Abstract
Infection of human urethral epithelial cells (UECs) with Neisseria gonorrhoeae increases the transcription of several host antiapoptotic genes, including bfl-1, cox-2, and c-IAP-2. In order to identify the bacterial factor(s) responsible for eliciting these changes, the transcriptional status of apoptotic machinery was monitored in UECs challenged with certain gonococcal membrane components. Initially, we observed that infection of UECs with gentamicin-killed gonococci increased the expression of the antiapoptotic Bcl-2 family member, bfl-1. This observation indicated that viable, replicating bacteria are not required for induction of antiapoptotic gene expression. Confirming this observation, treatment of UECs with purified gonococcal membrane increased the expression of bfl-1, cox-2, and c-IAP-2. This finding suggested that a factor or multiple factors present in the outer membrane (OM) are responsible for altering UEC antiapoptotic gene expression. Interestingly, treatment of UECs with gonococcal porin IB (PorB IB), a major constituent of the OM, significantly increased the transcription of bfl-1, cox-2, and c-IAP-2. The upregulation of these genes by PorB IB was determined to be dependent on NF-κB activation, as inhibiting NF-κB blocked induced expression of these genes. This work demonstrates the altered expression of host apoptotic factors in response to gonococcal PorB IB and supports a model whereby UEC cell death may be modulated as a potential mechanism of bacterial survival and proliferation.
Neisseria gonorrhoeae is the causative agent of the sexually transmitted disease gonorrhea and targets the urethral epithelium in men. The interaction of the gonococcus with male urethral epithelium elicits a dramatic cellular response, resulting in alterations in host gene expression and uptake of the bacteria within membrane vacuoles (13). Previously we reported that the response of urethral epithelial cells (UECs) to infection with N. gonorrhoeae includes a significant increase in the transcription of host antiapoptotic genes (2). These genes include bfl-1, cox-2, and c-IAP-2, each coding for a product that acts to inhibit programmed cell death (PCD). Bfl-1 is a member of the Bcl-2 family of apoptotic regulators and has been characterized to have a protective effect on host cells when overexpressed (10, 22). Like its well-studied homologue, Bcl-2, Bfl-1 has the capacity to heterodimerize with proapoptotic members of the Bcl-2 family, including Bax and Bid (45, 46). In this way, Bfl-1 can inhibit the function of proapoptotic factors and block the initiation of the apoptotic cascade by preventing the release of cytochrome c (cyt c) from the mitochondria (45).
Similarly, elevated expression of cox-2 and c-IAP-2 genes has been demonstrated to inhibit PCD (9, 21), albeit by functionally distinct mechanisms from Bfl-1-mediated protection. Cox-2 overexpression activates the phosphatidylinositol 3-kinase/Akt pathway, which subsequently leads to the increased expression of mcl-1 (24). Mcl-1 is an antiapoptotic member of the Bcl-2 family and can interact with and inhibit the function of proapoptotic factor Bok (16). The inhibitor of apoptosis protein 1 (HIAP-1), encoded by c-IAP-2, blocks PCD by binding directly to caspases, the central executioner proteases involved in apoptosis (8). Specifically, HIAP-1 targets caspases 3, 7, and 9 and inhibits their capacity to function as proteolytic enzymes after cyt c release (9, 36).
The expression of bfl-1, cox-2, and c-IAP-2 genes is controlled by the key transcription factor NF-κB (6, 19, 43, 47). NF-κB is a major inflammatory and survival mediator in host cells, controlling the transcription of a diverse array of genes, including genes encoding cytokines and antiapoptotic machinery. Interestingly, infection with N. gonorrhoeae has been demonstrated to activate NF-κB in some cell systems (33). Furthermore, bacterial porins have been identified as factors that can influence NF-κB activity (34), and neisserial porins, specifically, have been shown to modulate the apoptotic response of host cells (26, 28, 30).
The porins of the pathogenic Neisseria species, PorA or PorB from Neisseria meningitidis and PorB IA or PorB IB from N. gonorrhoeae, are the most highly represented proteins in the bacterial outer membrane (OM) (3). These proteins form a trimeric β-pleated barrel and serve as channels allowing the exchange of nutrients and ions between bacteria and their environment. Porins also display a unique property, in which they appear to have the capacity to translocate from the bacterial membrane to host membranes (44). It is believed that the translocation of neisserial porins to the mitochondrial membrane of infected cells has a dramatic effect on the host apoptotic response (26, 29). However, the effect seems to depend on a number of factors, including the source of the porin, the porin purification method, the cell system used, and possibly the presence or absence of serum in the cell culture medium. While translocation of neisserial porins to the mitochondria appears to be a critical step in the modulation of host cell death, the exact mechanism involved in regulating apoptosis after infection is not well defined. In addition, the potential role that neisserial porins may play in modulating other aspects of apoptosis regulation has yet to be addressed.
On the basis of our previous observations that N. gonorrhoeae increases the expression of antiapoptotic genes in infected UECs, we set out to identify the bacterial factor(s) responsible for inducing this response. Using reverse transcription-PCR (RT-PCR) and real-time PCR, we monitored the expression of bfl-1, cox-2, and c-IAP-2 genes in UECs infected with gonococci deficient in certain OM structures or UECs treated with purified gonococcal membrane components. Specifically, this report addresses the potential role of gonococcal porin IB (PorB IB) in the modulation of apoptotic regulator gene expression in the urethral epithelium.
MATERIALS AND METHODS
Epithelial cell culture.
Human papillomavirus E6- or E7-transformed human urethral epithelial cells (THUECs), described previously (14), were used in these studies. THUECs have been characterized to express the receptor to which the gonococcus binds, and the cellular response to a gonococcal infection is similar to that of the primary human urethral cells (PHUECs) from which they are derived (14). PHUECs originating from membranous urethral tissue explants obtained from male patients undergoing prostate surgery were also used to confirm studies performed with THUECs.
UECs were grown, maintained, and passaged as previously described (2, 12). Briefly, UECs were thawed from liquid nitrogen storage in a 37°C water bath, and 1-ml cell suspensions were added to 5 ml of prostate epithelial growth medium (PrEGM; Cambrex, San Diego, Calif.) supplemented with 5% fetal bovine serum (FBS). Diluted UEC suspensions were seeded to 25-cm2 tissue culture-treated flasks (Corning, Cambridge, Mass.). Twenty-four hours after seeding, cell cultures were maintained in FBS-free PrEGM unless noted otherwise in the text. Upon reaching the desired confluence, a mixture of 0.25% trypsin and 0.1% EDTA was used to lift UECs for passage. Cells were incubated with trypsin for 2 min at 25°C, the trypsin was removed, and the cells were incubated for 5 min at 37°C. UECs were suspended in PrEGM containing 5% FBS, centrifuged for 2 min at 1,380 × g, and suspended in the desired volume of PrEGM before seeding to 60- or 100-mm-diameter 24-well petri dishes.
Bacterial strains, PorB IB, and UEC challenge.
N. gonorrhoeae strain 1291 was primarily used in these studies. This strain was originally isolated from a male with gonococcal urethritis and has been characterized to express a single species of gonococcal lipooligosaccharide (LOS) (11, 17). In addition, N. gonorrhoeae strain FA1090 and the FA1090Δpil or Δopa mutants (a gift from Janne Cannon) were used as indicated. Strain FA1090 was isolated from a patient with disseminated gonococcal infection. Gonococcal PorB IB was isolated from N. gonorrhoeae strain MS11 and was given to us by Milan Blake. The method of porin isolation was originally adapted for use of the protein in vaccine development studies and therefore yields a high level of purity. Infections of UECs were performed as previously described (12). P+ (pilus-positive), Opa+ (opacity-associated protein-positive) organisms were selected on the basis of colony morphology. Bacteria were grown overnight (37°C, 5% CO2) on gonococcal (GC) agar, suspended in GC broth, and adjusted to an optical density at 600 nm of 0.16 (∼108 gonococci ml−1), before dilution in antibiotic-free PrEGM. Multiplicity of infection (MOI) ratios were approximately 10 or 100 gonococci per epithelial cell. For PorB IB studies, purified porin was diluted directly into antibiotic-free PrEGM at various concentrations, and UECs were treated for 4 h at 37°C. After the 4-h infection or PorB IB treatment, the cell monolayers were washed twice for 2 min each time in fresh antibiotic-free PrEGM and processed as described below.
