Abstract
Candida albicans is an opportunistic dimorphic fungus that inhabits various host mucosal sites. Conversion from the yeast to the hyphal form has been associated with increased virulence and mucosal invasiveness. C. albicans morphogenesis is regulated by multiple signals and signaling pathways. However, signals that control morphogenesis in vivo are unknown. We investigated the effects of host long chain fatty acids, eicosanoids, and bacterial short chain fatty acids on control of germination. None of the C18 or C20 fatty acids tested had an effect on enhancing germ tube formation (arachidonic acid, oleic acid, linolenic acid, or γ-linolenic acid). Among the different eicosanoids, both prostaglandin E2 and thromboxane B2 significantly enhanced serum-induced germination by C. albicans. Addition of antiprostaglandin or antithromboxane antibodies to serum alone inhibited germ tube formation by almost 30%, while control antibody had no effect, indicating that these eicosanoids are major morphogenic factors in the serum. Since these molecules also bind to albumin, this may also explain the hyphal transforming activity in serum that associates with albumin. Interestingly, short chain fatty acids (butyric acid), the product of lactic acid bacteria (LAB), inhibited germination. In addition, LAB culture supernatants as well as live LAB also inhibited C. albicans morphogenesis. Overall, these results indicate that fatty acid metabolites and fatty acid pathways can up-regulate and down-regulate germination in C. albicans.
Candida albicans inhabits various host mucosal surfaces, where it exists as both a member of the normal microflora and a potential opportunistic pathogen. C. albicans is a dimorphic fungus, with the ability to grow both as a yeast and as hyphae. Conversion to the hyphal form is required for virulence (40) and invasiveness (22) in vivo. Several signaling pathways regulating morphogenesis have been identified and well characterized in C. albicans (reviewed in reference 24). However, in vivo stimuli are still a subject of investigation. At mucosal surfaces, C. albicans is met by an environment dictated by the host and bacterial microflora. Both the host and bacterial microflora produce immunomodulatory fatty acid metabolites that may influence the behavior of C. albicans. One mechanism by which the host controls inflammatory responses is via a network of eicosanoids (20-carbon fatty acid metabolites), which include prostaglandins and leukotrienes (7, 27, 33, 34, 42). Members of the bacterial microflora also can participate in modulating local immune responses through production of short chain fatty acids (SCFA) (5, 9, 41) and even eicosanoids (43). The concept that the local fatty acid environment within the host influences virulence traits in C. albicans has not been examined. Therefore, the aim of these studies was to investigate the effects of fatty acids and fatty acid metabolites on C. albicans morphogenesis.
The observation that C. albicans germinates in serum was made four decades ago (4). However, the factors in serum responsible for inducing germination remain a subject of investigation. It has been suggested that serum albumin is the factor in serum involved in inducing morphogenesis (6). However, the inability of commercial preparations of albumin to induce morphogenesis prompted investigators to further explore the role of albumin. Experiments using serum from analbumic rats demonstrate that albumin is not required for induction of morphogenesis by serum. In addition, filtering serum through a 1-kDa membrane revealed that germination-inducing activity is also found in the hydrophobic compounds in the filtrate (14). The conflicting data concerning the ability of albumin may be due to the presence of small hydrophobic compounds that bind albumin in serum, such as fatty acids and fatty acid metabolites (35). Our laboratory and others have previously reported that prostaglandin E2 (PGE2), a cyclooxygenase product of arachidonic acid involved in control of inflammatory responses, enhances C. albicans morphogenesis (21, 30). C. albicans also produces a fatty acid metabolite similar to PGE2 that augments hyphal transformation (30). Similarly, cyclooxygenase inhibitors such as aspirin and etodolac inhibit morphogenesis (2). The latter two observations suggest the presence of an eicosanoid/oxylipin pathway in C. albicans that plays a role in control of germination.
