Abstract
Toll-like receptor 4 (TLR4) mediates the response to lipopolysaccharide, and its activation induces the expression of a large number of inflammatory genes, many of which are also induced by other pathogen-associated molecular patterns. Interestingly, the subset of genes that are dependent on TLR4 for optimal expression during gram-negative bacterial infection has not been determined. We have previously shown that TLR4-deficient mice rapidly develop acute pneumonia after inoculation with Bordetella bronchiseptica, suggesting that TLR4 is required for expression of early elicited gene products in this model. Microarray analysis with macrophages derived from wild-type and TLR4-deficient mice was used to identify genes whose expression, within 1 h of bacterial exposure, is dependent on TLR4. The results of this investigation suggest that TLR4 is not required for the majority of the transcriptional response to B. bronchiseptica. However, early tumor necrosis factor alpha (TNF-α) mRNA expression is primarily dependent on TLR4 and in vitro and in vivo protein levels substantiate this finding. TLR4-deficient mice and TNF-α−/− mice are similarly susceptible to infection with relatively low doses of B. bronchiseptica and in vivo neutralization studies indicate that it is the TLR4-dependent early elicited TNF-α response that is critical for preventing severe pneumonia and limiting bacterial growth. These results suggest that one critical role for TLR4 is the generation of a robust but transient TNF-α response that is critical to innate host defense during acute gram-negative respiratory infection.
Toll-like receptors (TLRs) are an evolutionarily ancient set of pattern recognition receptors that enable the host to detect a number of pathogen-associated molecular patterns (PAMPs) (19). Ligand binding to TLRs initiates intracellular signaling cascades that result in the nuclear translocation of transcription factors and the subsequent production of proinflammatory molecules that are involved in host defense (20). There are at least 10 known mammalian TLRs, of which TLR4 is the best studied. TLR4 is required for cytokine responses to most bacterial lipopolysaccharides (LPSs), and infection studies with mouse strains deficient or deleted in TLR4 have shown a requirement for TLR4 in host responses to, and control of, certain gram-negative bacterial respiratory infections (3, 4, 12, 18, 37). However, which TLR4-regulated gene products are critical to innate host defense has not been determined.
The bordetellae include three closely related subspecies of gram-negative respiratory pathogens. Bordetella pertussis and B. parapertussis cause whooping cough or pertussis in humans (36). B. bronchiseptica, which is believed to be the progenitor of the two human pathogens, infects a large range of mammals, including mice (26, 36). We have recently shown that TLR4-deficient mice are highly susceptible to B. bronchiseptica infection and rapidly develop severe pneumonia with initial doses as low as 103 CFU (18). Thus, this model provides an opportunity to examine which TLR4-regulated gene products are critical during a model of gram-negative bacterial respiratory infection.
Due to the rapid onset of severe disease observed in TLR4-deficient mice upon inoculation with B. bronchiseptica, we hypothesized that TLR4 regulates early inflammatory processes that are critical to innate host defense in this model. To investigate which early elicited responses to B. bronchiseptica are TLR4 dependent, we used microarray analysis to search for genes that are highly upregulated in wild-type (WT), but not TLR4-deficient, macrophages upon exposure to live bacteria. Interestingly, the global transcriptional response after bacterial exposure was similar in WT and TLR4-deficient cells. However, tumor necrosis factor alpha (TNF-α) was identified as a gene product whose expression is highly dependent on TLR4 shortly after exposure to B. bronchiseptica. The results of in vitro and in vivo infection experiments show that TLR4 regulates the early elicited TNF-α response and that early, but not late, TNF-α is critical to innate host defense against B. bronchiseptica. Collectively, these results suggest that a major reason for the increased susceptibility of TLR4-deficient mice to B. bronchiseptica infection is an impaired early elicited TNF-α response.
MATERIALS AND METHODS
BMMφ assays.
Bone marrow-derived macrophages (BMMφ) were established as previously described (8). For cytokine responses, cells were removed from the original plastic petri dishes (Corning) by gentle lifting and put in 24-well tissue culture-treated plates (Corning) at ∼105 cells per well. For microarray experiments, cell cultures were left in original dishes and used when monolayers of ∼107 cells were present. At 12 h before use, the medium was removed, the cells were gently washed with phosphate-buffered saline (PBS), and fresh Dulbecco modified Eagle medium was added. At the initiation of each experiment, the medium was again removed and fresh Dulbecco modified Eagle medium containing purified B. bronchiseptica LPS at concentrations of 1 to 10,000 ng/ml was added to the cells and incubated for 24 h. Alternatively, heat-killed B. bronchiseptica, which was killed by heating at 65°C for 30 min, was added at a multiplicity of infection (MOI) ranging from 1 to 1,000 CFU per cell. Live B. bronchiseptica RB50 was grown as previously described (11) and added at an MOI of about 5. The culture media was removed, centrifuged, divided into aliquots, and assayed for TNF-α by enzyme-linked immunosorbent assay (ELISA) (R&D Systems).
Mouse oligonucleotide arrays.
Mouse oligonucleotide arrays were printed as previously described (34). The Mouse Genome Oligo Set Version 1 was purchased from Operon (Alameda, Calif.) and contains 6,800 optimized 70-mers plus 24 controls with the melting temperature normalized to 78°C.. Sequences were optimized by the manufacturer by using BLAST against all known mouse genes to minimize cross-hybridization. Oligonucleotides were printed onto glass slides by using GeneMachines Omnigrid (San Carlos, Calif.) with additional controls obtained from Stratagene (SpotReport System, La Jolla, Calif.) at the Penn State University microarray core facility.
Microarray RNA extraction and sample preparation.