RT-PCR analysis.
After the cells were infected or treated, total RNA was harvested from unchallenged and challenged cells with Tri reagent (Sigma) by standard protocols. RNA samples were then DNase treated and column purified using the RNeasy minikit (Qiagen). After subsequent spectrophotometric quantification and resolution on a 1% agarose gel, cDNA synthesis was performed using the First Strand cDNA synthesis kit for RT-PCR (avian myeloblastosis virus) (Roche Biochemical). Primers specific for bfl-1 (forward, 5′-TGC AGT GCG TCC TAC AGA TAC-3′; reverse, 5′-CGT TTT GCC TTA TCC ATT CTC-3′), cox-2 (forward, 5′-CCT CCT GTG CCT GAT GAT TG-3′; reverse, 5′-CCG TAG ATG CTC AGG GAC TT-3′), c-IAP-2 (forward, 5′-AGT CTT GCT CGT GCT GGT T-3′; reverse, 5′-TTC ATT GGA CAG GGG TAG G-3′), bax (forward, 5′-ATG CGT CCA CCA AGA AGC-3′; reverse, 5′-GTG AGT GAG GCG GTG AGC-3′), and bak (forward, 5′-TTA CCG CCA TCA GCA GGA A-3′; reverse, 5′-GGT AGC CGA AGC CCA GAA G-3′) were designed for the amplification of a 363-, 396-, 356-, 400-, or 290-bp amplicon, respectively. The constitutively expressed 18S rRNA gene control was analyzed for comparison to the indicated apoptotic regulators of mRNA levels. QuantumRNA 18S internal standards (Ambion) were used according to the manufacturer's instructions, with amplification resulting in a 488-bp PCR product. PCR products (5 to 15 μl) were run on a 1% agarose gel and analyzed after standard ethidium bromide staining.
Real-time PCR analysis.
The expression of bfl-1 and cox-2 genes was monitored by real-time PCR using the TaqMan One Step PCR kit (Roche) according to the manufacturer's protocol. Primers specific for bfl-1 (forward, 5′-GAC TAT CTG CAG TGC GTC CTA CAG-3′; reverse, 5′-AAC ATT TTG TAG CAC TCT GGA CGT T-3′; TaqMan probe, 5′-CCA CAA CCT GGA TCA GGT CCA AGC A-3′) and cox-2 (forward, 5′-GAA TCA TTC ACC AGG CAA ATT G-3′; reverse, 5′-TCT GTA CTG CGG GTG GAA CA-3′; TaqMan probe, 5′-TGG CAG GGT TGC TGG TGG TAG GA-3′) were designed to amplify a 77- or 66-bp amplicon within the bfl-1 or cox-2 coding regions, respectively. Primers were designed for the 18S rRNA gene (forward, 5′-CGC CGC TAG AGG TGA AAT TC-3′; reverse, TCT TGG CAA ATG CTT TCG CT-3′; TaqMan probe, 5′-TGG ACC GGC GCA AGA CGG AC-3′), and the resulting 63-bp amplicon was monitored as a constitutively expressed internal control. Real-time PCR was performed using an ABI Prism 7700 sequence detector (Perkin-Elmer) with ABI Prism 7700 v1.7 analysis software at the University of Iowa DNA facility.
Immunoprecipitation and Western panel analysis.
After treatment of the urethral epithelium with gonococcal PorB IB, cells were lysed with ice-cold radioimmunoprecipitation assay (RIPA) buffer supplemented with a protease inhibitor cocktail (Calbiochem). Complete lysis was facilitated by passing the cell lysate through a 21-gauge needle. The cell lysate was then centrifuged at 10,000 × g for 10 min (4°C), and the supernatant was collected. Lysates were incubated overnight with gentle rotation (4°C) in the presence of a 1:200 dilution of rabbit anti-Bfl-1 immunoglobulin G (IgG) (sc-8351; Santa Cruz). The next day, the lysates were incubated with Gammabind G (Amersham) for 24 h with gentle rotation (4°C). Immunocomplexes were recovered by centrifugation at 2,500 × g for 5 min (4°C), and the pellet was subsequently washed four times with ice-cold RIPA buffer. The final pellet was resuspended in 30 μl of 1 M Tris-1% sodium dodecyl sulfate (SDS), diluted in gel loading buffer, boiled for 5 min, and loaded onto an SDS-polyacrylamide gel (4 to 12% polyacrylamide). After separation by SDS-polyacrylamide gel electrophoresis, proteins were transferred to a nitrocellulose membrane, and the membrane was subsequently blocked with two 1-h incubations in a solution containing Tris-buffered saline (TBS), 5% bovine serum albumin (BSA), and Tween 20. The membrane was then incubated for 1 h in the presence of a 1:200 dilution of rabbit anti-Bfl-1 IgG (sc-8351; Santa Cruz), washed five times for 5 min each time in TBS containing Tween 20, and finally incubated for 1 h in the presence of a 1:25,000 dilution of goat anti-rabbit IgG conjugated to horseradish peroxidase (Bio-Rad). Detection was achieved with SuperSignal (Pierce).
Isolation of gonococcal membrane.
Membrane preparations were performed as previously described (35). Briefly, 10-ml cultures grown overnight in broth were harvested by centrifugation, and the pellet was suspended in OM preparation buffer (50 mM Tris-chloride, 150 mM NaCl, 10 mM EDTA [pH 7.4]). The sample was then warmed to 56°C for 30 min, cooled to room temperature, and then passed through a series of syringes using different gauge needles to shear the cells. Lysates were then centrifuged at 16,000 × g for 15 min. The supernatant was removed and centrifuged at 25,000 × g for 20 min (20°C). This supernatant was then removed and centrifuged at 30,000 × g for 20 min (20°C). Finally, this supernatant was removed and centrifuged at 100,000 × g for 2 h (20°C). The final glass-like pellet was suspended in 0.1% SDS in endotoxin-free H2O.
LOS isolation, NaOH treatment, and coating of magnetic beads.
Bacteria were grown on 10 GC agar plates, and colonies were harvested in phosphate-buffered saline (PBS) and pelleted by centrifugation. The pellets were then washed twice with 0.15 M NaCl and suspended in 25 ml of double-distilled water (ddH2O). The cell suspension and 90% phenol were equilibrated to 65°C, and 25 ml of 90% phenol was then added to the bacterial suspension. The sample was incubated at 65°C for 1 h and then at 4°C for 1 h. The sample was then centrifuged at 3,300 × g for 10 min at 4°C, and the aqueous layer was collected, while the organic phase was back extracted with 25 ml of ddH2O. The back-extracted sample was again centrifuged as described above, and the aqueous layer was collected and combined with the first extraction. The combined sample was precipitated with 0.3 M sodium acetate and 3 volumes of cold absolute ethanol, flash cooled in a dry ice-ethanol bath, and incubated for 2 h at −20°C. The precipitate was centrifuged for 10 min at 12,000 × g (4°C), and the pellet was suspended in 6 ml of deionized water and precipitated again as described above. This precipitate was centrifuged, and the pellet was suspended in 4 ml of 0.06 M Tris base, 10 mM EDTA, and 2.0% SDS (pH 6.8). The sample was boiled for 5 min and cooled to room temperature.