MATERIALS AND METHODS
C. albicans germ tube assay.
A crystal violet-based germ tube assay was used to measure germination as previously described (1, 31, 46). C. albicans strain CHN1 was grown in sabouraud dextrose broth (SDB) at 22°C (room temperature) while shaking for 48 to 72 h. Samples were washed in 1× phosphate-buffered saline (PBS) and resuspended in 100% fetal bovine serum (FBS) to give a final concentration of 106 yeast cells/ml. C. albicans diluted in FBS was then plated into a 96-well flat-bottom plate at a volume of 100 μl/well. Additions or carrier was added (10 μl), and plates were incubated at 37°C for 2 h to induce germination. Adherent germ tubes formed were fixed, and nonadherent yeast forms were removed by sequential washes with 70% ethanol and 0.25% sodium dodecyl sulfate (SDS). Plates were washed additionally two or three times with distilled water. Plates were examined microscopically to ensure removal of nonadherent yeast forms. Remaining germ tube forms were then stained with 0.1% crystal violet for 5 min. Plates were then washed three times with distilled water, once with 0.25% SDS, and twice with distilled water. Crystal violet that stained germ tube forms was resolubilized by adding 200 μl of isopropanol-0.04 N HCl and 50 ml of 0.25% SDS. A spectrophotometer was used to read the A590.
Antiprostaglandin antibody treatment.
Prostaglandin screening antibody (Cayman Chemicals, Ann Arbor, Mich.) was resuspended in 2 ml of 1× PBS added in 10-μl amounts to wells. This broad-spectrum antibody recognizes the prostaglandins PGE2, PGD2, PGE1, PGE3, PGF1α, PGF2α, and PGF3α. This antibody does not recognize thromboxane B2 (TxB2), leukotrienes, or free fatty acids. TxB2 antibody (Cayman Chemicals, Ann Arbor, Mich.) was also resuspended in 2 ml of 1× PBS and added in 10-μl amounts to wells. For control wells, rabbit immunoglobulin G (IgG) (BD PharMingen, San Diego, Calif.) was added at an equal protein concentration as an experimental antibody.
Fatty acid treatment.
Long chain fatty acids and SCFA were resuspended or diluted in 1× PBS and brought to pH 7.0 prior to addition to C. albicans germ tube assay.
LAB treatment.
The lactic acid bacteria (LAB) Lactobacillus casei (ATCC 393), Lactobacillus paracasei (ATCC 27092), and Lactobacillus rhamnosus GG (ATCC 53103) were grown in Lactobacillus deMan, Rogosa, and Sharpe (MRS) broth (Becton-Dickson Microbiology Systems, Sparks, Md.) under microaerophilic conditions (10% H2, 5% N2, 85% CO2) at 37°C for 24 h. An equal amount of MRS broth, live lactobacilli, or Lactobacillus culture supernatant (100 μl) was added to each well in the C. albicans germ tube assay prior to incubation at 37°C, resulting in a final concentration of 50% FBS.
Statistical analysis.
Student's t test (two-tailed, unequal variance) was used to analyze the significance of differences between experimental groups. Data with a P value of ≤ 0.05 were considered to be significant.
RESULTS AND DISCUSSION.
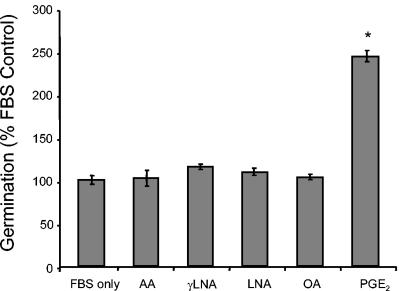
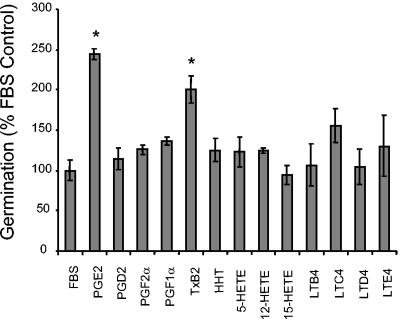
We first investigated whether unmodified fatty acids or specific eicosanoids present in serum could be largely responsible for the candidal morphogenesis activity of serum. Various long chain fatty acids were added to C. albicans diluted in serum. Interestingly, none had a significant effect on enhancing germ tube formation (Fig. 1). Varying the concentration of these fatty acids between 0.1 and 10 mM did not increase or decrease their ability to affect germination (data not shown). A variety of eicosanoids, including cyclooxygenase and lipoxygenase products, were also tested for their ability to affect C. albicans morphogenesis (Fig. 2). Of the eicosanoids tested, only PGE2 and TxB2 significantly enhanced serum-mediated germination by C. albicans (Fig. 2).
