Abstract
The pathogens Streptococcus pyogenes and Moraxella catarrhalis colonize overlapping regions of the human nasopharynx. We have found that M. catarrhalis can dramatically increase S. pyogenes adherence to human epithelial cells and that species-specific coaggregation of these bacteria correlates with this enhanced adherence.
In most environments, the interaction of individual microbes with other microbial species can have a profound effect on the colonization, persistence, and survival of the microorganisms. The importance of polymicrobial interactions has also been documented in human and animal diseases (5). In the case of periodontal disease, the binding or coaggregation of Porphyromonas gingivalis to Streptococcus gordonii enhances the colonization of the tooth surface by P. gingivalis (19). This coaggregation is complex and is mediated by at least two adhesin-receptor pairs (9, 19). Although much is known about polymicrobial interactions in the human colon and oral cavity, little work has been done investigating polymicrobial interactions among bacteria that colonize or infect the human nasopharynx.
As a model for possible polymicrobial interactions in the human nasopharynx, we investigated interactions between Streptococcus pyogenes and Moraxella catarrhalis for several reasons. First, S. pyogenes and M. catarrhalis are human-specific pathogens that colonize the nasopharynx, causing a variety of diseases (4, 7, 8, 13, 17, 21, 23, 28, 33). Their carriage rates among asymptomatic individuals can be very high, and the adherence of both organisms to epithelial cells is critical for their pathogenesis and they adhere to the same human epithelial cell lines (11, 15, 16, 22, 31).
M. catarrhalis increases S. pyogenes adherence to human epithelial cells.
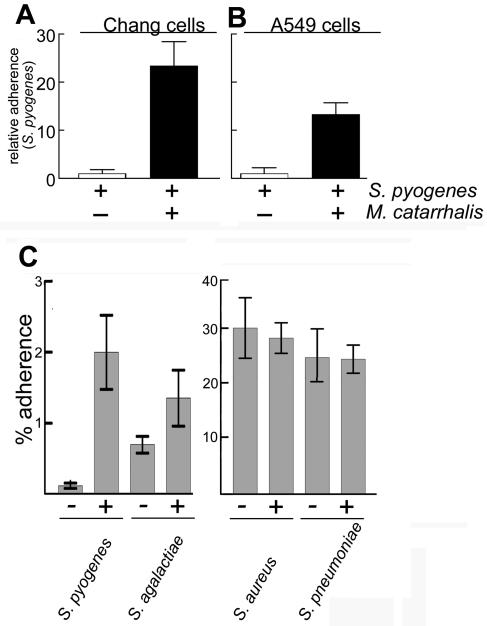
To assess whether S. pyogenes (strain 1881, serotype M1) (Table 1) and M. catarrhalis (strain 035E) interact, we measured their adherence to A549 (lung) and Chang (conjunctival) human epithelial cells alone or in combination with a quantitative adherence assay that we previously described (18). The presence of S. pyogenes had a small (less-than-threefold) negative influence on the binding of M. catarrhalis to both cell lines. However, M. catarrhalis substantially increased S. pyogenes adherence to Chang cells (22-fold) and to A549 cells (15-fold) (Fig. 1A and B). In contrast, a nonadherent Escherichia coli strain had no effect on S. pyogenes adherence. M. catarrhalis had a modest effect (threefold) on the adherence of the closely related species S. agalactiae. In addition, we observed that the adherence of two other gram-positive cocci (S. aureus and S. pneumoniae) was not significantly affected by M. catarrhalis (Fig. 1C). Thus, the dramatic effect of M. catarrhalis on S. pyogenes adherence is not shared by other gram-positive human pathogens.
TABLE 1.
