Abstract
Although Legionella pneumophila can multiply in diverse cell types from a variety of species, macrophages from most inbred mouse strains are nonpermissive for intracellular replication and allow little or no growth of the bacteria. This phenomenon is likely genetically controlled by the mouse naip5 (birc1e) gene located within the Lgn1 locus. In this study, we have investigated the resistance of C57BL/6J macrophages to L. pneumophila infection by examining the fate of both the bacterium and the infected cells compared to that in macrophages from the permissive A/J strain. Our results indicate that although the trafficking of the L. pneumophila-containing vacuole is partially disrupted in C57BL/6J macrophages, this cannot account for the severity of the defect in intracellular growth observed in this strain. Infected macrophages are lost shortly after infection, and at later times a larger fraction of the C57BL/6J macrophages in which L. pneumophila undergoes replication are apoptotic compared to those derived from A/J mice. Finally, a loss of bacterial counts occurs after the first round of growth. Therefore, the resistance mechanism of C57BL/6J macrophages to L. pneumophila infection appears to be multifactorial, and we discuss how early and late responses result in clearing the infection.
Legionella pneumophila is a facultative intracellular gram-negative bacterium able to replicate within freshwater amoebae (48). When humans are infected by inhalation of contaminated aerosols, the bacteria colonize and multiply within their alveolar macrophages and cause a pneumonia, known as Legionnaires' disease, that can be fatal if the host defenses are impaired (15, 37).
Internalization of the bacterium by the host cell is dependent on the dot/icm genes and occurs via macropinocytosis (22, 49). Shortly after uptake, the bacterium is found in a membrane-bound compartment that does not interact with the endocytic pathway (24, 25). Vesicles containing the bacterium neither acidify nor fuse with lysosomes and exclude endosomal or lysosomal markers such as Rab5, Lamp1, and cathepsin D (7, 28, 38). The bacterium then establishes an intracellular niche that supports replication. First, the vacuole is surrounded with mitochondria and small vesicles (23). Based on the thickness of their membranes (45) and the presence of calnexin around the nascent L. pneumophila vacuoles (9), these vesicles are thought to be derived from the endoplasmic reticulum (ER). In addition, the L. pneumophila vacuole intercepts vesicular traffic from ER exit sites (29), and proteins of the early secretory pathway are found about the vacuole and appear to be required for the establishment of intracellular replication (9). A few hours later, rough ER accumulates around the vacuole and the bacteria start to divide (44, 45). The bacteria continue to multiply within a perinuclear ER-like compartment that bears similarity to an autophagous vacuole which may eventually enter the endocytic pathway (43, 44). Finally, the host cell is lysed, allowing the bacteria to reinfect neighboring cells.
Various genetic screens have revealed that 25 L. pneumophila dot/icm genes, located in two separate chromosomal regions, are essential for the bypass of the endocytic pathway and intracellular growth of the bacterium (1, 3, 4, 13, 39, 47). The majority of these genes exhibit sequence similarity to genes required for conjugative DNA transfer and are therefore hypothesized to encode components of a type IV secretion machinery (41, 46). Consistent with this model, the Dot/Icm complex is required for the transfer of plasmid DNA or effector proteins (30) from one L. pneumophila cell to another and also for the translocation of the bacterial effector molecules RalF (36), LidA (8), SidC (30), LepB, and LepC (6) across the surface of the L. pneumophila vacuole. These proteins, and others that have recently been identified (30), presumably manipulate the host cell to allow formation of the replication vacuole.
Although L. pneumophila can multiply within different cell types, such as amoebae or human alveolar macrophages, most cells from inbred mouse strains are nonpermissive for intracellular replication. With the exception of a few strains such as A/J, macrophages isolated from these strains yield little or no growth of the bacterium (52, 53). The restrictive phenotype is controlled by the Lgn1 locus, located on chromosome 13 (2, 11, 51, 55), which contains a number of neuronal apoptosis inhibitory protein (naip) genes that vary from one inbred mouse strain to another (21, 54). The product of the naip5 (birc1e) gene likely confers resistance to L. pneumophila intracellular multiplication in the C57BL/6J mouse strain (12, 50). The function of Naip5 (Birc1e) is unknown; however, the protein shows sequence similarity to the Nod proteins (26), which are cytosolic factors related to the apoptosis regulator Apaf1 and to a class of plant disease resistance proteins. The Nod proteins are capable of activating the NF-κB pathway upon intracellular stimulation by bacterial products (20, 27). Naip and Nod proteins share a carboxy-terminal regulatory domain composed of a leucine-rich repeats domain and a central nucleotide-binding oligomerization domain, also known as the NOD domain. However, the Naip proteins contain additional baculovirus inhibitor of apoptosis domains that are not found in the Nod proteins (26). The role Naip plays in responding to intracellular pathogens and whether it is involved in inhibition of apoptosis are still unknown.
In this study, we have investigated the resistance of C57BL/6J macrophages to L. pneumophila infection by examining the fate of both the bacterium and the macrophages. Our results suggest a multifactorial mechanism involving loss of infected macrophages in the early stage of the infection and apoptosis in the later stage, as well as loss of bacterial counts after the first round of intracellular growth.
MATERIALS AND METHODS
Bacterial strains, mice, and media.
L. pneumophila Lp02 (thyA hsdR rpsL) and Lp03 (Lp02 dotA) strains are derivatives of Philadelphia 1 strain Lp01 (hsdR rpsL) (3). L. pneumophila strains were maintained as described previously (14, 16, 44). Charcoal-yeast extract-thymidine medium (CYET) and ACES yeast extract broth were prepared as described previously (3). All mice were purchased from the Jackson Laboratory (Bar Harbor, Maine).
Cell culture and antibodies.
