Abstract
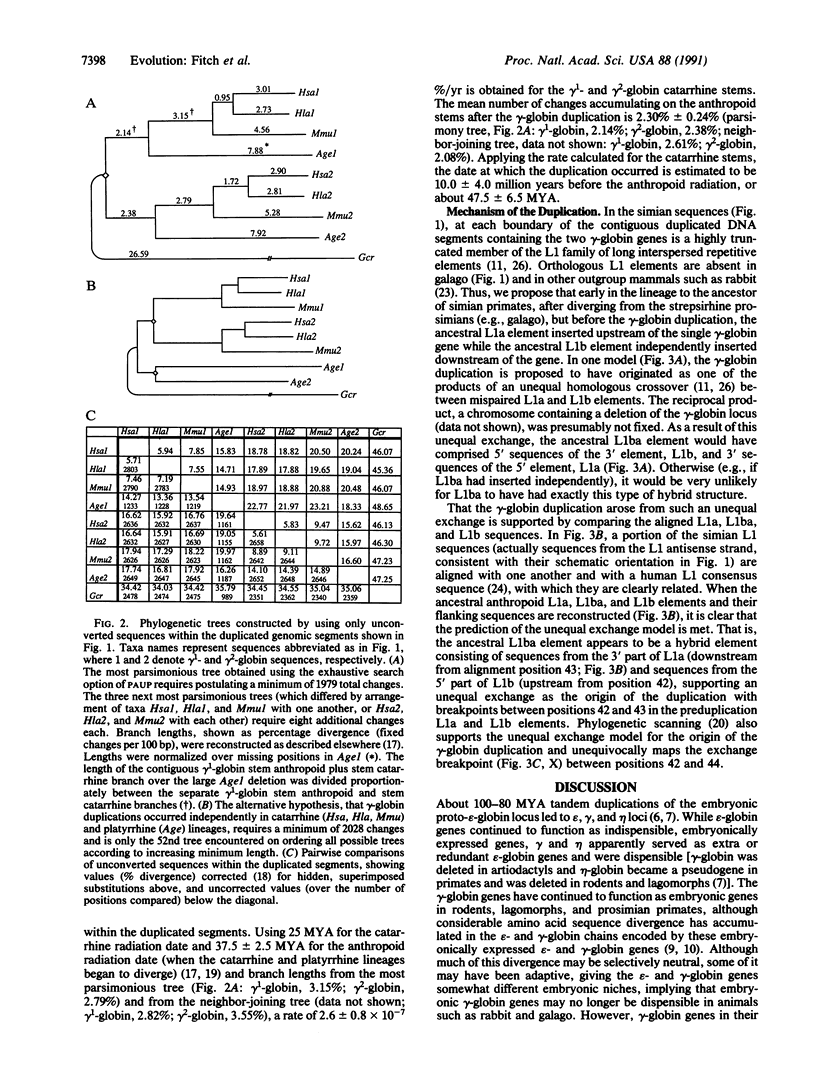
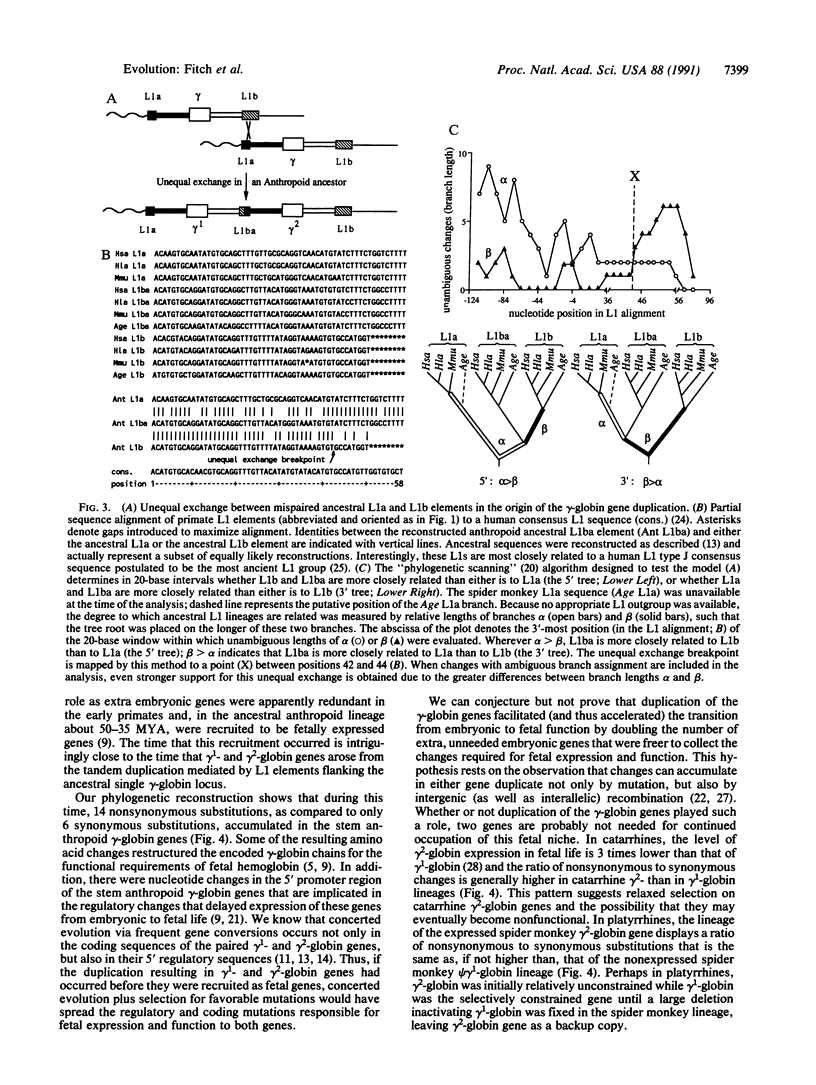
Regions surrounding the single gamma-globin gene of galago and the duplicated gamma 1- and gamma 2-globin genes of gibbon, rhesus monkey, and spider monkey were sequenced and aligned with those from humans. Contrary to previous studies, spider monkey was found to have not one but two gamma-globin genes, only one of which (gamma 2) is functional. The reconstructed evolutionary history of the gamma-globin genes and their flanking sequences traces their origin to a tandem duplication of a DNA segment approximately 5.5 kilobases long that occurred before catarrhine primates (humans, apes, and Old World monkeys) diverged from platyrrhines (New World monkeys), much earlier than previously thought. This reconstructed molecular history also reveals that the duplication resulted from an unequal homologous crossover between two related L1 long interspersed repetitive elements, one upstream and one downstream of the single ancestral gamma-globin gene. Perhaps facilitated by the redundancy resulting from the duplication, the gamma-globin genes escaped the selective constraints of embryonically functioning genes and evolved into fetally functioning genes. This view is supported by the finding that a burst of nonsynonymous substitutions occurred in the gamma-globin genes while they became restructured for fetal expression in the common ancestor of platyrrhines and catarrhines.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey W. J., Fitch D. H., Tagle D. A., Czelusniak J., Slightom J. L., Goodman M. Molecular evolution of the psi eta-globin gene locus: gibbon phylogeny and the hominoid slowdown. Mol Biol Evol. 1991 Mar;8(2):155–184. doi: 10.1093/oxfordjournals.molbev.a040641. [DOI] [PubMed] [Google Scholar]
- Barrie P. A., Jeffreys A. J., Scott A. F. Evolution of the beta-globin gene cluster in man and the primates. J Mol Biol. 1981 Jul 5;149(3):319–336. doi: 10.1016/0022-2836(81)90476-9. [DOI] [PubMed] [Google Scholar]
- Barsh G. S., Seeburg P. H., Gelinas R. E. The human growth hormone gene family: structure and evolution of the chromosomal locus. Nucleic Acids Res. 1983 Jun 25;11(12):3939–3958. doi: 10.1093/nar/11.12.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czelusniak J., Goodman M., Hewett-Emmett D., Weiss M. L., Venta P. J., Tashian R. E. Phylogenetic origins and adaptive evolution of avian and mammalian haemoglobin genes. Nature. 1982 Jul 15;298(5871):297–300. doi: 10.1038/298297a0. [DOI] [PubMed] [Google Scholar]
