Abstract
Persistent nasal carriers and noncarriers of Staphylococcus aureus were inoculated with a mixture of different S. aureus strains. The majority of noncarriers and nearly all persistent carriers returned to their original carrier state after artificial inoculation. Furthermore, the majority of persistent carriers tested positive again for their original resident strain. Using a human nasal inoculation model, we here demonstrate that the human factor is an important determinant of S. aureus nasal carriage.
Staphylococcus aureus nasal carriage is a major risk factor for S. aureus infections (10, 22). Recently, cell wall lipoteichoic acid was described as the core factor for S. aureus nasal colonization (23). However, in earlier studies, no single staphylococcal factor essential for nasal colonization could be identified (1, 6, 15, 19). Furthermore, host factors (7, 8, 10) as well as environmental factors are recognized determinants of the S. aureus nasal carrier state (3, 16).
Three human nasal S. aureus carriage patterns can be distinguished: persistent carriage, intermittent carriage, and noncarriage (17). S. aureus density in the anterior nares is higher in persistent carriers (13), which may partly explain their increased risk for S. aureus infections (5). Variation among colonizing strains is higher for intermittent carriers (21), suggesting that the basic determinants of persistent and intermittent carriage are different. The biology of S. aureus nasal carriage remains incompletely understood, although the importance of various host factors has been demonstrated (2, 8, 9, 12). In seeking further clarification, we performed a study in which persistent carriers and noncarriers were inoculated intranasally with a mixture of S. aureus strains.
(Parts of these results have been presented at the International Society for Staphylococci & Staphylococcal Infections meeting, June 2000, Kolding, Denmark.)
In 1988, a cohort of healthy volunteers (staff members of the Departments of Medical Microbiology & Infectious Diseases and Virology of the Erasmus MC) was formed to investigate bacterial and human factors associated with S. aureus nasal carriage (21). The composition of this volunteer cohort was not fixed, in that outgoing personnel were considered lost to follow-up and were replaced by incoming personnel. All volunteers were screened initially with 12 quantitative nasal swab cultures performed at 1-week intervals. After this initial establishment of S. aureus nasal carriage status, the volunteers were rescreened regularly with four quantitative nasal swab cultures at 1-week intervals. For the present study, only volunteers in follow-up for at least 2 years and with at least 16 nasal swab cultures done were included. Long-term persistent carriers were defined as those with all preceding cultures positive, and long-term noncarriers were defined as those with all preceding cultures negative. Participants were excluded if they suffered from diabetes mellitus, skin diseases, chronic obstructive pulmonary disease, or cardiac valve abnormalities or if they were taking immunosuppressive agents. Eleven persistent carriers and eight noncarriers agreed to participate in the present study. All participants gave written informed consent, and the study was approved by the Medical Ethics Review Committee of the Erasmus Medical Center, Rotterdam, The Netherlands (METC Erasmus MC decision no. 156.137/1996/186).
For the noncarriers, a mixture of four different S. aureus strains was prepared; the mixture consisted of S. aureus 502A (a strain used in intervention studies in the 1960s and 1970) (11), S. aureus DU 5819 (a protein A-deficient Dublin strain, courtesy of T. Foster), S. aureus 274 (a strain from a persistent carrier), and S. aureus 1036 (a strain from an intermittent carrier). Strains were selected from different carriage classes to analyze whether they had different colonization capacities (21). The strains did not produce superantigens and did not show different in vitro growth characteristics (data not shown). For the persistent carriers, the same mixture of four S. aureus strains was used, but with each carrier's own resident strain added.
Nasal swabs were obtained with cotton-wool swabs (Transwab, Corsham, United Kingdom) (21). The left and right anterior nares were swabbed four times around. The swabs were immediately placed in Stuart's transport medium (Transwab) and kept at 4°C until quantitative culture on phenol red-mannitol-salt agar (PHMA) and in phenol red-mannitol-salt broth (PHMB). The PHMB was incubated at 37°C (7 days); the PHMA culture plates were incubated at 37°C (48 h) and at room temperature (5 days). Identification of S. aureus was based upon colony morphology and a catalase and latex agglutination test (Staphaurex Plus; Murex, Dartford, United Kingdom). The geometric mean CFU in the 26 postinoculation cultures was calculated by the formula [10 log(CFU1 + 1) + 10 log(CFU2 + 1) +… + 10 log(CFU26 + 1)]/26. For each culture, 16 S. aureus colonies (maximum amount allowing for efficient molecular characterization), including all S. aureus morphotypes, were stored at −70°C. To obtain bacterial DNA, S. aureus isolates were grown overnight at 37°C on brucella blood agar and processed as described by Boom et al. (4). DNA was stored at −20°C. Restriction fragment length polymorphisms of the coagulase and protein A genes were determined for strain identification purposes in the four S. aureus strains and all resident S. aureus strains from persistent carriers before inoculation (20). Furthermore, all S. aureus strains isolated 2 and 13 weeks after inoculation and/or from the last positive culture were genotyped by this method. Pulsed-field gel electrophoresis was performed to confirm the results (14).