Bone marrow-derived macrophages from A/J and C57BL/6J female mice were prepared as described previously (44). After culturing cells from mouse femurs in L cell-conditioned medium, the macrophages were harvested and resuspended in RPMI 1640 containing 10% heat-inactivated fetal bovine serum and glutamine.
Anti-L. pneumophila polyclonal rabbit serum was described previously (1). Goat anti-rabbit immunoglobulin G (IgG)-Cascade blue and goat anti-rabbit IgG-Texas red were obtained from Molecular Probes (Eugene, Oreg.). Goat anti-rabbit IgG-fluorescein isothiocyanate (FITC) was obtained from Jackson Laboratory.
Assay for intracellular growth.
Postexponential-phase L. pneumophila strains grown in AYET to high motility (except where noted) were introduced onto monolayers of 4 × 105 bone marrow macrophages in 24-well dishes at a multiplicity of infection (MOI) of 0.05 by centrifugation for 5 min at 1,000 rpm (Hermle Z360K centrifuge). After incubation for 2 h at 37°C in the presence of 5% CO2, the monolayers were washed three times to remove any extracellular bacteria and the infection was allowed to continue. To quantify the level of growth at each time point, the monolayers were lysed by adding 0.05% saponin directly to the culture medium containing the infected cells, and CFU were determined by plating dilutions of the lysate onto CYET plates.
LAMP-1 targeting assay.
A total of 2 × 105 macrophages were seeded onto glass coverslips in 24-well dishes and were infected at an MOI of 1 as described for the intracellular growth assay. After incubation for 1 h at 37°C in the presence of 5% CO2, the monolayers were fixed in periodate-lysine-paraformaldehyde (PLP)-sucrose for 1 h at 37°C (33). The samples were stained for extracellular and total bacteria as previously described (44), and LAMP-1 was detected using rat monoclonal antibody IB4D (49). Coverslips were mounted using Fluoroguard antifade reagent (Bio-Rad, Hercules, Calif.) and processed for immunofluorescence microscopy. Data were quantified by counting 100 cell-associated bacteria per coverslip. The average and standard deviation were calculated from three coverslips.
Infectious center assay.
A total of 2 × 105 macrophages were seeded onto glass coverslips in 24-well dishes and were infected at an MOI of 1 as described for the intracellular growth assay. After incubation for 1 h at 37°C in the presence of 5% CO2, the monolayers were washed. Half of the coverslips were fixed with PLP fixative containing 5% sucrose for 1 h at 37°C (33). The other half was incubated for 13 more hours at 37°C in the presence of 5% CO2 before fixation. The samples were stained for extracellular and total bacteria as described previously (44). The number of intracellular bacteria within each vacuole was recorded by visual inspection. Data were quantified by counting 100 vacuoles per coverslip. The average and standard deviation were calculated from three coverslips.
TUNEL staining and apoptosis reagents.
The infection and staining were performed as described for the infectious center assay, except that an additional step of terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) (18) was performed according to the manufacturer's specifications (Roche Molecular Biochemical, Indianapolis, Ind.) before mounting the coverslips. The CaspACE FITC-VAD-FMK in situ marker was used according to the manufacturer's protocol (Promega, Madison, Wis.).
RESULTS
Biphasic growth of L. pneumophila in C57BL/6J macrophages.
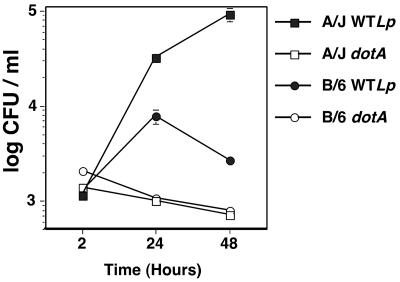
We first examined the behavior of L. pneumophila during incubation with bone marrow macrophages derived from either the Lgn1 permissive (A/J) or restrictive (C57BL/6J) mouse strain. As shown in Fig. 1, in permissive A/J macrophages, wild-type L. pneumophila grew about 100-fold over a 48-h period of time, with a 30-fold increase in growth during the first 24 h of infection. Consistent with previous results (2, 11, 52), when wild-type L. pneumophila was incubated with C57BL/6J macrophages, we only observed a sixfold increase in growth during the first 24 h of infection. Moreover, we observed a threefold decrease in the number of bacteria during the following 24 h. The dotA mutant failed to multiply in either A/J or C57BL/6J macrophages.
FIG. 1.
Biphasic growth of L. pneumophila in macrophages from C57BL/6J mice. A/J or C57BL/6J (B/6) bone marrow-derived macrophages were incubated with either wild-type L. pneumophila (WTLp) or the dotA mutant (dotA) at an MOI of 0.05. The level of growth was determined by measuring CFU at noted time points. The experiment was performed three times. Data are means and standard errors of three cultures from one representative experiment.
The behavior of L. pneumophila in C57BL/6J macrophages is consistent with the model that there are at least two levels of growth restriction: an early restriction which results in low bacterial yields, and a second blockage which prevents propagation of the bacteria after the initial burst of replication.
L. pneumophila escape from the endocytic pathway is dependent on the growth phase of the bacterium in C57BL/6J macrophages.
We hypothesized that the limited amount of intracellular growth of L. pneumophila in C57BL/6J macrophages during the initial 24-h incubation could be due to the fusion of the bacterium-containing vacuole with endocytic compartments. In a previous report, our investigators showed that wild-type L. pneumophila-containing vacuoles interacted with the endocytic pathway in C57BL/6J macrophages, whereas they did not in A/J macrophages (49). But during this study, we noticed variation in the efficiency of intracellular growth of the bacterium that was dependent on relatively small differences in the growth phase of the bacterium in culture prior to introduction onto macrophages. As these small variations in culture could have a significant impact on the relative efficiencies of intracellular growth in macrophages from different mouse strains, this was further investigated. L. pneumophila initiates intracellular growth most efficiently in postexponential phase (A600 of ∼3.2 to 3.7), which also corresponds to the stage of maximum motility (5). We found that the density corresponding to maximum intracellular growth could vary from A600 of 3 to 4.5 and correlated primarily with the degree of motility of the bacterium in culture rather than the culture density. Indeed, we found that a population of postexponential bacteria that contained 50% motile bacteria (as assessed visually by microscopy) was less efficient at establishing a replication vacuole than a population of bacteria containing close to 100% motile bacteria (data not shown).
