets of wild-type controls (Table 2). Antiparasite IgA was found in the intestinal fluid of all infected IL-9-deficient mice, similar to wild-type mice (data not shown). Strikingly, the number of mast cells in the small intestines of IL-9-deficient mice following infection was roughly one-third of that found in infected control BALB/c mice and was similar to that seen in the C57BL/6 mice (Fig. 4). In all cases, this number was much greater than in uninfected mice (Fig. 5A and data not shown). This is consistent with recent reports showing that IL-9 is not absolutely required for intestinal mast cell responses, although it does appear to enhance these responses (9).
TABLE 2.
G. lamblia infections in C57BL/6, BALB/c, and IL-9-deficient BALB/c mice
| Mouse straina | Day postinfection | 104 Parasites (avg ± SD)b |
|---|---|---|
| C57BL/6J | 5 | 0.7 ± 0.9 |
| 12 | ND | |
| BALB/c | 5 | 62.1 ± 49.2c |
| 12 | ND | |
| BALB/c IL-9−/− | 5 | 0.6 ± 0.3d |
| 12 | ND |
Four- to 6-week-old female mice were infected with 106 G. lamblia on day 0, and groups of 4 mice were analyzed on the indicated days postinfection.
Average and standard deviation of the number of parasites found in the small intestines. ND, none detected. Data are representative of the results from two separate experiments.
P < 0.05 compared to C57BL/6 mice by the Mann-Whitney test.
P < 0.05 compared to BALB/c controls by the Mann-Whitney test.
FIG. 4.
Mast cell responses in IL-9-deficient mice. BALB/c, IL-9-deficient, and C57BL/6 mice were infected with 106 G. lamblia trophozoites, and mast cell numbers were determined at day 14 postinfection. Mast cell numbers were determined on coded slides for 4 mice/genotype. Numbers of mast cells per three microscope fields were counted rather than mast cells per 10 villus-crypt units, as in Fig. 5, since tissue sections from many of these mice did not contain complete villus-crypt units but were sagittal sections through villi. Means and standard deviations are shown. *, P < 0.05 compared to BALB/c mice. Data are representative of the results from two independent experiments.
FIG. 5.
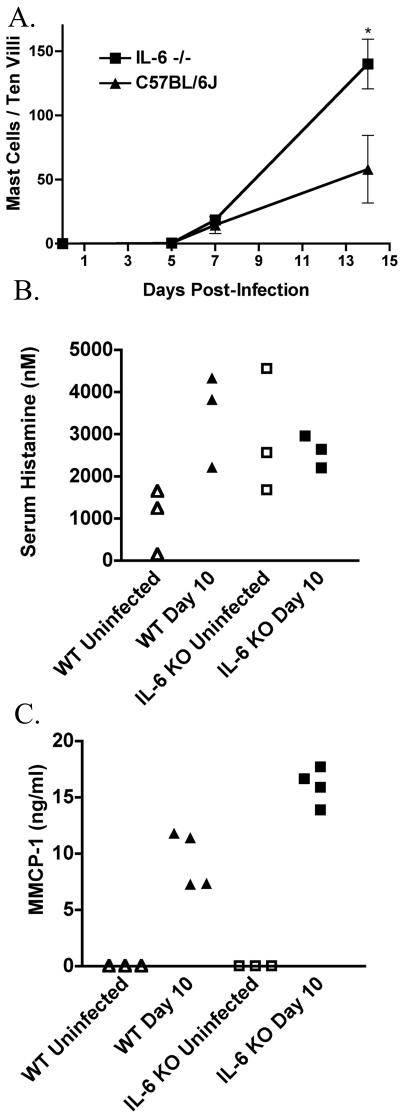
Mast cell responses in IL-6-deficient mice. C57BL/6J (triangles) and B6.129S2-Il6tm1Kopf (squares) mice were infected with 500,000 G. lamblia trophozoites and sacrificed at different times postinfection. Mast cells were visualized as described for Fig. 2. The number of mast cells are shown as means and standard deviations for 4 mice/time point (A). Sera were collected from C57BL/6J (triangles) and B6.129S2-Il6tm1Kopf (squares) mice on day 10 postinfection (filled symbols) and uninfected mice (open symbols) and assayed for histamine (B) and MMCP-1 (C). *, P < 0.05 compared to wild-type controls. Data from panel A are representative of the results from three independent experiments.