WT and TLR4-deficient BMMφ were incubated with B. bronchiseptica (MOI = 10) or purified B. bronchiseptica LPS (10 ng/ml) for 30 min or 1 h. RAW 264.7 murine macrophage cells were left untreated for use as a reference. Previous homotypical hybridization experiments show no significant differences in the basal transcription between BMMφ and RAW cells (data not shown). Total RNA was extracted by using Qiagen RNeasy kit, according to kit instructions. Reverse transcription reactions were carried out for all samples as follows. Then, 20 μg of total RNA in 10.5 μl of RNase-free water was incubated with 5 μl of Oligo-DT (0.5 μg/μl; Invitrogen) for 10 min at 65°C. A reaction mix of 6 μl of 5× first-strand buffer, 3 μl of 0.1 M dithiothreitol, 3 μl of deoxynucleoside triphosphate mix (2 mM amino-ally dUTP, 3 mM dTTP; 5 mM each dATP, dCTP, and dGTP), 0.5 μl of RNase inhibitor (10 U/μl; Invitrogen), and 2 μl of SuperScript II RNase H− reverse transcriptase (200 U/μl; Invitrogen) were added, and the reaction was incubated at 42°C for 2 h. The reaction was neutralized by the addition of 1.6 μl of 5 M NaOH and 20 μl of 0.5 M EDTA (pH 8.0) and incubated at 65°C for 15 min. The mixture was neutralized with the addition of 10 μl of 2 M HEPES (pH 8.0). The resulting cDNA samples were purified with a Nanosep 30K concentrator (Pall) and washed thrice with 450 μl of sterile water. Concentrated cDNA was dried in a speed vacuum (Savant) and reconstituted in 4.5 μl of 0.1 M NaHCO3 (pH 9.0). Treated WT and TLR4-deficient samples were coupled with Cy5 fluorescent dye and untreated samples were coupled with Cy3 dye. 4.5 μl of dye (reconstituted in 72 μl of dimethyl sulfoxide; Amersham) was added to the sample, followed by incubation in the dark at room temperature for 1 h. Coupling reactions were quenched with 4.5 μl of 4 M hydroxylamine and incubated for an additional 15 min. Each Cy5-coupled treated sample was combined with a Cy3-coupled reference sample. To the combined samples, 70 μl of sterile water was added, and the mixture purified by using QIAquick PCR purification kit according to kit instructions. Eluted cDNA was dried in a dark speed vacuum for 30 min and reconstituted in 20 μl of hybridization buffer (10 μl of formamide, 5 μl of 20× SSC, 2 μl of H2O, 1 μl of 2% sodium dodecyl sulfate [SDS], and 2 μl of human Cot1 DNA (1 mg/ml; Invitrogen).
Microarray slide preparation and hybridization.
Microarray slides used were preprinted by the Penn State University Microarray Facility using the Operon gene set (34). Slides were moistened in a humid chamber for 10 s, snap-dried, and cross-linked at 1,900 μJ × 100. Slides were washed for 30 s in 0.1% SDS and for 2 min in 70% ethanol, centrifuged at 500 rpm for 5 min to dry them, and then incubated in prehybridization buffer (0.5 g of bovine serum albumin [Sigma], 12.5 ml of 20× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], and 250 μl of 20% SDS to a final volume of 50 ml with H2O) at 42°C for 45 min. Slides were then rinsed for 1 min in H2O and isopropanol alcohol, respectively, and centrifuged at 5,000 rpm for 5 min to dry. Prepared DNA samples were boiled for 2 min, loaded under the lifter slip (Erie Scientific), and incubated in a humid chamber for 24 h at 42°C. Slides were washed in 2× SSC with 0.1% SDS (3 min), 1× SSC (2 min), 0.2× SSC (1 min), and 0.05× SSC (10 s) and then dried by centrifugation at 500 rpm for 5 min.
Analysis of microarray results.
Arrays were scanned with an Axon 4000a scanner and processed by using GenePix software.
Normalization and analysis of the gene expression profiles were performed as follows. For each gene, a Z test was performed to determine that the spot intensity was significantly different from background. For spots that passed the Z test for either the 532-nm or the 635-nm channels, the mean background intensity for each wavelength was subtracted from the mean value of the spot intensity for the corresponding wavelength, resulting in an adjusted spot intensity for each channel. Spots whose adjusted intensities for either channel were ≤0 were discarded. Each gene's adjusted mean intensity for the 625-nm channel was divided by the adjusted mean for the 532-nm channel. This value was multiplied by the normalization factor and converted to a log2 scale. The normalization factor was calculated by averaging the 632-nm channel spot intensities for the entire slide and dividing this by the average of the 532-nm channel spot intensities for the entire slide. This is based on the assumption that the average red/green ratio over the entire gene set should be equal to 1. These log2 ratios were then compared to those of related experiments. Genes that were not present in all experiments were filtered, and the remaining genes were clustered hierarchically by using Genesis software.
Animal infections.
WT C57BL/6 and C57BL/10ScSn mice, TLR4-deficient C3H/HeJ and C57BL/10ScNCr mice, and TNF-α−/− mice were obtained from Jackson Laboratories. WT C3H/HeN mice were obtained from Charles River Laboratories. All mice were maintained in the animal care facility at The Pennsylvania State University in accordance with institutional guidelines. Mice were lightly sedated with isofluorane and inoculated intranasally with the indicated CFU of B. bronchiseptica in 50 μl of PBS (Merck) by pipetting the inoculum onto the tip of the external nares. B. bronchiseptica strain RB50 was grown as previously described (11). Groups of three to four mice were sacrificed at the indicated time point, and colonization of the lungs was quantified as previously described (11). For in vivo TNF-α measurements, the lungs were homogenized in 1 ml of PBS and stored at −80°C until assayed. The lung homogenate aliquots were subsequently thawed and assayed by ELISA. For TNF-α neutralization experiments, 1 ml of PBS containing 1 mg of anti-TNF-α, from clone MP6-XT3, was injected intraperitoneally at the indicated times postinoculation (14). For survival studies, mice were observed for increased signs and symptoms of bordetellosis to include ruffled fur, labored breathing, and diminished responsiveness, moribund mice were euthanized to alleviate suffering.