To digest contaminating protein, the sample was incubated with 10 μg of proteinase K per ml for 1 h at 65°C and then incubated overnight at 37°C. To remove SDS, precipitation was performed six more times with 0.3 M sodium acetate and 3 volumes of absolute ethanol. After the final precipitation, pellets were suspended in ddH2O and centrifuged at 120,000 × g for 1 h and 15 min. The glass-like pellet was suspended in high-performance liquid chromatography (HPLC)-grade water and centrifuged at 120,000 × g for 1.5 h. The pellet was suspended in HPLC-grade water and lyophilized overnight. The lyophilized sample was suspended in endotoxin-free H2O. For deacylation of the purified sample, 2 mg of LOS was incubated in the presence of 50 mM NaOH at 80°C for 20 min. After the incubation, an equal volume of 1 M Tris-HCl (pH 7) was added, and the neutralized sample was dialyzed (Slide-A-lyzer 10K cassette; Pierce) with three exchanges against PBS. The sample was removed from the cassette and filtered in a sterile manner.
To coat magnetic beads, the prepared LOS was sonicated for 20 min and incubated in the presence of magnetic beads (7 × 107 tosylactivated Dynabeads M-280; Dynal A.S., Oslo, Norway) with 0.1 M sodium carbonate buffer (pH 9.5) for 16 to 24 h (37°C) with gentle rotation. Coated beads were then pelleted with a magnet, washed four times, suspended in PBS containing 0.1% BSA (pH 7.4) and stored at 4°C.
Dual-luciferase assay to measure NF-κB activation.
UECs were seeded into 24-well tissue culture-treated plates and transiently transfected the next day with 1 μg of pGL2 (NF-κB-luciferase) (Promega) and 0.1 μg of pRLnull (Promega) per well using FuGene-6 (Roche) according to the manufacturer's instructions. After transfection overnight, the medium was removed, and UECs were either infected with N. gonorrhoeae (MOI of 100:1) or treated with 5 μg of PorB IB per ml for 4 h. After the treatment period, cells were washed once in fresh antibiotic-free PrEGM, and cell lysates were prepared and assayed for luciferase activity using the dual-luciferase reporter kit (Promega) according to the manufacturer's instructions. The assay was performed three times for each condition, and the data are reported as the ratio of relative light units of firefly (Photinus pyralis) to Renilla (Renilla reniformis) luciferase. Renilla luciferase is included as an internal control reporter, providing a baseline response and allowing for more reliable interpretation of the experimental data.
NF-κB inhibition studies.
UECs were treated for 1 h at 37°C with MG-132 (Calbiochem) diluted directly into antibiotic-free PrEGM to the desired concentration. MG-132 is a cell-permeable proteasome inhibitor that inhibits NF-κB activation by preventing the degradation of IκB. This sequesters NF-κB in a bound state to IκB and prevents its nuclear translocation. After the 1-h incubation, the medium overlying the cells was removed, and UECs were infected with N. gonorrhoeae or treated with PorB IB for 4 h.
RESULTS
Treatment of UECs with gonococcal OM increases the expression of host antiapoptotic genes.
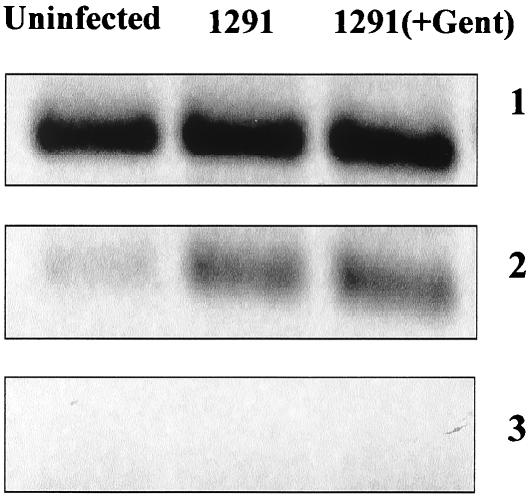
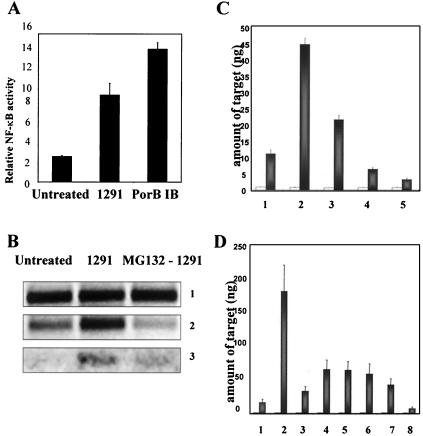
In order to identify the bacterial factor(s) responsible for the alteration in host apoptotic regulator gene expression, we first analyzed whether viable, replicating gonococci are required. UECs were infected for 4 h with viable gonococci or with bacteria incubated for 1 h in the presence of 100 μg of gentamicin per ml. Bacteria were plated after this 1-h incubation to assure efficient and complete killing (data not shown). RT-PCR analysis then demonstrated that expression of bfl-1 was increased in UECs infected with either live or gentamicin-killed gonococci (Fig. 1, panel 2). This indicated that viable bacteria are not required for altering host apoptotic regulator gene expression. For a control, the expression of the 18S rRNA gene was monitored in both uninfected and infected cells, and the level of the 18S rRNA gene product did not change with the two conditions (Fig. 1, panel 1). In addition, reverse transcriptase was excluded during cDNA synthesis to control for possible DNA contamination, and no amplification was observed (Fig. 1, panel 3).
FIG. 1.
Increased expression of bfl-1 in urethral epithelium infected with live or gentamicin-killed N. gonorrhoeae. UECs received medium alone (uninfected) or were challenged for 4 h with live (1291) or gentamicin-killed (1291+Gent) gonococci. After infection, total RNA was harvested, and cDNA was synthesized. PCR analysis then demonstrated that expression of the antiapoptotic Bcl-2 family member bfl-1 is increased in UECs infected with either live or gentamicin-killed gonococci. Panel 1, 18S rRNA internal control; panel 2, bfl-1; panel 3, reverse transcriptase-negative controls.
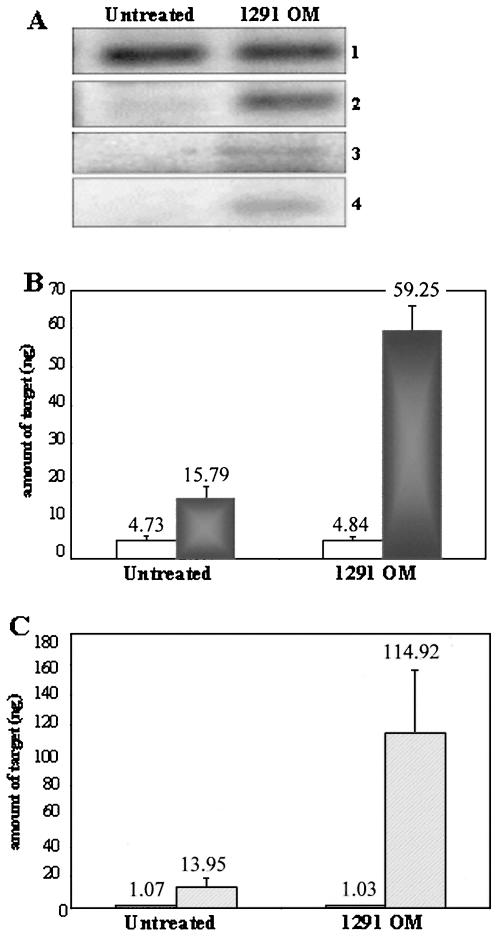
The lack of a requirement for viable bacteria in eliciting the observed host response suggested that a gonococcal surface component may be sufficient for altering UEC apoptotic regulator gene expression. To address this, gonococcal membrane was isolated, and UECs were treated for 4 h with 10 μg of the purified membrane preparation per ml. Untreated cells received membrane suspension buffer (0.1% SDS-H2O) diluted in cell culture medium as a baseline control. We initially observed by RT-PCR that the transcription of the antiapoptotic genes bfl-1, c-IAP-2, and cox-2 was increased in UECs treated with purified gonococcal membrane (Fig. 2A, panels 2 to 4). Subsequently, real-time PCR was performed to obtain a quantitative representation of bfl-1 and cox-2 expression in cells treated with gonococcal membrane. Real-time PCR analysis demonstrated that expression of bfl-1 (Fig. 2B, dark gray bars) and cox-2 (Fig. 2C, hatched bars) is significantly higher (bfl-1, P < 0.001; cox-2, P = 0.025) in membrane-treated cells, while no change in expression occurred with the 18S rRNA control (Fig. 2B and C, white bars). These results confirmed our previous observation that viable gonococci are not required to activate host cell gene expression and indicated that a factor or multiple factors present in the OM of N. gonorrhoeae are responsible for the increase in the transcription of host antiapoptotic genes.