- Davis P. S., Shen M. W., Judd B. H. Asymmetrical pairings of transposons in and proximal to the white locus of Drosophila account for four classes of regularly occurring exchange products. Proc Natl Acad Sci U S A. 1987 Jan;84(1):174–178. doi: 10.1073/pnas.84.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover G. A. DNA turnover and the molecular clock. J Mol Evol. 1987;26(1-2):47–58. doi: 10.1007/BF02111281. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fitch D. H., Goodman M. Phylogenetic scanning: a computer-assisted algorithm for mapping gene conversions and other recombinational events. Comput Appl Biosci. 1991 Apr;7(2):207–215. doi: 10.1093/bioinformatics/7.2.207. [DOI] [PubMed] [Google Scholar]
- Fitch D. H., Mainone C., Goodman M., Slightom J. L. Molecular history of gene conversions in the primate fetal gamma-globin genes. Nucleotide sequences from the common gibbon, Hylobates lar. J Biol Chem. 1990 Jan 15;265(2):781–793. [PubMed] [Google Scholar]
- Giebel L. B., van Santen V. L., Slightom J. L., Spritz R. A. Nucleotide sequence, evolution, and expression of the fetal globin gene of the spider monkey Ateles geoffroyi. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6985–6989. doi: 10.1073/pnas.82.20.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. L., Sheen J. Y., Gehring W. J., Green M. M. Unequal crossing-over associated with asymmetrical synapsis between nomadic elements in the Drosophila melanogaster genome. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5017–5021. doi: 10.1073/pnas.80.16.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M., Czelusniak J., Koop B. F., Tagle D. A., Slightom J. L. Globins: a case study in molecular phylogeny. Cold Spring Harb Symp Quant Biol. 1987;52:875–890. doi: 10.1101/sqb.1987.052.01.096. [DOI] [PubMed] [Google Scholar]
- Goodman M., Koop B. F., Czelusniak J., Weiss M. L. The eta-globin gene. Its long evolutionary history in the beta-globin gene family of mammals. J Mol Biol. 1984 Dec 25;180(4):803–823. doi: 10.1016/0022-2836(84)90258-4. [DOI] [PubMed] [Google Scholar]
- Hardison R. C. Comparison of the beta-like globin gene families of rabbits and humans indicates that the gene cluster 5'-epsilon-gamma-delta-beta-3' predates the mammalian radiation. Mol Biol Evol. 1984 Sep;1(5):390–410. doi: 10.1093/oxfordjournals.molbev.a040326. [DOI] [PubMed] [Google Scholar]
- Jurka J. Subfamily structure and evolution of the human L1 family of repetitive sequences. J Mol Evol. 1989 Dec;29(6):496–503. doi: 10.1007/BF02602921. [DOI] [PubMed] [Google Scholar]
