Abstract
Cell surface hydrophobicity contributes to the pathogenesis of the opportunistic fungal pathogen Candida albicans. Previous work demonstrated a correlation between hydrophobicity status and changes in the acid-labile, phosphodiester-linked β-1,2-oligomannoside components of the N-linked glycans of cell wall mannoprotein. Glycan composition also defines the two major serotypes, A and B, of C. albicans strains. Here, we show that the cell surface hydrophobicity of the two serotypes is qualitatively different, suggesting that the serotypes may differ in how they modulate cell surface hydrophobicity status. The cell wall mannoproteins from hydrophilic and hydrophobic cells of both serotypes were compared to determine whether the glycan differences due to serotype affect the glycan differences due to hydrophobicity status. Composition analysis showed that the protein, hexose, and phosphate contents of the mannoprotein fraction did not differ significantly among the strains tested. Electrophoretic profiles of the acid-labile mannan differed only with hydrophobicity status, not serotype, though some strain-specific differences were observed. Furthermore, a newly available β-1,2-oligomannoside ladder allowed unambiguous identification of acid-labile mannan components. Finally, to assess whether the acid-stable mannan also affects cell surface hydrophobicity status, this fraction was fragmented into its component branches by acetolysis. The electrophoretic profiles of the acid-stable branches were very similar regardless of hydrophobicity status. However, differences were observed between serotypes. These results support and extend our current model that modification of the acid-labile β-1,2-oligomannoside chain length but not modification of the acid-stable region is one common mechanism by which switching of cell surface hydrophobicity status of C. albicans strains occurs.
Mycoses are becoming a more common underlying cause of death in the United States (36). This trend is reflected in the increasing frequency of nosocomial fungal bloodstream infections observed in the United States and elsewhere (3, 43, 54). The majority of these nosocomial fungal infections (78.3%) are due to Candida species, with C. albicans being the most frequently isolated species (3, 41).
Cell surface hydrophobicity plays an important role in the pathogenicity of microorganisms, including C. albicans. Hydrophobic C. albicans cells are more adherent than hydrophilic cells to a variety of host tissues, and the pattern of adherence is more widespread (16, 18). Hydrophobic cells are also more resistant than hydrophilic cells to phagocytic killing (2, 22). C. albicans is the only pathogenic fungal species observed so far to be able to regulate cell surface hydrophobicity status (15, 23), which changes with growth phase and conditions (13, 21).
Cell surface hydrophobicity status has been linked to the character of the cell wall architecture. The surface layer of the C. albicans cell wall contains fibril structures composed of mannoproteins (6, 42, 50, 53). The ability of C. albicans to regulate cell surface hydrophobicity apparently corresponds to an ability to alter the conformation of the mannoprotein fibrils (19, 33). Fibrils on the surface of hydrophobic cells are short (either truncated or folded over), aggregated, and variable. Those on the surface of hydrophilic cells are longer, evenly spaced, and radiating (19).
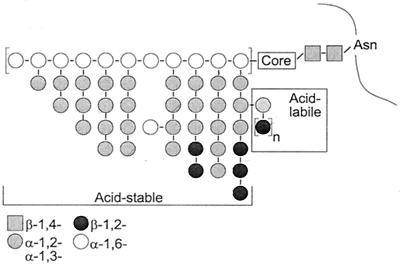
Our previous studies of the C. albicans cell wall proteins indicated that the differences in fibril conformation correlate with changes in cell wall protein N-linked glycosylation, in particular the phosphodiester-linked, acid-labile β-1,2-oligomannoside tertiary branches (Fig. 1). There is no difference in the overall composition (protein, hexose, phosphate, or electrophoretic profile) of mannoprotein from hydrophobic versus hydrophilic cells (33, 34). However, the acid-labile mannan from hydrophobic cells is composed of longer oligosaccharides than that from hydrophilic cells (34).
FIG. 1.
Simplified schematic of C. albicans N-linked glycans. The Man8 core group is attached to the asparagine through two N-acetylglucosamine residues (grey squares). The acid-stable fraction consists of the extended outer chain decorated with α-1,2- and α-1,3-linked oligomannoside branches (grey circles). Acid-labile oligomannosides are linked to the acid-stable branches through a phosphodiester (P), n = 1 to 14. The β-1,2-linked mannose units in the acid-stable region are present only in serotype A strains.
These analyses were carried out primarily on a single serotype B strain, A9. However, serotype does appear to have clinical significance beyond strain identification. For example, while the prevalence of both serotypes, A and B, in the oral flora of hospitalized and nonhospitalized individuals appears to be roughly equal, serotype B strains are more commonly associated with symptomatic oral infections in immunocompromised patients (4, 5).
Suzuki's group and others have shown that serotype is defined by surface mannan composition. Specifically, serotype A cells possess additional β-1,2-linked mannoside epitopes in the acid-stable region (Fig. 1) (49). The presence of acid-stable β-1,2-oligomannosides also correlates with differences in serotype A and B cell surface hydrophobicity. Under conditions where serotype B strains are highly hydrophilic, serotype A strains remain moderately hydrophobic (13, 21).
In this report, we extend our previous studies to assess the relationship between cell surface hydrophobicity and surface protein mannosylation in both serotypes of C. albicans. Mannoprotein elemental composition and acid-labile mannan electrophoretic profiles of mannoproteins extracted from the cell walls of several strains were compared. The latter analysis was facilitated by the availability of a β-1,2-oligomannoside ladder. This ladder allowed us, for the first time, to unambiguously assign identities to the bands in the gel. Finally, we addressed a major question that remained from our initial reports: what changes, if any, occur in the acid-stable mannan with changes in cell surface hydrophobicity status? Our current results support and extend our previous conclusions that cell wall protein glycosylation differences between hydrophobic and hydrophilic cells are confined to the acid-labile β-1,2-oligomannosides and that this condition is common to all strains studied thus far. In addition, the presence of β-1,2-linked mannose units in the acid-stable branches of serotype A mannoproteins correlate with both oligomannoside electrophoretic profile and cell surface hydrophobicity differences between the serotypes. These results suggest that regulation of cell surface hydrophobicity status is linked to subtle changes in wall protein glycosylation.
