Abstract
The pathogenicity of enteropathogenic Escherichia coli (EPEC) is linked to the locus of enterocyte effacement, or LEE, encoding a type III secretion system (T3SS) that directly transfers bacterial effector proteins into eukaryotic cells. Atypical diffusely adhering EPEC (DA-EPEC) strains that harbor homologues of the LEE but lack the EPEC adherence factor plasmid have been increasingly associated with outbreaks of diarrhea. In this study, we have completely sequenced and functionally characterized LEE pathogenicity islands derived from the clinical DA-EPEC isolates 3431 (O8:H−) and 0181 (O119:H9:K61). LEE3431 and LEE0181 exhibit genetic organization analogous to that of the prototype LEEE2348/69. Genes constituting the T3SS apparatus are highly conserved. However, LEE-encoded effector proteins exhibit major differences. Transfer and functional expression of LEE0181 in an E. coli XL1 blue MR background demonstrated that LEE0181 contains all the information for signal transduction and pedestal formation.
Enteropathogenic Escherichia coli (EPEC) is a common cause of persistent diarrhea among infants, primarily in developing countries (7, 8, 23). Characteristic features of EPEC are the presence of the EPEC adherence factor (EAF) plasmid encoding bundle-forming pili (BFP), the lack of the known enterotoxins (heat-stable enterotoxin, heat-labile enterotoxin, and Stx), and the induction of attaching and effacing (A/E) lesions. A/E lesions are characterized by the destruction of brush border microvilli, the rearrangement of host cytoskeletal proteins, and the apical formation of an actin-raised platform (“pedestal”) directly underneath the adherent bacteria. All factors responsible for the A/E phenotype are encoded by a chromosomal pathogenicity island, the locus of enterocyte effacement, or LEE (20, 21). LEE homologues have been characterized in other pathogenic E. coli strains, such as the EHEC strain EDL933 and the RDEC-1 strain, and also in Citrobacter rodentium. The LEE is a 35.6-kb cluster of genes containing five polycistronic operons (LEE1 to LEE5) that encode a type III secretion system (T3SS) which transfers effector proteins into the target host cells (18). These proteins are responsible for the ensuing pathology.
In recent years, atypical EPEC strains that harbor homologues of the LEE but lack the EAF plasmid and thus BFP as well as the regulator Per (plasmid-encoded regulator) have been identified (3, 19, 29). Due to the lack of BFP, these strains exhibit a diffuse adherence pattern and therefore have been described as diffusely adhering EPEC (DA-EPEC) (3). As epidemiological studies show that these DA-EPEC strains are increasingly associated with outbreaks of diarrhea (5, 15, 30), they have been recognized as emerging human and animal pathogens (6, 7, 25, 29). Nonetheless, LEE pathogenicity islands derived from the emerging DA-EPEC strains have not been investigated yet. In this study, we have characterized and comparatively analyzed the LEEs derived from the clinical DA-EPEC strains 3431 (O8:H−) and 0181 (O119:H9:K61) (courtesy of L. R. Trabulsi, Saõ Paulo, Brazil).
(This study was conducted in partial fulfillment of the requirements for a Ph.D. from the University of Münster, Münster, Germany, to J. F. Gärtner.)
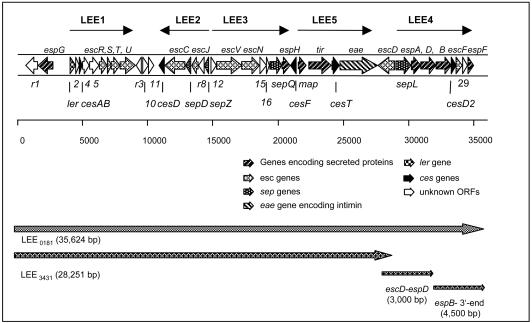
To characterize and compare the LEEs, cosmid libraries of the DA-EPEC strains 0181 and 3431 employing the SuperCos1 cosmid vector (Stratagene) were generated. LEE-harboring cosmid clones were identified by colony hybridization. Cosmid clones used for sequencing encompassed the LEEs of the two DA-EPEC strains as depicted in Fig. 1. In addition, the sequence between the eae and espD genes of LEE3431 was obtained using a 3-kb PCR fragment generated with the primer pair JG31-JG32 (Fig. 1; Table 1). The 3′ sequence of LEE3431 from the espD gene to the 3′ end had been determined previously (3).