IL-6 is not necessary for mast cell responses.
We and others have recently shown that IL-6 is required for efficient control of Giardia lamblia infections in mice (2, 33). Furthermore, while IL-6 was first described as a B cell growth factor, it is also a growth factor for human mast cells and is commonly used to culture these cells from peripheral or cord blood. We therefore asked whether mast cell responses during Giardia infections were dependent on IL-6 by examining responses in IL-6-deficient mice. As we have previously reported, wild-type C57BL/6 mice 2 weeks postinfection reduced the number of parasites in the small intestine to levels below the limits of detection, whereas IL-6-deficient mice were still heavily infected at this time (Table 3) (36). Mast cells began to accumulate in the small intestines of both wild-type and IL-6-deficient mice 7 days postinfection, coincident with the beginning of parasite elimination in the wild-type mice (Fig. 5A). By day 14 postinfection, the IL-6-deficient mice had accumulated roughly three times as many mast cells as wild-type mice, even though these mice still had large numbers of parasites. Thus, the mast cell response during Giardia infection occurs independently of IL-6. The accumulation of mast cells in IL-6-deficient mice, however, is insufficient to effectively control the infection. To determine whether the enhanced mast cell accumulation seen in IL-6-deficient mice was actually responsible for the failure of these mice to control infections, IL-6-deficient mice infected with Giardia were treated with anti-c-kit antibody to block the accumulation of mast cells. Anti-c-kit-treated IL-6-deficient mice had just as many parasites as mice treated with control antibody (5.0 × 106 ± 0.2 × 106 versus 5.5 × 106 ± 0.5 × 106, n = 4 per group). As expected, the anti-c-kit treatment did indeed block the accumulation of mast cells in IL-6-deficient mice (data not shown). Thus, the enhanced mast cell responses in IL-6-deficient mice do not contribute to their failure to control infections.
TABLE 3.
G. lamblia infections in IL-6-deficient mice
| Mouse straina | Day postinfection | 104 Parasites (avg ± SD)b |
|---|---|---|
| C57BL/6J | 5 | 9 ± 19 |
| 7 | 19 ± 30 | |
| 14 | ND | |
| C57BL/6J IL-6−/− | 5 | 156 ± 246c |
| 7 | 378 ± 216c | |
| 14 | 619 ± 463c |
Four- to 6-week-old female mice were infected with 5 × 105 G. lamblia on day 0, and groups of 4 mice were analyzed on the indicated days postinfection.
Average and standard deviation of the number of parasites found in the small intestines. ND, none detected. Data are representative of the results from three separate experiments.
P < 0.05 compared to wild-type controls by the Mann-Whitney test.
If mast cells contribute to the control of infections in wild-type mice, why don't they control the infection in IL-6-deficient mice? A possible answer is that mast cells require IL-6 to produce mediators required to control infections. Indeed, it has recently been shown that IL-6 enhances the production of histamine by cultured human peripheral blood mast cells (18). We therefore measured serum levels of two mast cell mediators, histamine and mouse mast cell protease 1 (MMCP-1), in wild-type and IL-6-deficient mice before and after infection with Giardia. Sera from wild-type mice had three times more histamine at 10 days postinfection than sera from uninfected mice (Fig. 5B) (3,451 ± 638 nM versus 1,009 ± 448 nM, n = 3, P < 0.05), consistent with the observed mast cell response. IL-6-deficient mice had elevated serum histamine levels in the absence of infection, and these levels were unchanged by infection (Fig. 5B) (2,929 ± 1,474 nM versus 2,592 ± 379 nM, n = 3, P > 0.05). Similarly, while MMCP-1 was not detectable in the sera of uninfected mice, both wild-type mice and IL-6-deficient mice showed elevated levels of MMCP-1 in the sera 10 days postinfection (Fig. 5C). The response in wild-type mice was lower than that seen in IL-6-deficient mice (9.4 ± 2.5 ng/ml and 16.0 ± 1.6 ng/ml, n = 4, P < 0.01), consistent with the increased numbers of mast cells seen in these animals. As expected, this response was completely blocked by treatment with anti-c-kit antibody. Thus, neither production of histamine nor MMCP-1 was lacking in the IL-6-deficient mice.
Mast cells as source of IL-6.