FIG. 1.
Effect of long chain fatty acids on C. albicans morphogenesis. Long chain fatty acids (Caymen Chemicals) or carrier (1× PBS) was added to C. albicans diluted in 100% FBS to a final concentration of 0.1 nM at neutral pH. Cultures were incubated at 37°C to induce germination, and germ tube formation was measured after 2 h with the crystal violet germ tube assay. Results are expressed as percent control (% control = A540 for experimental well/A540 for control well). Background absorbances (A450 of the well containing 100% PBS) were subtracted out. The assay was performed in triplicate and repeated two times with similar results. *, P < 0.05 as determined by the Student's t test. AA, arachidonic acid; LNA, linolenic acid; OA, oleic acid.
FIG. 2.
Effect of eicosanoids on C. albicans morphogenesis. Eicosanoids (Caymen Chemicals) or carrier (1× PBS) was added to C. albicans diluted in 100% FBS to a final concentration of 0.1 nM at neutral pH. Germination was measured as described in the legend to Fig. 1. Background absorbances (A450 of the well containing 100% PBS) were subtracted out. The assay was performed in triplicate and repeated two times with similar results. PG, prostaglandin; Tx, thromboxane; HHT, hydroxyheptadecatrienoic acid; LT, leukotriene. *, P < 0.05 as determined by Student's t test.
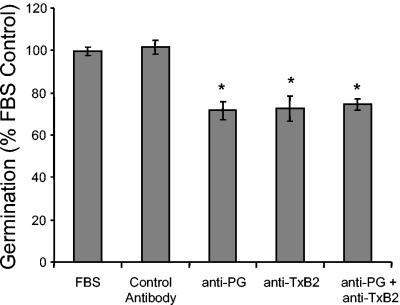
To neutralize the prostaglandins in serum alone, polyclonal antiprostaglandin antibodies (Caymen Biochemicals) were added to cultures of C. albicans plus serum and changes in germ tube formation were measured (Fig. 3). Polyclonal antiprostaglandin antibodies bind to the E, D, and F series prostaglandins. They do not recognize free fatty acids, TxB2, or leukotrienes. Antiprostaglandin antibodies inhibited germ tube formation by 29%, while control antibody had no effect (Fig. 3). These data indicate that prostaglandins are major morphogenic factors in the serum and are consistent with previous studies that PGE2 can augment germ tube formation (21, 30).
FIG. 3.
Effect of antiprostaglandin or antithromboxane antibody on C. albicans morphogenesis in FBS. Polyclonal antiprostaglandin antibody, anti-TxB2 antibody (Cayman Chemicals), control rabbit IgG (BD Pharmingen, San Diego, Calif.), or carrier (1× PBS) was added to C. albicans yeast diluted in 100% FBS. Germination was measured as described in the legend to Fig. 1. The assay was performed in triplicate and repeated two times with similar results. Anti-PG, antiprostaglandin antibody *, P < 0.05 as determined by Student's t test.
TxB2 is an arachidonic acid metabolite found in serum and not recognized by the prostaglandin screening antibody (3). We therefore tested anti-TxB2 antibody in the germ tube assay to determine if we could further inhibit germination. Addition of anti-TxB2 antibody also inhibited germ tube formation by 28% (Fig. 3). However, addition of both antiprostaglandin and anti-TxB2 antibodies was not additive (Fig. 3). Since thromboxanes and prostaglandins are both found bound to albumin in the serum (15, 25, 26, 36, 37, 47), we speculate that the bioactivity we are blocking in the serum with antiprostaglandin and anti-TxB2 antibodies may be found on the same “carrier” molecule, albumin. There is a precedent for this in the literature: the hyphal transformation activity in serum copurifies with the albumin fraction, and purified albumin also contains hyphal transformation activity (14).