FIG. 1.
Organization of the prototype LEEE2348/69 and analogous segments of atypical LEEs covered by cosmid clones. The cosmid clone LEE0181 covers the complete LEE of the DA-EPEC strain 0181, and the cosmid LEE3431 encompasses 28,251 bp of the LEE of the DA-EPEC strain 3431. The sequence between the eae and the espD genes of LEE3431 was obtained using a 3-kb PCR fragment; the 3′ sequence of LEE3431 from the espD gene to the 3′ end had been determined previously.
TABLE 1.
Oligonucleotides used as primer pairs and probes
| Primer (reference) | Primer sequence (5′ to 3′) | Product (no. of bp) | TAa (°C) |
|---|---|---|---|
| EspB3431(+) (16a) | TCC CCC GGG ATG AAT ACT ATT GAT AAT AAT | espB (924) | 56 |
| EspB3431(−) (16a) | TCC CCC GGG TTA CCC GGC TAA GCG ACC CGA | ||
| EspD3431(+) (16a) | AAT CTG TTC ACG CTA GGC GGA TCC GCG ATG CTT AAT | espD (1200) | 60 |
| EspD3431(−) (16a) | TAA ACC AAT TCC CCC GGG GGA TTA AAT TCG ACC ACT | ||
| K260 (20) | GAG CGA ATA TTC CGA TAT CTG GTT | orf394/selC (527) | 60 |
| K261 (20) | CCT GCA AAT AAA CAC GGG GCA T | ||
| JG31 | CGTCTGGGCGGGAGATGTTGATACCATCTT | Strain 3431 eaeA-espD (3 kb) | 64 |
| JG32 | CATCTGAAGTAGCCGAAGCAGCATTAGCCC | ||
| K295 (20) | CGC CGA TTT TTC TTA GCC CA | orf394/LEE (405) | 60 |
| K296 (20) | CAT CTC GAA ACA AAC TGG TC | ||
| C6 (2a) | GAT ATA TAA GGG ATT AGA AGG GG | LEE/selC (381) | 50 |
| K260 (20) | GAG CGA ATA TTC CGA TAT CTG GTT | ||
| C14 (2a) | CGA ACT GTT AAC CAC ACT G | LEE/pheU (2,006) | 53 |
| C23 (2a) | CCT ATG AGC AAT CGA AGA AAG G | ||
| Ler R1 (22a) | GTT AAA TAT TTT TCA GCG GTA | ler (390) | 52 |
| Ler F1 (22a) | CAT GCG GAG ATT ATT TAT TAT | ||
| SK1 | CCC GAA TTC GGC ACA AGC ATA AGC | eaeA (863) | 52 |
| SK2 | CCC GGA TCC GTC TCG CCA GTA TTC G | ||
| Tir 19R (22a) | CGG AAT TCT TAA ACG AAA CGT ACT GGT | tir (1650) | 58 |
| Tir 19F (22a) | AAG GAT CCA TGC CTA TTG GTA ACC TTG G |
TA, annealing temperature.
G+C contents, insertion sites, and flanking regions of the LEEs of the DA-EPEC strains 0181 and 3431.
Pathogenicity islands are large clusters of functionally cooperative virulence genes that are horizontally transferred between different species, resulting in significant changes in fitness and also in pathogenicity (“quantum leaps in evolution”) (16). The G+C contents of the atypical LEEs were determined to be 38.3% (LEE0181) and 39.5% (LEE3431). These are considerably lower than that of the E. coli chromosome but similar to those of LEEs of other Enterobacteriaceae, such as the EPEC strain E2348/69 (38.4%), the enterohemorrhagic E. coli (EHEC) strain EDL933 (39.6%), Citrobacter rodentium (38.1%), and the RDEC-1 strain (41.3%) (10, 12, 31).