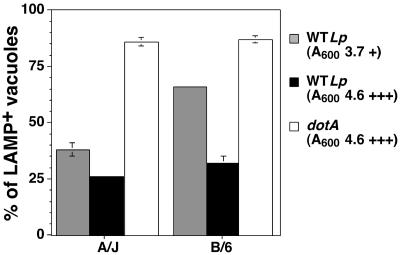
Therefore, we examined the fate of the L. pneumophila-containing vacuole as a function of bacterial motility. For this purpose, postexponential L. pneumophila cells grown to densities showing either some evidence of motility (A600 of ∼3.5) or a high degree of motility (A600 of ∼4.5) were incubated for 1 h with A/J or C57BL/6J bone marrow-derived macrophages and assayed for colocalization with the late endosomal marker LAMP-1 to measure defective targeting. As shown in Fig. 2, a population of bacteria showing evidence of motility displayed defective targeting that was more pronounced in C57BL/6J macrophages. A total of 65% of the wild-type L. pneumophila were found in a LAMP-1-positive compartment in C57BL/6J macrophages compared to 35% in A/J macrophages. This result is similar to what our group has previously observed when using bacteria grown to a set density (49). On the other hand, bacteria grown to a high degree of motility gave almost indistinguishable targeting to a LAMP-1-positive compartment in A/J and C57BL/6J macrophages (Fig. 2). A similar trend was observed with a wild-type L. pneumophila strain harboring the backbone vector (pMMB207) used to construct the GFP strain that we used in our previous study (49) (data not shown). As expected, no matter what growth condition or mouse strain was used, the dotA mutant was found in a LAMP-1-positive compartment.
FIG. 2.
L. pneumophila escape from the endocytic pathway in C57BL/6J (B6) macrophages is dependent on the growth phase of the infecting bacteria. Wild-type L. pneumophila (WTLp) or the dotA mutant (dotA) cells were grown to late exponential phase until they showed evidence of motility (+) or a majority of the bacteria were motile (+++). The bacteria were introduced into macrophages derived from either A/J or C57BL/6J mice and incubated for 1 h prior to fixation and assay for localization with the lysosomal marker LAMP-1. The experiment was performed three times. Data were quantified by counting 100 vacuoles per coverslip. Displayed are means and standard errors for three coverslips from a representative experiment.
Based on these results, all of the studies were then performed using cultures that were determined to be postexponential based on high bacterial motility. As the growth curve displayed in Fig. 1 used such bacteria, it is unlikely that inappropriate targeting to a late endosomal compartment during the first few hours after initial bacterial uptake could be the sole cause of the intracellular growth defect observed in macrophages derived from the restrictive mouse strains.
A small population of L. pneumophila establishes a replication vacuole in C57BL/6J macrophages.
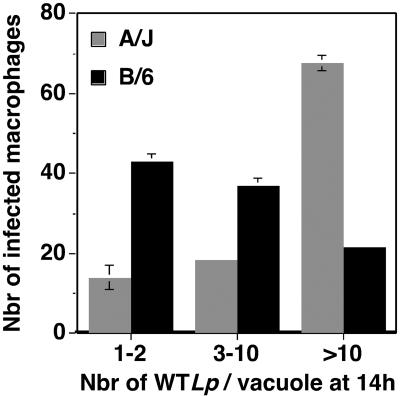
Since we observed a sixfold increase in L. pneumophila CFU during the first 24 h of infection of C57BL/6J macrophages that was not maintained on further incubation, we performed a microscopic analysis of the infected cells to determine what portion of the bacterial population was able to efficiently establish a replication vacuole. For this purpose, A/J or C57BL/6J bone marrow-derived macrophages were incubated with either wild-type L. pneumophila or the dotA mutant for 1 or 14 h, and the number of intracellular bacteria in individual phagosomes was recorded at each time point.
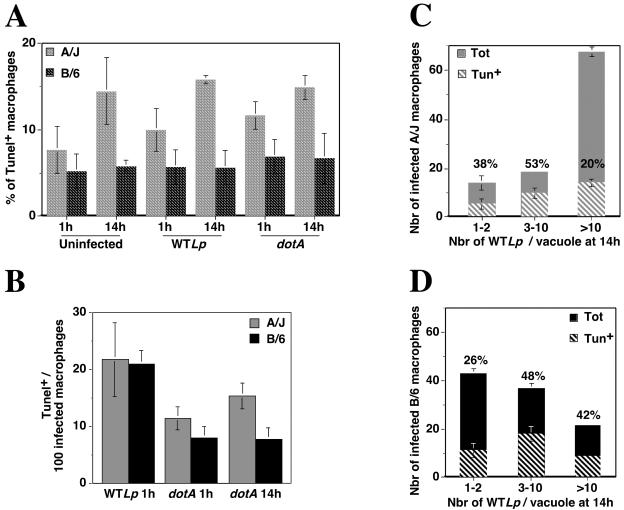
Under all conditions, all the infected macrophages from the two mouse strains contained 1 to 2 bacteria per vacuole 1 h postinfection, and this was also observed for the dotA mutant 14 h postinfection (data not shown). In contrast, 14 h postinfection with wild-type L. pneumophila, 65% of the infected A/J macrophages contained more than 10 bacteria per vacuole, as opposed to only 20% of the infected C57BL/6J macrophages showing the same-sized vacuoles (Fig. 3). Moreover, 40% of the macrophages from C57BL/6J mice showed replication vacuoles that were limited to two or three bacterial divisions (3 to 10 bacteria per vacuole), compared to only 20% in A/J. In addition, 40% of the infected C57BL/6J macrophages were apparently unable to support replication (1 to 2 bacteria per vacuole), compared to only 15% in A/J.