Histologic evaluation.
The lungs from treated mice were removed and inflated with 1.5 ml of 10% neutral buffered formalin, embedded in paraffin, and sectioned. Slides were prepared and stained with hematoxylin and eosin (H&E). An assessment of microscopic lesions was made by one of the authors (M.J.K.) experienced in rodent pathology and blinded to experimental treatment. Descriptive evaluations of the lesions were recorded, and lung lesions were graded by using a scale of 0 to 5. Sections with no lesions and no inflammation were given a score of 0, a score of 1 indicated slight inflammation with few or scattered lesions and fewer than 10% of lung fields affected, a score of 2 indicated mild lesions with 10 to 20% of lung fields affected, a score of 3 indicated moderate lesions with 20 to 30% of the lung fields affected, and those given a score of 4 were characterized by extensive lesions, marked inflammation, and 31 to 50% of the lung was affected. A score of 5 indicated there were extensive lesions with >50% of the lung fields affected. Plus marks indicated scores with a severity that fell between categories.
Statistics.
The mean ± the standard error was determined for each treatment group in the individual experiments. Statistical significance was calculated by using a paired Student's t test, with a significance level set at P values of <0.05 for a single comparison. In vitro cytokine experiments were performed at least twice, and animal infection experiments for cytokine or CFU assays were repeated two or three times.
RESULTS
Role of TLR4 in the global transcriptional response to B. bronchiseptica.
Previous studies have shown that TLR4 is critical for innate host defense during respiratory infection with B. bronchiseptica and that TLR4-deficient mice rapidly develop severe suppurative pneumonia shortly after inoculation (18). We hypothesized that the rapid onset of severe disease observed in this model is due to diminished or absent early elicited gene products that require TLR4 for optimal expression. Using microarray technology, we examined which specific genes were upregulated early during exposure to B. bronchiseptica in TLR4-sufficient cells but not in TLR4-deficient cells. We chose macrophages as a prototypical cell type since the transfer of TLR4 sufficient macrophages protects TLR4-deficient mice against Pasteurella pneumotropica infection, suggesting an important role for macrophages in TLR4-mediated respiratory innate immunity (10). WT and TLR4-deficient BMMφ were exposed to live B. bronchiseptica at an MOI of 10 for 30 min or 1 h or to purified B. bronchiseptica LPS at a concentration of 10 ng/ml for 1 h. RNA was extracted, reversed transcribed, and analyzed by microarray. A complete set of array data is available for viewing at http://www.vetsci.psu.edu/personnel/faculty/harvill.cfm. LPS stimulation of WT BMMφ induced a global transcriptional response (Fig. 1A) that is similar in scope to what has been previously shown (7). In addition, 77% of the genes induced by LPS were also induced by live bacteria, whereas 59% of the genes induced by live bacteria were also induced by LPS. Therefore, there is considerable overlap in the responses to LPS and live bacteria (Fig. 1B). In TLR4-deficient cells, LPS induced no significant transcriptional response (Fig. 1A); however, the bacteria provoked a transcriptional response similar to that observed in WT cells, in that 83% of genes upregulated in WT cells were also induced in TLR4-deficient cells (Fig. 1B). This suggests that the majority of inflammatory genes expressed in response to B. bronchiseptica are not dependent on TLR4 expression but are likely induced by other PAMPs. Since TLR4 is apparently not required for the majority of the inflammatory response to this bacterium but is required to control infection, we searched for genes that were highly upregulated in WT, but not TLR4-deficient, cells. The microarray data was filtered to select genes that consistently were upregulated (≥4-fold), in response to live bacteria, in WT BMMΦ, but not in TLR4-deficient BMMφ. This process yielded 15 genes (Fig. 2), one of which was TNF-α, which is known to play an important role in regulating inflammatory processes (27, 32). Since our results suggest that the early elicited expression of TNF-α upon exposure to B. bronchiseptica is dependent on TLR4 and since the role of TNF-α in host defense during Bordetella infection has not been described, we chose to further investigate its role in limiting severe acute bordetellosis.
FIG. 1.
Display of the global transcriptional response of WT and TLR4d BMMΦ exposed to 10 ng of purified B. bronchiseptica LPS/ml for 1 h (A) and live B. bronchiseptica at an MOI of 10 for 1 h or 30 min (B). Red indicates an upregulated gene, whereas green indicates a downregulated gene. A complete set of array data is available at http://www.vetsci.psu.edu/personnel/faculty/harvill.cfm.
FIG. 2.
Fold induction of genes whose mean fold induction was 4 or greater in WT but not in TLR4-deficient BMMφ after 1 h of exposure to B. bronchiseptica. Bars: □, WT; ▪, TLR4 deficient.
TLR4 is required for the TNF-α response to B. bronchiseptica in BMMφ.