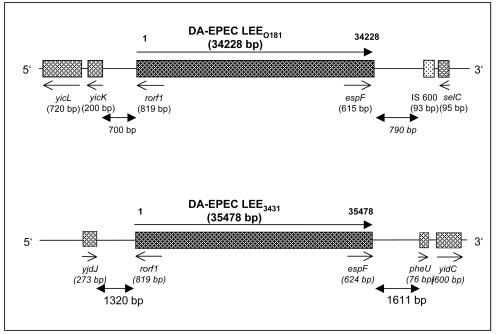
LEE0181 is inserted into the selC tRNA gene of the E. coli K-12 genome (Fig. 2), as has been reported for LEEE2348/69 and the LEE of the EHEC strain EDL933 as well as those of several EPEC 1 and EHEC 1 serovars (12, 20). The 5′- and 3′-flanking regions of LEE0181 are identical to the flanking regions of LEEE2348/69. The 5′-flanking region of about 700 bp begins in the yicK gene of the E. coli K-12 genome, which as a result of the integration is largely truncated (250 instead of 1,185 bp). The 3′-flanking region of about 790 bp is followed by an insertion element, which is identical to the IS600 flanking the 3′ end of LEEE2348/69, as well as by the selC tRNA gene of the E. coli K-12 genome (12).
FIG. 2.
Schematic overview of the chromosomal junctions of the LEEs of the DA-EPEC strains 0181 and 3431.
LEE3431 is inserted next to the pheU tRNA gene of the E. coli genome (Fig. 2). At the 5′ end, LEE3431 has been incorporated in the yjdK gene, resulting in the deletion of the gene. The 3′ end of LEE3431 is followed by a 240-bp fragment, which is identical to the 3′-flanking region of LEEE2348/69. Downstream (1,260 bp) is a 134-bp repeat region, which has also been found at the 3′ junction of the LEE of the Shiga toxin-producing E. coli strain 413/89-1 (O26:H−) (27). Directly adjacent to the 3′ end of LEE3431, the pheU gene followed by the yjdC gene has been identified, indicating a deletion of the intervening 10-kb chromosomal DNA encompassing the lysU, ydlL, cadA, and cadC genes.
Sequence characteristic of the cloned LEE.
The nucleotide sequence of the LEE of the DA-EPEC strain 0181 (35,624 bp) covers the core region of the LEE, defined as the region encompassing the first (rorf1) and the last (espF) genes of the LEE as well as 675 and 797 bp of flanking sequences at the 5′ and 3′ ends. The nucleotide sequence of the LEE of the DA-EPEC strain 3431 (38,235 bp) contains the core region of the LEE as well as 777 and 1,904 bp of noncoding sequences at the 5′- and 3′-flanking regions, respectively.
LEE pathogenicity islands are highly conserved in terms of size and organization of genes. Each LEE harbors 41 open reading frames (ORFs) organized into five polycistronic operons (LEE1, LEE2, LEE3, LEE5, and LEE4) that exhibit identical orders and orientations. It is noteworthy that all of the identified ORFs in LEE0181 and LEE3431 correspond to genes located on the LEEs of the prototype EPEC strain E2348/69, the EHEC strain EDL933, Citrobacter rodentium, and the RDEC-1 strain, indicating that these LEEs may have descended from a common ancestor. The increased size of LEE3431 (35,478 bp) compared to LEEE2348/69 (34,066 bp) is due to the insertion of a 1,431-bp Tn5 transposon into the ler gene. The functional significance of the ler-mediated regulation is currently under examination in our laboratory. With 34,228 bp, LEE0181 is only slightly (162 bp) larger than LEEE2348/69. Comparisons of sequences of specific operons indicated a closer relationship of LEE0181 with LEEE2348/69 than with LEE3431 (Table 2).
TABLE 2.