Since IL-6 was not necessary for mast cell accumulation or activation, we next examined whether mast cells are in fact a source of IL-6 during Giardia infection. RT-PCR analysis of IL-6 mRNA from infected wild-type mice treated with anti-c-kit antibody to block the mast cell response shows that IL-6 mRNA levels are reduced in the absence of mast cells compared to infected mice treated with control IgG or not treated with antibody (Fig. 6). However, IL-6 mRNA levels in the anti-c-kit-treated infected mice still appear higher than those in uninfected mice. Mast cells are not the only source of this cytokine in the intestinal tract, and these data suggest that multiple cell types contribute to the IL-6 response in vivo. These data are consistent with the idea that mast cells are a source of IL-6 during Giardia infection and that this IL-6 production is important for control of the infection.
FIG. 6.

IL-6 mRNA expression in mast cell-deficient mice. RNA was isolated from uninfected C57BL/6 mice (lane 1) and infected mice treated with no antibody (lane 2), with anti-c-kit antibody (lane 3) or with control IgG (lane 4). RNA was converted to cDNA, and IL-6 levels were determined from pooled samples (n = 4).
DISCUSSION
Our results clearly demonstrate that mast cells are important for control of murine infections with G. lamblia. Mice infected with Giardia accumulated mast cells in the small intestine as they eliminated the infection. Furthermore, mice which failed to mount a mast cell response, either due to mutation in the c-kit gene or due to treatment with anti-c-kit antibodies, also failed to eliminate the infection. Results also show that mast cell responses do not require IL-6 or IL-9. IL-6-deficient mice infected with Giardia accumulated more mast cells in the small intestines than wild-type controls. IL-9-deficient mice also accumulated significant numbers of mast cells compared to uninfected mice, although there was reduced mast cell accumulation compared to wild-type controls in this model. Finally, our data indicate that IL-6 is not necessary for the production of mast cell mediators such as histamine and MMCP-1 but instead suggest that IL-6 production by mast cells is an important element of the immune response to Giardia.
A previous study with c-kitw/wf mice infected with G. muris observed prolonged infections in this model (8). They were unable to restore immunity, however, by adoptive transfer of bone marrow-derived mast cells. We have found that c-kitw/wv mice also fail to eliminate G. lamblia infections as well as control mice. We have also documented a defect in parasite-specific IgA production in these mice. To our knowledge, this is the first report of a defect in antibody production in c-kit mutant mice. Furthermore, while the failure of these mice to eliminate parasites does not exclude a role for mast cells in this infection, it requires that a different approach be used to demonstrate a requirement for mast cells. Treatment with anti-c-kit antibodies clearly inhibited the elimination of parasites and blocked mast cell responses without reducing parasite-specific antibodies. This result supports the idea that mast cells are required, since ACK-2 treatment of adult mice did not inhibit the production of parasite-specific IgA nor does it interfere with intestinal motility coordinated by the interstitial cells of Cajal that innervate the intestinal musculature (17, 35). While ACK-2 treatment of adult mice does inhibit the development of spermatogonia, it is difficult to see how this could affect Giardia infection in the intestine. In addition, these experiments were done in female mice and ACK-2 treatment does not interfere with oocyst development.
Immunity to Giardia occurs in distinct phases. It was previously shown that IL-6 is important in the early immune response (36), and the data presented here argue that mast cells are also involved in this phase. It is therefore likely that mast cell responses function in the same pathway as IL-6. IL-6 is clearly not required for the accumulation of mast cells, since the number of mast cells observed in infected IL-6-deficient mice was roughly threefold greater than in wild-type mice. IL-6 is also not required for production of histamine or MMCP-1, as both were found in IL-6-deficient mice. It remains possible that IL-6 is required for the production of some other mediator made by mast cells and that this mediator is required for the control of infections. Alternatively, mast cell mediators in conjunction with IL-6 from an unknown source are required in concert to control this infection. The simplest connection between mast cells and IL-6 in this system, however, is that mast cell production of IL-6 itself is involved in the elimination of Giardia. Consistent with this idea, mice treated with anti-c-kit antibodies to prevent mast cell responses had lower levels of IL-6 mRNA in the small intestine than did controls. Other cell types may also be producing IL-6 during Giardia infections, however, and a blockade of mast cell responses did not completely eliminate the induction of IL-6 mRNA levels.