To determine whether the transcriptional response observed in microarray gene expression studies reflects differences in protein levels, we performed in vitro cell culture experiments and measured TNF-α protein levels by ELISA. WT and TLR4-deficient BMMφ were exposed to purified B. bronchiseptica LPS for 24 h at concentrations from 1 to 10,000 ng/ml, and the cell culture supernatants were then assayed for TNF-α. Upon exposure to LPS, WT BMMφ produced substantial amounts of TNF-α, whereas there was no detectable response from TLR4-deficient cells (Fig. 3A). Both WT and TLR4-deficient BMMφ secreted similar levels of TNF-α upon exposure to S. aureus peptidoglycan, a TLR2 agonist, indicating that TLR4-deficient cells are not universally deficient in TNF-α production. These results verify that TLR4 is required for the TNF-α response to purified B. bronchiseptica LPS. The fact that this bacterium is highly cytotoxic in vitro (40) prevents the use of large doses of live bacteria in cell culture experiments. In order to evaluate the requirement for TLR4 in the TNF-α response to increasing concentrations of B. bronchiseptica, we exposed both cell types to heat-killed bacteria at MOIs ranging from 0.1 to 1,000 (ca. 104 to 108 killed bacteria per well) for a 24-h period. WT cells produced TNF-α in response to MOIs as low as 0.1, whereas production of TNF-α by TLR4-deficient BMMφ was not detected at MOIs that were less than 1,000 (Fig. 3C). To evaluate the role of TLR4 in the TNF-α response to live B. bronchiseptica, we exposed WT and TLR4-deficient BMMφ to live bacteria at an MOI of ∼5 for 2 h before substantial cytotoxicity was observed. WT BMMφ produced a significant TNF-α response upon exposure to live bacteria, whereas there was no significant increase in the response produced by TLR4-deficient cells (Fig. 3B). Collectively, these results indicate that in vitro the early elicited TNF-α response to B. bronchiseptica is largely dependent on TLR4.
FIG. 3.
In vitro TNF-α response from BMMΦ at 24 h after exposure to the indicated concentrations (in nanograms/milliliter) of purified B. bronchiseptica LPS or 10 μg of Staphylococcus aureus PGN/ml (M = medium control) (A), at 24 h after exposure to the indicated MOIs of heat-killed B. bronchiseptica (Bb) (C), and at 2 h after exposed to live B. bronchiseptica at an MOI of ca. 5 (B). Bars: □, WT; ▪, TLR4 deficient (n = 3 to 4). The data are representative of three different experiments.
TLR4 is required for the early elicited TNF-α response to B. bronchiseptica in vivo.
To determine whether TLR4 is important in regulating the TNF-α response to B. bronchiseptica during respiratory tract infection, we inoculated WT and TLR4-deficient mice with ∼105 CFU. The mice were sacrificed at 2 h postinoculation, the lungs were excised and homogenized in PBS, and TNF-α levels were measured by ELISA. The lungs of WT mice, but not those of TLR4-deficient mice, inoculated with B. bronchiseptica contained significantly elevated TNF-α levels compared to lungs from sham-infected mice (Fig. 4A). To determine the period in which TNF-α was elevated in WT mice compared to TLR4-deficient mice, we measured TNF-α protein levels in the lungs at time points ranging from 30 min to 72 h postinoculation (Fig. 4B). The TNF-α levels in the lungs of WT mice were elevated as early as 30 min postinoculation, peaked at ca. 2 h, and remained elevated, compared to that of TLR4-deficient mice, for up to 12 h. There was no significant difference between WT and TLR4-deficient lung TNF-α levels at 24, 48, or 72 h postinoculation; however, these levels remained higher than that of sham-infected mice (data not shown), suggesting a TLR4-independent mechanism for TNF-α production at these time points. Similar differences in early TNF lung levels were observed in WT C57BL/10ScSn and TLR4-deficient C57BL/10ScNCR mice (data not shown), further supporting that the initial TNF-α response after infection occurs within the first 12 h and that this robust but transient response is largely dependent on TLR4.
FIG. 4.
(A) Lung TNF-α levels at 2 h postinoculation with 5 × 105 CFU of B. bronchiseptica (Inf) or a PBS control (sham) mice. (B) Lung TNF-α levels at 0.5, 1, 2, 6, 12, 24, 48, and 72 h following inoculation with 5 × 105 CFU of B. bronchiseptica. Symbols: □, WT; ▪, TLR4 deficient. n = 3 to 4 per group. The data are representative of 3 different experiments.
Neutralization of TNF-α during infection.
In order to determine whether TNF-α is required for protection during infection with B. bronchiseptica, we inoculated WT C3H/HeN mice with 5 × 105 CFU, as described above, and injected either 1 mg of monoclonal antibody MP6-XT3 (anti-TNF-α) in 1 ml of PBS or PBS alone at the time of infection. This treatment has previously been shown to neutralize serum and lung TNF-α for ca. 7 days (14), and we were able to confirm that this treatment reduced TNF-α levels in the lungs of infected mice to near that seen in sham-infected mice (data not shown). Mice treated with anti-TNF-α at the time of infection developed lethal bordetellosis within 96 h (Table 1). In order to determine the period in which TNF-α expression is important for innate host defense, we repeated this procedure but delayed the time of anti-TNF-α treatment for 0, 2, 6, 12, 24, 48, or 72 h after inoculation. Neutralization at the time of infection, or within 2 h, resulted in 100% of the mice developing lethal disease. Neutralization of TNF-α at 6 or 12 h postinoculation resulted in 25 or 12.5%, respectively, of the mice developing lethal bordetellosis. Neutralization at 24, 48, or 72 h postinoculation had no measurable impact on the survival of these animals. Interestingly, the time points at which neutralization of TNF-α affects disease outcome correlate with the time points at which the expression of TNF-α is dependent on TLR4 signaling, suggesting that the later TLR4-independent TNF-α expression is not required for innate host defense. However, it appears that the early TLR4-dependent TNF-α expression is critical for innate host defense during acute Bordetella respiratory infection.
TABLE 1.