Bacterial strains used in this study
| Strain | Description | Source or reference(s) |
|---|---|---|
| M. catarrhalis O35E | Wild type | 2 |
| M. catarrhalis O35E hag (O35E.TN2) | Adhesin mutant; decreased adherence to A549 cells | 16 |
| M. catarrhalis O35E uspA1 (O35E.1) | Adhesin mutant; decreased adherence to Chang cells | 1 |
| M. catarrhalis O35E uspA2 | Decreased serum resistance | 1 |
| S. pyogenes 1881 | Opacity factor (−); emm1 | 27 |
| S. pyogenes 90-226 | Opacity factor (−); emm1 | 10, 12 |
| S. pyogenes SF370 | Opacity factor (−); emm1 | 30 |
| S. pyogenes CS101 | Opacity factor (+); emm49 | 25 |
| S. aureus RN6390 | Wild type | 24 |
| S. agalactiae | Wild type | Laboratory stock |
| S. pneumoniae | Wild type | 20 |
FIG. 1.
M. catarrhalis specifically enhances the binding of S. pyogenes to human cells. S. pyogenes, S. agalactiae, S. pneumoniae, or S. aureus (∼2 × 107 CFU) was incubated with human cells alone or mixed with M. catarrhalis (∼2 × 107 CFU). Results represent the mean of two to four independent experiments done in duplicate. Error bars represent the standard deviations. (A and B) M. catarrhalis enhanced S. pyogenes binding to A549 and Chang cells (closed bars) >15-fold compared to S. pyogenes alone (open bars). (C). M. catarrhalis had no effect on S. agalactiae, S. pneumoniae, or S. aureus adherence to A549 cells.
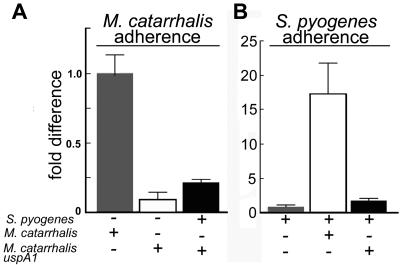
Microscopic examination of M. catarrhalis and S. pyogenes bound to human cells suggested that the bacteria were colocalized on the eukaryotic cell surface (data not shown). We therefore hypothesized that M. catarrhalis was acting as an adapter, binding to both human cells and S. pyogenes. To test this, we measured the ability of an M. catarrhalis adherence-negative uspA1 mutant to modulate the binding of S. pyogenes to Chang cells. As previously reported (18), the uspA1 mutation decreased the binding of M. catarrhalis to Chang cells 10-fold (Fig. 2A). When S. pyogenes was coinfected with the M. catarrhalis wild-type strain, S. pyogenes adherence was increased by 18-fold (Fig. 2B) while coinfection of monolayers with the M. catarrhalis uspA1 mutant increased S. pyogenes adherence only 2-fold (Fig. 2B). Similar results were seen following coinfection of A549 cells with an adherence-negative M. catarrhalis hag mutant that was recently demonstrated to be a major M. catarrhalis adhesin for A549 cells (data not shown) (16).
FIG. 2.
The M. catarrhalis adhesin UspA1 was necessary for enhanced adherence to Chang cells. S. pyogenes (∼2 × 107 CFU) and M. catarrhalis (∼2 × 107 CFU) were incubated with Chang cells either alone or mixed as described in Materials and Methods. Results represent the mean of two to four independent experiments done in duplicate. Error bars represent the standard deviations. (A) The adherence of a uspA1 mutant of M. catarrhalis to Chang cells (open bar) was only 10% of that of the isogenic wild-type strain (gray bar), as previously reported (1). Coinfection with S. pyogenes had a small effect on the adherence of the M. catarrhalis uspA1 mutant (closed bar). (B) Wild-type M. catarrhalis enhanced the adherence of S. pyogenes 18-fold (gray bar versus open bar). The M. catarrhalis uspA1 mutant enhanced the adherence of S. pyogenes only twofold (closed bar).
M. catarrhalis and S. pyogenes form coaggregates.
To test whether these bacteria directly interact, we developed a quantitative assay to measure their coaggregation. First, bacterial cell surfaces were labeled with biotin with the membrane-impermeable biotin derivative sulfo-NHS-LC-biotin (EZ-Link; Pierce, Rockford, Ill.). Freshly prepared 1.7 mM sulfo-NHS-LC-biotin solution in water (0.5 ml) was added to plate-grown bacteria (108 CFU in 1 ml of PBSG [11.9 mM phosphate, 137 mM NaCl, 2.7 mM KCl, 0.15% gelatin type B from bovine skin, pH 7.4]), and the cells were incubated at room temperature for 30 min. Excess biotinylation reagent was removed by four washes with PBSG.