Comparison of ORFs of the LEEs identified in DA-EPEC strains 0181 and 3431 with the corresponding ORFs of the prototype EPEC strain E2348/69
| ORF | No. of aa corresponding to ORF of LEE from:
|
Gene designation | Function of protein | % Similarity of protein sequences from strains:
|
||||
|---|---|---|---|---|---|---|---|---|
| Prototype EPEC strain E2348/69 | DA-EPEC strain 3431 | DA-EPEC strain 0181 | 3431 and E2348/69 | 0181 and E2348/69 | 3431 and 0181 | |||
| rorf1 | 272 | 272 | 272 | Unknown | 81.2 | 98.9 | 81.6 | |
| rorf2 | 398 | 387 | 398 | espG | Secreted protein | 69.5 | 99.7 | 69.5 |
| orf1 | 129 | 86 | 129 | ler | LEE regulator | 93.0 | 99.2 | 93.0 |
| orf2 | 72 | 72 | 72 | cesAB | Chaperone for EspA and EspB | 93.1 | 98.6 | 91.7 |
| orf3 | 107 | 107 | 107 | Type III secretion? | ||||
| orf4 | 199 | 199 | 199 | Type III secretion? | 93.5 | 100 | 93.5 | |
| orf5 | 231 | 231 | 231 | Type III secretion? | 79.7 | 98.7 | 79.7 | |
| orf6 | 217 | 217 | 217 | escR | Type III secretion | 95.9 | 99.5 | 96.3 |
| orf7 | 89 | 89 | 89 | escS | Type III secretion | 95.5 | 100 | 95.5 |
| orf8 | 258 | 258 | 258 | escT | Type III secretion | 93.8 | 99.6 | 93.4 |
| orf9 | 345 | 345 | 345 | escU | Type III secretion | 95.1 | 100 | 95.1 |
| rorf3 | 152 | 154 | 152 | Unknown | 99.3 | |||
| orf10 | 123 | 112 | 123 | Unknown | 85.7 | 99.2 | 85.7 | |
| orf11 | 137 | 137 | 137 | Unknown | 94.2 | 100 | 94.2 | |
| rorf4 | 151 | 151 | 151 | cesD | Chaperone for EspD | 96.7 | 100 | 96.7 |
| rorf5 | 512 | 512 | 512 | escC | Type III secretion | 97.5 | 99.6 | 97.9 |
| rorf6 | 151 | 151 | 151 | sepD | Type III secretion | 95.4 | 99.3 | 96.0 |
| rorf7 | 190 | 190 | 190 | escJ | Type III secretion | 97.9 | 99.5 | 97.4 |
| rorf8 | 142 | Type III secretion? | ||||||
| rorf9 | 98 | 99 | 98 | sepZ | Type III secretion | 59.2 | 96.9 | 59.2 |
| orf12 | 117 | Type III secretion? | ||||||
| orf13 | 675 | 675 | 675 | escV | Type III secretion | 96 | 99.7 | 96.1 |
| orf14 | 446 | 446 | 446 | escN | Type III secretion | 95.7 | 99.6 | 96.0 |
| orf15 | 125 | 125 | 125 | Type III secretion? | 93.6 | 100 | 93.6 | |
| orf16 | 138 | 138 | 138 | Type III secretion? | 89.1 | 97.1 | 90.6 | |
| orf17 | 305 | 305 | 305 | sepQ | Type III secretion | 87.9 | 100 | 87.9 |
| orf18 | 176 | 177 | espH | Type III secretion | ||||
| rorf10 | 120 | 120 | 120 | cesF | Chaperone for EspF | 78.3 | 98.3 | 77.5 |
| orf19 | 203 | 203 | 203 | map | Secreted protein | 74.9 | 88.7 | 76.4 |
| orf20 | 550 | 549 | 550 | tir | Intimin receptor | 56.5 | 95.8 | 56.8 |
| orf21 | 156 | 156 | 156 | cesT | Chaperone for Tir | 96.8 | 100 | 96.8 |
| orf22 | 939 | 937 | 948 | eae | Intimin | 79.5 | 83.2 | 79.8 |
| rorf11 | 406 | 406 | 406 | escD | Type III secretion | 92.9 | 97.8 | 94.1 |
| orf23 | 351 | 351 | 351 | sepL | Type III secretion | 94.6 | 97.7 | 94.3 |
| orf24 | 192 | 192 | 190 | espA | Secreted protein | 81.2 | 74.2 | 73.7 |
| orf25 | 380 | 380 | 379 | espD | Secreted protein | 75.8 | 85.5 | 71.1 |
| orf26 | 321 | 307 | 321 | espB | Secreted protein | 61.9 | 100 | 61.9 |
| orf27 | 135 | 135 | 135 | cesD2 | Chaperone for EspD | 92.6 | 100 | 92.6 |
| orf28 | 73 | 73 | 73 | escF | Type III secretion | 97.3 | 100 | 97.3 |
| orf29 | 92 | 92 | 92 | Unknown | ||||
| orf30 | 206 | 207 | 204 | espF | Secreted protein | 75.7 | 85.8 | 73.0 |
Genes involved in the T3SS.