Results of neutralization of TNF-α
| Time (h)a | % Lethal bordetellosisb | Time to death (days) | n |
|---|---|---|---|
| 0 | 100 | 3 | 14 |
| 2 | 100 | 3 | 10 |
| 6 | 25 | 4 | 8 |
| 12 | 12.5 | 4 | 8 |
| 24 | 0 | 6 | |
| 48 | 0 | 6 | |
| 72 | 0 | 6 |
That is, the time after infection of anti-TNF administration.
That is, the percentage of mice developing lethal bordetellosis.
That is, the time to death (in days) after inoculation.
Critical requirement for TLR4 and TNF-α during B. bronchiseptica infection.
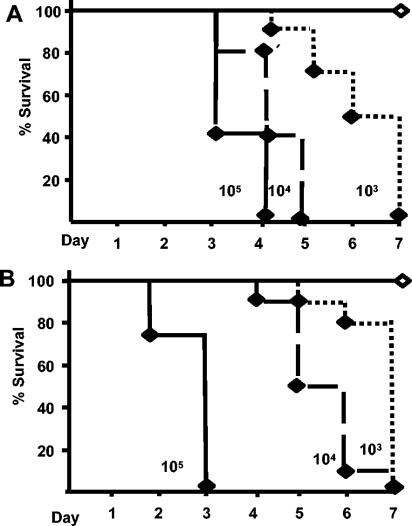
To compare the requirements for TLR4 and TNF-α in protecting against bordetellosis, we inoculated WT C3H/HeN, TLR4-deficient C3H/HeJ, and WT C57BL/6 and TNF-α−/− mice intranasally with ca. 5 × 105, 5 × 104, or 5 × 103 CFU of B. bronchiseptica in a volume of 50 μl of PBS. As expected, WT C3H/HeN and C57BL/6 mice infected with 5 × 105 CFU endured infection with no visible signs of distress (11). Consistent with earlier results (18), TLR4-deficient mice rapidly succumb to severe bordetellosis (Fig. 5A) after inoculation with as few as 5 × 103 CFU. Within 48 to 72 h, TNF-α−/− mice inoculated with 5 × 105 CFU also exhibited signs of extreme bordetellosis, including hunched backs, ruffled fur, inflamed external nares, and labored breathing. The health of these animals rapidly deteriorated and, as a result, they were all euthanized by day 3 postinoculation to prevent suffering (Fig. 5B). Initial doses as low as 5 × 103 CFU were also fatal to TNF-α−/− mice by day 7 postinoculation, whereas doses as high as 106 CFU are tolerated with few symptoms in WT mice (Fig. 5B and (11). The severe disease symptoms observed in TNF-α−/− mice were very similar to those of TLR4-deficient mice. These results indicate that both TLR4 and TNF-α are critical to innate host defense and suggest that both have important roles in preventing severe bordetellosis.
FIG. 5.
Survival curve of WT C3H and TLR4-deficient mice (A) or WT C57BL/6 and TNF-α−/− mice (B) inoculated with the indicated CFU of B. bronchiseptica. Symbols: ⋄, WT; ♦, TLR4 deficient and TNF-α−/−. n = 8 to 10 per group.
TLR4 and TNF-α deficiency result in severe suppurative pneumonia during B. bronchiseptica infection.
Since we had previously shown B. bronchiseptica infection in TLR4-deficient mice resulted in severe lung pathology characterized by suppurative bronchopneumonia (18), we sought to determine whether TNF-α−/− mice exhibited a similar phenotype. Mice were sacrificed 3 days postinoculation, and histological examination of the lungs was performed. The lungs of both strains of WT mice on day 3 postinoculation contained scattered moderate perivascular and peribronchiolar lymphoid cuffing with mixed inflammatory cell infiltrates (Fig. 6B). The mean lung pathology score of C57BL/6 WT mice was 2.1 on a 5 point scale, and in C3H/HeN mice it was 1.1 (Fig. 6A). The lungs of both TLR4-deficient and TNF-α−/− mice contained extensive perivascular and peribronchiolar lymphoid aggregates, consolidated areas of necrosis, and severe suppurative alveolar pneumonia with predominant neutrophilic infiltrates (Fig. 6B). Lungs of both TLR4-deficient and TNF-α−/− mice contained significantly higher numbers of lesions, as well as increased lesion severity, with mean lesion scores of 3.5 and 3.9, respectively (Fig. 6A). These results indicate that during acute infection with B. bronchiseptica, deficiencies in either TLR4 or TNF-α result in severe pneumonia characterized by an exaggerated neutrophil response and necrotic lung tissue damage.
FIG. 6.
(A) Lung pathology scores of H&E-stained lung sections on day 3 postinoculation with 5 × 105 CFU of B. bronchiseptica; (B) representative field of H&E-stained lung sections; (C) CFU of B. bronchiseptica recovered from the lungs of mice on day 3 postinoculation. n = 3 to 4 per group. The data are representative of three different experiments.
To establish whether increased lung pathology during B. bronchiseptica infection correlates with increased bacterial burden, we sacrificed TLR4-deficient C3H/HeJ, WT C3H/HeN, WT C3H/HeN mice treated with anti-TNF-α at the time of inoculation, as well as TNF-α−/− C57BL/6 and WT C57BL/6 mice, 3 days after inoculation with 5 × 105 CFU of B. bronchiseptica and measured lung colonization levels. The lungs of both strains of WT mice harbored ∼106 CFU on day 3 (Fig. 6C). The lungs of TLR4-deficient, TNF-α−/−, and anti-TNF-α C3H/HeN treated mice contained nearly 10,000-fold-higher bacterial numbers. Similar differences in lung bacterial burdens were observed in WT C57BL10/ScSn, TLR4-deficient C57BL/10ScNCr, and anti-TNF-α treated C57BL/10ScSn mice (data not shown). These results indicate that both TLR4 and TNF-α are required to control bacterial numbers during acute infection with B. bronchiseptica.