FIG. 3.
Large replication vacuole formation is defective in C57BL/6J (B/6) macrophages. Bone marrow-derived macrophages from A/J or C57BL/6J mice were incubated for 14 h with wild-type L. pneumophila (WTLp) at an MOI of 1. The samples were fixed and stained for extracellular and total bacteria. The number of intracellular bacteria in each vacuole was recorded by visual inspection. The experiment was performed three times. Data were quantified by counting 100 vacuoles per coverslip. Displayed are means and standard errors for three samples from one representative experiment under each condition, showing the number of vacuoles having the noted number of bacteria.
Taken together, these results indicate that L. pneumophila replication in C57BL/6J macrophages appeared blocked at multiple steps. A population of infected macrophages stopped the initiation of bacterial replication either by targeting the bacterium-containing vacuole to a LAMP-1-positive compartment (Fig. 2) or by an unknown mechanism, which led to a lack of bacterial replication in 40% of the macrophages. In addition, in macrophages in which replication was established there was limited bacterial proliferation.
Loss of infected C57BL/6J macrophages over time.
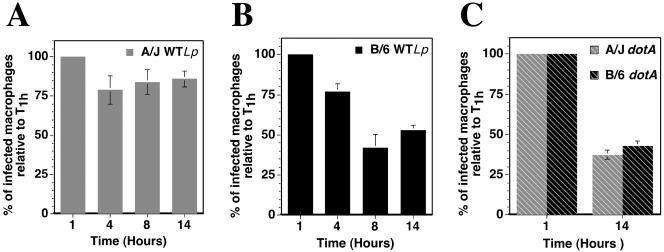
During the microscopic analysis of the intracellular growth of wild-type L. pneumophila in C57BL/6J macrophages, we noticed that the total number of macrophages harboring bacteria was reduced after the 14-h incubation period, whereas the absolute number of macrophages appeared constant. This observation suggested degradation of the bacteria over time or loss of infected cells. To investigate this further, bone marrow-derived macrophages from A/J and C57BL/6J mice were incubated with L. pneumophila for various times. We first determined that 8 h postinfection, the number of wild-type L. pneumophila-containing vacuoles that interacted with late endosomal compartments in C57BL/6J macrophages was unchanged compared to that at 1 h postinfection. In addition, at 14 h postinfection a population of large vacuoles acquired the late endocytic marker LAMP-1, but the proportion was not higher than in macrophages from A/J mice (data not shown). We next determined the number of infected macrophages for each time point. The results are displayed in Fig. 4 as the percentage of infected macrophages relative to the 1-h time point. When A/J macrophages were incubated for 14 h with wild-type L. pneumophila, the number of infected macrophages was reduced by only 20%, with all of the loss occurring during the first 4 h of the infection (Fig. 4A). After incubation of wild-type L. pneumophila with C57BL/6J macrophages, a similar loss of infected macrophages occurred during the first 4 h. By 8 h postinfection, however, the number of infected C57BL/6J macrophages was reduced to 40% of that observed after initial incubation with the bacteria. The number of infected cells then appeared to stabilize (Fig. 4B). When A/J or C57BL/6J macrophages were incubated with the dotA mutant, there was a 60% reduction in the number of infected macrophages over the 14-h period, probably as a result of degradation of the bacteria due to improper targeting of the vacuole containing this mutant (Fig. 4C).
FIG. 4.
Loss of infected C57BL/6J (B/6) macrophages over time. A/J or C57BL/6J bone marrow-derived macrophages were incubated with either wild-type L. pneumophila (WTLp) or the dotA mutant (dotA) at an MOI of 1 for 1, 4, 8, or 14 h. The number of infected macrophages was determined by counting 300 macrophages per coverslip and identifying macrophages that had internalized bacteria. The experiment was preformed three times. Displayed are means (normalized to the 1-h time point) and standard errors of percentages of macrophages with internalized bacteria for three samples under each condition from one representative experiment.
As the bacterial growth conditions that were used resulted in the bacteria-containing vacuoles avoiding the late endocytic pathway, it seems likely that the lowered number of infected cells was due to the death of the macrophages.
Infected C57BL/6J macrophages show altered levels of apoptosis 14 h postinfection.
As the products of the Lgn1 locus that confers the resistance of C57BL/6J macrophages to L. pneumophila infection show sequence similarity to baculovirus inhibitors of apoptosis (10), we investigated the survival of the macrophages after infection by using the TUNEL assay for DNA fragmentation, a typical hallmark of the later stages of apoptosis.