MATERIALS AND METHODS
Strains and culture conditions.
All studies were conducted with C. albicans. Strain A9 is serotype B and was obtained from Richard Calderone at Georgetown University. The strain is one of several originally isolated from the oral cavities of AIDS patients by Philip Smith of the National Institute of Dental Research, Bethesda, Md. (56). LGH1095 (ATCC MYA-2719) is a serotype B clinical isolate from Lafayette General Hospital, Lafayette, La., and has been described previously (21). SC5314 is a serotype A strain originally obtained from a patient with disseminated candidiasis (10). B311 was obtained from the American Type Culture Collection (ATCC 32354) and is serotype A.
All strains were maintained as frozen stocks (−80°C) in 100 mM sucrose-2 mM ZnSO4 (17). Yeast cells from frozen stocks were subcultured three times in yeast nitrogen base (Difco) buffered with 0.055 M sodium phosphate, pH 7.2, and supplemented with 2% (wt/vol) glucose.
Cell surface hydrophobicity.
Cell surface hydrophobicity was measured by the hydrophobic microsphere assay developed in our laboratory (14, 20). Washed cells were suspended in cold 0.05 M sodium phosphate buffer (pH 7.2) to a concentration of 2 × 106 cells ml−1. From a stock 10% solids suspension of polystyrene microspheres (0.825-μm diameter; Bangs Laboratories), 6 μl was removed and added to 2 ml of buffer. Equal volumes (100 μl) of the cell and microsphere suspensions were combined in polycarbonate tubes, rapidly equilibrated to room temperature, and mixed in a vortex mixer for 30 s. Microsphere attachment was assessed by bright-field microscopy at 400× magnification. The percentage of cells with three or more attached spheres was recorded as the percent hydrophobicity.
Mannoprotein preparation.
Glycans (glucan and mannoprotein) were extracted from the cell wall with a protocol based on that of Peat et al. (40) and described previously (34). The previously described protocol was modified so that cells were not dried with acetone. The washed cell pellet was immediately suspended in 50 ml of distilled H2O, and the cell suspension was autoclaved at 121°C for 90 min. Mannoprotein was separated from glucan with a previously described differential precipitation strategy based on that of Lloyd (32, 33).
Biochemical assays.
Prior to compositional analysis, samples of purified mannoprotein were dried to constant mass, then dissolved in distilled H2O to 10 mg ml−1. Hexose content was determined by the phenol-sulfuric acid method of Dubois et al. (8). Sample absorbances were compared to a mannose standard curve. Protein concentration was determined by the bicinchoninic acid assay (BCA; Pierce Chemical) (48). Bovine serum albumin was used as the standard protein to generate a standard curve. Phosphate was determined with the method of Ames and Dubin (1) with K2HPO4 to make a standard curve and d-glucose-6-phosphate as the control. Oligosaccharide concentration was determined by measuring the concentration of reducing ends as reported by Dygert et al. (9). This assay detects reducing sugars under conditions mild enough that oligosaccharides are not degraded into their monosaccharide components. Composition results were compared by analysis of variance, with a significance level of α = 0.05.
Fractionation of mannan.
For acid-labile mannan, the phosphodiester-linked acid-labile oligomannosides were released with a method modified from that of Okubo et al. (37). Purified mannoprotein was dissolved (10 mg ml−1) in 10 mM HCl and heated in a boiling water bath for 60 min. The solution was neutralized with 1 N NaOH, and the acid-stable mannoprotein remnant was separated by precipitation with 3 volumes of ice-cold absolute ethanol. Precipitation was continued overnight at −20°C. Precipitates were pelleted by centrifugation at 10,000 × g for 10 min. The supernatant fluid containing the acid-labile mannan was removed, dried by lyophilization, dissolved in distilled H2O, and stored at −20°C.
Acetolysis.
Several variations of the general procedure have appeared in the literature over the years. We adapted a consensus procedure that fit both our scale of preparation and the glassware and equipment at hand in the laboratory or readily available commercially. In general, the strategy followed the mild conditions described by Suzuki et al. (30), under which the α-1,6-glycosidic bonds linking the outer chain backbone are preferentially cleaved and the side branches are released intact. The mannoprotein pellet from the step above was dissolved in 2 ml of formamide (24, 38, 55) with mild heating (30 to 35°C). The alcohol groups were then acetylated by the addition of 5 ml of pyridine and 5 ml of acetic anhydride, and incubating for 18 h at 40°C in a sand bath. This reaction was carried out in a 50-ml Pyrex screw-top tube. Acetates were precipitated by pouring into 5 volumes (50 ml) of distilled H2O, followed by centrifugation at 3,000 × g for 10 min. The supernatant fluid was removed and discarded. Pellets were washed once with 5 ml of distilled H2O and dried to a dark brown glass by heating for 5 h in a sand bath at 40°C under a gentle stream of nitrogen.
The dried acetates underwent acetolysis as follows: the pellets were dissolved in 5 ml of acetic anhydride, 5 ml of acetic acid-sulfuric acid (100:1) was added, and the mixture was incubated at 40°C for 36 to 40 h in a sand bath. Acetolysates were poured over a minimal volume of wet ice, and the solution was extracted twice with an equal volume of chloroform. The chloroform extract was separated from the aqueous phase with a separatory funnel, and the chloroform was removed with a rotary evaporator. The remaining residue was dried to completion under a gentle stream of nitrogen gas. Deacetylation was carried out by dissolving the dried material in 10 ml of methanol and raising the pH to alkaline with methanolic sodium methoxide. The solution was allowed to stand at room temperature for 20 min, during which time the deacetylated acetolysates precipitated. The samples were neutralized with glacial acetic acid and then centrifuged at 3,000 × g for 10 min. The pellets were washed twice with 5 ml of methanol, dissolved in 2 ml of distilled H2O, and stored at −20°C.