FIG. 2.
Analysis of antiapoptotic gene expression in UECs treated with purified gonococcal membrane. (A) RT-PCR analysis of antiapoptotic gene expression in UECs either left untreated or treated for 4 h with 10 μg of purified gonococcal outer membrane (1291 OM) per ml. Panel 1, 18S rRNA gene; panel 2, bfl-1; panel 3, c-IAP-2; panel 4, cox-2. (B) Real-time PCR analysis of bfl-1 expression showing an approximately fourfold increase in expression (P < 0.001) in 1291 OM-treated UECs (gray bars), while no change in expression occurred with the 18S rRNA control (white bars). (C) Real-time PCR analysis of cox-2 expression (hatched bars) demonstrating an approximately eightfold upregulation (P = 0.025) in 1291 OM-treated UECs. The 18S rRNA control (white bars) showed no change in expression in the different conditions. Real-time PCR data are shown as the mean from three independent reactions per condition.
Gonococcal PorB IB transcriptionally activates antiapoptotic genes in UECs.
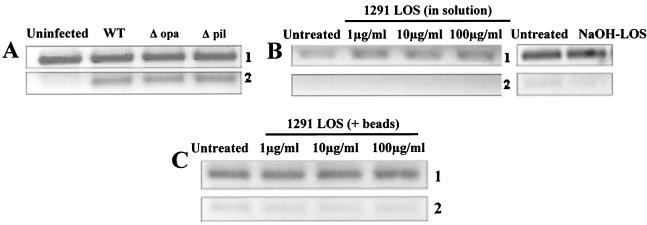
N. gonorrhoeae expresses a variety of surface factors that may engage the host cell and stimulate alterations in host gene expression. Therefore, we analyzed the expression of apoptotic regulators in UECs that were either infected with mutant gonococci lacking key surface structures or treated with purified gonococcal membrane components. The pili and opacity-associated (Opa) proteins are gonococcal membrane factors that are largely responsible for allowing the bacteria to adhere to host cells (4, 32). Infection of UECs with gonococci lacking pili (Δpil) or Opa proteins (Δopa) increased the transcription level of bfl-1 to a level comparable to that of wild-type organisms (Fig. 3A, panel 2). These findings suggested that pili and Opa proteins are not required for the alteration in host apoptotic regulator gene expression.
FIG. 3.
Analysis of the effects of various gonococcal OM components on the expression of bfl-1. (A) UECs received medium alone (Uninfected) or were infected for 4 h with either wild-type N. gonorrhoeae strain FA1090 (WT), a FA1090 mutant lacking expression of the opacity-associated proteins (Δopa), or a FA1090 mutant lacking pili (Δpil). RT-PCR revealed the increased expression of bfl-1 (panel 2) in UECs infected with wild-type, Δopa, or Δpil gonococci, indicating that Opa and pili are not required to elicit the increase in host antiapoptotic gene expression. No change in expression was observed in the 18S rRNA control (panel 1). (B) UECs received medium alone (Untreated) or were treated for 4 h with either untreated gonococcal LOS diluted directly into culture medium [1291 LOS (in solution)] or LOS treated with 50 mM NaOH (NaOH-LOS). RT-PCR analysis of bfl-1 (panel 2) and 18S rRNA gene (panel 1) was performed. C) UECs were left untreated or treated for 4 h with magnetic beads coated with 1, 10, or 100 μg of purified gonococcal LOS [1291 LOS (+beads)] per ml. RT-PCR demonstrated that 18S rRNA gene (panel 1) and bfl-1 (panel 2) expression is not altered in UECs treated with gonococcal LOS.
Gonococcal LOS, another key membrane constituent, emanates from the bacterial surface and has been identified to engage the asialoglycoprotein receptor on UECs, leading to the uptake of gonococci by a receptor-mediated endocytic event (12). To analyze the effect of LOS on host cell gene expression, we initially treated UECs with purified gonococcal LOS suspended directly in cell culture medium. At LOS concentrations of 1, 10, or 100 μg/ml, no alteration in the transcription level of bfl-1 was observed in LOS-treated cells as measured by RT-PCR (Fig. 3B, panel 2). Due to the hydrophobic nature of gonococcal LOS and its propensity to form micelles in solution, it is possible that aggregate formation may prevent LOS from stimulating certain host cell responses. To address this possibility, we treated the purified LOS with 50 mM NaOH to deacylate the lipid A core and subsequently inhibit micelle formation. RT-PCR analysis again demonstrated that the expression of bfl-1 is not increased in UECs treated with deacylated LOS (Fig. 3B, panel 2).
We then wished to determine whether the presentation of the LOS to the host cell surface is critical in eliciting alterations in apoptotic regulator gene expression. Therefore, purified LOS was coated onto 2-μm-diameter magnetic beads (Dynal A.S.) to mimic its presentation off the bacterial surface to the UEC. An enzyme-linked immunosorbent assay demonstrated efficient coating of the beads with LOS prior to treatment of UECs (data not shown). At concentrations of 1, 10, and 100 μg of LOS per ml, no effect on the transcription of either bfl-1 (Fig. 3C, panel 2) or the control gene 18S rRNA (Fig. 3C, panel 1) was observed. For a control, cells were treated with uncoated beads, and no alterations in host gene expression were observed (data not shown). In support of these findings, infection of UECs with a gonococcal mutant (1291 R6) expressing a completely truncated LOS resulted in increased levels of bfl-1 transcript (data not shown), indicating that a functional LOS is not required for eliciting the observed response. Taken together, the results of these studies suggest that LOS, pili, and Opa proteins are not required for modulating the expression of the antiapoptotic gene bfl-1.