Sequence analysis clearly demonstrated that the proteins constituting the T3SS apparatus (Esc and Sep proteins) are highly conserved. The Sep (secretion of E. coli proteins) and Esc (E. coli secretion) proteins of the DA-EPEC strain 0181 and the EPEC strain E2348/69 are nearly identical (>96.9% identity). The corresponding proteins of the DA-EPEC strain 3431 and those of the EPEC strain E2348/69 and the DA-EPEC strain 0181 share lower identities of 87.9 to 97.9% (Table 2). This remarkable conservation of Esc and Sep proteins is paralleled in other A/E pathogens, such as the EHEC strain EDL933, the RDEC-1 strain, and Citrobacter rodentium and—once again—emphasizes the stringent requirements for the T3SS (10, 12, 31).
In contrast, the SepZ protein turned out to be almost hypervariable among T3SS proteins and to represent one of the most divergent proteins of the LEE (Table 2) (10, 12, 31). Interestingly, the SepZ proteins of the DA-EPEC strain 0181 and the EPEC strain E2348/69 are almost identical (96.9% identity). In contrast, the SepZ proteins of the DA-EPEC strain 3431 and the EPEC strain E2348/69 as well as that of the DA-EPEC strain 0181 exhibit very low identity of only 59.2%. These differences point to a possible role for SepZ in the specificity of the T3SS.
LEE-encoded effector proteins.
In contrast to the proteins involved in the T3SS, the LEE-encoded secreted effector proteins exhibit differences which are larger than would have been expected for clonal divergence among E. coli strains (Table 2) (12, 31). These variations may reflect greater evolutionary pressure on the secreted effector proteins both from the host immune system and from differences among hosts. We found a considerably large variance among the Esp's (E. coli secreted proteins), namely, EspA, EspB, EspD, and EspF (Table 2).
The sequence analysis of the EspA proteins of the two DA-EPEC strains as well as that of the EPEC prototype strain E2348/69 revealed identities of 73.7 to 81.4% (Table 2). EspB together with EspD forms a translocation pore in the target cell membrane and has been described as a cytosolic effector protein tampering with cellular signaling processes (17, 28). The LEE3431-encoded EspB is 14 amino acids (aa) shorter (307 aa) than the E2348/69 counterpart and shares only 61.9% identity with that counterpart. In contrast, EspB0181 is completely identical to EspBE2348/69. EspD3431 is the same size as and shares 75.8% identity with EspDE2348/69, whereas EspD0181 is 1 aa shorter than EspD2348/69 and shares 85.5% identity with that protein. These findings reflect a relatively close relationship of LEE0181 with LEE2348/69 that is also corroborated by the shared inability of the Esp's of E2348/69 and 0181 to induce hemolysis. In contrast, the Esp proteins of the DA-EPEC strain 3431 are sufficient for erythrocyte lysis (17).
The impairment of gastrointestinal barrier functions is regarded as an important step in EPEC pathogenesis. In this process, the T3SS-secreted effector protein EspF has been suggested to play a major role (22). EspF exhibits differences in amino acid sequences and also in protein sizes in different A/E pathogens. EspF contains 301 aa residues in Citrobacter rodentium, 248 aa in the EHEC strain EDL933, 206 aa in the EPEC strain E2348/69, 204 aa in the DA-EPEC strain 0181, 207 aa in the DA-EPEC strain 3431, and only 160 aa in the RDEC-1 strain (10, 12, 31). These size differences in EspF are largely due to the number of proline-rich repeats, and it has been speculated that these repeats may be involved in host specificity (10) (Fig. 3). While RDEC-1 has two repeats, the EPEC and DA-EPEC strains have three, the EHEC strain EDL933 carries four, and Citrobacter rodentium harbors five repeats. The EspF0181 protein is again more closely related to EspFE2348/69 (85.8% identity) than to the EspF protein from DA-EPEC strain 3431 (73% identity) (Fig. 3).
FIG. 3.
Alignments of the EspF protein of the EPEC protoype strain E2348/69 with those of the DA-EPEC strains 0181 and 3431. Residues differing from those of EspFE2348/69 are boxed in black. The three proline-rich repeats in EspF are shaded in grey.