Labeling with fluorophore.
The reducing ends of acid-labile oligosaccharides and acid-stable acetolysates were measured as described above. Aliquots were drawn from each sample so that equivalent moles of oligosaccharides could be compared. These aliquots were then dried in a centrifugal vacuum evaporator (Virtis) and dissolved in 5 μl of acetic acid (5%) containing 8-aminonaphthalene-1,3,6-trisulfonic acid (ANTS, 0.15 M). Sodium cyanoborohydride (5 μl of a 1 M solution in dimethyl sulfoxide) was added, and the solutions were incubated at 37°C overnight. Labeled samples were dried in a centrifugal vacuum evaporator and stored at −20°C until use. Labeled and dried samples were then dissolved in distilled H2O to give 2 nmol of reducing ends per 10 μl.
Dextran (α-1,6-glucose; Fluka 31391), maltooligosaccharides (α-1,4-glucose; Sigma M-3639), and β-1,4-oligomannosides (V-Labs) were used as electrophoretic mobility markers. Dextran was subjected to acid hydrolysis prior to labeling (57). β-1,2-Oligomannosides were provided by Bert Fraser-Reid and coworkers (Natural Products and Glycotechnology Research Institute, Durham, N.C.) (35).
FACE.
Fluorophore-assisted carbohydrate electrophoresis (FACE) was performed essentially as described by Jackson (26) and modified by Goins and Cutler (11). Because the cleanliness of the glass plates is critical to satisfactory gels, the plates were soaked in 10% (wt/vol) methanolic KOH, washed with detergent (7X, ICN), rinsed with deionized water, and finally rinsed with 95% ethanol. Electrophoresis was carried out in a Hoeffer SE600 gel electrophoresis apparatus (Amersham) cooled to 4°C with a recirculating water bath. Gels were run at 600 V for 4 to 6 h or until the ANTS front reached 1 cm from the bottom of the gel, and then imaged with a Kodak EDAS 290 system containing a DC290 digital camera and UV transilluminator (302 nm). Image contrast and brightness were optimized with Photoshop 7.0 (Adobe).
RESULTS
Comparison of cell surface hydrophobicity and cell wall composition.
Both serotype B strains, A9 and LGH1095, were highly hydrophobic at 23°C and highly hydrophilic at 37°C (Table 1). The serotype A strains, SC5314 and B311, were also highly hydrophobic at 23°C but remained moderately hydrophobic at 37°C. This characteristic of strain B311 has been observed previously (13). Assay of additional serotype A (ATCC 10231) and serotype B (LGH870) strains confirmed the observed cell surface hydrophobicity expression differences (data not shown).
TABLE 1.
Compositional analysis of mannan from C. albicans strainsa
| Strain | Serotype | nb | Mean (SD)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hydrophobic cells
|
Hydrophilic cells
|
|||||||||
| CSH (%) | % Protein | % Hexose | % PO4 | CSH (%) | % Protein | % Hexose | % PO4 | |||
| A9 | B | 3/3 | 93.6 (3.1) | 6.0 (0.9) | 74.3 (3.6) | 3.9 (0.1)1 | 4.0 (1.0) | 5.1 (0.5) | 79.9 (13.7) | 3.2 (0.1)1,2 |
| LGH1095 | B | 3/3 | 99.2 (0.7) | 4.9 (0.9) | 77.6 (5.8) | 4.7 (0.5)3 | 1.4 (1.7) | 5.6 (1.1) | 80.9 (2.6) | 3.7 (0.1)3 |
| SC5314 | A | 3/3 | 99.6 (0.7) | 4.0 (0.5) | 84.1 (5.4) | 4.3 (0.3) | 30.2 (18.4) | 4.6 (1.3) | 84.5 (7.6) | 3.8 (0.2) |
| B311 | A | 2/3 | 98.0 | 4.9 | 78.8 | 4.5 | 27.0 | 4.9 (1.0) | 82.4 (6.5) | 3.9 (0.4)2 |
Data are presented as the mean (sample standard deviation) where applicable. Values with the same superscript were significantly different (P = 0.05). CSH, cell surface hydrophobicity.
Assays for each n were run in duplicate.
Protein, hexose, and phosphate contents were determined for all four strains (Table 1). The protein and hexose percentages for strain A9 were consistent with previously published results (34). Phosphate percentages for all strains examined and both growth conditions were higher than have been reported (12, 31, 34, 37, 44, 45), ranging from 0 to 2.4% depending on the strain and growth conditions.
Analysis of variance comparisons were carried out across cell surface hydrophobicity status, serotype, and strain. A second difference from our previous report (34) was that comparison of individual components indicated a difference in phosphate content between hydrophobic and hydrophilic cells of strains A9 (P = 0.001) and LGH1095 (P = 0.03). This difference was not observed for the serotype A strains (B311 and SC5314). Similarly, as was previously observed for A9, none of the strains tested showed differences in protein or hexose composition between hydrophobic and hydrophilic cells. When the composition results were compared by serotype, the only difference observed was the phosphate content of hydrophilic cells. This was also the only parameter to show a difference between strains. Subsequent analysis of the analysis of variance results with Bonferroni post hoc tests indicated that in both cases the statistical result was due to differences between B311 and A9. Overall, however, there appeared to be no gross difference in mannan composition among the four C. albicans strains under investigation.
Comparison of acid-labile mannan from A and B serotype strains.
