Abstract
Pneumocystis carinii is an opportunistic fungal pathogen that causes life-threatening pneumonia in immunocompromised individuals. Infants appear to be particularly susceptible to infection with Pneumocystis. We have previously shown that there is a significant delay in clearance of the organisms from the lungs of neonatal mice compared to adults. Since alveolar macrophages are the effector cells responsible for killing and clearance of Pneumocystis, we have examined alveolar macrophage activity in neonatal mice. We found that alveolar macrophage activation is delayed about 1 week in Pneumocystis-infected neonates compared to adults. Opsonization of the organism by Pneumocystis-specific antibody resulted in increased clearance of the organism in neonatal mice; however, there was decreased expression of activation markers on neonatal alveolar macrophages and reduced levels of cytokines associated with macrophage activation. Mice born to immunized dams had significant amounts of Pneumocystis-specific immunoglobulin G in their lungs and serum at day 7 postinfection, whereas mice born to naïve dams had merely detectable levels. This difference correlated with enhanced Pneumocystis clearance in mice born to immunized dams. The increase in specific antibody, however, did not result in significant inflammation in the lungs, as no differences in numbers of activated CD4+ cells were observed. Furthermore, there was no difference in cytokine or chemokine concentrations in the lungs of pups born to immune compared to naïve dams. These findings indicate that specific antibody plays an important role in Pneumocystis clearance from lungs of infected neonates; moreover, this process occurs without inducing inflammation in the lungs.
Pneumocystis carinii f. sp. hominis is an opportunistic fungal pathogen that is a significant cause of life-threatening pneumonia in immunosuppressed individuals. It has recently been appreciated that Pneumocystis can either colonize or infect immunocompetent individuals, particularly young children (12, 39-41). Since Pneumocystis is thought to be ubiquitous in the environment (4, 42), it is not surprising that a large percentage of children have been shown to have serum antibodies specific to these organisms by 2 years of life (31). Infants seem to be particularly susceptible to primary Pneumocystis infection, which is likely due to an immature immune system. In this regard, it has been shown that neonatal mice infected with P. carinii f. sp. muris (the mouse-specific species) have a delayed pulmonary immune response compared to adult mice (8, 9). This delay in the immune response to Pneumocystis was characterized by depressed lung proinflammatory cytokines and chemokines, T-cell migration, and antibody production in neonates over the first 3 weeks of life (8, 9, 33).
It is well known that CD4+ T cells are required for clearance of Pneumocystis infection (16, 36) and that specific antibody can be very effective at mediating clearance of the organisms (11, 13, 15, 36). However, neonates have deficiencies in humoral and cell-mediated immunity which have been well documented (1, 26). Under conditions of weak costimulation, neonatal CD4+ T cells tend to be biased toward Th2-type responses (2, 3). Moreover, airway dendritic cells tend to have an immature phenotype and therefore do not provide adequate costimulation to T cells (29, 30). The lack of strong T-cell help would undoubtedly contribute to deficiencies in T-cell-dependent antibody responses that are seen in infants less than 2 months of age. In addition, it has recently been shown that delayed antibody responses in developing mice correspond to delayed maturation of follicular dendritic cell networks in germinal centers (32). The maturational process of adaptive immunity in newborns likely provides a permissive environment for Pneumocystis growth.
A number of studies over the years have shown that passive administration of Pneumocystis-specific antibodies are effective at controlling Pneumocystis growth in the lungs of animals (10, 11, 36). In addition, it has previously been shown that immunization of dams with Pneumocystis resulted in enhanced clearance kinetics of the organisms from infected pups, which was assumed to be due to elevated levels of specific antibody passively acquired from the dams (8). However, clearance of organisms from the lungs of pups born to immunized dams still lagged behind that of adult mice (8). One possibility may be that alveolar macrophage function was immature in neonates compared to that of adults. To address this hypothesis, we have examined alveolar macrophage phenotype in Pneumocystis-infected and uninfected neonatal mice compared to that in adult mice and in the presence of Pneumocystis-specific antibody. We have now expanded previous observations to determine that although Pneumocystis-specific antibody can be found in the lungs of pups born to immunized dams, control of infection precedes infiltration of activated CD4+ T cells into the lungs. However, rates of clearance of Pneumocystis organisms still lags behind rates in adult mice, which is likely due to differences in the activation of alveolar macrophages.
MATERIALS AND METHODS
Mice and experimental design.