The ability of C. albicans to establish colonization at various mucosal surfaces is highly dependent on the presence or absence of members of the normal bacterial microflora. Antibiotic treatment often promotes increased C. albicans carriage and infection in the oral cavity, intestinal tract, and vaginal mucosa. The ability of the bacterial flora to control or prevent C. albicans colonization is due in part to competitive exclusion of favored niches. Oral bacteria including Escherichia coli and Streptococcus spp. can inhibit C. albicans morphogenesis in vitro, which indicates that the bacterial flora may produce compounds that may inhibit germination and epithelial adherence (29).
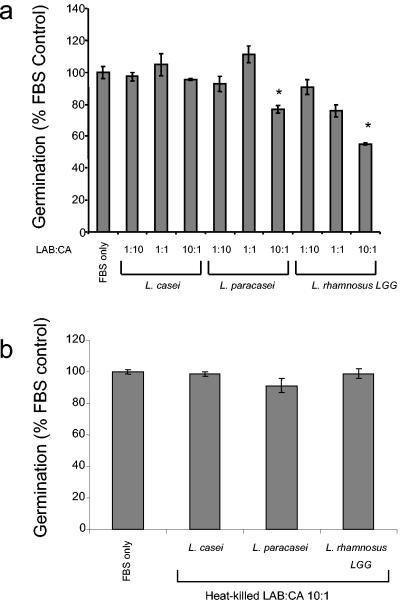
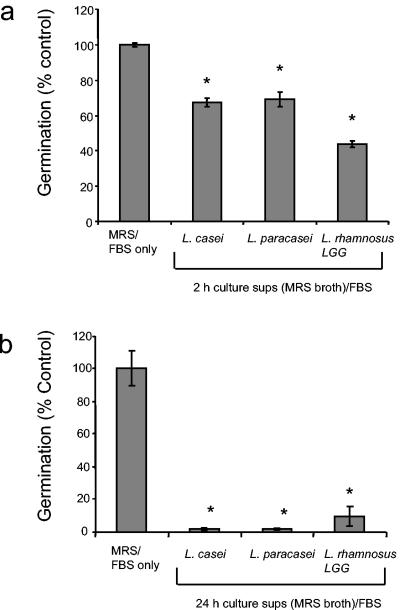
LAB are also known to inhibit C. albicans colonization of the epithelium of the gastrointestinal tract in mice and subsequent hyphal invasion and systemic infection (45). L. rhamnosus LGG is a commonly used probiotic strain with immunomodulatory activity (16, 23, 28, 32). In a mouse model of gastrointestinal candidiasis, prior inoculation with L. rhamnosus LGG reduced C. albicans levels and invasion (45). We tested whether L. rhamnosus LGG could inhibit C. albicans germ tube formation. Addition of live L. rhamnosus LGG at a 10:1 bacterium/yeast ratio significantly inhibited germ tube formation in serum (23 to 44% inhibition) (Fig. 4a). This inhibition is not due to competitive exclusion, because LAB do not adhere to the tissue culture plastic. In addition, MRS broth or LAB culture supernatant alone does not cause an increase in background above PBS and therefore does not interfere with the assay. Incubation of heat-killed bacteria did not inhibit germination, suggesting that metabolically active bacteria are required for this effect (Fig. 4b). To investigate whether a soluble product from Lactobacillus could inhibit C. albicans germ tube formation, lactobacilli were cultured under anaerobic conditions in MRS broth for 2 or 24 h and supernatants were collected and added to C. albicans diluted in 100% FBS. Supernatants from 2-h cultures of L. casei, L. paracasei, and L. rhamnosus LGG all inhibited germ tube formation (30 to 55% inhibition) (Fig. 5a). However, the addition of 24-h cultures of LAB almost completely inhibited germination (92 to 98% inhibition) (Fig. 5b), suggesting that accumulation of a soluble compound in the culture supernatant is responsible for the inhibition. The inhibitory effect of a secreted product from L. rhamnosus LGG on C. albicans germ tube formation may represent a novel probiotic effect of this strain.
FIG. 4.