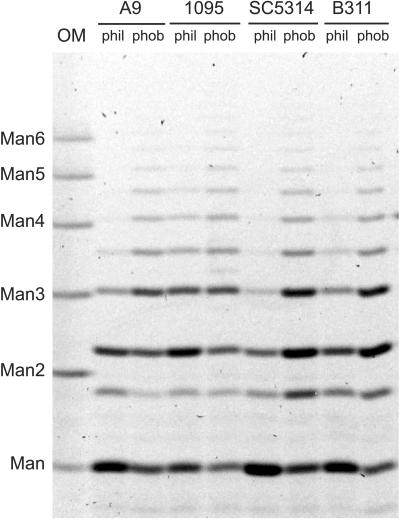
Acid-labile mannan from hydrophilic and hydrophobic cells was extracted and fluorescently labeled, and the components were separated electrophoretically (Fig. 2). The results for strain A9 match those reported earlier (34). Acid-labile mannan from hydrophobic cells contains longer oligosaccharides than that from hydrophilic cells. Because these previously published results were conducted with a single wild-type strain, we extended our analysis of acid-labile mannan to another serotype B strain, LGH1095, and two serotype A strains, SC5314 and B311. There were some strain-specific qualities to the electrophoretic profiles. For example, acid-labile mannan from hydrophilic LGH1095 and B311 cells contains longer oligosaccharides than the corresponding fractions from A9 and SC5314. However, the overall comparison between hydrophobic and hydrophilic cells remained as was previously published: acid-labile mannan from hydrophobic cells contains longer chains that that from hydrophilic cells. Furthermore, the results indicated that this characteristic is independent of serotype (Fig. 2).
FIG. 2.
Representative comparison of acid-labile oligomannosides released from mannan extracted from hydrophilic (phil) and hydrophobic (phob) C. albicans yeast cells. OM, β-1,4-oligomannoside standards. Degrees of polymerization (Man to Man6) are indicated on the left.
Assignment of acid-labile mannan component identity.
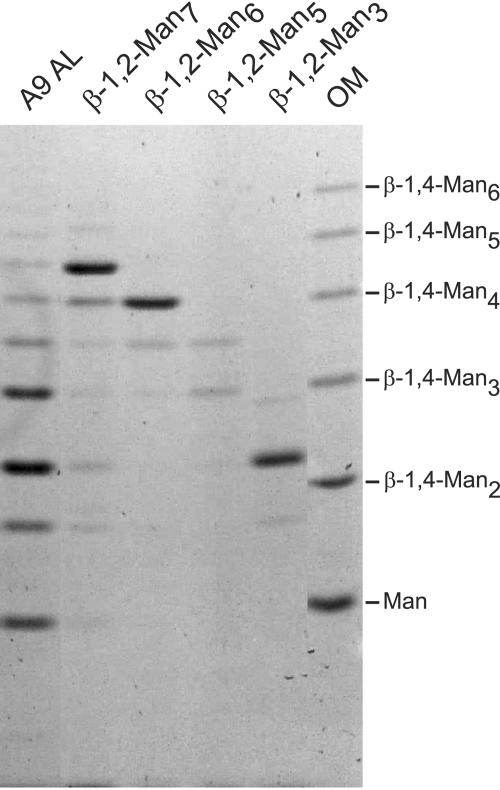
Synthetic β-1,2-oligomannosides were labeled with ANTS, and their electrophoretic mobilities were compared to that of acid-labile mannan released from C. albicans mannoprotein (Fig. 3). This comparison confirmed that β-1,2-oligomannosides have a different electrophoretic mobility than β-1,4-oligomannosides. Furthermore, the comparison confirmed that our sample preparation produced acid-labile mannan containing only β-1,2-oligomannosides, free of any contaminant.
FIG. 3.
Assignment of acid-labile oligomannoside component identity. Acid-labile mannan released from hydrophobic C. albicans yeast cells (A9 AL) was compared to synthesized β-1,2-oligomannosides (β-1,2-Man2 to β-1,2-Man7). OM, β-1,4-oligomannoside standards. Difficulty in solubilizing β-1,2-Man5 prohibited loading equivalent amounts of mannopentaose. Degrees of polymerization for the standards are indicated on the right.
Comparison of acid-stable mannan from hydrophilic and hydrophobic yeast cells.
Acid-stable mannan from hydrophobic and hydrophilic C. albicans yeast cells of both serotypes was subjected to acetolysis under mild conditions that preferentially cleave the more labile α-1,6-bonds joining the backbone sugars (Fig. 1) (30, 39). Compared to acetolysates prepared with a higher sulfuric acid concentration (acetic acid-acetic anhydride-sulfuric acid, 10:10:1), the milder conditions (100:100:1) produced longer oligosaccharides (27, 30) (data not shown). Acetolysate components were labeled with ANTS and separated by FACE.
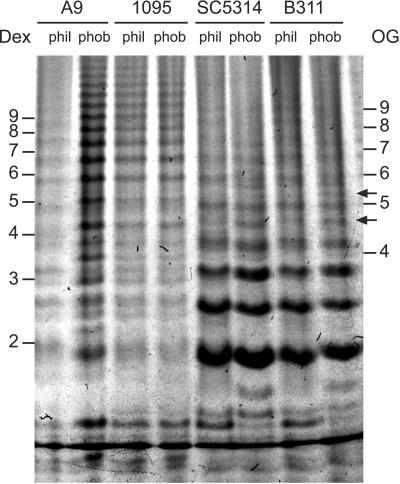
A representative gel demonstrating separation of acid-stable acetolysates is shown in Fig. 4. The relatively greater intensity of bands in the A9 hydrophobic cell sample over the hydrophilic cell sample resulted from slight differences in loading enhanced by the high sensitivity of fluorescence detection in FACE. While other replicate gels did not show this disparity in A9 band intensity, the gel used for Fig. 4 most clearly showed the resolution of bands in the upper part of the gel.