Our studies further support a role for antibody during the later phase, but not during the early phase, of control of G. lamblia infections in mice. Like IL-6-deficient mice, anti-c-kit-treated mice produced antiparasite IgA but failed to rapidly control this infection, indicating that antibodies are not sufficient early on. The failure of c-kitw/wv mice to produce antiparasite IgA and to control infections with G. lamblia, even after 4 months, highlights the importance of IgA in this second pathway. Similarly, Langford et al. recently found that IgA-deficient mice developed chronic infections with G. muris and G. lamblia, although a sharp drop in parasite numbers was seen early in these infections (19). In humans, chronic giardiasis is associated with hypogammaglobulinemias, including Bruton's agammaglobulinemia and common variable immunodeficiency (11), suggesting that IgA may also be important for the control of chronic infections in humans.
IL-9, but not IL-6, is involved in generating the mast cell response to Giardia infection. IL-9-deficient mice had approximately one-third as many mast cells in the small intestine as control mice following infection. This was far less of a difference than was previously seen in the lungs of IL-9-deficient mice after challenge with S. mansoni eggs (33). However, the absence of IL-9 had less of an effect on intestinal mast cell responses to Nippostrongylus brasiliensis (9). IL-4 was shown to compensate for the absence of IL-9 in these experiments. The elevated mast cell response seen in the IL-6-deficient mice is also consistent with a role for IL-4 in promoting mast cell responses, as increased expression of IL-4 was previously seen in G. lamblia-infected IL-6 mutant mice (2). Finally, the absence of mast cell responses in STAT-6-deficient mice infected with G. lamblia (S. M. Singer and Z. Petrin, unpublished data) indicates that IL-4 signaling is necessary for intestinal mast cell responses in this model.
Variable outcomes of infection have long been noted in human giardiasis. We observed that, at 5 days postinfection, the number of parasites observed in BALB/c mice was greater than that seen in C57BL/6 mice. Both strains, however, were able to reduce parasite loads below the limits of detection by 12 days postinfection. Similarly, G. muris infections resolve more quickly in C57BL/10 mice than in BALB/c mice (34). Indeed, anti-gamma interferon treatment delayed G. muris elimination in C57BL/10 mice but not in BALB/c mice (34). Differences between these strains in response to several other infections have been noted and usually correlate with elevated Th2 responses in BALB/c mice compared to Th1-biased responses in C57BL/6 or C57BL/10 mice (1). This is also consistent with the elevated IL-4 production noted in IL-6-deficient mice (2; E. Li and S. M. Singer, unpublished data).
Mast cell responses may contribute to the pathophysiology of giardiasis in addition to the control of infections. Symptoms of giardiasis include malabsorptive diarrhea, cramps, and nausea and are similar to those noted in food allergies, celiac disease, and other intestinal disorders. In several of these diseases and in several animal models, mast cells have been implicated in contributing to this pathology (reviewed in reference 10). In addition, analysis of human giardiasis patients has suggested a possible involvement of mast cell responses during symptomatic infections (5, 7). The increase in serum MMCP-1 and histamine levels observed after infection of C57BL/6 mice suggests that symptoms of giardiasis may be related to allergic processes. Consistent with this idea, giardiasis in humans has been reported to be associated with both allergic responses and chronic urticaria (6, 7, 22). The contribution of mast cells to the pathophysiology of giardiasis in patients clearly warrants further study.
We have shown that mast cells are important for rapidly controlling murine infection with the protozoan parasite G. lamblia. We have also shown that IL-6 controls this infection early on and that mast cells are likely an important source of IL-6 during this infection. Identification of these responses provides correlates of protection to enhance the design and evaluation of new vaccines. The importance of mast cell responses in this infection also suggests new strategies for coping with the symptoms of this infection. Finally, further study of the regulation of these responses should provide novel insights into the regulation of immunity and inflammation in the gastrointestinal tract.
Acknowledgments
This work was supported by Public Health Service grant AI-49565 from the National Institute of Allergy and Infectious Diseases (to S.M.S.) and by a Crohn's and Colitis Foundation of America Summer Student Research Award (to Z.P.).