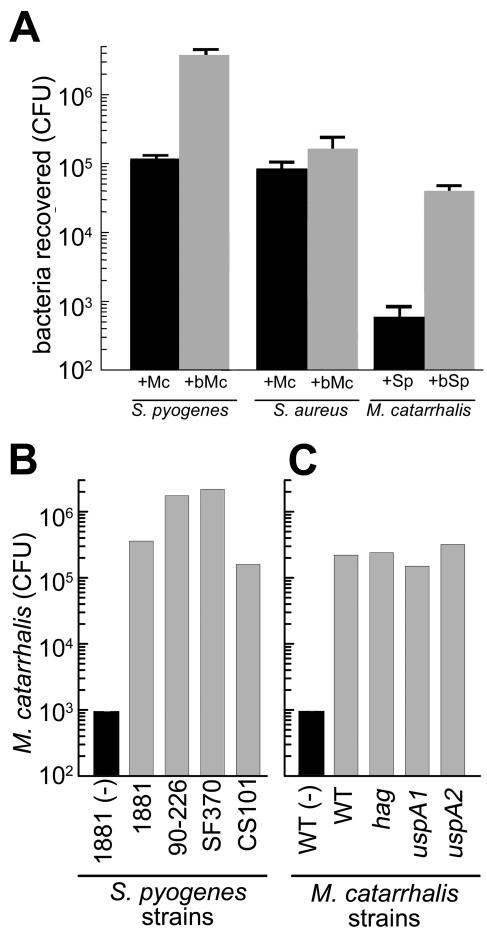
To measure coaggregation, biotinylated (107 CFU) or nonbiotinylated M. catarrhalis cells were mixed with streptavidin-magnetic beads (10 μl of a 50% slurry; Cortex Biochem, San Leandro, Calif.) and incubated with gentle agitation for 30 min at 37°C in microcentrifuge tubes. The tubes were placed in a magnetic separator (Cortex Biochem), and after 30 s, the supernatant was aspirated and the beads were resuspended in 1 ml of PBSG. The beads were washed four more times and resuspended in 1 ml of PBSG. Unlabeled S. pyogenes was mixed with M. catarrhalis-magnetic beads and incubated for 30 min. Unbound bacteria were removed by five rounds of washing (changing tubes each time), and bound bacteria were enumerated by serial dilution and outgrowth on selective medium. We recovered (3.8 ± 0.72) × 106 S. pyogenes CFU when using biotinylated M. catarrhalis cells (Fig. 3A, +bMc). In contrast, about 40-fold less S. pyogenes [(1.0 ± 0.17) × 105 CFU] was recovered when the cells were mixed with unlabeled M. catarrhalis (Fig. 3A, +Mc). The reverse experiment, with biotinylated S. pyogenes and measurement of M. catarrhalis recovery, gave a comparable result (Fig. 3A). These results are not due to growth effects during the outgrowth step since similar results were obtained when bacterial numbers were directly quantitated by quantitative real-time PCR and an S. pyogenes-specific probe (29) (data not shown). Coaggregation was not due to nonspecific clumping of the bacteria since Staphylococcus aureus was not copurified with biotinylated M. catarrhalis (Fig. 3A). It is worth noting that neither S. pyogenes nor M. catarrhalis coaggregated with biotinylated E. coli (data not shown), ruling out the possibility of a surface avidin-like protein mediating the coaggregation.
FIG. 3.