All persistent carriers were treated with mupirocin nasal ointment (Bactroban; GlaxoSmithKline, Zeist, The Netherlands) two times daily for 5 days. The noncarriers did not receive mupirocin treatment. Ten weeks later, with nasal swab cultures negative, all participants were inoculated. Inoculation was performed using cotton-wool swabs drenched in PHMB containing 109 CFU of each strain/ml. For each nostril, one swab was firmly applied against the inner side of the anterior nares and turned around four times. In this way, the strains were inoculated in a total amount of 109 CFU. At the time of inoculation, blood was drawn for the determination of the erythrocyte sedimentation rate, C-reactive protein, leukocyte count and differentiation, and antistaphylococcal antibodies. These tests were repeated when required. Nasal cultures were performed weekly during the study period. All participants with positive cultures at the end of the study were offered mupirocin nasal ointment (Bactroban).
The primary end point was survival of S. aureus in the nose after artificial colonization. Survival was considered ended when at least two consecutive nasal swab cultures were negative. Kaplan-Meier curves and the log rank test were used to compare S. aureus survival curves. Participants still carrying S. aureus in their noses at the end of the study were considered censored in the analysis. The secondary end point was the geometric mean count of CFU over 26 weeks. Percentages and continuous data were compared by Fisher's exact test and Mann-Whitney's test, respectively.
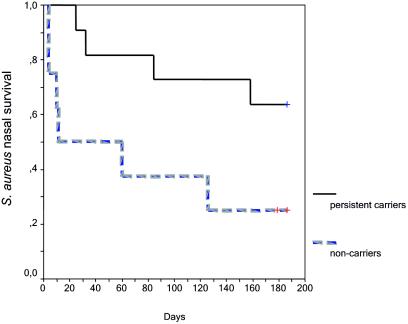
After artificial inoculation with a mixture of S. aureus strains, median nasal survival of S. aureus was 186 days in persistent carriers versus 35.5 days in noncarriers (P = 0.0427) (Fig. 1). Also, the 26-week log geometric mean number of CFU was higher in persistent carriers (median, 2.9 [range, 0.6 to 3.7] versus 0.7 [range, 0 to 3.6]), nearly reaching statistical significance (P = 0.069).
FIG. 1.
S. aureus survival after artificial nasal inoculation in long-term persistent S. aureus nasal carriers and nasal noncarriers. Kaplan-Meier curves of S. aureus nasal survival in persistent carriers (solid line) and in noncarriers (broken grey line) are shown. Survival ended when at least two consecutive nasal swab cultures were negative. After artificial inoculation with a mixture of S. aureus strains, the median nasal survival of S. aureus was 186 days in persistent carriers versus 35.5 days in noncarriers (P = 0.0427; log rank test).
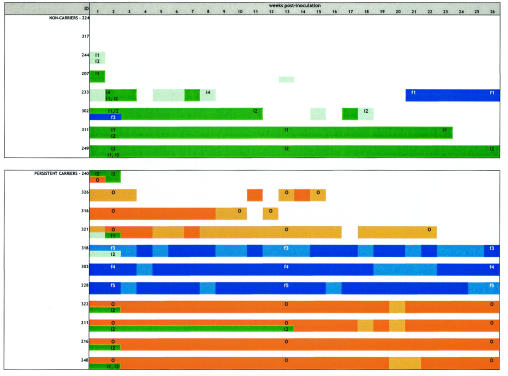
Six out of eight noncarriers became noncarriers again: four within 2 weeks after inoculation (noncarriers 224, 317, 244, and 207), and two after 19 (noncarrier 302) and 23 (noncarrier 311; inoculation strain S. aureus 502A) weeks (Fig. 2). Noncarrier 302 remained persistently positive with inoculation S. aureus strain 274 until week 11, was intermittently positive between weeks 12 and 18, and reverted to the noncarrier state again at week 19 (Fig. 2). Two noncarriers still had positive nasal cultures at the end of the study. Noncarrier 233 had positive cultures up to week 8, negative cultures between weeks 9 and 20, and then cultured positive again until the end of the study. This person first tested positive for S. aureus DU 5819, while ultimately a foreign S. aureus strain not included in the inoculation mixture was identified. Noncarrier 249 remained persistently positive after inoculation until the end of the study with inoculation S. aureus strain 274 and thus had become a persistent S. aureus nasal carrier (Fig. 2). Two of the noncarrier volunteers developed minor self-limiting skin lesions, noncarrier 311 colonized with S. aureus 502A and noncarrier 249 colonized with S. aureus strain 274. No antibiotic treatment was necessary, and all laboratory parameters remained completely normal. No side effects were noted in the persistent-carrier group (P = 0.1637).