DISCUSSION
TLRs play a central role in the initiation of innate immune responses, as well as in the generation of adaptive immunity (1). Therefore, it has been hypothesized that the manipulation of TLR responses may be a prime target for therapies aimed at preventing acute disease, improving adaptive responses, and controlling inflammatory disorders (41). Since the discovery of tlr4 as the gene mutated in LPS-resistant mice, a role for TLR4 in innate host defense to certain gram-negative bacteria has been recognized (28, 30). We have previously demonstrated an essential requirement for TLR4 in innate host defense to B. bronchiseptica, a natural mouse pathogen, in that inoculation of TLR4-deficient mice with doses that closely mimic a natural infection results in the development of severe bordetellosis and lethal pneumonia (18). This presents an opportunity to investigate which TLR4 regulated immune functions are vital to innate host defense in the context of a natural host-pathogen model.
Due to the rapid development of severe and fatal disease in this model, we hypothesized that TLR4 regulates early elicited gene products that are vital to innate host defense against B. bronchiseptica. Since macrophages are believed to coordinate early inflammatory events, we sought to examine gene expression with this cell type. Using microarray analysis, we searched for gene products that were highly dependent on TLR4 shortly after exposure to this bacterium. To minimize the potential for secondary effects on gene expression, we examined the transcriptional response at 1 h after exposure to live B. bronchiseptica and its LPS. In WT macrophages, the majority of the genes induced by LPS were also induced by live bacteria. Interestingly, a small subset of LPS-induced genes were not upregulated in response to live bacteria, suggesting that the bordetellae may have a mechanism to inhibit certain host responses, a concept that is supported by previous array experiments (2). There was no significant transcriptional response to LPS detected in the absence of intact TLR4 signaling. However, the gene expression pattern induced by live bacteria in TLR4-deficient cells was similar in scope to that observed in WT cells. This would suggest that additional PAMPs expressed by B. bronchiseptica are able to induce gene expression, presumably via other TLRs. This conclusion is also supported by the observation that live bacteria induced a small subset of genes that were not induced by LPS. These findings are consistent with the results of previous studies that have shown that the gene expression patterns induced by different classes of pathogens, such as gram-positive and gram-negative bacteria, are largely overlapping (2, 21, 22). Interestingly, gram-negative bacteria generally induce more intense and longer lasting responses, potentially due to the increased complexity in TLR4 signaling, compared to other TLRs (2, 7, 21, 22). These results suggest specificity in innate immune responses to different classes of pathogens, an idea recently proposed by Netea et al. (23). This concept is further supported by the work of Weiss et al., which shows that there are temporal differences in the activation of TLR4 compared to TLR2 during infection (38). Interestingly, these previous studies did not examine how individual TLRs contribute to the overall gene expression patterns to bacterial infection. Therefore, we examined the role of TLR4 in the global transcriptional response to a gram-negative pathogen. Although the majority of the transcriptional response to B. bronchiseptica was not dependent on TLR4, we identified TNF-α as a potential contributor to the specificity of innate immune responses to this particular class of pathogens.
To determine whether the results of our gene array assays were relevant in an infection model, we performed more traditional experiments. In vitro assays measuring secreted protein levels confirmed that TLR4 is required for the TNF-α response to B. bronchiseptica. In vivo studies demonstrated that it is the early-elicited TNF-α response to B. bronchiseptica infection that is TLR4 dependent, whereas the TNF-α response at later time points is not. Since TNF-α is a well-known mediator of acute inflammatory responses, we sought to determine whether the early-elicited TLR4-dependent TNF-α response is critical to innate host defense against B. bronchiseptica. We neutralized TNF-α at the time of infection and at later time points. The results of these experiments indicate that neutralization of TNF-α at time points when its expression is TLR4 dependent resulted in the development of severe respiratory disease, a finding similar to that seen in TLR4-deficient mice. Although previous studies have used antibody-mediated neutralization as a method to demonstrate a critical role for TNF-α during pulmonary infection (16), here we explored the period during which TNF-α expression is critical. The results of the present study demonstrate that in this model it is the early-elicited TLR4-dependent TNF-α that is vital to innate host defense. This finding suggests that a major reason why TLR4-deficient mice are highly susceptible to B. bronchiseptica infection is an impaired early-elicited TNF-α response.
It has been hypothesized that a major role for TNF-α may be in augmenting LPS-induced inflammatory responses (5, 35, 39). Previous studies have shown that TLR4-deficient mice have impaired cytokine and chemokine responses to gram-negative bacteria. Interestingly, many of these observations were at time points significantly later than 2 h postinoculation. Our observation of a large transient peak in TNF-α production very early in infection suggests that a reason that TLR4-deficient mice show diminished cytokine and chemokine production during infection may be due in part to a diminished early TNF-α response. This is supported by studies which show that optimal IL-8 and MIP-2 responses after LPS exposure require TNF-α expression (5, 39). Wang et al., Higgins et al., and our own unpublished results suggest that TLR4-deficient mice exhibit impaired early neutrophil responses after gram-negative bacterial challenge in TLR4-deficient mice (12, 37). Correspondingly, TNF-α appears to be important for neutrophil responses to several microbial pathogens, as well as intratracheally instilled LPS (17, 31, 33). Doyle et al. have recently implicated TLRs in the induction of phagocytic gene programs and, similarly, a role for TLR4 in phagocytosis has also been described (6, 13) In addition, several studies have shown an association between diminished TNF-α levels and impaired phagocytosis (15, 24, 25). Collectively, these findings support the hypothesis that a critical role of TLR4 in host defense against gram-negative bacteria is the induction of an early robust TNF-α response which induces additional, or augments existing, host defense mechanisms. Further studies are needed to determine which immune functions are primarily dependent on TLR4 versus TNF-α, and current experiments are focused on investigating the contribution of TNF-α to the induction of gene expression patterns after exposure to B. bronchiseptica.