- Koop B. F., Goodman M. Evolutionary and developmental aspects of two hemoglobin beta-chain genes (epsilon M and beta M) of opossum. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3893–3897. doi: 10.1073/pnas.85.11.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop B. F., Tagle D. A., Goodman M., Slightom J. L. A molecular view of primate phylogeny and important systematic and evolutionary questions. Mol Biol Evol. 1989 Nov;6(6):580–612. doi: 10.1093/oxfordjournals.molbev.a040574. [DOI] [PubMed] [Google Scholar]
- Langley C. H., Montgomery E., Hudson R., Kaplan N., Charlesworth B. On the role of unequal exchange in the containment of transposable element copy number. Genet Res. 1988 Dec;52(3):223–235. doi: 10.1017/s0016672300027695. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Russell D. W., Brown M. S. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell. 1987 Mar 13;48(5):827–835. doi: 10.1016/0092-8674(87)90079-1. [DOI] [PubMed] [Google Scholar]
- Maeda N., Smithies O. The evolution of multigene families: human haptoglobin genes. Annu Rev Genet. 1986;20:81–108. doi: 10.1146/annurev.ge.20.120186.000501. [DOI] [PubMed] [Google Scholar]
- Maizels N. Might gene conversion be the mechanism of somatic hypermutation of mammalian immunoglobulin genes? Trends Genet. 1989 Jan;5(1):4–8. doi: 10.1016/0168-9525(89)90004-8. [DOI] [PubMed] [Google Scholar]
- Margot J. B., Demers G. W., Hardison R. C. Complete nucleotide sequence of the rabbit beta-like globin gene cluster. Analysis of intergenic sequences and comparison with the human beta-like globin gene cluster. J Mol Biol. 1989 Jan 5;205(1):15–40. doi: 10.1016/0022-2836(89)90362-8. [DOI] [PubMed] [Google Scholar]
- Ohta T. Time for acquiring a new gene by duplication. Proc Natl Acad Sci U S A. 1988 May;85(10):3509–3512. doi: 10.1073/pnas.85.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Scott A. F., Schmeckpeper B. J., Abdelrazik M., Comey C. T., O'Hara B., Rossiter J. P., Cooley T., Heath P., Smith K. D., Margolet L. Origin of the human L1 elements: proposed progenitor genes deduced from a consensus DNA sequence. Genomics. 1987 Oct;1(2):113–125. doi: 10.1016/0888-7543(87)90003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Koop B. F., Xu P. L., Goodman M. Rhesus fetal globin genes. Concerted gene evolution in the descent of higher primates. J Biol Chem. 1988 Sep 5;263(25):12427–12438. [PubMed] [Google Scholar]
- Tagle D. A., Koop B. F., Goodman M., Slightom J. L., Hess D. L., Jones R. T. Embryonic epsilon and gamma globin genes of a prosimian primate (Galago crassicaudatus). Nucleotide and amino acid sequences, developmental regulation and phylogenetic footprints. J Mol Biol. 1988 Sep 20;203(2):439–455. doi: 10.1016/0022-2836(88)90011-3. [DOI] [PubMed] [Google Scholar]