FIG. 2.
Postartificial nasal inoculation culture results for 11 long-term persistent and 8 non-S. aureus nasal carriers during 26 weeks of follow-up. ID, identification number of individual participants. At time points 2 and 13 days postinoculation of the last positive culture, all S. aureus strains cultured were genotyped. The various genotypically distinct S. aureus strains cultured are identified by colors and codes. The original resident strains of the persistent carriers are colored orange and coded O (“own”). The four S. aureus strains used in the inoculation mixture are colored (mint) green and coded i1 (inoculation S. aureus strain 502A), i2 (inoculation S. aureus strain 274 [persistent-carrier strain]), i3 (inoculation S. aureus strain 1036 [intermittent-carrier strain]), and i4 (inoculation S. aureus strain DU 5819 [protein A-deficient strain]). Five unique foreign S. aureus strains, which were neither resident strains from persistent carriers nor inoculation strains from the inoculation mixture, were cultured in five participants. These foreign S. aureus strains are colored blue and coded f1, f2, f3, f4, and f5. Multiple genotypically distinct S. aureus strains can thus be cultured at each point in time during follow-up. The shading of colors indicates the total number of CFU of S. aureus per culture: dark coloring, more than 100 CFU; light coloring,1 to 99 CFU; no coloring, cultures negative at that point in time.
In the persistent-carrier group, seven persons became persistent carriers again after artificial nasal inoculation: four carrying their own resident strains (persistent carriers 322, 211, 216, and 248) and three carrying unique foreign strains not included in the inoculation mixture (persistent carriers 318, 303, and 228). These new strains were all genetically different and did not represent a laboratory contamination. Three persistent carriers became intermittent carriers with their own resident strains (persistent carriers 326, 316, and 321), and one person reverted to the noncarrier state (persistent carrier 240) (Fig. 2).
The present results identify the importance of host factors in determining the S. aureus nasal carrier state in healthy adults. Half of the noncarriers became noncarriers again within 2 weeks after inoculation. Only one noncarrier became a persistent carrier, which coincided with the occurrence of minor self-limiting skin lesions. These data suggest that most noncarriers are inherently resistant to colonization, but when S. aureus carriage is imposed, minor skin lesions can develop. Bacterial interference may be an explanation of the noncarrier state: when an ecological niche is already occupied by other bacteria, S. aureus does not seem to have the means to establish a local population (18). Recent data indicate that when the noncarriers were treated with mupirocin prior to inoculation, elimination was as efficient: only 1 out of 16 volunteers was found to be still colonized after 16 weeks (data not shown). This finding suggests that noncarriers are not protected by a mupirocin-susceptible resident population of bacteria.
Among the 11 persistent carriers, 7 became persistent carriers again: 4 with their own resident strains and 3 with genetically unique foreign strains not included in the mixture. Three persons became intermittent carriers, all with their own resident strains. Only one person reverted to the noncarrier state. Given the opportunity, persistent carriers will select for an optimally fitting S. aureus strain, either from the inoculation mixture or from their environment (21). Including the intermittent carriers, 7 out of 11 volunteers were colonized with their original resident strains again.
So far, no single common genetic or phenotypic characteristic segregating successful from less successful or nonsuccessful colonizing S. aureus strains has been identified (1, 7, 8, 15, 19). However, lipoteichoic acid has been recently implicated as an essential bacterial factor for S. aureus nasal colonization in a rat model (23). Here we conclude that, in addition, host characteristics significantly codetermine the S. aureus carrier state and that optimal fit between host and bacteria seems to be important. Further research should focus on identifying the specific host and bacterial factors involved. New strategies for the prevention of S. aureus nasal carriage and endogenous S. aureus infection could then be developed.
Acknowledgments
We gratefully acknowledge the volunteers that agreed to participate in this study. Without their willing participation, none of what is presented here could actually have been documented in such detail.