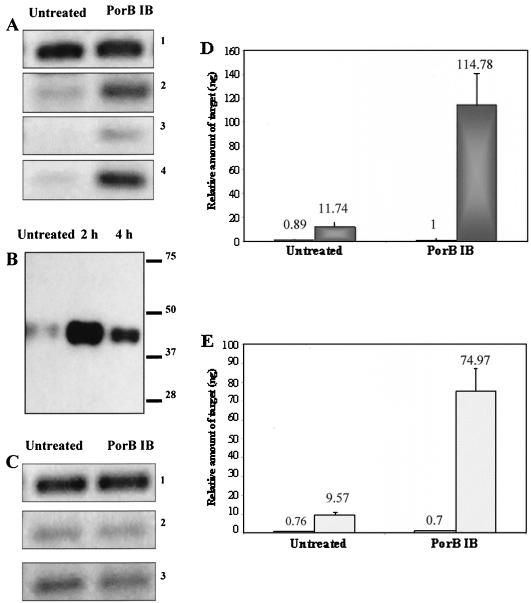
Porins constitute nearly 60% of total surface protein of the pathogenic Neisseria species (3) and are essential for bacterial viability. Interestingly, neisserial porins have recently been identified to modulate host cell apoptosis (28, 29). Therefore, we chose to analyze the effect of purified gonococcal PorB IB on UEC apoptotic regulator gene expression. Treatment of UECs with 5 μg of purified PorB IB per ml increased the expression of bfl-1, c-IAP-2, and cox-2 genes when analyzed by RT-PCR (Fig. 4A, panels 2 to 4). This effect was not dependent on the absence of serum in the cell culture medium, as UECs maintained in the presence of 5% FBS also demonstrated increased levels of bfl-1 after treatment with PorB IB (data not shown). Transcript levels of bfl-1 were not altered in UECs treated with porin suspension buffer alone (data not shown). The upregulation of bfl-1 gene expression correlated with an increase in Bfl-1 protein levels, as Western panel analysis demonstrated that Bfl-1 expression is elevated after treatment of UECs with PorB IB (Fig. 4B). Real-time PCR analysis of untreated and PorB IB-treated UECs confirmed the results of our RT-PCR studies, showing high levels of statistical significance corresponding to the increases in expression for bfl-1 (P < 0.01; dark gray bars) and cox-2 (P < 0.01; hatched bars) in PorB IB-treated cells (Fig. 4D and E). Interestingly, the alterations in antiapoptotic gene expression after PorB IB treatment were not accompanied by increases in the transcription levels of major proapoptotic members of the Bcl-2 family, such as bax and bak (Fig. 4C, panels 2 and 3). This work provides evidence that gonococcal PorB IB may be a key factor in regulating UEC apoptosis after infection and demonstrates for the first time that a bacterial porin can modulate the expression of host antiapoptotic machinery.
FIG. 4.
Apoptotic regulator expression in UECs treated with purified gonococcal PorB IB. (A) UECs (maintained in the absence of serum) received medium alone (Untreated) or were treated for 4 h with 5 μg of gonococcal porin IB (PorB IB) per ml. RT-PCR analysis demonstrated the increased expression of antiapoptotic genes in PorB IB-treated UECs. Panel 1, 18S rRNA gene; panel 2, bfl-1; panel 3, c-IAP-2; panel 4, cox-2. (B) Western panel analysis of Bfl-1 in untreated urethral epithelium and cells treated for 2 or 4 h with 5 μg of PorB IB per ml. Molecular size markers (in kilodaltons) are indicated on the right. (C) RT-PCR analysis of proapoptotic gene expression in untreated and PorB IB-treated UECs. Panel 1, 18S rRNA control; panel 2, bax; panel 3, bak. (D) Real-time PCR analysis of 18S rRNA gene (white bars) and bfl-1 (gray bars; P < 0.01) in untreated and PorB IB-treated UECs. (E) Real-time PCR analysis of the 18S rRNA gene (white bars) and cox-2 (hatched bars; P < 0.01) expression in untreated and PorB IB-treated UECs.
NF-κB is activated in gonococcus-infected and PorB IB-treated UECs and regulates the transcription of antiapoptotic genes.
The genes showing increased expression in our studies have been demonstrated to be under the control of the transcription factor NF-κB in other systems (5, 6). NF-κB activation is required for induced expression of the bfl-1, c-IAP-2, and cox-2 genes and represents a major survival and inflammatory signal in host cells (19, 42, 43, 47). In addition, both gonococcal infection and neisserial porins have been identified to activate NF-κB in other cell systems (27, 33, 34). Therefore, we looked at whether NF-κB is activated in UECs that are infected with the gonococcus or treated with PorB IB. Dual-luciferase assays (Promega) were performed, which demonstrated a nearly 3.5-fold activation (P = 0.006) of NF-κB in cells infected for 4 h with N. gonorrhoeae (Fig. 5A). These studies also showed that treatment of UECs with PorB IB for 4 h activated NF-κB approximately fivefold (P = 0.0006) (Fig. 5A), while mock-transfected cells showed no stimulation in NF-κB activity (data not shown).
FIG. 5.
Activation of NF-κB in gonococcus-infected and PorB IB-treated UECs. (A) UECs were left untreated, infected with N. gonorrhoeae (1291), or treated for 4 h with purified gonococcal porin IB (PorB IB). Dual-luciferase assays were then performed to measure the activity of NF-κB in UECs. The relative NF-κB activity, plotted as the ratio of the firefly luciferase to Renilla luciferase reporter expression, is graphed. The values are the means ± standard errors of the means (error bars) for three experiments. (B) UECs received medium alone (untreated) or were infected for 4 h with N. gonorrhoeae (1291) or were pretreated for 1 h with 10 μM MG-132 to inhibit NF-κB activation prior to the 4-h infection with the gonococcus (MG132 - 1291). RT-PCR analysis was then performed to analyze the expression of the 18S rRNA control (panel 1), bfl-1 (panel 2), or cox-2 (panel 3). (C) Real-time PCR analysis of 18S rRNA (white bars) and bfl-1 (gray bars) gene expression in (bar 1) untreated UECs, (bar 2) gonococcus-infected cells (1291), (bar 3) cells treated with 1 μM MG-132 prior to infection with 1291, (bar 4) cells treated with 10 μM MG-132 prior to infection with 1291, or (bar 5) cells treated with 10 μM MG-132 alone. (D) Real-time PCR analysis of 18S rRNA (white bars) and bfl-1 (gray bars) gene expression in (bar 1) untreated UECs, (bar 2) cells treated with 5 μg of PorB IB per ml for 4 h, (bar 3) cells treated with 10 μM MG-132 prior to treatment with 5 μg of PorB IB per ml, (bar 4) cells treated with 50 ng of PorB IB per ml, (bar 5) cells treated with 0.1 μM MG-132 prior to PorB IB treatment, (bar 6) cells treated with 1 μM MG-132 prior to PorB IB treatment, (bar 7) cells treated with 10 μM MG-132 prior to PorB IB treatment, or (bar 8) cells treated with 10 μM MG-132 alone.
We then wished to demonstrate a correlation between the activation of NF-κB and the increased expression of UEC antiapoptotic genes. To accomplish this, we determined whether inhibiting NF-κB activity would subsequently block the induced expression of antiapoptotic genes. UECs were treated with the NF-κB inhibitor MG-132 (Calbiochem) for 1 h prior to a 4-h infection with the gonococcus or treatment with purified PorB IB. RT-PCR analysis initially demonstrated that while infection with N. gonorrhoeae increases the expression of antiapoptotic genes in UECs, pretreatment of cells with MG-132 blocks the induction of host cell antiapoptotic gene transcription (Fig. 5B, panels 2 and 3). Similar results were achieved when the activation of NF-κB was inhibited using the cell-permeable NF-κB inhibitor peptide SN50 (Calbiochem) (data not shown). Real-time PCR analysis confirmed the results of our RT-PCR studies and showed a dose-dependent decrease in the expression of bfl-1 in infected cells as the concentration of MG-132 was increased (Fig. 5C, gray bars). The inhibition of NF-κB by MG-132 also blocked PorB IB induction of UEC antiapoptotic gene expression. Real-time PCR indicated that 10 μM MG-132 almost completely blocked the increased expression of bfl-1 after PorB IB treatment. In addition, as little as 50 ng of PorB IB per ml significantly upregulated bfl-1 (P = 0.01), and this increase could be reduced (P = 0.05) by the addition of 10 μM MG-132 prior to PorB IB treatment (Fig. 5D, gray bars). As expected, treatment of UECs with MG-132 alone lowered the basal expression of antiapoptotic genes below that of untreated cells (Fig. 5D). These findings provide evidence that infection of UECs with N. gonorrhoeae, and specifically, the interaction of host cells with PorB IB, activates NF-κB to regulate the expression of genes whose products function to inhibit PCD.