Additional highly variable genes include the eae gene encoding the outer membrane adhesin intimin and the tir gene encoding the intimin receptor Tir (translocated intimin receptor). The interaction of intimin and Tir mediates the intimate attachment between bacterium and host cell and plays an important role in EPEC pathogenesis (11, 23). The intimin proteins of the three strains compared in this study differ both in their sequences and their lengths. While intimin of the DA-EPEC strain 0181 comprises 948 aa, the corresponding proteins of the DA-EPEC strain 3431 and the EPEC strain E2348/69 have 937 and 939 aa. Moreover, intiminE2348/69 is 79.5 and 83.2% identical to intimin3431 and intimin0181, which are only 79.8% identical to each other (Table 2). Differences in intimin sequences are thought to reflect the evolutionary lineages of the various LEEs (2, 26) and have been employed to classify A/E E. coli isolates. Several genetically and serologically distinct intimin types (intimin α to intimin λ) have been identified. The comparison of the intimin sequences of the DA-EPEC strains showed that the intimin of the DA-EPEC strain 3431 is 99.8% identical to intimin ϕ2 and that the intimin of strain 0181 is identical to intimin ɛ2. The intimin of the EPEC prototype strain E2348/69 shows an identity of 94.4% to intimin α2. Based on the intimin sequences, the relationship among the intimins derived from the DA-EPEC strains and the prototype EPEC strain E2348/69 can be displayed as a phylogenetic tree (Fig. 4) that again reflects the relatively close relationship of LEE0181 to LEEE2348/69.
FIG. 4.
Phylogenetic tree of intimin proteins of DA-EPEC strains, the prototype EPEC strain E2348/69, the EHEC strain EDL933, and the rabbit EPEC strain RDEC-1 as a visualization of sequence alignments. The tree was generated using DNASTAR MegAlign software.
The genes encoding the chaperones involved in the stabilization and secretion of the effector proteins EspB, EspD, Tir, and Map as well as EspF, CesD, CesD2, CesT, and CesF (9, 14, 24) turned out to be highly conserved. These proteins are unique to the LEE and have not been found in other T3SSs. The high level of sequence identity between chaperones of different strains supports a prominent role in the maintenance of effector functions. Interestingly, whereas the Tir effector proteins differ in the three EPEC strains, the corresponding chaperone CesT, which binds to the N-terminal region of Tir (1), is highly conserved among the three LEEs examined in this study (Table 2). The Tir of LEE0181 is 95.8% identical to the Tir of LEEE2348/69. In contrast, the Tir of LEE3431 shows an identity of 56.5% to the Tir of LEEE2348/69.
Functional analysis of the cloned LEE0181.
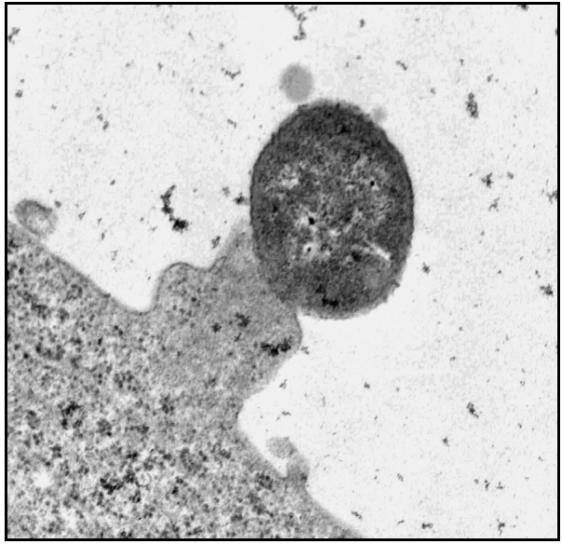
The recombinant E. coli JG-LEE0181/XL1 blue MR strain harboring the complete LEE0181 adhered to host cells and, furthermore, induced the formation of pedestal-like structures underneath adherent bacteria in HeLa cells (Fig. 5). In addition, the recombinant strain secreted EspB and induced a fluorescence actin staining-positive reaction as well as the accumulation of tyrosine-phosphorylated proteins (presumably Tir) at the site of infection (data not shown). These results indicate that the LEE of DA-EPEC also encodes all information necessary for bacterial adherence to the host cell and the activation of signal transduction pathways leading to A/E lesions and pedestal formation.
FIG. 5.
Transmission electron microscopy of a HeLa cell infected with JG-LEE0181/XL1 blue MR (magnification, ×19,000). The recombinant strain is able to adhere to HeLa cells and induces the formation of host cell protrusions (pedestals) underneath adherent bacteria.