Effect of live or heat-killed LAB on C. albicans morphogenesis. Lactobacillus spp. were grown in MRS broth for 24 h under microaerophilic conditions at 37°C at neutral pH. Live or heat-killed lactobacilli diluted in MRS broth or carrier (MRS broth) were added to C. albicans diluted in 100% FBS at various ratios of LAB to C. albicans. Germination was measured as described in the legend to Fig. 1. The assay was performed in triplicate and repeated two times with similar results. *, P < 0.05 as determined by Student's t test. CA, C. albicans.
FIG. 5.
Effect of LAB culture supernatants on C. albicans morphogenesis. Lactobacillus spp. were grown in Lactobacillus MRS broth for (a) 2 h or (b) 24 h under microaerophilic conditions at 37°C. Lactobacillus culture supernatants (sups) from these two time points or carrier (MRS broth) was added to C. albicans diluted in 100% FBS at neutral pH. Germination was measured as described in Materials and Methods. The assay was performed in triplicate and repeated two times with similar results. *, P < 0.05 as determined by Student's t test.
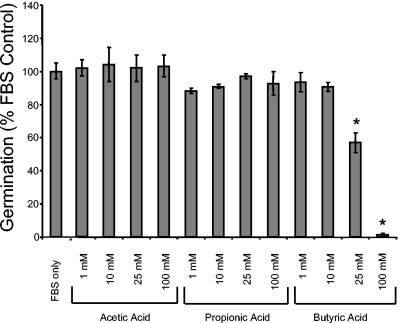
Members of the normal flora such as LAB produce large quantities of biologically active SCFA. These fatty acids, which are by-products of anaerobic fermentation, possess an anti-inflammatory function (reviewed in reference 39). Therefore, examination of the effect of SCFA on morphogenesis may provide a mechanism by which LAB prevent candidal colonization. To test the effect of SCFA on C. albicans morphogenesis, commercially available acetic, propionic, and butyric acid (C2, C3, and C4 SCFA, respectively) were used (Fig. 6). These were all buffered to pH 7.0 to eliminate the effect of pH on morphogenesis (12). When added to C. albicans diluted in 100% FBS, 25 mM butyric acid (buffered to pH 7.0) significantly inhibited germ tube formation by 40%, while addition of 100 mM butyric acid almost completely abrograted germ tube formation (98% inhibition) (Fig. 6). Interestingly, butyric acid is the SCFA that is most well known for its immunomodulatory activities (10, 38, 44). The inhibitory effect of sodium butyrate on C. albicans has also been reported and been linked with inhibition of chitin synthesis (11, 17). SCFA are produced in large quantities by LAB, as a by-product of fermentation (19, 39). Therefore, the levels of SCFA used in the assay are physiologically relevant. Depending on the substrate used, in vitro SCFA production can range from 8 to 80 mol/100 ml within a 24-h incubation period (8). In vivo, the SCFA concentration ranges from 20 to 140 mM in the cecum and large intestine (39). Our data indicate that one mechanism by which L. casei LGG inhibits C. albicans colonization may be via inhibition of morphogenesis via butyric acid production.
FIG. 6.
Effect of SCFA on C. albicans morphogenesis. SCFA at various concentrations (Sigma Chemical Co., St. Louis, Mo.) or carrier (1× PBS) was added to C. albicans diluted in 100% FBS and adjusted to neutral pH. Germination was measured as described in the legend to Fig. 1. The assay was performed in triplicate and repeated two times with similar results. *, P < 0.05 as determined by Student's t test.