DISCUSSION
The work outlined in this report has identified that treatment of human urethral epithelium with gonococcal PorB IB increases the expression of antiapoptotic genes but does not affect the transcriptional status of several key proapoptotic Bcl-2 family members. By using RT-PCR and real-time PCR analysis, PorB IB was demonstrated to induce the expression of bfl-1, cox-2, and c-IAP-2 genes, while other key gonococcal OM components, such as LOS, pili, and Opa proteins are not required for the activation of these antiapoptotic genes. The increase in bfl-1 gene expression was shown to correlate with an increase in Bfl-1 protein levels, demonstrating that the interaction of porin with the urethral epithelium does not merely alter the transcriptional status of these apoptotic regulators. Furthermore, the upregulation of these genes was determined to be dependent on NF-κB activation, as inhibiting NF-κB activity blocked induced expression of the genes. This work demonstrates, for the first time, the regulation of host apoptotic machinery expression by a bacterial porin and supports a model whereby gonococcal invasion of the urethral epithelium may modulate apoptosis to potentiate bacterial survival and proliferation of the infection.
Apoptosis, also known as regulated (or programmed) cell death, is highly complex and can be initiated or inhibited through multiple defined processes, and most likely many undefined, cellular processes. In general, it is believed that apoptosis occurs after the activation of the extrinsic or intrinsic death pathway (15). The extrinsic pathway, otherwise referred to as the death receptor pathway, is triggered after binding of a ligand (i.e., Fas) to a surface receptor (i.e., CD95). This ligand-receptor interaction results in a receptor-clustering event that stimulates the autoactivation of procaspase 8 to active caspase 8 (Fig. 6A) (31). Caspase 8 can then activate downstream effector caspases (i.e., caspase 3) or can activate the proapoptotic Bcl-2 family member, Bid (25). Proteolytic processing of Bid by caspase 8 results in the translocation of Bid from the cytosol to the mitochondria, where it can promote the release of cyt c.
FIG. 6.
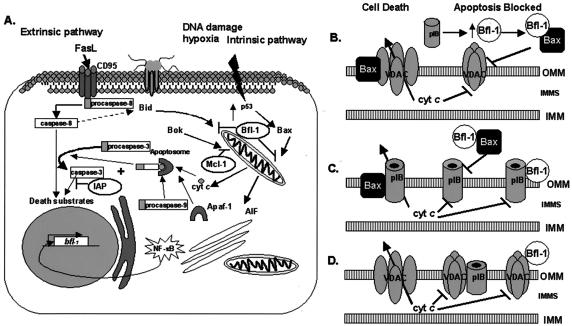
Model of the regulation of urethral epithelial programmed cell death after infection with N. gonorrhoeae. (A) Infection of UECs with N. gonorrhoeae or treatment with purified gonococcal PorB IB activates NF-κB and increases the expression of antiapoptotic factors that can function to regulate apoptosis initiated by either the extrinsic or intrinsic death pathway. Infection with the gonococcus does not alter the expression status of key proapoptotic members of the Bcl-2 family. IAP, inhibition of apoptosis; AIF, apoptosis-inducing factor. (B) The increased expression of antiapoptotic factors (e.g., Bfl-1) by gonococcal porin (pIB) may prevent the opening of a Bax-mediated VDAC pore, sequestering cyt c in the intermitochondrial membrane space (IMMS). (C) In the absence of key antiapoptotic regulators, proapoptotic Bcl-2 family members (i.e., Bax) may interact with and promote the open conformation of gonococcal pIB, allowing for the release of cyt c. Increased levels of antiapoptotic factors (i.e., Bfl-1) may maintain pIB in a closed conformation either by interacting directly with pIB or by preventing its association with Bax or other proapoptotic factors. OMM and IMM, outer and inner mitochondrial membranes, respectively. (D) Gonococcal pIB may translocate to the outer mitochondrial membrane (OMM) of UECs and interact directly with VDAC, preventing the release of cyt c through an otherwise open VDAC pore. It is also possible, but unlikely, that Bfl-1 may interact directly with VDAC to influence the size of the VDAC channel.
The intrinsic pathway, also termed the mitochondrial pathway, can be triggered by certain extracellular (e.g., hypoxia) or intracellular (e.g., DNA damage) signals (15). Activation of the intrinsic pathway by these cues promotes the convergence of proapoptotic Bcl-2 family members on the mitochondria. For example, stimulation through the intrinsic pathway can lead to the activation of Bax, a key promoter of cell death. Activated Bax translocates from its cytosolic localization to the mitochondrial membrane where it is proposed that Bax may independently (18) or cooperatively with another factor (38, 40) open the mitochondrial permeability transition (PT) pore through which cyt c can escape into the cytosol.
Interestingly, infection of UECs with N. gonorrhoeae, and specifically the interaction of PorB IB with host cells, increases the expression of genes whose products act to block apoptosis triggered by either the intrinsic or extrinsic death pathway (Fig. 6A). NF-κB is central to the transcriptional regulation of these apoptotic regulators. In most cell types, NF-κB activity is kept under tight control through its interaction with IκB and is prevented from undergoing nuclear translocation in conditions that support IκB stability. However, upon stimulation that may result from a diverse range of signals (1), IκB is phosphorylated and targeted for degradation by proteasomes. This releases NF-κB, allowing it to translocate to the nucleus and activate the transcription of a great diversity of genes, including genes whose products are involved in inflammation and the regulation of apoptosis. It should be noted that in some cases, NF-κB may act to promote apoptosis depending on the stimulus and cell type (20). Previous studies have identified that signaling through Toll-like receptors (TLRs) (e.g., TLR2) can induce NF-κB nuclear translocation (27). In addition, certain neisserial OM components, including porin, H8, and other lipoproteins, can serve as ligands for TLRs, thereby influencing the activity of NF-κB (27). It is possible that TLR signaling mediated by gonococcal PorB IB may also serve a key regulatory event in the urethral epithelium by modulating the activity of NF-κB and subsequent expression of UEC antiapoptotic machinery.
Of interest to us was the inability of gonococcal LOS to increase the expression of antiapoptotic genes, despite reports that lipopolysaccharide from gram-negative bacteria is a potent inducer of NF-κB activity (7, 34). There are several possibilities that may explain this observation. First, the Rel/NF-κB transcription factor family is comprised of a number of cellular proteins that can complex in a variety of different ways depending on the cell type and stimulus (1). For instance, distinct signals (e.g., LOS versus PorB IB) may promote the formation of different Rel/NF-κB complexes that interact with different transcription factors (e.g., AP-1) and regulatory proteins (IκBα, IκBβ, etc.), and regulate the transcription of a completely distinct set of genes. Recent studies by Lin et al. (23) would support this idea, as these researchers identified that within a single cell type, NF-κB activation yielded opposite effects, depending on the inducing signal. A second possibility is that additional bacterial factors may be required for LOS to activate NF-κB and subsequently alter UEC apoptotic regulator gene expression. However, this seems unlikely, as treatment of UECs with purified LOS leads to elevated levels of certain inflammatory cytokines (e.g., interleukin-8) that are under the transcriptional regulation of NF-κB (14). These studies demonstrate that LOS alone can at least modulate the expression of a subset of NF-κB-regulated genes. Therefore, the work outlined in this report provides evidence that gonococcal LOS is not the bacterial factor responsible for altering UEC apoptotic regulator gene expression but points instead to PorB IB as the candidate necessary for increasing the transcription of host antiapoptotic genes.