We thank Andrew McKenzie for providing IL-9-deficient mice, Fred Finkelman for providing ACK-2 and J1.2 antibodies, the Lombardi Comprehensive Cancer Center Tissue Resource for the preparation of paraffin-embedded sections, Joseph Scofi for technical assistance, and Navreet Nanda and Ted Nash for helpful comments on the manuscript.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Bienz, M., W. J. Dai, M. Welle, B. Gottstein, and N. Muller. 2003. Interleukin-6-deficient mice are highly susceptible to Giardia lamblia infection but exhibit normal intestinal immunoglobulin A responses against the parasite. Infect. Immun. 71:1569-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt, E. B., R. T. Strait, D. Hershko, Q. Wang, E. E. Muntel, T. A. Scribner, N. Zimmermann, F. D. Finkelman, and M. E. Rothenberg. 2003. Mast cells are required for experimental oral allergen-induced diarrhea. J. Clin. Investig. 112:1666-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd, L. G., J. T. Conrad, and T. E. Nash. 1994. Giardia lamblia infections in adult mice. Infect Immun. 62:3583-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Anchino, M., D. Orlando, and L. De Feudis. 2002. Giardia lamblia infections become clinically evident by eliciting symptoms of irritable bowel syndrome. J. Infect. 45:169-172. [DOI] [PubMed] [Google Scholar]
- 6.Di Prisco, M. C., I. Hagel, N. R. Lynch, R. M. Barrios, N. Alvarez, and R. Lopez. 1993. Possible relationship between allergic disease and infection by Giardia lamblia. Ann. Allergy 70:210-213. [PubMed] [Google Scholar]
- 7.Di Prisco, M. C., I. Hagel, N. R. Lynch, J. C. Jimenez, R. Rojas, M. Gil, and E. Mata. 1998. Association between giardiasis and allergy. Ann. Allergy Asthma Immunol. 81:261-265. [DOI] [PubMed] [Google Scholar]
- 8.Erlich, J. H., R. F. Anders, I. C. Roberts-Thomson, J. W. Schrader, and G. F. Mitchell. 1983. An examination of differences in serum antibody specificities and hypersensitivity reactions as contributing factors to chronic infection with the intestinal protozoan parasite, Giardia muris, in mice. Aust. J. Exp. Biol. Med. Sci. 61:599-615. [DOI] [PubMed] [Google Scholar]
- 9.Fallon, P. G., H. E. Jolin, P. Smith, C. L. Emson, M. J. Townsend, R. Fallon, and A. N. McKenzie. 2002. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17:7-17. [DOI] [PubMed] [Google Scholar]
- 10.Farthing, M. J. 2003. Immune response-mediated pathology in human intestinal parasitic infection. Parasite Immunol. 25:247-257. [DOI] [PubMed] [Google Scholar]
- 11.Faubert, G. 2000. Immune response to Giardia duodenalis. Clin. Microbiol. Rev. 13:35-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friend, D. S., N. Ghildyal, M. F. Gurish, J. Hunt, X. Hu, K. F. Austen, and R. L. Stevens. 1998. Reversible expression of tryptases and chymases in the jejunal mast cells of mice infected with Trichinella spiralis. J. Immunol. 160:5537-5545. [PubMed] [Google Scholar]
- 13.Galli, S. J., M. Tsai, J. R. Gordon, E. N. Geissler, and B. K. Wershil. 1992. Analyzing mast cell development and function using mice carrying mutations at W/c-kit or Sl/MGF (SCF) loci. Ann. N. Y. Acad. Sci. 664:69-88. [DOI] [PubMed] [Google Scholar]
- 14.Grencis, R. K., K. J. Else, J. F. Huntley, and S. I. Nishikawa. 1993. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 15:55-59. [DOI] [PubMed] [Google Scholar]
- 15.Hardin, J. A., A. G. Buret, M. E. Olson, M. H. Kimm, and D. G. Gall. 1997. Mast cell hyperplasia and increased macromolecular uptake in an animal model of giardiasis. J. Parasitol. 83:908-912. [PubMed] [Google Scholar]
- 16.Heyworth, M. F., J. R. Carlson, and T. H. Ermak. 1987. Clearance of Giardia muris infection requires helper/inducer T lymphocytes. J. Exp. Med. 165:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huizinga, J. D., L. Thuneberg, M. Kluppel, J. Malysz, H. B. Mikkelsen, and A. Bernstein. 1995. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373:347-349. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi, T., S. Ishida, T. Kinoshita, S. Sakuma, N. Sugawara, T. Yamashita, and K. Koike. 2002. IL-6 enhances IgE-dependent histamine release from human peripheral blood-derived cultured mast cells. Cytokine 20:200-209. [DOI] [PubMed] [Google Scholar]