We first analyzed the overall population of macrophages, including both macrophages harboring bacteria and those that had no apparent associated bacteria (Fig. 5A). In general, A/J macrophages had two to three times more TUNEL-positive cells than C57BL/6J macrophages (Fig. 5A). Further incubation of A/J macrophages for 14 h showed some increase in TUNEL-positive cells (Fig. 5A, compare A/J uninfected at 1 h to uninfected at 14 h), whereas continued culture of C57BL/6J macrophages resulted in no increase in the TUNEL reaction (Fig. 5A, compare C57BL/6J uninfected at 1 h to uninfected at 14 h). This effect was independent of whether bacteria had been added to the culture (Fig. 5A, compare uninfected to wild-type L. pneumophila and dotA). Since analysis of the entire population showed an increased number of TUNEL-positive A/J macrophages that was independent of added bacteria, we focused on the population of macrophages harboring bacteria. One hour postinfection, all the A/J and C57BL/6J macrophages incubated with wild-type L. pneumophila contained 1 to 2 bacteria per vacuole (data not shown), and about 20% of the infected cells showed a TUNEL-positive reaction with both mouse strains (Fig. 5B, A/J and C57BL/6J wild-type L. pneumophila at 1 h). A similar result was observed at 1 or 14 h postinfection with the dotA mutant, with 10 to 15% of the infected cells being TUNEL positive (Fig. 5B, A/J and C57BL/6J dotA at 1 and 14 h). However, at 14 h postinfection, the most striking finding was that C57BL/6J cells harboring greater than 10 bacteria showed a much higher proportion of TUNEL-positive nuclei than was seen with A/J macrophages (compare Fig. 5C and D, >10 bacteria/vacuole). A total of 20% of A/J macrophages harboring greater than 10 bacteria appeared apoptotic at 14 h postinfection, which was similar to what was observed in uninfected macrophages at this time point (Fig. 4A and C). On the other hand, the proportion of C57BL/6J macrophages harboring greater than 10 bacteria that were apoptotic was increased approximately eightfold compared to macrophages that had not seen bacteria, with 42% of this population of macrophages showing a TUNEL-positive reaction (Fig. 5A and D). In the macrophages harboring fewer bacteria, there were no apparent differences in the proportion of TUNEL-positive cells of the two mouse strains (Fig. 5C and D, 1 to 2 and 3 to 10 bacteria/vacuole). Similar results were obtained when the fluorescent caspase substrate FITC-VAD-FMK was used to detect caspase activity, an enzymatic activity important for apoptosis (34), although addition of a 20 μM concentration of the general caspase inhibitor z-VAD-FMK (Promega) was unable to restore growth of L. pneumophila in C57BL/6J macrophages as assessed by the efficiency of formation of large replication vacuoles (data not shown). Therefore, in addition to the fact that bacterial replication initiates poorly and appears to proceed either slowly or to terminate prematurely in macrophages from a restrictive mouse strain, there also appears to be premature cell death specifically in the population of macrophages harboring large vacuoles.
FIG. 5.
A large portion of infected C57BL/6J (B/6) macrophages is apoptotic 14 h postinfection. (A) A/J or C57BL/6J bone marrow-derived macrophages were incubated with either wild-type L. pneumophila (WTLp) or the dotA mutant (dotA) at an MOI of 1 for 1 or 14 h. The overall population of cells (including both macrophages harboring bacteria and those with no apparent associated bacteria) was assayed for apoptosis by visual inspection (TUNEL assay). Macrophages cultured in the absence of bacteria but processed as the culture incubated with L. pneumophila, were used as a control (uninfected). In panels B to D, the experiment was performed as described for panel A except that only the population of cells harboring bacteria was examined. (B) Conditions that led to 1 to 2 bacteria/vacuole. Displayed is the number of TUNEL-positive cells per 100 infected macrophages from a population of A/J or C57BL/6J bone marrow-derived macrophages incubated for 1 h with wild-type L. pneumophila (WTLp) or for 1 or 14 h with the dotA mutant (dotA). (C and D) Conditions that led to intracellular replication. Displayed are the number of macrophages having the noted number of bacteria per vacuole (Tot) and the relative number of cells that were apoptotic (Tun+) from a population of A/J (C) or C57BL/6J (D) bone marrow-derived macrophages incubated for 14 h with wild-type L. pneumophila (WTLp). The percentage of TUNEL-positive cells for each category is indicated above each bar. All experiments were performed three times, and each figure displays the results from one representative experiment.
DISCUSSION
In this study, we have investigated the resistance of macrophages from C57BL/6J mice having the restrictive Lgn1 allele to L. pneumophila infection. As previously reported, our results indicate that in macrophages from A/J mice, L. pneumophila grew logarithmically for 48 h following initial infection. This is in contrast to C57BL/6J macrophages, in which one cycle of bacterial replication was initiated and maintained inefficiently for 24 h prior to an absolute block in further replication. In fact, after the initial replication cycle there was loss of bacterial viability, indicating that the bacteria were killed, perhaps during reinitiation of the second round of infection.
The poor growth of L. pneumophila in C57BL/6J macrophages observed during the first replication cycle was probably not due to the degradation of the bacterium in a lysosomal compartment, as we used growth conditions that maximized bypass of the endocytic pathway. This finding is in contrast with our group's previous study (49), in which we reported that L. pneumophila-containing vacuoles colocalized with the lysosomal marker LAMP-1 in C57BL/6J macrophages. We believe that the growth stage of the bacterium at the time of the infection can account for the difference in results. With the L. pneumophila strain used in this study, highly motile bacteria were able to escape the endocytic pathway in either permissive or restrictive macrophages, although the necessity to obtain high degrees of motility seemed to be less critical in A/J macrophages (Fig. 2). As motility is well correlated with the infectious stage of the bacterium (5), these results indicate that escape from the endocytic pathway in C57BL/6J macrophages requires maximal expression of proteins that are necessary for intracellular growth. Therefore, at least some of the resistance of C57BL/6J macrophages to L. pneumophila infection appears to be due to a more robust endocytic pathway than that of A/J macrophages. The role of the Lgn1 restrictive allele in this process remains to be elucidated.
Another phenomenon that contributes to the resistance of C57BL/6J macrophages to L. pneumophila infection is the loss of infected macrophages over time. The number of infected A/J macrophages was only reduced by 20% in the course of a single L. pneumophila replication cycle (14 h), whereas 50% of the infected C57BL/6J macrophages were lost within 8 h. Loss of macrophages during the first 8 h after infection is unlikely due to apoptosis, because we did not observed a significant increase in the amount of TUNEL-positive C57BL/6J macrophages during this period of time, although the nuclei of the infected cells looked more condensed (data not shown). We cannot exclude that the apoptotic cells lifted from the coverslip during the immunostaining procedure, but this is unlikely because we successfully observed infected cells that were apoptotic at the late times of infection. It is possible that the bacterial effector molecules injected in the cytoplasm of the host cell during the establishment of the replication vacuole mediate the elimination of the infected cells during the early time of the infection.