It is well recognized that, in humans and domestic animals, infants are at increased risk of severe bordetellosis; however, the reasons for this are not well understood. Our study suggests a possible explanation. Airway TLR expression in young mice is lower than that of adults (9); correspondingly, infant mice have been shown to produce lower TNF-α levels, during respiratory infection, in comparison to adults (29). These findings coupled with our results which show a critical role for TLR4 and TNF-α in preventing severe bordetellosis raise the possibility that diminished TNF-α responses may contribute to exacerbated disease in infants. Recently, Higgins et al. described a role for TLR4-dependent IL-10 in the development of T-regulatory-cell-mediated protection during B. pertussis infection (12). Their model shows a vastly different role for TLR4 than what we describe here. Whereas TLR4 appears to play a more important role in adaptive immunity to B. pertussis, it is vital to innate immunity during B. bronchiseptica infection. The reason for this disparity may be related to differential virulence factor expression between these two subspecies. However, our unpublished observations suggest that TNF-α is also critical to host defense against B. pertussis, as well as against B. bronchiseptica.
Acknowledgments
We thank G. Kirimanjeswara, M. Pilione, and A. Henderson for assistance in preparing the manuscript. A. Preston, University of Guelph, kindly provided the purified B. bronchiseptica LPS.
This study was supported by U.S. Department of Agriculture grant 2002-35204-11684 and NIH grant AI 053075. P.B.M. is supported by the U.S. Army Medical Service Corps LTHET program.
Editor: D. L. Burns
REFERENCES
- 1.Beutler, B., K. Hoebe, X. Du, and R. J. Ulevitch. 2003. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 74:479-485. [DOI] [PubMed] [Google Scholar]
- 2.Boldrick, J. C., A. A. Alizadeh, M. Diehn, S. Dudoit, C. L. Liu, C. E. Belcher, D. Botstein, L. M. Staudt, P. O. Brown, and D. A. Relman. 2002. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proc. Natl. Acad. Sci. USA 99:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapes, S. K., D. A. Mosier, A. D. Wright, and M. L. Hart. 2001. MHCII, Tlr4, and Nramp1 genes control host pulmonary resistance against the opportunistic bacterium Pasteurella pneumotropica. J. Leukoc. Biol. 69:381-386. [PubMed] [Google Scholar]
- 4.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 5.DeForge, L. E., J. S. Kenney, M. L. Jones, J. S. Warren, and D. G. Remick. 1992. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood: separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J. Immunol. 148:2133-2141. [PubMed] [Google Scholar]
- 6.Doyle, S. E., R. M. O'Connell, G. A. Miranda, S. A. Vaidya, E. K. Chow, P. T. Liu, S. Suzuki, N. Suzuki, R. L. Modlin, W. C. Yeh, T. F. Lane, and G. Cheng. 2004. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 199:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao, J. J., V. Diesl, T. Wittmann, D. C. Morrison, J. L. Ryan, S. N. Vogel, and M. T. Follettie. 2003. Bacterial LPS and CpG DNA differentially induce gene expression profiles in mouse macrophages. J. Endotoxin Res. 9:237-243. [DOI] [PubMed] [Google Scholar]
- 8.Gerber, J. S., and D. M. Mosser. 2001. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J. Immunol. 166:6861-6868. [DOI] [PubMed] [Google Scholar]
- 9.Harju, K., V. Glumoff, and M. Hallman. 2001. Ontogeny of Toll-like receptors Tlr2 and Tlr4 in mice. Pediatr. Res. 49:81-83. [DOI] [PubMed] [Google Scholar]
- 10.Hart, M. L., D. A. Mosier, and S. K. Chapes. 2003. Toll-like receptor 4-positive macrophages protect mice from Pasteurella pneumotropica-induced pneumonia. Infect. Immun. 71:663-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 67:6109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins, S. C., E. C. Lavelle, C. McCann, B. Keogh, E. McNeela, P. Byrne, B. O'Gorman, A. Jarnicki, P. McGuirk, and K. H. Mills. 2003. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J. Immunol. 171:3119-3127. [DOI] [PubMed] [Google Scholar]
- 13.Honstettre, A., E. Ghigo, A. Moynault, C. Capo, R. Toman, S. Akira, O. Takeuchi, H. Lepidi, D. Raoult, and J. L. Mege. 2004. Lipopolysaccharide from Coxiella burnetii is involved in bacterial phagocytosis, filamentous actin reorganization, and inflammatory responses through Toll-like receptor 4. J. Immunol. 172:3695-3703. [DOI] [PubMed] [Google Scholar]
- 14.Huffnagle, G. B., G. B. Toews, M. D. Burdick, M. B. Boyd, K. S. McAllister, R. A. McDonald, S. L. Kunkel, and R. M. Strieter. 1996. Afferent phase production of TNF-α is required for the development of protective T-cell immunity to Cryptococcus neoformans. J. Immunol. 157:4529-4536. [PubMed] [Google Scholar]
- 15.Hultgren, O., H. P. Eugster, J. D. Sedgwick, H. Korner, and A. Tarkowski. 1998. TNF/lymphotoxin-alpha double-mutant mice resist septic arthritis but display increased mortality in response to Staphylococcus aureus. J. Immunol. 161:5937-5942. [PubMed] [Google Scholar]