The lack of the EAF plasmid and thus of the BFP reduces the ability of DA-EPEC to form biofilm-embedded microcolonies or “bioclips” on the host cell surface and consequently impairs quorum sensing, inducing the activation of LEE and the following signal transduction. Besides the F1845 fimbriae (4), which have been identified in strain 0181 (3), little is known about possible adhesins expressed by DA-EPEC. In addition, Per, which is largely regulated by the LEE-encoded Ler, has been shown to be responsible for maximal expression of LEE-encoded proteins (13). Therefore, due to the lack of per, regulation of LEE expression in DA-EPEC strains lacking the EAF may be quite different. In further studies it will be interesting to see whether an alternative regulatory system may be involved in DA-EPEC strains.
Nucleotide sequence accession numbers. Sequences of the LEEs of DA-EPEC strains 0181 and 3431 have been deposited in GenBank under accession numbers AJ633129 and AJ633130, respectively.
Acknowledgments
We are indebted to J. B. Kaper (Baltimore, Md.) for the kind gift of the EPEC prototype strain E2348/69 and to F. Ebel (Munich, Germany) for providing anti-Tir antibodies. Furthermore, we thank L. Greune for expert electron microscopy, V. Humberg for practical help, and T. Ide, G. Heusipp, and C. Cichon for helpful discussions.
This study was supported by grants from the BMBF Project Network of Competence Pathogenomics Alliance [Functional Genomic Research on Enterohaemorrhagic, Enteropathogenic and Enteroaggregative Escherichia coli (EHEC, EPEC, EAEC)], project group Karch/Schmidt, Universitätsklinikum Münster (BD 119523/207800), and the Deutsche Forschungsgemeinschaft (DFG: SFB 293/TPB5) and by a personal grant from the VolkswagenStiftung (I 79/078 to M.A.S.).
Editor: J. B. Bliska
REFERENCES
- 1.Abe, A., M. de Grado, R. A. Pfuetzner, C. Sanchez-Sanmartin, R. Devinney, J. L. Puente, N. C. Strynadka, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli translocated intimin receptor, Tir, requires a specific chaperone for stable secretion. Mol. Microbiol. 33:1162-1175. [DOI] [PubMed] [Google Scholar]
- 2.Adu-Bobie, J., G. Frankel, C. Bain, A. G. Goncalves, L. R. Trabulsi, G. Douce, S. Knutton, and G. Dougan. 1998. Detection of intimins alpha, beta, gamma, and delta, four intimin derivatives expressed by attaching and effacing microbial pathogens. J. Clin. Microbiol. 36:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Beinke, C. 1999. Ph.D. thesis. University of Münster, Münster, Germany.
- 3.Beinke, C., S. Laarmann, C. Wachter, H. Karch, L. Greune, and M. A. Schmidt. 1998. Diffusely adhering Escherichia coli strains induce attaching and effacing phenotypes and secrete homologs of Esp proteins. Infect. Immun. 66:528-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilge, S. S., C. R. Clausen, W. Lau, and S. L. Moseley. 1989. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J. Bacteriol. 171:4281-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouzari, S., M. N. Jafari, F. Shokouhi, M. Parsi, and A. Jafari. 2000. Virulence-related DNA sequences and adherence patterns in strains of enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 185:89-93. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho, V. M., C. L. Gyles, K. Ziebell, M. A. Ribeiro, J. L. Catao-Dias, I. L. Sinhorini, J. Otman, R. Keller, L. R. Trabulsi, and A. F. Pestana de Castro. 2003. Characterization of monkey enteropathogenic Escherichia coli (EPEC) and human typical and atypical EPEC serotype isolates from neotropical nonhuman primates. J. Clin. Microbiol. 41:1225-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke, S. C., R. D. Haigh, P. P. Freestone, and P. H. Williams. 2003. Virulence of enteropathogenic Escherichia coli, a global pathogen. Clin. Microbiol. Rev. 16:365-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cravioto, A., R. E. Reyes, F. Trujillo, F. Uribe, A. Navarro, J. M. De La Roca, J. M. Hernandez, G. Perez, and V. Vazquez. 1990. Risk of diarrhea during the first year of life associated with initial and subsequent colonization by specific enteropathogens. Am. J. Epidemiol. 131:886-904. [DOI] [PubMed] [Google Scholar]
- 9.Creasey, E. A., R. M. Delahay, A. A. Bishop, R. K. Shaw, B. Kenny, S. Knutton, and G. Frankel. 2003. CesT is a bivalent enteropathogenic Escherichia coli chaperone required for translocation of both Tir and Map. Mol. Microbiol. 47:209-221. [DOI] [PubMed] [Google Scholar]