The microscopic analysis of L. pneumophila intracellular growth indicated that in A/J macrophages 65% of the bacteria undergo successful replication, resulting in vacuoles containing greater than 10 bacteria, whereas in C57BL/6J macrophages only 20% of the intracellular bacteria were able to establish similar sized vacuoles. We found that this specific population of C57BL/6J macrophages bearing large vacuoles had a higher proportion of apoptotic cells compared to A/J macrophages. The presence of caspase inhibitors, however, did not affect L. pneumophila growth in A/J macrophages, in contrast to published results obtained with infected U937 cells (35), and did not restore the growth of the bacterium in C57BL/6J macrophages.
The results obtained with A/J macrophages are in contradiction with observations made by others studying apoptosis induction upon L. pneumophila infection of a susceptible cell line such as U937 cells or human peripheral blood monocytes. Apoptosis induction and caspase 3 activation have been reported after high-multiplicity L. pneumophila infection of U937 cells (17, 56), and a recent study suggested that the caspase 3-dependent cleavage of rabaptin 5 is essential for the maturation of the replication vacuole, since the L. pneumophila-containing vacuole interacts with the endocytic pathway in the presence of a caspase 3 inhibitor (35). Those authors also reported that in the early stage of infection of human peripheral blood monocytes none of the infected cells was apoptotic, whereas 100% of the cells that contained large vacuoles were apoptotic at a late stage of the infection. In A/J (permissive) macrophages, there were clearly apoptotic nuclei at early times after infection, but the proportion did not significantly increase over the next 14 h. In C57BL/6J (restrictive) macrophages, although there was a higher percentage of TUNEL-positive cells among macrophages having large vacuoles, the proportion never reached 100% (Fig. 5).
We believe that the differences in cell lines, L. pneumophila strains, and MOI used in the different studies may account for the differences in results. Induction of apoptosis during L. pneumophila infection of mouse bone marrow-derived macrophages has never been reported. Our results indicate that permissive A/J macrophages do not undergo extensive apoptosis upon L. pneumophila infection or replication, although there are slightly more TUNEL-positive cells among the infected macrophages than the uninfected. However, a significant fraction of the resistant C57BL/6J macrophages that contain large L. pneumophila vacuoles do display features of apoptotic cells. We hypothesize that in response to L. pneumophila infection and replication, the host cell initiates apoptosis but the bacteria are able to inhibit or reverse the process, perhaps by injecting effectors molecules dedicated to this function. It is possible that such bacterial proteins are more efficient in macrophages having the permissive Lgn1 allele than in restrictive macrophages.
It is possible that the loss of viable counts observed after a first round of infection of C57BL/6J macrophages is due to premature lysis of the macrophages harboring replication vacuoles, releasing bacteria that have not reached postexponential phase. Bacteria in this stage of growth are probably not fully competent to reinitiate a second round of infection and may be degraded if they encounter a macrophage (28). This may be a profound restriction, given that in C57BL/6J macrophages bacteria grown less than optimally are particularly susceptible to mistargeting to late endocytic compartments.
An alternative explanation for the loss in viable counts after a first round of infection is that intracellular replication of L. pneumophila in C57BL/6J macrophages induces the activation of neighboring cells, resulting in degradation of bacteria unable to counteract this activation during the second round of infection. It has been reported that L. pneumophila infection of A/J macrophages induces interleukins, including interleukin-1α (IL-1α), IL-6, and IL-10 production but not IL-12, and L. pneumophila can suppress lipopolysaccharide-dependent IL-12 production by macrophages (31, 32). These results indicate that during infection, L. pneumophila may suppress the activation of A/J macrophages in order to escape the innate immune response and replicate inside the cell. This hypothesis is supported by the fact that macrophages from the restrictive BALB/c mouse strain, but not from A/J mice, produce interferon gamma (IFN-γ) after the first round of infection with L. pneumophila and that treatment of BALB/c macrophages with anti-IFN-γ restores bacterial growth (40). In addition, L. pneumophila is more pathogenic for BALB/c IFN-γ null mice after intranasal inoculation (42). It has also been shown that L. pneumophila growth in A/J macrophages is inhibited by IFN-γ treatment and that this effect was partially due to iron limitation (19). Taken together, these results suggest that IFN-γ production is associated with anti-L. pneumophila activity, at least partially via iron limitation. These results reinforce the idea that macrophage activation may be part of the mechanism of resistance to L. pneumophila infection in C57BL/6J mice, particularly after 24 h of bacterial incubation with macrophages.
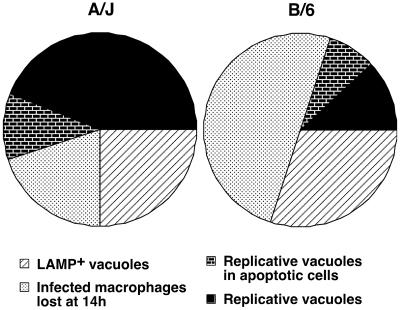
To summarize, we believe that the resistance of C57BL/6J macrophages to L. pneumophila infection appears to be multifactorial. In mouse bone marrow-derived macrophages, the combination of the targeting of L. pneumophila to a LAMP-1-positive compartment of suboptimally cultured bacteria, the loss of infected macrophages, and the apoptosis of the macrophages in which L. pneumophila undergoes robust replication results in a bacterial replication cycle that is completed in only 10% of the initially infected C57BL/6J macrophages, compared to 50% of the infected A/J macrophages (Fig. 6). Even under conditions in which proper targeting of the bacterium-containing vacuole is maximized, an initial restriction takes place in the early stage of the infection that leads to the elimination of the infected cells via a mechanism that is yet to be identified. A second response, potentially mediated by the intracellular replication of L. pneumophila, appears to induce more apoptosis compared to permissive macrophages, specifically in cells harboring large numbers of bacteria. Finally, there is loss of bacterial viability after the initial infection cycle. This could be due to enhanced restriction of the macrophages caused by replication of the bacteria in neighboring cells or due to premature lysis of macrophages harboring replicating vacuoles, liberating L. pneumophila that are not fully competent to initiate new rounds of replication.