- 16.Laichalk, L. L., S. L. Kunkel, R. M. Strieter, J. M. Danforth, M. B. Bailie, and T. J. Standiford. 1996. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect. Immun. 64:5211-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malaviya, R., T. Ikeda, E. Ross, and S. N. Abraham. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature 381:77-80. [DOI] [PubMed] [Google Scholar]
- 18.Mann, P. B., M. J. Kennett, and E. T. Harvill. 2004. Toll-like receptor 4 is critical to innate host defense in a murine model of bordetellosis. J. Infect. Dis. 189:833-836. [DOI] [PubMed] [Google Scholar]
- 19.Medzhitov, R., and C. Janeway, Jr. 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 8:452-456. [DOI] [PubMed] [Google Scholar]
- 20.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 21.Nau, G. J., J. F. Richmond, A. Schlesinger, E. G. Jennings, E. S. Lander, and R. A. Young. 2002. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA 99:1503-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nau, G. J., A. Schlesinger, J. F. Richmond, and R. A. Young. 2003. Cumulative Toll-like receptor activation in human macrophages treated with whole bacteria. J. Immunol. 170:5203-5209. [DOI] [PubMed] [Google Scholar]
- 23.Netea, M. G., C. Van Der Graaf, J. W. Van Der Meer, and B. Jan-Kullberg. 2004. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J. Leukoc. Biol. 75:749-755. [DOI] [PubMed]
- 24.Netea, M. G., L. J. van Tits, J. H. Curfs, F. Amiot, J. F. Meis, J. W. van der Meer, and B. J. Kullberg. 1999. Increased susceptibility of TNF-alpha lymphotoxin-alpha double knockout mice to systemic candidiasis through impaired recruitment of neutrophils and phagocytosis of Candida albicans. J. Immunol. 163:1498-1505. [PubMed] [Google Scholar]
- 25.Ojielo, C. I., K. Cooke, P. Mancuso, T. J. Standiford, K. M. Olkiewicz, S. Clouthier, L. Corrion, M. N. Ballinger, G. B. Toews, R. Paine III, and B. B. Moore. 2003. Defective phagocytosis and clearance of Pseudomonas aeruginosa in the lung following bone marrow transplantation. J. Immunol. 171:4416-4424. [DOI] [PubMed] [Google Scholar]
- 26.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis, and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer, K. 2003. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 14:185-191. [DOI] [PubMed] [Google Scholar]
- 28.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 29.Qureshi, M. H., J. Cook-Mills, D. E. Doherty, and B. A. Garvy. 2003. TNF-α-dependent ICAM-1- and VCAM-1-mediated inflammatory responses are delayed in neonatal mice infected with Pneumocystis carinii. J. Immunol. 171:4700-4707. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi, S. T., and R. Medzhitov. 2003. Toll-like receptors and their role in experimental models of microbial infection. Genes Immun. 4:87-94. [DOI] [PubMed] [Google Scholar]
- 31.Schelenz, S., D. A. Smith, and G. J. Bancroft. 1999. Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus: obligatory role of TNF-alpha and GM-CSF in neutrophil recruitment. Med. Mycol. 37:183-194. [DOI] [PubMed] [Google Scholar]
- 32.Schluter, D., and M. Deckert. 2000. The divergent role of tumor necrosis factor receptors in infectious diseases. Microbes Infect. 2:1285-1292. [DOI] [PubMed] [Google Scholar]
- 33.Skerrett, S. J., T. R. Martin, E. Y. Chi, J. J. Peschon, K. M. Mohler, and C. B. Wilson. 1999. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am. J. Physiol. 276:L715-L727. [DOI] [PubMed] [Google Scholar]
- 34.Tien, E. S., J. P. Gray, J. M. Peters, and J. P. Vanden Heuvel. 2003. Comprehensive gene expression analysis of peroxisome proliferator-treated immortalized hepatocytes: identification of peroxisome proliferator-activated receptor alpha-dependent growth regulatory genes. Cancer Res. 63:5767-5780. [PubMed] [Google Scholar]
- 35.Ulich, T. R., L. R. Watson, S. M. Yin, K. Z. Guo, P. Wang, H. Thang, and J. del Castillo. 1991. The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am. J. Pathol. 138:1485-1496. [PMC free article] [PubMed] [Google Scholar]
- 36.van der Zee, A., F. Mooi, J. Van Embden, and J. Musser. 1997. Molecular evolution and host adaptation of Bordetella spp.: phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. J. Bacteriol. 179:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, X., C. Moser, J. P. Louboutin, E. S. Lysenko, D. J. Weiner, J. N. Weiser, and J. M. Wilson. 2002. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J. Immunol. 168:810-815. [DOI] [PubMed] [Google Scholar]
- 38.Weiss, D. S., B. Raupach, K. Takeda, S. Akira, and A. Zychlinsky. 2004. Toll-like receptors are temporally involved in host defense. J. Immunol. 172:4463-4469. [DOI] [PubMed] [Google Scholar]
- 39.Xavier, A. M., N. Isowa, L. Cai, E. Dziak, M. Opas, D. I. McRitchie, A. S. Slutsky, S. H. Keshavjee, and M. Liu. 1999. Tumor necrosis factor-alpha mediates lipopolysaccharide-induced macrophage inflammatory protein-2 release from alveolar epithelial cells: autoregulation in host defense. Am. J. Respir. Cell Mol. Biol. 21:510-520. [DOI] [PubMed] [Google Scholar]
- 40.Yuk, M. H., E. T. Harvill, and J. F. Miller. 1998. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol. Microbiol. 28:945-959. [DOI] [PubMed] [Google Scholar]
- 41.Zuany-Amorim, C., J. Hastewell, and C. Walker. 2002. Toll-like receptors as potential therapeutic targets for multiple diseases. Nat. Rev. Drug Discov. 1:797-807. [DOI] [PubMed] [Google Scholar]