FIG. 4.
Representative comparison of acid-stable mannan acetolysates released from mannan extracted from hydrophilic (phil) and hydrophobic (phob) C. albicans yeast cells. Dex, acid hydrolysis products of dextran. OG, maltooligosaccharides resulting from acid hydrolysis of amylose. Numbers indicate degrees of polymerization. The relative intensities of the A9 lanes reflect slight loading differences heightened by the detection sensitivity of FACE. A replicate gel confirmed the equivalence of the A9 samples but did not as clearly show resolution of bands in the upper portion of the gel.
In general, no differences in the electrophoretic profiles between hydrophilic and hydrophobic cells were seen regardless of serotype. Several specific differences were evident with regard to the electrophoretic profiles of acid-stable mannan acetolysates. Mannan from serotype A cells (SC5314 and B311) contained more of the smaller branches than mannan from serotype B cells (A9 and LGH1095). Also, a few bands in the very-low-molecular-weight range appeared to show changes in mobility between hydrophobic and hydrophilic cells, perhaps due to alterations in chain structure, but these were not present in all strains. Other bands, migrating at an α-1,4-oligoglucoside degree of polymerization of approximately 4.5 and 5.5, appear more pronounced in mannoprotein from hydrophobic cells but were not present in all strains (Fig. 4, arrows).
DISCUSSION
Taken together, these results demonstrate that the only significant difference between mannoprotein from hydrophobic and hydrophilic cells is in the acid-labile component (33, 34). There was no overall difference in protein, hexose, or phosphate composition of mannoproteins extracted from hydrophobic versus hydrophilic yeast cells (Table 1). The sum of these three components accounted for 84.2 to 92.8% of the dried mannoprotein mass. Because the mannoprotein samples were dried to constant mass prior to analysis, it is unlikely that the remaining mass is due to residual water. A more likely candidate is lipid, specifically the phospholipomannan described by Trinel et al. (51).
The mannoprotein preparations described in earlier reports by us and others included an initial step of drying in acetone, which may have unintentionally extracted releasable lipid wall components. The oligomannoside component of phospholipomannan (PLM) would cause any PLM released during hot water extraction of cell wall glycans to cofractionate with the mannoprotein. Thus, the presence of PLM in the samples would not only provide at least some of the residual mass of the dried samples but also explain the higher phosphate content measured in the samples here. We consider the phosphate data valid because the assay was internally consistent. The potassium phosphate standard curve was linear over the range in which the experimental samples were measured, and a standard solution of glucose 6-phosphate was measured to within 1% of the expected value.
In addition to the compositional analyses, we extended our previous results by comparing the acid-labile mannan from hydrophobic and hydrophilic cells of several strains. As was expected, based on our current understanding of C. albicans N-glycans, the difference in acid-labile mannan between hydrophobic and hydrophilic cells held true for all strains tested; acid-labile mannan from hydrophobic cells contained longer oligosaccharides than that from hydrophilic cells (Fig. 2). This difference was independent of serotype. As observed previously, the degree of polymerization of clearly visible acid-labile branches reached 10 to 11 units in some cases. The longest acid-labile oligosaccharide described by Suzuki's group was seven (28, 46, 47), however, Trinel et al. reported acid-labile β-1,2-oligomannosides up to 14 units in length (52).
An issue left unresolved from previous studies was confirmation of the identity of the bands observed in the acid-labile fraction. The studies conducted on C. albicans mannoproteins by Suzuki and coworkers used fractionated mannan for the express purpose of component structure identification by nuclear magnetic resonance. Since we subjected our mannoprotein samples to the same treatment they did and they saw only β-1,2-oligomannosides in the acid-labile fraction, we assumed that our acid-labile fractions contained only the β-1,2-oligomannosides as well. However, we had to consider the possibility that that was not the case, since most bands did not comigrate with the β-1,4-oligomannose standards as we expected they would. Fraser-Reid and coworkers were able to synthesize β-1,2-oligomannosides without a reducing-end conjugate. Thus, these oligosaccharides could be labeled with ANTS and compared directly to our acid-labile samples by FACE. This allowed unambiguous identification of the bands in acid-labile mannan samples and confirmed that the acid-labile fraction comprises only β-1,2-oligomannosides (Fig. 3).
The comparisons of mannan elemental composition presented both here and previously showed no obvious differences between samples from hydrophilic and hydrophobic yeast cells. However, differences in oligosaccharide distribution would not be apparent from these comparisons. That is, the elemental composition analyses only reveal the kind and amount of the components, not how they are assembled. Acid-stable mannan from hydrophobic and hydrophilic cells could differ in how the monosaccharides are arranged into a complete glycan. However, analysis of the acetolysates of the acid-stable regions revealed no common differences among strains between hydrophobic and hydrophilic cells.
Differences were seen, however, between mannan from serotype A and serotype B cells. The fluorescent signal from the smaller acetolysates from serotype A cell mannan was much more intense than that from serotype B mannan, suggesting that serotype A acid-stable mannan is biased towards having these shorter side chains. This seems contradictory to what would be predicted based on the molar ratios reported by Suzuki and coworkers for side chain composition. These molar ratios suggest that the serotype B strains would have more of the shorter groups and the serotype A strains would have more of the longer groups (27, 28, 31, 46). In addition, the longest side chain reported by Suzuki and coworkers was eight units in length (29). If each successive band up the length of the gel represents an incremental increase in degree of polymerization, then each sample clearly had oligosaccharides 10 units or longer. One explanation for this observation is that the acetolysis was incomplete. This would mean that some side chains were still connected to each other through the α-1,6-linked oligomannoside backbone and would migrate as a larger species.