FIG. 6.
Schematic representation of the fate of the bacterium or the host cell upon L. pneumophila infection of A/J or C57BL/6J macrophages. Shown are bacteria that colocalize with the lysosomal marker LAMP-1, infected macrophages that are lost over time, infected cells that undergo apoptosis as L. pneumophila replicates, and infected macrophages in which L. pneumophila undergoes successful replication.
Acknowledgments
We thank Susan Van Rheenen, Molly Bergman, Marion Shonn, and Matthias Machner for review of the manuscript.
R.I. is an Investigator of the Howard Hughes Medical Institute (HHMI). I.D. was supported by an EMBO fellowship and is currently a Human Frontier Science Program Fellow. This work was supported by the HHMI.
Editor: D. L. Burns
REFERENCES
- 1.Andrews, H. L., J. P. Vogel, and R. R. Isberg. 1998. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect. Immun. 66:950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckers, M. C., S. Yoshida, K. Morgan, E. Skamene, and P. Gros. 1995. Natural resistance to infection with Legionella pneumophila: chromosomal localization of the Lgn1 susceptibility gene. Mamm. Genome 6:540-545. [DOI] [PubMed] [Google Scholar]
- 3.Berger, K. H., and R. R. Isberg. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7:7-19. [DOI] [PubMed] [Google Scholar]
- 4.Brand, B. C., A. B. Sadosky, and H. A. Shuman. 1994. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol. Microbiol. 14:797-808. [DOI] [PubMed] [Google Scholar]
- 5.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, J., K. S. de Felipe, M. Clarke, H. Lu, O. R. Anderson, G. Segal, and H. A. Shuman. 2004. Legionella effectors that promote nonlytic release from protozoa. Science 303:1358-1361. [DOI] [PubMed] [Google Scholar]
- 7.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2000. Deviant expression of Rab5 on phagosomes containing the intracellular pathogens Mycobacterium tuberculosis and Legionella pneumophila is associated with altered phagosomal fate. Infect. Immun. 68:2671-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conover, G. M., I. Derré, J. P. Vogel, and R. R. Isberg. 2003. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol. Microbiol. 48:305-321. [DOI] [PubMed] [Google Scholar]
- 9.Derré, I., and R. R. Isberg. 2004. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect. Immun. 72:3048-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deveraux, Q. L., and J. C. Reed. 1999. IAP family proteins—suppressors of apoptosis. Genes Dev. 13:239-252. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich, W. F., D. M. Damron, R. R. Isberg, E. S. Lander, and M. S. Swanson. 1995. Lgn1, a gene that determines susceptibility to Legionella pneumophila, maps to mouse chromosome 13. Genomics 26:443-450. [DOI] [PubMed] [Google Scholar]
- 12.Diez, E., S. H. Lee, S. Gauthier, Z. Yaraghi, M. Tremblay, S. Vidal, and P. Gros. 2003. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat. Genet. 33:55-60. [DOI] [PubMed] [Google Scholar]
- 13.Edelstein, P. H., M. A. Edelstein, F. Higa, and S. Falkow. 1999. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc. Natl. Acad. Sci. USA 96:8190-8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feeley, J. C., R. J. Gibson, G. W. Gorman, N. C. Langford, J. K. Rasheed, D. C. Mackel, and W. B. Baine. 1979. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser, D. W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, C. C. Shepard, and P. S. Brachman. 1977. Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297:1189-1197. [DOI] [PubMed] [Google Scholar]
- 16.Gabay, J. E., M. Blake, W. D. Niles, and M. A. Horwitz. 1985. Purification of Legionella pneumophila major outer membrane protein and demonstration that it is a porin. J. Bacteriol. 162:85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao, L. Y., and Y. Abu Kwaik. 1999. Activation of caspase 3 during Legionella pneumophila-induced apoptosis. Infect. Immun. 67:4886-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavrieli, Y., Y. Sherman, and S. A. Ben-Sasson. 1992. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119:493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebran, S. J., Y. Yamamoto, C. Newton, T. W. Klein, and H. Friedman. 1994. Inhibition of Legionella pneumophila growth by gamma interferon in permissive A/J. mouse macrophages: role of reactive oxygen species, nitric oxide, tryptophan, and iron(III). Infect. Immun. 62:3197-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girardin, S. E., L. H. Travassos, M. Herve, D. Blanot, I. G. Boneca, D. J. Philpott, P. J. Sansonetti, and D. Mengin-Lecreulx. 2003. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 278:1702-1708. [DOI] [PubMed] [Google Scholar]
- 21.Growney, J. D., and W. F. Dietrich. 2000. High-resolution genetic and physical map of the Lgn1 interval in C57BL/6J implicates Naip2 or Naip5 in Legionella pneumophila pathogenesis. Genome Res. 10:1158-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilbi, H., G. Segal, and H. A. Shuman. 2001. icm/dot-dependent upregulation of phagocytosis by Legionella pneumophila. Mol. Microbiol. 42:603-617. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwitz, M. A. 1983. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz, M. A. 1984. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell 36:27-33. [DOI] [PubMed] [Google Scholar]
- 26.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 27.Inohara, N., Y. Ogura, F. F. Chen, A. Muto, and G. Nunez. 2001. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J. Biol. Chem. 276:2551-2554. [DOI] [PubMed] [Google Scholar]