Quantitation of M. catarrhalis and S. pyogenes coaggregation. (A) biotinylated (+bMc, gray bar) or nonbiotinylated (+M, dark bar) M. catarrhalis was incubated with S. pyogenes or S. aureus. Nonbiotinylated S. pyogenes (+Sp, dark bar) or biotinylated S. pyogenes (+bSp, gray bar) was incubated with M. catarrhalis. Avidin-conjugated magnetic beads were added, and biotinylated bacteria were purified by extensive washing on a magnetic separator. Purified aggregates were serially diluted and plated on appropriate media. The bars represent CFU of the nonbiotinylated bacteria recovered after purification. Error bars represent the standard deviation of three independent experiments. In each case, the difference between biotinylated and nonbiotinylated bacteria was significant (P < 0.001) as determined by Student's t test. More than 30-fold more S. pyogenes was recovered with the streptavidin-magnetic beads in the presence of biotinylated M. catarrhalis (gray bars) than in the presence of nonbiotinylated M. catarrhalis (dark bars). No enhancement of binding was observed with M. catarrhalis and S. aureus (data not shown). Biotinylated S. pyogenes (gray bar) was also able to enhance the recovery of nonbiotinylated M. catarrhalis (dark bar) by 100-fold. (B) S. pyogenes strains (gray bars) were biotinylated and incubated with M. catarrhalis O35E, and coaggregation was measured as described above. M. catarrhalis was also incubated with beads in the absence of S. pyogenes (dark bar). M. catarrhalis coaggregated with each of the S. pyogenes strains. (C) Wild-type (WT) and adhesin mutant M. catarrhalis O35E bacteria were incubated with biotinylated S. pyogenes 1881, and coaggregation was measured as described above. Wild-type M. catarrhalis O35E was also incubated with beads in the absence of S. pyogenes (dark bar). All of the M. catarrhalis adhesin mutants coaggregated with S. pyogenes, suggesting that these adhesin proteins do not mediate coaggregation of these bacteria.
M. catarrhalis also coaggregated with three other S. pyogenes strains (serotype M1 strains SF370 and 90-226 [10, 12, 30] and serotype M49 strain CS101 [25]), suggesting that coaggregation with M. catarrhalis is widespread among S. pyogenes strains (Fig. 3B).
To test whether the M. catarrhalis adhesins necessary for increasing S. pyogenes adherence (Fig. 2) are also necessary for coaggregation, we used the coaggregation assay to measure the binding of M. catarrhalis hag, uspA1, and uspA2 mutants to biotinylated S. pyogenes. These mutants bound as well as wild-type M. catarrhalis (100- to 1,000-fold increased recovery) to biotinylated S. pyogenes (Fig. 3C). This supports our hypothesis that M. catarrhalis acts as an adapter that can mediate S. pyogenes adherence to human cells. In addition, these results indicate that the M. catarrhalis surface molecules UspA1, UspA2, and Hag do not mediate coaggregation with S. pyogenes.
Killed M. catarrhalis coaggregates with S. pyogenes.
To further investigate the M. catarrhalis modulation of S. pyogenes adherence, we tested whether heat-killed (60°C for 10 min) or formalin-killed (10% formalin in PBSG for 10 min, followed by extensive washing) M. catarrhalis eliminates this effect. Heat-killed M. catarrhalis no longer coaggregated with S. pyogenes or enhanced its adherence to A549 cells (Fig. 4). Microscopic examination showed few heat-killed M. catarrhalis bacteria bound to lung cells (data not shown), suggesting that the M. catarrhalis molecule(s) involved in this process was heat labile. Formalin-killed M. catarrhalis cells coaggregated with S. pyogenes and enhanced adherence to levels similar to those observed with viable M. catarrhalis (Fig. 4). Thus, neither de novo protein synthesis by M. catarrhalis nor a soluble factor produced by M. catarrhalis was necessary for enhancing S. pyogenes adherence or coaggregation.
FIG. 4.
Effects of heat and formalin on the coaggregation and coadherence of M. catarrhalis and S. pyogenes. S. pyogenes (Sp) was incubated alone or with biotinylated M. catarrhalis (bMc), heat-killed biotinylated M. catarrhalis, or formalin-killed, biotinylated M. catarrhalis. Cells were washed, and half of the cells were purified with streptavidin- magnetic beads and the other half were used in an adherence assay with A549 cells as described in the text. (A) S. pyogenes coaggregated with the live and formalin-killed, biotinylated M. catarrhalis cells but not with the heat-killed, biotinylated M. catarrhalis cells. (B) S. pyogenes adherence to A549 cells was also enhanced by the live and formalin-killed biotinylated M. catarrhalis cells but not by the heat killed, biotinylated M. catarrhalis cells. Strep., streptococcal.