However, another explanation for both of the seemingly contradictory observations has presented itself. We and others have noted that linkage position and anomericity have an effect on electrophoretic mobility (7, 25, 34), so that a heterogenous oligomannoside (in terms of linkage position and anomericity) will likely migrate at a different rate than a homogenous oligomannoside of equivalent length. This migration variability does not seem to occur with chromatographic separations. For example, the chromatographic peaks identified by Kobayashi et al. (27, 30) contained oligomannosides with more than one structure. Enzymatic or chemical treatments were required to differentiate the separate structures of the same degree of polymerization. Thus, it is possible that the band intensity in serotype A acetolysate samples at the lower end of the gel is due not to an increase in a single species but to comigration of two or more species of slightly different structure. At the other end of the gel, bands that at first seem to have a degree of polymerization of 12 to 14 may be shorter chains that have decreased mobility due to their specific composition.
We have now compared the two main components (acid labile and acid stable) of N-linked glycans from hydrophobic and hydrophilic C. albicans cells of both serotypes. We compared overall composition in terms of protein and hexose. We also directly compared the side chains present in the most easily removed N-glycan subcomponent, the acid-labile mannan, and those released by fragmentation of the acid-stable mannan outer chain backbone by acetolysis. The results of these comparisons support our previous conclusions that only the acid-labile mannan differs significantly between N-glycans from hydrophobic and hydrophilic cells.
The reason that serotype A cells are unable to exhibit similar extreme levels of hydrophilicity as serotype B cells is unclear. However, if the assumption is made that the different serotype strains produce similar levels of acid-labile groups in their mannan, then the presence of β-1,2-oligomannosides in the acid-stable mannan may have an effect on the ability to exhibit high levels of hydrophilicity. We have speculated that β-1,2-oligomannosides form a tight, inflexible helix that has a hydrophobic face (34). Such a structure would facilitate interactions between other β-1,2-oligomannoside groups or perhaps between the glycan and hydrophobic protein sequences, thus altering fibril protein structure (34). It may be that the additional β-1,2-linked mannose groups in the acid-stable mannan of serotype A cells increases these interactions and does not allow complete extension of the fibril proteins. This would prevent complete masking of the underlying hydrophobic proteins.
Testing this hypothesis through comparison of serotype A and B cells by freeze-fracture, freeze-etch electron microscopy remains to be completed. Nevertheless, the results of our studies thus far indicate that regulation of β-1,2-mannosyltransferase activity, particularly that involved in acid-labile mannan synthesis, is one mechanism by which the cell surface hydrophobicity status of C. albicans cells is determined and consequently affects the relative pathogenicity of serotype A and B strains in immunocompromised patients.
Acknowledgments
This work was supported by U.S. Public Health Service grant AI 043997 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank Bert Fraser-Reid for synthesizing and providing us with the ladder of underivatized β-1,2-oligomannosides.
Editor: T. R. Kozel
REFERENCES
- 1.Ames, B. N., and D. T. Dubin. 1960. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J. Biol. Chem. 235:769-775. [PubMed] [Google Scholar]
- 2.Antley, P. P., and K. C. Hazen. 1988. Role of yeast cell growth temperature on Candida albicans virulence in mice. Infect. Immun. 56:2884-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck-Sagué, C. M., W. R. Jarvis, and the National Nosocomial Infection Surveillance System. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 4.Brawner, D. L., G. L. Anderson, and K. Y. Yuen. 1992. Serotype prevalence of Candida albicans from blood culture isolates. J. Clin. Microbiol. 30:149-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brawner, D. L., and J. E. Cutler. 1989. Oral Candida albicans isolates from nonhospitalized normal carriers, immunocompetent hospitalized patients, and immunocompromised patients with or without acquired immunodeficiency syndrome. J. Clin. Microbiol. 27:1335-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassone, A., E. Mattia, and L. Boldrini. 1978. Agglutination of blastospores of Candida albicans by concanavalin A and its relationship with the distribution of mannan polymers and the ultrastructure of the cell wall. J. Gen. Microbiol. 105:263-273. [DOI] [PubMed] [Google Scholar]
- 7.Chen, F. A., and R. A. Evangelista. 1995. Analysis of mono- and oligosaccharide isomers derivatized with 9-aminopyrene-1,4,6-trisulfonate by capillary electrophoresis with laser-induced fluorescence. Anal. Biochem. 230:273-280. [DOI] [PubMed] [Google Scholar]
- 8.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 9.Dygert, S., L. H. Li, D. Florida, and J. A. Thoma. 1965. Determination of reducing sugar with improved precision. Anal. Biochem. 13:367-374. [DOI] [PubMed] [Google Scholar]
- 10.Gillum, A. M., E. Y. H. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 11.Goins, T. L., and J. E. Cutler. 2000. Relative abundance of oligosaccharides in Candida species as determined by fluorophore-assisted carbohydrate electrophoresis. J. Clin. Microbiol. 38:2862-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamada, T., T. Nakajima, K. Izaki, and K. Matsuda. 1981. Comparison of the mannan structure from cell-wall mutant Candida sp. M-7002 and its wild type. I. Characterization of the proteo-mannan from the mutant and the wild-type cells. Eur. J. Biochem. 119:365-371. [DOI] [PubMed] [Google Scholar]