- 28.Joshi, A. D., S. Sturgill-Koszycki, and M. S. Swanson. 2001. Evidence that Dot-dependent and -independent factors isolate the Legionella pneumophila phagosome from the endocytic network in mouse macrophages. Cell Microbiol. 3:99-114. [DOI] [PubMed] [Google Scholar]
- 29.Kagan, J. C., and C. R. Roy. 2002. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 4:945-954. [DOI] [PubMed] [Google Scholar]
- 30.Luo, Z. Q., and R. R. Isberg. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA 101:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsunaga, K., T. W. Klein, C. Newton, H. Friedman, and Y. Yamamoto. 2001. Legionella pneumophila suppresses interleukin-12 production by macrophages. Infect. Immun. 69:1929-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsunaga, K., H. Yamaguchi, T. W. Klein, H. Friedman, and Y. Yamamoto. 2003. Legionella pneumophila suppresses macrophage interleukin-12 production by activating the p42/44 mitogen-activated protein kinase cascade. Infect. Immun. 71:6672-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean, I. W., and P. K. Nakane. 1974. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J. Histochem. Cytochem. 22:1077-1083. [DOI] [PubMed] [Google Scholar]
- 34.Mehmet, H. 2000. Caspases find a new place to hide. Nature 403:29-30. [DOI] [PubMed] [Google Scholar]
- 35.Molmeret, M., S. D. Zink, L. Han, A. Abu-Zant, R. Asari, D. M. Bitar, and Y. Abu Kwaik. 2004. Activation of caspase-3 by the Dot/Icm virulence system is essential for arrested biogenesis of the Legionella-containing phagosome. Cell Microbiol. 6:33-48. [DOI] [PubMed] [Google Scholar]
- 36.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679-682. [DOI] [PubMed] [Google Scholar]
- 37.Nash, T. W., D. M. Libby, and M. A. Horwitz. 1984. Interaction between the Legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J. Clin. Investig. 74:771-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy, C. R., K. H. Berger, and R. R. Isberg. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663-674. [DOI] [PubMed] [Google Scholar]
- 39.Sadosky, A. B., L. A. Wiater, and H. A. Shuman. 1993. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61:5361-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salins, S., C. Newton, R. Widen, T. W. Klein, and H. Friedman. 2001. Differential induction of gamma interferon in Legionella pneumophila-infected macrophages from BALB/c and A/J mice. Infect. Immun. 69:3605-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA 95:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shinozawa, Y., T. Matsumoto, K. Uchida, S. Tsujimoto, Y. Iwakura, and K. Yamaguchi. 2002. Role of interferon-γ in inflammatory responses in murine respiratory infection with Legionella pneumophila. J. Med. Microbiol. 51:225-230. [DOI] [PubMed] [Google Scholar]
- 43.Sturgill-Koszycki, S., and M. S. Swanson. 2000. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 192:1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilney, L. G., O. S. Harb, P. S. Connelly, C. G. Robinson, and C. R. Roy. 2001. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J. Cell Sci. 114:4637-4650. [DOI] [PubMed] [Google Scholar]
- 46.Vogel, J. P., H. L. Andrews, S. K. Wong, and R. R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873-876. [DOI] [PubMed] [Google Scholar]
- 47.Vogel, J. P., C. Roy, and R. R. Isberg. 1996. Use of salt to isolate Legionella pneumophila mutants unable to replicate in macrophages. Ann. N. Y. Acad. Sci. 797:271-272. [DOI] [PubMed] [Google Scholar]
- 48.Wadowsky, R. M., L. J. Butler, M. K. Cook, S. M. Verma, M. A. Paul, B. S. Fields, G. Keleti, J. L. Sykora, and R. B. Yee. 1988. Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl. Environ Microbiol. 54:2677-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watarai, M., I. Derré, J. Kirby, J. D. Growney, W. F. Dietrich, and R. R. Isberg. 2001. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J. Exp. Med. 194:1081-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright, E. K., S. A. Goodart, J. D. Growney, V. Hadinoto, M. G. Endrizzi, E. M. Long, K. Sadigh, A. L. Abney, I. Bernstein-Hanley, and W. F. Dietrich. 2003. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr. Biol. 13:27-36. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto, Y., T. W. Klein, and H. Friedman. 1991. Legionella pneumophila growth in macrophages from susceptible mice is genetically controlled. Proc. Soc. Exp. Biol. Med. 196:405-409. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto, Y., T. W. Klein, C. A. Newton, and H. Friedman. 1988. Interaction of Legionella pneumophila with peritoneal macrophages from various mouse strains. Adv. Exp. Med. Biol. 239:89-98. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto, Y., T. W. Klein, C. A. Newton, R. Widen, and H. Friedman. 1988. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect. Immun. 56:370-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yaraghi, Z., E. Diez, P. Gros, and A. MacKenzie. 1999. cDNA cloning and the 5′ genomic organization of Naip2, a candidate gene for murine Legionella resistance. Mamm. Genome 10:761-763. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida, S., Y. Goto, Y. Mizuguchi, K. Nomoto, and E. Skamene. 1991. Genetic control of natural resistance in mouse macrophages regulating in-tracellular Legionella pneumophila multiplication in vitro. Infect. Immun. 59:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zink, S. D., L. Pedersen, N. P. Cianciotto, and Y. Abu-Kwaik. 2002. The Dot/Icm type IV secretion system of Legionella pneumophila is essential for the induction of apoptosis in human macrophages. Infect. Immun. 70:1657-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]