The PorB IB-induced upregulation of the bfl-1, cox-2, and c-IAP-2 genes may protect host cells from PCD through several mechanisms, each targeting different points in the apoptotic pathway. For example, the increased expression of Bfl-1 may inhibit UEC apoptosis by titrating out the activity of proapoptotic factors, such as Bax. Although it is not completely defined how Bax functions to promote apoptosis at the mitochondria, accumulating evidence suggests that its interactions with the voltage-dependent anion channel (VDAC) may be a critical regulatory event (40). VDAC, also termed the mitochondrial porin, is a component of the PT pore and has been identified to complex with several members of the Bcl-2 family (38, 39, 41). Shimizu et al. (40) demonstrated that VDAC interacts with the proapoptotic proteins Bax and Bak, and this association potentiates the opening of a VDAC-mediated pore through which cyt c is released. Therefore, in the absence of antiapoptotic expression, apoptosis may be initiated by a Bax-induced VDAC pore. However, elevated levels of Bfl-1 resulting from PorB IB stimulation may inhibit Bax from associating with VDAC, maintaining VDAC in a closed conformation (Fig. 6B). Interestingly, our attempts to demonstrate an inhibition in staurosporine-induced apoptosis by purified PorB IB have not yielded the significant protection from PCD that is observed after infection with whole gonococci. Therefore, the proposed mechanisms of PorB IB action in regulating apoptosis remain undefined. It is possible that the continual production of PorB IB by viable gonococci during infection may be an important factor in maintaining the antiapoptotic response. In addition, the increased expression of bfl-1, cox-2, and c-IAP-2 genes resulting from PorB IB treatment may not be sufficient to allow for complete protection from staurosporine-induced apoptosis. Other factors, including additional bacterial and/or host proteins, may complement the activity of PorB IB, thereby playing a key synergistic role in the inhibition of urethral cell death.
While the work outlined in this report suggests an antiapoptotic role for PorB IB in UECs, recent studies by Müller et al. (29) have demonstrated a proapoptotic function for gonococcal porin in other cell types. The discrepancies in these findings are certainly intriguing but may serve to highlight the potential opposing effects that gonococcal porin may have in different cell types and at different times during the infection. Recently, we observed that infection of Chang cells with N. gonorrhoeae did not result in an increased level of bfl-1, cox-2, or c-IAP-2 (2). Furthermore, infection of Chang cells with the gonococcus did not provide the protection from staurosporine-induced apoptosis that we observed in UECs (2). Therefore, it is highly possible that the gonococcus, and specifically PorB IB, may elicit dramatically different effects in different cell types. This may involve the potential interaction of gonococcal PorB IB with pro- or antiapoptotic Bcl-2 family members, which may act to regulate the action of PorB IB during the course of the infection (Fig. 6B to D).
In addition to eliciting different effects based on cell type, PorB IB may also display opposing function based on the time point of infection. The level of purine nucleotides in the cytoplasm represents one mechanism of regulating the size of the PorB IB channel, so that at high concentrations of ATP or GTP, the channel remains closed (37). Therefore, it is interesting to speculate that early during the infection, while nucleotide and nutrient levels are high, PorB IB may play a role in inhibiting host cell death. This would allow the bacteria to utilize host cell nutrient stores and enhance the capability of the gonococcus to replicate in its intracellular niche. Subsequently, at later points during the infection when nutrient and nucleotide levels are limiting, the PorB IB channel may open, thereby promoting cell death. This would allow for the infection of surrounding, uninfected cells and contribute to the persistence of the infection. Our studies have focused primarily on the early response of UECs to infection with the gonococcus. It will be interesting in future studies to examine the response of the urethral epithelium to infection and PorB IB treatment at later time points. In addition, work will be directed at analyzing the putative interactions of gonococcal porin with Bcl-2 family proteins, as well as the potential synergistic roles of other host and bacterial proteins in the modulation of urethral cell apoptosis.
In summary, we have demonstrated that gonococcal PorB IB significantly increases the expression of genes whose products function to inhibit apoptosis. Furthermore, this work provides evidence that infection of UECs with N. gonorrhoeae or treatment with PorB IB activates NF-κB to regulate the transcription of host antiapoptotic genes. These studies highlight the diverse role that bacterial porins play in infection and contribute to our understanding of gonococcal disease and the intricate regulatory mechanisms that control PCD.
Acknowledgments
We thank Kevin Knutson and Jessica Linton at the University of Iowa DNA facility for assistance with the analysis of the real-time PCR experiments. We also thank members of the Gail Bishop laboratory for technical assistance with the dual-luciferase assay. We thank Janne Cannon for supplying us with the gonococcal Opa and pili deletion mutants and Milan Blake for providing the purified gonococcal porin. Additionally, we thank the members of the Apicella lab for helpful discussions and technical assistance.
Research in M. A. Apicella's laboratory was supported in part by NIH grants AI45728 and AI43924.
Editor: J. T. Barbieri
REFERENCES
- 1.Barkett, M., and T. D. Gilmore. 1999. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 18:6910-6924. [DOI] [PubMed] [Google Scholar]
- 2.Binnicker, M. J., R. D. Williams, and M. A. Apicella. 2003. Infection of human urethral epithelium with Neisseria gonorrhoeae elicits an upregulation of host anti-apoptotic factors and protects cells from staurosporine-induced apoptosis. Cell. Microbiol. 5:549-560. [DOI] [PubMed] [Google Scholar]
- 3.Blake, M. S., and E. C. Gotschlich. 1986. Functional and immunological properties of pathogenic Neisseria surface proteins, p. 377-400. In M. Inouye (ed.), Bacterial outer membranes as model systems. John Wiley and Sons, New York, N.Y.