- 13.Hazen, B. W., and K. C. Hazen. 1988. Dynamic expression of cell surface hydrophobicity during initial yeast cell growth and before germ tube formation of Candida albicans. Infect. Immun. 56:2521-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazen, B. W., and K. C. Hazen. 1988. Modification and application of a simple, surface hydrophobicity detection method to immune cells. J. Immunol. Methods 107:157-163. [DOI] [PubMed] [Google Scholar]
- 15.Hazen, B. W., R. E. Liebert, and K. C. Hazen. 1988. Relationship of cell surface hydrophobicity to morphology of monomorphic and dimorphic fungi. Mycologia 80:348-355. [Google Scholar]
- 16.Hazen, K. C. 1989. Participation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cells. Infect. Immun. 57:1894-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazen, K. C., L. D. Bourgeois, and J. F. Carpenter. 1988. Cryoprotection of antibody by organic solutes and organic solute/divalent cation mixtures. Arch. Biochem. Biophys. 267:363-371. [DOI] [PubMed] [Google Scholar]
- 18.Hazen, K. C., D. L. Brawner, M. H. Riesselman, M. A. Jutila, and J. E. Cutler. 1991. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect. Immun. 59:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazen, K. C., and B. W. Hazen. 1992. Hydrophobic surface protein masking by the opportunistic fungal pathogen Candida albicans. Infect. Immun. 60:1499-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazen, K. C., and B. W. Hazen. 1987. A polystyrene microsphere assay for detecting cell surface hydrophobicity within Candida albicans populations. J. Microbiol. Methods 6:289-299. [Google Scholar]
- 21.Hazen, K. C., and B. W. Hazen. 1987. Temperature-modulated physiological characteristics of Candida albicans. Microbiol. Immunol. 31:497-508. [DOI] [PubMed] [Google Scholar]
- 22.Hazen, K. C., G. Mandell, E. Coleman, and G. Wu. 2000. Influence of fluconazole at subinhibitory concentrations on cell surface hydrophobicity and phagocytosis of Candida albicans. FEMS Microbiol. Lett. 183:89-94. [DOI] [PubMed] [Google Scholar]
- 23.Hazen, K. C., J. G. Wu, and J. Masuoka. 2001. Comparison of the hydrophobic properties of Candida albicans and Candida dubliniensis. Infect. Immun. 69:779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman, J., B. Lindberg, and S. Svensson. 1972. Determination of the anomeric configuration of sugar residues in acetylated oligo- and polysaccharides by oxidation with chromium trioxide in acetic acid. Acta Chem. Scand. 26:661-666. [Google Scholar]
- 25.Jackson, P. 1994. High-resolution polyacrylamide gel electrophoresis of fluorophore-labeled reducing saccharides. Methods Enzymol. 230:250-265. [DOI] [PubMed] [Google Scholar]
- 26.Jackson, P. 1990. The use of polyacrylamide-gel electrophoresis for the high-resolution separation of reducing saccharides labelled with the fluorophore 8-aminonaphthalene-1,3,6-trisulphonic acid. Biochem. J. 270:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi, H., N. Shibata, H. Mitobe, Y. Ohkubo, and S. Suzuki. 1989. Structural study of phosphomannan of yeast-form cells of Candida albicans J.-1012 strain with special reference to application of mild acetolysis. Arch. Biochem. Biophys. 272:364-375. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi, H., N. Shibata, M. Nakada, S. Chaki, K. Mizugami, Y. Ohkubo, and S. Suzuki. 1990. Structural study of cell wall phosphomannan of Candida albicans NIH B-792 (serotype B) strain, with special reference to 1H and 13C NMR analyses of acid-labile oligomannosyl residues. Arch. Biochem. Biophys. 278:195-204. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi, H., N. Shibata, T. Osaka, Y. Miyagawa, Y. Ohkubo, and S. Suzuki. 1992. Structural study of cell wall mannan of a Candida albicans (serotype A) strain. Phytochemistry 31:1147-1153. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi, H., N. Shibata, and S. Suzuki. 1986. Acetolysis of Pichia pastoris IFO 0948 strain mannan containing α-1,2 and α-1,2 linkages using acetolysis medium of low sulfuric acid concentration. Arch. Biochem. Biophys. 245:494-503. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi, H., S. Takahashi, N. Shibata, M. Miyauchi, M. Ishida, J. Sato, K. Maeda, and S. Suzuki. 1994. Structural modification of cell wall mannans of Candida albicans serotype A strains grown in yeast extract-Sabouraud liquid medium under acidic conditions. Infect. Immun. 62:968-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lloyd, K. O. 1970. Isolation, characterization, and partial structure of peptido galactomannans from the yeast form of Cladosporium werneckii. Biochemistry 9:3446-3453. [DOI] [PubMed] [Google Scholar]
- 33.Masuoka, J., and K. C. Hazen. 1997. Cell wall protein mannosylation determines Candida albicans cell surface hydrophobicity. Microbiology 143:3015-3021. [DOI] [PubMed] [Google Scholar]
- 34.Masuoka, J., and K. C. Hazen. 1999. Differences in the acid-labile component of Candida albicans mannan from hydrophobic and hydrophilic yeast cells. Glycobiology 9:1281-1286. [DOI] [PubMed] [Google Scholar]
- 35.Mathew, F., M. Mach, K. C. Hazen, and B. Fraser-Reid. 2003. Orthoester-based strategy for efficient synthesis of virulent antigenic-1,2-linked oligomannans of Candida albicans. Synlett 2003:1319-1322. [Google Scholar]
- 36.McNeil, M. M., S. L. Nash, R. A. Hajjeh, M. A. Phelan, L. A. Conn, B. D. Plikaytis, and D. W. Warnock. 2001. Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clin. Infect. Dis. 33:641-647. [DOI] [PubMed] [Google Scholar]
- 37.Okubo, Y., N. Shibata, T. Ichikawa, S. Chaki, and S. Suzuki. 1981. Immunochemical study on bakers' yeast mannan prepared by fractional precipitation with cetylmethylammonium bromide. Arch. Biochem. Biophys. 212:204-215. [DOI] [PubMed] [Google Scholar]
- 38.Okubo, Y., and S. Suzuki. 1978. Fractional precipitation of D-mannan from bakers' yeast with concanavalin A. Carbohydr. Res. 62:135-141. [Google Scholar]
- 39.Pazur, J. H. 1986. Neutral polysaccharides, p. 55-96. In M. F. Chaplin and J. F. Kennedy (ed.), Carbohydrate analysis: a practical approach. IRL Press, Washington, D.C.