- 19.Langford, T. D., M. P. Housley, M. Boes, J. Chen, M. F. Kagnoff, F. D. Gillin, and L. Eckmann. 2002. Central importance of immunoglobulin A in host defense against Giardia spp. Infect. Immun. 70:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leitch, G. J., I. A. Udezulu, Q. He, and G. S. Visvesvara. 1993. Effects of protein malnutrition on experimental giardiasis in the Mongolian gerbil. Scand. J. Gastroenterol. 28:885-893. [DOI] [PubMed] [Google Scholar]
- 21.Maeda, H., A. Yamagata, S. Nishikawa, K. Yoshinaga, S. Kobayashi, and K. Nishi. 1992. Requirement of c-kit for development of intestinal pacemaker system. Development 116:369-375. [DOI] [PubMed] [Google Scholar]
- 22.McKnight, J. T., and P. E. Tietze. 1992. Dermatologic manifestations of giardiasis. J. Am. Board Fam. Pract. 5:425-428. [PubMed] [Google Scholar]
- 23.Nash, T. E. 1997. Antigenic variation in Giardia lamblia and the host's immune response. Philos. Trans. R. Soc. Lond. Ser. B 352:1369-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishikawa, S., M. Kusakabe, K. Yoshinaga, M. Ogawa, S. Hayashi, T. Kunisada, T. Era, and T. Sakakura. 1991. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit-dependency during melanocyte development. EMBO J. 10:2111-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada, S., H. Nakauchi, K. Nagayoshi, S. Nishikawa, Y. Miura, and T. Suda. 1991. Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood 78:1706-1712. [PubMed] [Google Scholar]
- 26.Puddington, L., S. Olson, and L. Lefrancois. 1994. Interactions between stem cell factor and c-Kit are required for intestinal immune system homeostasis. Immunity 1:733-739. [DOI] [PubMed] [Google Scholar]
- 27.Roberts-Thomson, I. C., and G. F. Mitchell. 1978. Giardiasis in mice. I. Prolonged infections in certain mouse strains and hypothymic (nude) mice. Gastroenterology 75:42-46. [PubMed] [Google Scholar]
- 28.Sanders, K. M. 1996. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 111:492-515. [DOI] [PubMed] [Google Scholar]
- 29.Singer, S. M., and T. E. Nash. 2000. T-cell-dependent control of acute Giardia lamblia infections in mice. Infect. Immun. 68:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snider, D. P., J. Gordon, M. R. McDermott, and B. J. Underdown. 1985. Chronic Giardia muris infection in anti-IgM-treated mice. I. Analysis of immunoglobulin and parasite-specific antibody in normal and immunoglobulin-deficient animals. J. Immunol. 134:4153-4162. [PubMed] [Google Scholar]
- 31.Snider, D. P., D. Skea, and B. J. Underdown. 1988. Chronic giardiasis in B-cell-deficient mice expressing the xid gene. Infect. Immun. 56:2838-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stager, S., and N. Muller. 1997. Giardia lamblia infections in B-cell-deficient transgenic mice. Infect. Immun. 65:3944-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Townsend, M. J., P. G. Fallon, D. J. Matthews, P. Smith, H. E. Jolin, and A. N. McKenzie. 2000. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 13:573-583. [DOI] [PubMed] [Google Scholar]
- 34.Venkatesan, P., R. G. Finch, and D. Wakelin. 1997. A comparison of mucosal inflammatory responses to Giardia muris in resistant B10 and susceptible BALB/c mice. Parasite Immunol. 19:137-143. [DOI] [PubMed] [Google Scholar]
- 35.Ward, S. M., A. J. Burns, S. Torihashi, and K. M. Sanders. 1994. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J. Physiol. (London) 480:91-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou, P., E. Li, N. Zhu, J. Robertson, T. Nash, and S. M. Singer. 2003. Role of interleukin-6 in the control of acute and chronic Giardia lamblia infections in mice. Infect. Immun. 71:1566-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]