- 4.Blake, M. S., C. M. Blake, M. A. Apicella, and R. E. Mandrell. 1995. Gonococcal opacity: lectin-like interactions between Opa proteins and lipooligosaccharide. Infect. Immun. 63:1434-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, Q., H. H. Lee, Y. Li, T. P. Parks, and G. Cheng. 2000. Upregulation of Bcl-x and Bfl-1 as a potential mechanism of chemoresistance, which can be overcome by NF-κB inhibition. Oncogene 19:4936-4940. [DOI] [PubMed] [Google Scholar]
- 6.Chu, Z. L., T. A. McKinsey, L. Liu, J. J. Gentry, M. H. Malim, and D. W. Ballard. 1997. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc. Natl. Acad. Sci. USA 94:10057-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordle, S. R., R. Donald, M. A. Read, and J. Hawiger. 1993. Lipopolysaccharide induces phosphorylation of MAD3 and activation of c-Rel and related NF-κB proteins in human monocytic THP-1 cells. J. Biol. Chem. 268:11803-11810. [PubMed] [Google Scholar]
- 8.Deveraux, Q. L., and J. C. Reed. 1999. IAP family proteins-suppressors of apoptosis. Genes Dev. 13:239-252. [DOI] [PubMed] [Google Scholar]
- 9.Deveraux, Q. L., N. Roy, H. R. Stennicke, T. Van Arsdale, Q. Zhou, S. M. Srinivasula, E. S. Alnemri, G. S. Salvesen, and J. C. Reed. 1998. IAPs block apoptotic events induced by caspase 8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Souza, B., M. Rowe, and D. Walls. 2000. The bfl-1 gene is transcriptionally upregulated by the Epstein-Barr virus LMP1, and its expression promotes the survival of a Burkitt's lymphoma cell line. J. Virol. 74:6652-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudas, K. C., and M. A. Apicella. 1988. Selection and immunochemical analysis of lipooligosaccharide mutants of Neisseria gonorrhoeae. Infect. Immun. 56:499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey, H. A., M. P. Jennings, C. A. Campbell, R. Williams, and M. A. Apicella. 2001. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol. Microbiol. 42:659-672. [DOI] [PubMed] [Google Scholar]
- 13.Harvey, H. A., M. R. Ketterer, A. Preston, D. Lubaroff, R. Williams, and M. A. Apicella. 1997. Ultrastructural analysis of primary human urethral epithelial cell cultures infected with Neisseria gonorrhoeae. Infect. Immun. 65:2420-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey, H. A., D. M. Post, and M. A. Apicella. 2002. Immortalization of human urethral epithelial cells: a model for the study of the pathogenesis of and the inflammatory cytokine response to Neisseria gonorrhoeae infection. Infect. Immun. 70:5808-5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 16.Hsu, S. Y., A. Kaipia, E. McGee, M. Lomeli, and A. J. Hsueh. 1997. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc. Natl. Acad. Sci. USA 94:12401-12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John, C. M., J. M. Griffiss, M. A. Apicella, R. E. Mandrell, and B. W. Gibson. 1991. The structural basis for pyocin resistance in Neisseria gonorrhoeae lipooligosaccharides. J. Biol. Chem. 266:19303-19311. [PubMed] [Google Scholar]
- 18.Jurgensmeier, J. M., Z. Xie, Q. Deveraux, L. Ellerby, D. Bredesen, and J. C. Reed. 1998. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95:4997-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaltschmidt, B., R. A. Linker, J. Deng, and C. Kaltschmidt. 4 December 2002, posting date. Cyclooxygenase-2 is a neuronal target gene of NF-κB. BMC Mol. Biol. 3:16. [Online.] http://www.biomedcentral.com/1471-2199/3/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasibhatla, S., L. Genestier, and D. R. Green. 1999. Regulation of Fas-ligand expression during activation-induced cell death in T lymphocytes via nuclear factor κB. J. Biol. Chem. 274:987-992. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J. M., J. S. Kim, H. C. Jung, I. S. Song, and C. Y. Kim. 2000. Upregulated cyclooxygenase-2 inhibits apoptosis of human gastric epithelial cells infected with Helicobacter pylori. Dig. Dis. Sci. 45:2436-2443. [DOI] [PubMed] [Google Scholar]
- 22.Lee, H. H., H. Dadgostar, Q. Cheng, J. Shu, and G. Cheng. 1999. NF-κB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc. Natl. Acad. Sci. USA 96:9136-9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, B., C. Williams-Skipp, Y. Tao, M. S. Schleicher, L. L. Cano, R. C. Duke, and R. I. Scheinman. 1999. NF-κB functions as both a proapoptotic and antiapoptotic regulatory factor within a single cell type. Cell Death Differ. 6:570-582. [DOI] [PubMed] [Google Scholar]
- 24.Lin, M. T., R. C. Lee, P. C. Yang, F. M. Ho, and M. L. Kuo. 2001. Cyclooxygenase-2 inducing Mcl-1-dependent survival mechanism in human lung adenocarcinoma CL1.0 cells. Involvement of phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 276:48997-49002. [DOI] [PubMed] [Google Scholar]
- 25.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 26.Massari, P., Y. Ho, and L. M. Wetzler. 2000. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc. Natl. Acad. Sci. USA 97:9070-9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 28.Massari, P., C. A. King, A. Y. Ho, and L. M. Wetzler. 2003. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cell. Microbiol. 5:99-109. [DOI] [PubMed] [Google Scholar]
- 29.Müller, A., D. Gunther, V. Brinkmann, R. Hurwitz, T. F. Meyer, and T. Rudel. 2000. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. EMBO J. 19:5332-5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller, A., D. Gunther, F. Dux, M. Naumann, T. F. Meyer, and T. Rudel. 1999. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 18:339-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muzio, M., B. R. Stockwell, H. R. Stennicke, G. S. Salvesen, and V. M. Dixit. 1998. An induced proximity model for caspase-8 activation. J. Biol. Chem. 273:2926-2930. [DOI] [PubMed] [Google Scholar]
- 32.Nassif, X., and M. So. 1995. Interaction of pathogenic neisseriae with nonphagocytic cells. Clin. Microbiol. Rev. 8:376-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naumann, M., S. Wessler, C. Bartsch, B. Wieland, and T. F. Meyer. 1997. Neisseria gonorrhoeae epithelial cell interaction leads to the activation of the transcription factors nuclear factor κB and activator protein 1 and the induction of inflammatory cytokines. J. Exp. Med. 186:247-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perfetto, B., G. Donnarumma, D. Criscuolo, I. Paoletti, E. Grimaldi, M. A. Tufano, and A. Baroni. 2003. Bacterial components induce cytokine and intercellular adhesion molecules-1 and activate transcription factors in dermal fibroblasts. Res. Microbiol. 154:337-344. [DOI] [PubMed] [Google Scholar]
- 35.Post, D. M., M. R. Ketterer, N. J. Phillips, B. W. Gibson, and M. A. Apicella. 2003. The msbB mutant of Neisseria meningitidis strain NMB has a defect in lipooligosaccharide assembly and transport to the outer membrane. Infect. Immun. 71:647-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy, N., Q. L. Deveraux, R. Takahashi, G. S. Salvesen, and J. C. Reed. 1997. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 16:6914-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudel, T., A. Schmid, R. Benz, H. A. Kolb, F. Lang, and T. F. Meyer. 1996. Modulation of Neisseria porin (PorB) by cytosolic ATP/GTP of target cells: parallels between pathogen accommodation and mitochondrial endosymbiosis. Cell 85:391-402. [DOI] [PubMed] [Google Scholar]
- 38.Shi, Y., J. Chen, C. Weng, R. Chen, Y. Zheng, Q. Chen, and H. Tang. 2003. Identification of the protein-protein contact site and interaction mode of human VDAC1 with Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 305:989-996. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu, S., A. Konishi, T. Kodama, and Y. Tsujimoto. 2000. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc. Natl. Acad. Sci. USA 97:3100-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu, S., M. Narita, and Y. Tsujimoto. 1999. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399:483-487. [DOI] [PubMed] [Google Scholar]
- 41.Vander Heiden, M. G., X. X. Li, E. Gottleib, R. B. Hill, C. B. Thompson, and M. Colombini. 2001. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J. Biol. Chem. 276:19414-19419. [DOI] [PubMed] [Google Scholar]
- 42.Wang, C. Y., D. C. Guttridge, M. W. Mayo, and A. S. Baldwin, Jr. 1999. NF-κB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol. Cell. Biol. 19:5923-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 44.Weel, J. F., and J. P. van Putten. 1991. Fate of the major outer membrane protein P.IA in early and late events of gonococcal infection of epithelial cells. Res. Microbiol. 142:985-993. [DOI] [PubMed] [Google Scholar]
- 45.Werner, A. B., E. de Vries, S. W. Tait, I. Bontjer, and J. Borst. 2002. Bcl-2 family member Bfl-1/A1 sequesters truncated Bid to inhibit its collaboration with pro-apoptotic Bak or Bax. J. Biol. Chem. 277:22781-22788. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, H., S. W. Cowan-Jacob, M. Simonen, W. Greenhalf, J. Heim, and B. Meyhack. 2000. Structural basis of BFL-1 for its interaction with BAX and its anti-apoptotic action in mammalian and yeast cells. J. Biol. Chem. 275:11092-11099. [DOI] [PubMed] [Google Scholar]
- 47.Zong, W. X., L. C. Edelstein, C. Chen, J. Bash, and C. Gelinas. 1999. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-κB that blocks TNFα-induced apoptosis. Genes Dev. 13:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]