M. catarrhalis inhibits streptococcal invasion of human epithelial cells.
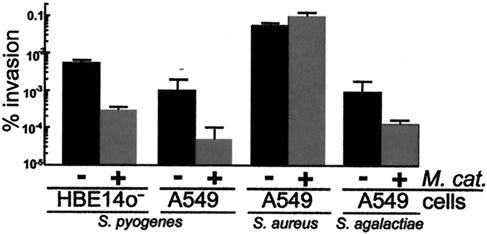
Since S. pyogenes is also an invasive pathogen (10, 12) and adherence is a necessary first step for invasion, we tested whether M. catarrhalis affects S. pyogenes invasion. With a gentamicin resistance invasion assay (26), we found that invasion of A549 cells by S. pyogenes was reduced 25-fold in the presence of M. catarrhalis (Fig. 5). Similar results were obtained with a human bronchial epithelial cell line that forms polarized monolayers (16HBE14o−), which are more similar to in vivo epithelial layers (31, 32) (Fig. 5). M. catarrhalis was not acting as a general inhibitor of invasion, since it had no effect on S. aureus invasion (Fig. 5). Interestingly, M. catarrhalis also decreased S. agalactiae invasion of A549 cells by 10-fold. Since M. catarrhalis has only a small effect on S. agalactiae adherence (Fig. 1), our results imply that the effects of M. catarrhalis on the invasion of S. agalactiae and S. pyogenes was at least partly independent of its ability to coaggregate.
FIG. 5.
M. catarrhalis inhibited S. pyogenes and S. agalactiae invasion of human epithelial cells. Human cell lines 16HBE14o− (polarized bronchial epithelial cells) and A549 (lung epithelial cells) were incubated with S. pyogenes, S. aureus, or S. agalactiae alone (light gray bar) or with M. catarrhalis (∼2 × 107 CFU/ml, dark bar). M. catarrhalis inhibited S. pyogenes (25- to 28-fold) and S. agalactiae (10-fold) invasion but not S. aureus invasion. None of the bacteria tested had a significant effect on the adherence or invasion of M. catarrhalis. Bar height is the mean of two or three independent experiments, each done in duplicate. Error bars represent the standard deviation.
Summary.
In this paper, we have shown that M. catarrhalis has striking effects on S. pyogenes adherence to and invasion of human epithelial cell lines. This remarkable enhancement was specific and widespread among S. pyogenes strains. Our results are consistent with the hypothesis that M. catarrhalis and S. pyogenes form coaggregates and that these coaggregates bind to human cells via M. catarrhalis adhesins. Together, our data suggest that prior colonization by M. catarrhalis could have a profound effect on the binding to, as well as the invasion of, mucosal surfaces by S. pyogenes. Since there are no reports of M. catarrhalis-S. pyogenes coinfections, we instead hypothesize that colonization by M. catarrhalis could increase the probability of colonization by S. pyogenes or the progression of disease. Because more than 90% of clinical isolates of M. catarrhalis produce β-lactamases (14), another significant issue is the possibility that in coaggregates S. pyogenes is more resistant to β-lactam antibiotics, the recommended antimicrobial treatment for S. pyogenes infections (3). This possibility is supported by the observation that M. catarrhalis increased the resistance of S. pneumoniae to β-lactam antibiotics in continuous-biofilm studies (6). Furthermore, because M. catarrhalis appears to inhibit streptococcal invasion in vitro, prior colonization by M. catarrhalis might promote an S. pyogenes surface infection rather than a deep-tissue, invasive disease. Thus, coaggregation with M. catarrhalis is one of many factors that could contribute to the pathogenicity of S. pyogenes.
In addition to the potential clinical consequences of this coaggregation and coadhesion, these results highlight the potential importance of microbial interactions among bacteria in the nasopharynx. We are currently identifying the bacterial molecules that mediate this polymicrobial interaction to better understand the mechanisms involved.