Our data demonstrate that both long chain fatty acid metabolites and SCFA influence C. albicans morphogenesis. Among the different eicosanoids, PGE2 and TxB2 enhance germination by C. albicans. Since cyclooxygenase inhibitors (aspirin, indomethacin) have been reported to inhibit germination and biofilm formation (2), this suggests the presence of an eicosanoid/oxylipin pathway that plays a role in morphogenesis. This also suggests that the immune response of the host (eicosanoid microenvironment) could influence C. albicans morphogenesis or virulence in vivo. Interestingly, SCFA (butyric acid) inhibit germination. This may represent a novel probiotic activity of this fermentation product in the gastrointestinal tract. Other examples of Candida-bacterial antagonism via fatty acid metabolites have been reported. Pseudomonas aeruginosa can produce 15-hydroxyeicosatetraenoic acid (15-HETE) from exogenous arachidonic acid and can grow on and kill hyphal C. albicans (18, 43). Pseudomonas is also found in low levels as part of the bacterial microflora and increases in number after antibiotic treatment (13, 20). The mechanism of Pseudomonas-Candida antagonism has been proposed to be multifactorial. Since we have demonstrated that 15-HETE does not decrease or augment germination, it seems likely that this fatty acid metabolite does not play a role in the bacterial-fungal antagonism of these two microbes. Overall, these results indicate that fatty acid metabolic pathways can regulate germination of C. albicans and the local fatty acid environment can influence whether C. albicans exists as a commensal or pathogen.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health: National Institute of Allergy and Infectious Diseases grant R01-AI059201 (G.B.H.) and National Heart, Lung, and Blood Institute training grant T32HL007749 (M.C.N.).
Editor: T. R. Kozel
REFERENCES
- 1.Abe, S., T. Satoh, Y. Tokuda, S. Tansho, and H. Yamaguchi. 1994. A rapid colorimetric assay for determination of leukocyte-mediated inhibition of mycelial growth of Candida albicans. Microbiol. Immunol. 38:385-388. [DOI] [PubMed] [Google Scholar]
- 2.Alem, M. A. S., and L. J. Douglas. 2004. Effects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of Candida albicans. Antimicrob. Agents Chemother. 48:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessandrini, P., P. Avogaro, G. Bittolo Bon, P. Patrignani, and C. Patrono. 1985. Physiologic variables affecting thromboxane B2 production in human whole blood. Thromb. Res. 37:1-8. [DOI] [PubMed] [Google Scholar]
- 4.Andleigh, H. S. 1964. Rapid identification of Candida albicans. Mycopathol. Mycol. Appl. 23:81-84. [DOI] [PubMed] [Google Scholar]
- 5.Andoh, A., T. Tsujikawa, and Y. Fujiyama. 2003. Role of dietary fiber and short-chain fatty acids in the colon. Curr. Pharm. Des. 9:347-358. [DOI] [PubMed] [Google Scholar]
- 6.Barlow, A. J., T. Aldersley, and F. W. Chattaway. 1974. Factors present in serum and seminal plasma which promote germ-tube formation and mycelial growth of Candida albicans. J. Gen. Microbiol. 82:261-272. [DOI] [PubMed] [Google Scholar]
- 7.Betz, M., and B. S. Fox. 1991. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 146:108-113. [PubMed] [Google Scholar]
- 8.Birkbeck, J. 1999. Colon cancer: the potential involvement of the normal microflora, p. 262-294. In G. Tannock (ed.), Medical importance of the normal microflora. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 9.Blaut, M. 2002. Relationship of prebiotics and food to intestinal microflora. Eur. J. Nutr. 41(Suppl. 1):I11-I16. [DOI] [PubMed] [Google Scholar]
- 10.Bohmig, G. A., P. M. Krieger, M. D. Saemann, C. Wenhardt, E. Pohanka, and G. J. Zlabinger. 1997. n-Butyrate downregulates the stimulatory function of peripheral blood-derived antigen-presenting cells: a potential mechanism for modulating T-cell responses by short-chain fatty acids. Immunology 92:234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun, P. C., R. F. Hector, M. E. Kamark, J. T. Hart, and R. L. Cihlar. 1987. Effect of cerulenin and sodium butyrate on chitin synthesis in Candida albicans. Can. J. Microbiol. 33:546-550. [DOI] [PubMed] [Google Scholar]