- 10.Deng, W., Y. Li, B. A. Vallance, and B. B. Finlay. 2001. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 69:6323-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnenberg, M. S., and J. B. Kaper. 1992. Enteropathogenic Escherichia coli. Infect. Immun. 60:3953-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 13.Elliott, S. J., V. Sperandio, J. A. Giron, S. Shin, J. L. Mellies, L. Wainwright, S. W. Hutcheson, T. K. McDaniel, and J. B. Kaper. 2000. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 68:6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott, S. J., C. B. O'Connell, A. Koutsouris, C. Brinkley, M. S. Donnenberg, G. Hecht, and J. B. Kaper. 2002. A gene from the locus of enterocyte effacement that is required for enteropathogenic Escherichia coli to increase tight-junction permeability encodes a chaperone for EspF. Infect. Immun. 70:2271-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galane, P. M., and M. Le Roux. 2001. Molecular epidemiology of Escherichia coli isolated from young South African children with diarrhoeal diseases. J. Health Popul. Nutr. 19:31-38. [PubMed] [Google Scholar]
- 16.Hacker, J., and E. Carniel. 2001. Ecological fitness, genomic islands and bacterial pathogenicity. A Darwinian view of the evolution of microbes. EMBO Rep. 2:376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Ide, T. 2002. Ph.D. thesis. University of Münster, Münster, Germany.
- 17.Ide, T., S. Laarmann, L. Greune, H. Schillers, H. Oberleithner, and M. A. Schmidt. 2001. Characterization of translocation pores inserted into plasma membranes by type III-secreted Esp proteins of enteropathogenic Escherichia coli. Cell. Microbiol. 3:669-679. [DOI] [PubMed] [Google Scholar]
- 18.Kenny, B., and B. B. Finlay. 1997. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect. Immun. 65:2528-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyaw, C. M., C. R. De Araujo, M. R. Lima, E. G. Gondim, M. M. Brigido, and L. G. Giugliano. 2003. Evidence for the presence of a type III secretion system in diffusely adhering Escherichia coli (DAEC). Infect. Genet. Evol. 3:111-117. [DOI] [PubMed] [Google Scholar]
- 20.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23:399-407. [DOI] [PubMed] [Google Scholar]
- 22.McNamara, B. P., A. Koutsouris, C. B. O'Connell, J. P. Nougayrede, M. S. Donnenberg, and G. Hecht. 2001. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Investig. 107:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Michgehl, S. 2001. Diploma thesis. University of Münster, Münster, Germany.
- 23.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neves, B. C., R. Mundy, L. Petrovska, G. Dougan, S. Knutton, and G. Frankel. 2003. CesD2 of enteropathogenic Escherichia coli is a second chaperone for the type III secretion translocator protein EspD. Infect. Immun. 71:2130-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paciorek, J. 2002. Virulence properties of Escherichia coli faecal strains isolated in Poland from healthy children and strains belonging to serogroups O18, O26, O44, O86, O126 and O127 isolated from children with diarrhoea. J. Med. Microbiol. 51:548-556. [DOI] [PubMed] [Google Scholar]
- 26.Pelayo, J. S., I. C. Scaletsky, M. Z. Pedroso, V. Sperandio, J. A. Giron, G. Frankel, and L. R. Trabulsi. 1999. Virulence properties of atypical EPEC strains. J. Med. Microbiol. 48:41-49. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt, H., W. L. Zhang, U. Hemmrich, S. Jelacic, W. Brunder, P. I. Tarr, U. Dobrindt, J. Hacker, and H. Karch. 2001. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 69:6863-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor, K. A., P. W. Luther, and M. S. Donnenberg. 1999. Expression of the EspB protein of enteropathogenic Escherichia coli within HeLa cells affects stress fibers and cellular morphology. Infect. Immun. 67:120-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trabulsi, L. R., R. Keller, and T. A. Tardelli Gomes. 2002. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 8:508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yatsuyanagi, J., S. Saito, H. Sato, Y. Miyajima, K. Amano, and K. Enomoto. 2002. Characterization of enteropathogenic and enteroaggregative Escherichia coli isolated from diarrheal outbreaks. J. Clin. Microbiol. 40:294-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu, C., T. S. Agin, S. J. Elliott, L. A. Johnson, T. E. Thate, J. B. Kaper, and E. C. Boedeker. 2001. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infect. Immun. 69:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]