Editor: J. N. Weiser
REFERENCES
- 1.Aly, R., H. R. Shinefield, C. Litz, and H. I. Maibach. 1980. Role of teichoic acid in the binding of Staphylococcus aureus to nasal epithelial cells. J. Infect. Dis. 141:463-465. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong-Esther, C. A. 1976. Carriage patterns of Staphylococcus aureus in a healthy non-hospital population of adults and children. Ann. Hum. Biol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert, D., A. van Belkum, M. Sluijter, A. Luijendijk, R. de Groot, H. C. Rümke, H. A. Verbrugh, and P. W. Hermans. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363:1871-1872. [DOI] [PubMed] [Google Scholar]
- 4.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruun, J. N. 1970. Post-operative wound infection: predisposing factors and the effect of a reduction in dissemination of staphylococci. Acta Med. Scand. 514:1-89. [PubMed] [Google Scholar]
- 6.Carruthers, M. M., and W. J. Kabat. 1983. Mediation of staphylococcal adherence to mucosal cells by lipoteichoic acid. Infect. Immun. 40:444-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole, A. M., P. Dewan, and T. Ganz. 1999. Innate antimicrobial activity of nasal secretions. Infect. Immun. 67:3267-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole, A. M., S. Tahk, A. Oren, D. Yoshioka, Y. H. Kim, A. Park, and T. Ganz. 2001. Determinants of Staphylococcus aureus nasal carriage. Clin. Diagn. Lab. Immunol. 8:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoeksma, A., and K. C. Winkler. 1963. The normal flora of the nose in twins. Acta Leiden. 32:123-133. [PubMed] [Google Scholar]
- 10.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Light, I. J., R. L. Walton, J. M. Sutherland, H. R. Shinefield, and V. Brackvogel. 1967. Use of bacterial interference to control a staphylococcal nursery outbreak. Deliberate colonization of all infants with the 502A strain of Staphylococcus aureus. Am. J. Dis. Child. 113:291-300. [DOI] [PubMed] [Google Scholar]
- 12.Noble, W. C. 1974. Carriage of Staphylococcus aureus and beta haemolytic streptococci in relation to race. Acta Derm. Venereol. 54:403-405. [PubMed] [Google Scholar]
- 13.Nouwen, J. L., A. Ott, M. F. Q. VandenBergh, H. A. M. Boelens, A. Hofman, A. van Belkum, and H. A. Verbrugh. 2004. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule.” Clin. Infect. Dis. 39:806-811. [DOI] [PubMed]
- 14.Nouwen, J. L., A. van Belkum, S. de Marie, J. Sluijs, J. J. Wielenga, J. A. Kluytmans, and H. A. Verbrugh. 1998. Clonal expansion of Staphylococcus epidermidis strains causing Hickman catheter-related infections in a hemato-oncologic department. J. Clin. Microbiol. 36:2696-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 16.Peacock, S. J., A. Justice, D. Griffiths, G. D. De Silva, M. N. Kantzanou, D. Crook, K. Sleeman, and N. P. Day. 2003. Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J. Clin. Microbiol. 41:5718-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riewerts Eriksen, N. H., F. Espersen, V. Thamdrup Rosdahl, and K. Jensen. 1995. Carriage of Staphylococcus aureus among 104 healthy persons during a 19 month period. Epidemiol. Infect. 115:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinefield, H. R., J. C. Ribble, M. Boris, H. F. Eichenwald, R. Aly, and H. Maibach. 1974. Bacterial interference between strains of S. aureus. Ann. N. Y. Acad. Sci. 236:444-455. [DOI] [PubMed] [Google Scholar]
- 19.Shuter, J., V. B. Hatcher, and F. D. Lowy. 1996. Staphylococcus aureus binding to human nasal mucin. Infect. Immun. 64:310-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Belkum, A., N. H. Riewarts Eriksen, M. Sijmons, W. Van Leeuwen, M. Van den Bergh, J. Kluytmans, F. Espersen, and H. Verbrugh. 1997. Coagulase and protein A polymorphisms do not contribute to persistence of nasal colonisation by Staphylococcus aureus. J. Med. Microbiol. 46:222-232. [DOI] [PubMed] [Google Scholar]
- 21.VandenBergh, M. F. Q., E. P. F. Yzerman, A. van Belkum, H. A. M. Boelens, M. Sijmons, and H. A. Verbrugh. 1999. Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. J. Clin. Microbiol. 37:3133-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 23.Weidenmaier, C., J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond, and A. Peschel. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10:243-245. [DOI] [PubMed] [Google Scholar]