- 12.Buffo, J., M. A. Herman, and D. R. Soll. 1984. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia 85:21-30. [DOI] [PubMed] [Google Scholar]
- 13.Edlund, C., G. Beyer, M. Hiemer-Bau, S. Ziege, H. Lode, and C. E. Nord. 2000. Comparative effects of moxifloxacin and clarithromycin on the normal intestinal microflora. Scand. J. Infect. Dis. 32:81-85. [DOI] [PubMed] [Google Scholar]
- 14.Feng, Q., E. Summers, B. Guo, and G. Fink. 1999. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 181:6339-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folco, G., E. Granstrom, and H. Kindahl. 1977. Albumin stabilizes thromboxane A2. FEBS Lett. 82:321-324. [DOI] [PubMed] [Google Scholar]
- 16.Gorbach, S. L. 2000. Probiotics and gastrointestinal health. Am. J. Gastroenterol. 95:S2-S4. [DOI] [PubMed] [Google Scholar]
- 17.Hoberg, K. A., R. L. Cihlar, and R. A. Calderone. 1983. Inhibitory effect of cerulenin and sodium butyrate on germination of Candida albicans. Antimicrob. Agents Chemother. 24:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogan, D. A., and R. Kolter. 2002. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296:2229-2232. [DOI] [PubMed] [Google Scholar]
- 19.Hove, H., I. Nordgaard-Andersen, and P. B. Mortensen. 1994. Effect of lactic acid bacteria on the intestinal production of lactate and short-chain fatty acids, and the absorption of lactose. Am. J. Clin. Nutr. 59:74-79. [DOI] [PubMed] [Google Scholar]
- 20.Kager, L., I. Ljungdahl, A. S. Malmborg, C. E. Nord, R. Pieper, and P. Dahlgren. 1981. Antibiotic prophylaxis with cefoxitin in colorectal surgery: effect on the colon microflora and septic complications—a clinical model for prediction of the benefit and risks in using a new antibiotic in prophylaxis. Ann. Surg. 193:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalo-Klein, A., and S. S. Witkin. 1990. Prostaglandin E2 enhances and gamma interferon inhibits germ tube formation in Candida albicans. Infect. Immun. 58:260-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaposzta, R., L. Marodi, M. Hollinshead, S. Gordon, and R. P. da Silva. 1999. Rapid recruitment of late endosomes and lysosomes in mouse macrophages ingesting Candida albicans. J. Cell Sci. 112:3237-3248. [DOI] [PubMed] [Google Scholar]
- 23.Kirjavainen, P. V., H. S. ElNezami, S. J. Salminen, J. T. Ahokas, and P. F. Wright. 1999. Effects of orally administered viable Lactobacillus rhamnosus GG and Propionibacterium freudenreichii subsp. shermanii JS on mouse lymphocyte proliferation. Clin. Diagn. Lab. Immunol. 6:799-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, H. 2001. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 4:728-735. [DOI] [PubMed] [Google Scholar]
- 25.Maclouf, J., H. Kindahl, E. Granstrom, and B. Samuelsson. 1980. Interactions of prostaglandin H2 and thromboxane A2 with human serum albumin. Eur. J. Biochem. 109:561-566. [DOI] [PubMed] [Google Scholar]
- 26.Maclouf, J., H. Kindahl, E. Granstrom, and B. Samuelsson. 1980. Thromboxane A2 and prostaglandin H2 form covalently linked derivatives with human serum albumin. Adv. Prostaglandin Thromboxane Res. 6:283-286. [PubMed] [Google Scholar]
- 27.Matsuoka, T., M. Hirata, H. Tanaka, Y. Takahashi, T. Murata, K. Kabashima, Y. Sugimoto, T. Kobayashi, F. Ushikubi, Y. Aze, N. Eguchi, Y. Urade, N. Yoshida, K. Kimura, A. Mizoguchi, Y. Honda, H. Nagai, and S. Narumiya. 2000. Prostaglandin D2 as a mediator of allergic asthma. Science 287:2013-2017. [DOI] [PubMed] [Google Scholar]
- 28.Miettinen, M., A. Lehtonen, I. Julkunen, and S. Matikainen. 2000. Lactobacilli and streptococci activate NF-kappa B and STAT signaling pathways in human macrophages. J. Immunol. 164:3733-3740. [DOI] [PubMed] [Google Scholar]