Acknowledgments
We thank R. Blumenthal and R. Mark Wooten for reading and providing helpful comments on the manuscript. We also thank Sarah Coffman for performance of some of the coadherence assays. We also thank D. Prinz and M. Rezcallah for the gifts of some of the S. pyogenes and S. pneumoniae strains used.
The American Heart Association and The Ohio Board of Regents (D.D.S.) and the National Institutes of Health (E.R.L.) supported this work.
Editor: D. L. Burns
REFERENCES
- 1.Aebi, C., E. R. Lafontaine, L. D. Cope, J. L. Latimer, S. L. Lumbley, G. H. McCracken, Jr., and E. J. Hansen. 1998. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect. Immun. 66:3113-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi, C., I. Maciver, J. L. Latimer, L. D. Cope, M. K. Stevens, S. E. Thomas, G. H. McCracken, Jr., and E. J. Hansen. 1997. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect. Immun. 65:4367-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisno, A. L., M. A. Gerber, J. M. Gwaltney, Jr., E. L. Kaplan, and R. H. Schwartz. 2002. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin. Infect. Dis. 35:113-125. [DOI] [PubMed] [Google Scholar]
- 4.Bisno, A. L., and D. L. Stevens. 1996. Streptococcal infections of skin and soft tissues. N. Engl. J. Med. 334:240-245. [DOI] [PubMed] [Google Scholar]
- 5.Brogden, K. A., and J. M. Guthmiller (ed.). 2002. Polymicrobial diseases. ASM Press, Washington, D.C. [PubMed]
- 6.Budhani, R. K., and J. K. Struthers. 1998. Interaction of Streptococcus pneumoniae and Moraxella catarrhalis: investigation of the indirect pathogenic role of beta-lactamase-producing moraxellae by use of a continuous-culture biofilm system. Antimicrob. Agents Chemother. 42:2521-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaussee, M. S., J. Liu, D. L. Stevens, and J. J. Ferretti. 1996. Genetic and phenotypic diversity among isolates of Streptococcus pyogenes from invasive infections. J. Infect. Dis. 173:901-908. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, J. J. 1999. Moraxella (Branhamella) catarrhalis: clinical, microbiological and immunological features in lower respiratory tract infections. APMIS Suppl. 88:1-36. [PubMed] [Google Scholar]
- 9.Chung, W. O., D. R. Demuth, and R. J. Lamont. 2000. Identification of a Porphyromonas gingivalis receptor for the Streptococcus gordonii SspB protein. Infect. Immun. 68:6758-6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleary, P. P., and D. Cue. 2000. High frequency invasion of mammalian cells by beta hemolytic streptococci. Subcell. Biochem. 33:137-166. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dombek, P. E., D. Cue, J. Sedgewick, H. Lam, S. Ruschkowski, B. B. Finlay, and P. P. Cleary. 1999. High-frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol. Microbiol. 31:859-870. [DOI] [PubMed] [Google Scholar]
- 13.Faden, H. 2001. The microbiologic and immunologic basis for recurrent otitis media in children. Eur. J. Pediatr. 160:407-413. [DOI] [PubMed] [Google Scholar]
- 14.Fung, C. P., S. F. Yeo, and D. M. Livermore. 1994. Susceptibility of Moraxella catarrhalis isolates to beta-lactam antibiotics in relation to beta-lactamase pattern. J. Antimicrob. Chemother. 33:215-222. [DOI] [PubMed] [Google Scholar]
- 15.Holm, M. M., S. L. Vanlerberg, I. M. Foley, D. D. Sledjeski, and E. R. Lafontaine. 2004. The Moraxella catarrhalis porin-like outer membrane protein CD is an adhesin for human lung cells. Infect. Immun. 72:1906-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holm, M. M., S. L. Vanlerberg, D. D. Sledjeski, and E. R. Lafontaine. 2003. The Hag protein of Moraxella catarrhalis strain O35E is associated with adherence to human lung and middle ear cells. Infect. Immun. 71:4977-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karalus, R., and A. Campagnari. 2000. Moraxella catarrhalis: a review of an important human mucosal pathogen. Microbes Infect. 2:547-559. [DOI] [PubMed] [Google Scholar]