- 40.Peat, S., W. J. Whelan, and T. E. Edwards. 1961. Polysaccharides of baker's yeast. IV. Mannan. J. Chem. Soc. 1961:29-34. [Google Scholar]
- 41.Pfaller, M. A., and D. J. Diekema. 2002. Role of sentinel surveillance of candidemia: trends in species distribution and antifungal susceptibility. J. Clin. Microbiol. 40:3551-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poulain, D., G. Tronchin, J. F. Dubremetz, and J. Biguet. 1978. Ultrastructure of the cell wall of Candida albicans blastospores: study of its constitutive layers by the use of a cytochemical technique revealing polysaccharides. Ann. Microbiol. 129A:141-153. [PubMed] [Google Scholar]
- 43.Rangel-Frausto, M. S., T. Wiblin, H. M. Blumberg, L. Saiman, J. Patterson, M. Rinaldi, M. Pfaller, J. E. Edwards, Jr., W. Jarvis, J. Dawson, R. P. Wenzel, and the National Epidemiology of Mycoses Survey Study Group. 1999. National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin. Infect. Dis. 29:253-258. [DOI] [PubMed] [Google Scholar]
- 44.Shibata, N., S. Fukasawa, H. Kobayashi, M. Tojo, T. Yonezu, A. Ambo, Y. Ohkubo, and S. Suzuki. 1989. Structural analysis of phospho-D-mannan-protein complexes isolated from yeast and mold form cells of Candida albicans NIH A-207 serotype A strain. Carbohydr. Res. 187:239-253. [DOI] [PubMed] [Google Scholar]
- 45.Shibata, N., T. Ichikawa, M. Tojo, M. Takahashi, N. Ito, Y. Okubo, and S. Suzuki. 1985. Immunochemical study on the mannans of Candida albicans NIH A-207, NIH B-792, and J.-1012 strains prepared by fractional precipitation with cetyltrimethylammonium bromide. Arch. Biochem. Biophys. 243:338-348. [DOI] [PubMed] [Google Scholar]
- 46.Shibata, N., H. Kobayashi, S. Takahashi, Y. Okawa, K. Hisamichi, and S. Suzuki. 1991. Structural study on a phosphorylated mannotetraose obtained from the phosphomannan of Candida albicans NIH B-792 strain by acetolysis. Arch. Biochem. Biophys. 290:535-542. [DOI] [PubMed] [Google Scholar]
- 47.Shibata, N., N. Senbongi, T. Hosoya, K. Kawahara, R. Akagi, A. Suzuki, H. Kobayashi, S. Suzuki, and Y. Okawa. 1997. Demonstration of the presence of α-1,6-branched side chains in the mannan of Candida stellatoidea. Eur. J. Biochem. 246:477-485. [DOI] [PubMed] [Google Scholar]
- 48.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki, S. 1997. Immunochemical study on mannans of genus Candida. I. Structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34. Curr. Top. Med. Mycol. 8:57-70. [PubMed] [Google Scholar]
- 50.Tokunaga, M., M. Kusamichi, and H. Koike. 1986. Ultrastructure of outermost layer of cell wall in Candida albicans observed by rapid-freezing technique. J. Electron Microsc. 35:237-246. [PubMed] [Google Scholar]
- 51.Trinel, P.-A., M. Borg-von-Zepelin, G. Lepage, T. Jouault, D. Mackenzie, and D. Poulain. 1993. Isolation and preliminary characterization of the 14- to 18-kilodalton Candida albicans antigen as a phospholipomannan containing β-1,2-linked oligomannosides. Infect. Immun. 61:4398-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trinel, P. A., G. Lepage, T. Jouault, G. Strecker, and D. Poulain. 1997. Definitive chemical evidence for the constitutive ability of Candida albicans serotype A strains to synthesize β-1,2 linked oligomannosides containing up to 14 mannose residues. FEBS Lett. 416:203-206. [DOI] [PubMed] [Google Scholar]
- 53.Tronchin, G., D. Poulain, J. Herbaut, and J. Biguet. 1981. Cytochemical and ultrastructural studies of Candida albicans. II. Evidence for a cell wall coat using concanavalin A. J. Ultrastruct. Res. 75:50-59. [DOI] [PubMed] [Google Scholar]
- 54.Voss, A., J. A. J. W. Kluytmans, J. G. M. Koeleman, L. Spanjaard, C. M. J. E. Vandenbroucke-Grauls, H. A. Verbrugh, M. C. Vos, A. Y. L. Weersink, J. A. A. Hoogkamp-Korstanje, and J. F. G. M. Meis. 1996. Occurence of yeast bloodstream infections between 1987 and 1995 in five Dutch university hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 15:909-912. [DOI] [PubMed] [Google Scholar]
- 55.Wells, G. B., V. Kontoyiannidou, S. J. Turco, and R. L. Lester. 1982. Resolution of acetylated oligosaccharides by reverse-phase high pressure chromatography. Methods Enzymol. 83:132-137. [DOI] [PubMed] [Google Scholar]
- 56.Whelan, W. L., J. M. Delga, E. Wadsworth, T. J. Walsh, K. J. Kwon-Chung, R. Calderone, and P. N. Lipke. 1990. Isolation and characterization of cell surface mutants of Candida albicans. Infect. Immun. 58:1552-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamashita, K., T. Mizuochi, and A. Kobata. 1982. Analysis of oligosaccharides by gel filtration. Methods Enzymol. 83:105-126. [DOI] [PubMed] [Google Scholar]