- 29.Nair, R. G., S. Anil, and L. P. Samaranayake. 2001. The effect of oral bacteria on Candida albicans germ-tube formation. APMIS 109:147-154. [DOI] [PubMed] [Google Scholar]
- 30.Noverr, M. C., S. M. Phare, G. B. Toews, M. J. Coffey, and G. B. Huffnagle. 2001. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 69:2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okutomi, T., S. Abe, S. Tansho, H. Wakabayashi, K. Kawase, and H. Yamaguchi. 1997. Augmented inhibition of growth of Candida albicans by neutrophils in the presence of lactoferrin. FEMS Immunol. Med. Microbiol. 18:105-112. [DOI] [PubMed] [Google Scholar]
- 32.Pena, J. A., and J. Versalovic. 2003. Lactobacillus rhamnosus GG decreases TNF-alpha production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell Microbiol. 5:277-285. [DOI] [PubMed] [Google Scholar]
- 33.Peters-Golden, M. 2002. 'Good' lipids for asthma. Nat. Med. 8:931-932. [DOI] [PubMed] [Google Scholar]
- 34.Peters-Golden, M. 2002. Open mind, open airways: broadening the paradigm of prostaglandins and allergic airway inflammation. Am. J. Respir. Crit. Care Med. 165:1035-1036. [DOI] [PubMed] [Google Scholar]
- 35.Petitpas, I., T. Grune, A. A. Bhattacharya, and S. Curry. 2001. Crystal structures of human serum albumin complexed with monounsaturated and polyunsaturated fatty acids. J. Mol. Biol. 314:955-960. [DOI] [PubMed] [Google Scholar]
- 36.Raz, A. 1972. Interaction of prostaglandins with blood plasma proteins. Comparative binding of prostaglandins A 2, F 2 and E 2 to human plasma proteins. Biochem. J. 130:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raz, A. 1972. Interaction of prostaglandins with blood plasma proteins. I. Binding of prostaglandin E 2 to human plasma proteins and its effect on the physiological activity of prostaglandin E 2 in vitro and in vivo. Biochim. Biophys. Acta 280:602-613. [PubMed] [Google Scholar]
- 38.Saemann, M. D., G. A. Bohmig, C. H. Osterreicher, H. Burtscher, O. Parolini, C. Diakos, J. Stockl, W. H. Horl, and G. J. Zlabinger. 2000. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 14:2380-2382. [DOI] [PubMed] [Google Scholar]
- 39.Saemann, M. D., G. A. Bohmig, and G. J. Zlabinger. 2002. Short-chain fatty acids: bacterial mediators of a balanced host-microbial relationship in the human gut. Wien. Klin. Wochenschr. 114:289-300. [PubMed] [Google Scholar]
- 40.Saville, S. P., A. L. Lazzell, C. Monteagudo, and J. L. Lopez-Ribot. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schley, P. D., and C. J. Field. 2002. The immune-enhancing effects of dietary fibres and prebiotics. Br. J. Nutr. 87(Suppl. 2):S221-S230. [DOI] [PubMed] [Google Scholar]
- 42.Strassmann, G., V. Patil-Koota, F. Finkelman, M. Fong, and T. Kambayashi. 1994. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J. Exp. Med. 180:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vance, R. E., S. Hong, K. Gronert, C. N. Serhan, and J. J. Mekalanos. 2004. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc. Natl. Acad. Sci. USA 101:2135-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wachtershauser, A., and J. Stein. 2000. Rationale for the luminal provision of butyrate in intestinal diseases. Eur. J. Nutr. 39:164-171. [DOI] [PubMed] [Google Scholar]
- 45.Wagner, R. D., C. Pierson, T. Warner, M. Dohnalek, M. Hilty, and E. Balish. 2000. Probiotic effects of feeding heat-killed Lactobacillus acidophilus and Lactobacillus casei to Candida albicans-colonized immunodeficient mice. J. Food Prot. 63:638-644. [DOI] [PubMed] [Google Scholar]
- 46.Wakabayashi, H., S. Abe, S. Teraguchi, H. Hayasawa, and H. Yamaguchi. 1998. Inhibition of hyphal growth of azole-resistant strains of Candida albicans by triazole antifungal agents in the presence of lactoferrin-related compounds. Antimicrob. Agents Chemother. 42:1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, J., C. E. Petersen, C. E. Ha, and N. V. Bhagavan. 2002. Structural insights into human serum albumin-mediated prostaglandin catalysis. Protein Sci. 11:538-545. [DOI] [PMC free article] [PubMed] [Google Scholar]