- 18.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamont, R. J., A. El-Sabaeny, Y. Park, G. S. Cook, J. W. Costerton, and D. R. Demuth. 2002. Role of the Streptococcus gordonii SspB protein in the development of Porphyromonas gingivalis biofilms on streptococcal substrates. Microbiology 148:1627-1636. [DOI] [PubMed] [Google Scholar]
- 20.Lesinski, G. B., S. L. Smithson, N. Srivastava, D. Chen, G. Widera, and M. A. Westerink. 2001. A DNA vaccine encoding a peptide mimic of Streptococcus pneumoniae serotype 4 capsular polysaccharide induces specific anti-carbohydrate antibodies in Balb/c mice. Vaccine 19:1717-1726. [DOI] [PubMed] [Google Scholar]
- 21.Martin, D. R., and L. A. Single. 1993. Molecular epidemiology of group A streptococcus M type 1 infections. J. Infect. Dis. 167:1112-1117. [DOI] [PubMed] [Google Scholar]
- 22.Meier, P. S., R. Troller, I. N. Grivea, G. A. Syrogiannopoulos, and C. Aebi. 2002. The outer membrane proteins UspA1 and UspA2 of Moraxella catarrhalis are highly conserved in nasopharyngeal isolates from young children. Vaccine 20:1754-1760. [DOI] [PubMed] [Google Scholar]
- 23.Murphy, T. F. 1996. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol. Rev. 60:267-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novick, R. P., H. F. Ross, S. J. Projan, J. Kornblum, B. Kreiswirth, and S. Moghazeh. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Podbielski, A., A. Flosdorff, and J. Weber-Heynemann. 1995. The group A streptococcal virR49 gene controls expression of four structural vir regulon genes. Infect. Immun. 63:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podbielski, A., M. Woischnik, B. A. Leonard, and K. H. Schmidt. 1999. Characterization of nra, a global negative regulator gene in group A streptococci. Mol. Microbiol. 31:1051-1064. [DOI] [PubMed] [Google Scholar]
- 27.Rezcallah, M. S., M. D. Boyle, and D. D. Sledjeski. 2004. Mouse skin passage of Streptococcus pyogenes results in increased streptokinase expression and activity. Microbiology 150:365-371. [DOI] [PubMed] [Google Scholar]
- 28.Schlievert, P. M., A. P. Assimacopoulos, and P. P. Cleary. 1996. Severe invasive group A streptococcal disease: clinical description and mechanisms of pathogenesis. J. Lab. Clin. Med. 127:13-22. [DOI] [PubMed] [Google Scholar]
- 29.Smith, T. C., D. D. Sledjeski, and M. D. Boyle. 2003. Regulation of protein H expression in M1 serotype isolates of Streptococcus pyogenes. FEMS Microbiol. Lett. 219:9-15. [DOI] [PubMed] [Google Scholar]
- 30.Suvorov, A. N., and J. J. Ferretti. 1996. Physical and genetic chromosomal map of an M type 1 strain of Streptococcus pyogenes. J. Bacteriol. 178:5546-5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timpe, J. M., M. M. Holm, S. L. Vanlerberg, V. Basrur, and E. R. Lafontaine. 2003. Identification of a Moraxella catarrhalis outer membrane protein exhibiting both adhesin and lipolytic activities. Infect. Immun. 71:4341-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan, H., H. L. Winton, C. Soeller, G. A. Stewart, P. J. Thompson, D. C. Gruenert, M. B. Cannell, D. R. Garrod, and C. Robinson. 2000. Tight junction properties of the immortalized human bronchial epithelial cell lines Calu-3 and 16HBE14o. Eur. Respir. J. 15:1058-1068. [DOI] [PubMed] [Google Scholar]
- 33.Wessels, M. R., A. E. Moses, J. B. Goldberg, and T. J. DiCesare. 1991. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc. Natl. Acad. Sci. USA 88:8317-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]