A colony of C.B-Igh-1b/ICrTac-Prkdcscid (SCID) mice originally obtained from Taconic (Germantown, N.Y.) was used to maintain a source of P. carinii f. sp. muris. BALB/cAnNCr mice were obtained from the National Cancer Institute (Frederick, Md.) and bred in the Veterans Administration Medical Center Veterinary Medical Unit, Lexington, Ky. For some experiments, B6D2F1 hybrid mice (National Cancer Institute) were used for breeding experiments. There was no difference in the kinetics of clearance of Pneumocystis from B6D2F1 pups compared to that from BALB/c pups. Mice were maintained in microisolators and had free access to sterilized food and water. Female mice were inoculated intratracheally (i.t.) with 107 P. carinii on the same day that they were housed with males for breeding. Dams were given a booster inoculation of 107 organisms intranasally (i.n.) on day 15 of gestation. Neonatal mice (24 to 72 h old) and adult controls were infected i.n. with approximately 106 Pneumocystis organisms per g of body weight. Protocols for the use of mice were approved by the local institutional animal care and use committee.
Enumeration and inoculation of P. carinii organisms.
For isolation of organisms for inoculation, lungs were excised from Pneumocystis-infected SCID mice and pushed through stainless steel mesh in Hanks balanced salt solution (HBSS). Cell debris was removed by centrifugation at 100 × g for 2 min. Aliquots of lung homogenates were spun onto glass slides, fixed in methanol, and stained with Diff-Quik (Dade International, Miami, Fla.). Pneumocystis nuclei were counted by microscopy. Mice to be infected were anesthetized lightly with halothane gas and 106 to 107 Pneumocystis organisms injected either i.t. or i.n. in 10 to 100 μl of phosphate-buffered saline (PBS), depending on the size of the mouse. For determination of lung Pneumocystis burden, right lung lobes were excised, minced, and digested in RPMI medium supplemented with 2% fetal calf serum and 1 mg of collagenase A and 50 U of DNase/ml for 1 h at 37°C. Digested lung fragments were pushed through mesh screens, and aliquots were spun onto glass slides and stained with Diff-Quik for microscopic enumeration as previously described (16). Lung burden is expressed as log10 Pneumocystis nuclei per right lung lobe, and the limit of detection was 3.23 log10 Pneumocystis nuclei.
MAb and opsonization of Pneumocystis organisms.
Sera were collected from BALB/c mice immunized twice with i.t. inoculations of 107 Pneumocystis organisms and frozen prior to use. For antibody opsonization, organisms were purified as described above and incubated with either 200 μl of a 1:2 dilution of polyclonal antiserum or 200 μl of 0.5 mg of monoclonal antibody (MAb) 90-3-4F11(G1)/ml. MAb 90-3-4F11(G1) is an isotype switch variant (immunoglobulin G1 [IgG1]) of a MAb specific for a Pneumocystis kexin-like protein, KEX1 (11, 20). Opsonized organisms were washed to remove extraneous antibody and adjusted for i.n. inoculation of 2 × 106 organisms per neonatal mouse.
Isolation of cells from alveolar spaces, lungs, and lymph nodes.
Mice were killed by exsanguination under deep halothane anesthesia. The lungs were lavaged with HBSS containing 3 mM EDTA. Bronchial alveolar lavage fluid (BALF) from the first wash was saved for quantitation of cytokines. Lungs were digested as described above, and an aliquot for the enumeration of Pneumocystis organisms was removed. Erythrocytes were removed from lung digests by using a hypotonic lysing buffer, cells were washed, and single-cell suspensions were counted. Tracheobronchial lymph nodes (TBLN) were pushed through nylon mesh screens in HBSS and counted.
Flow cytometric analysis of lung and lymph node lymphocytes.
Lung lavage, lung digest, and TBLN cells were washed in PBS with 0.1% bovine serum albumin and 0.02% NaN3 and stained with appropriate concentrations of fluorochrome-conjugated antibodies specific for murine T cells (CD4, CD8, CD44, and CD62L), B cells (CD19, CD80, and CD86), and macrophages (F4/80, CD11c, CD11b, and FcγR or CD11c, CD11c, major histocompatibility [MHC] class II, and CD40). Antibodies were purchased from PharMingen (San Diego, Calif.) or eBioscience (San Diego, Calif.). Expression of these molecules on the surface of cells was determined by multiparameter flow cytometry using a FACSCaliber cytofluorimeter (Becton Dickinson, Mountain View, Calif.).
Pneumocystis-specific enzyme-linked immunosorbent assay (ELISA).
Blood was collected from the abdominal aorta under halothane anesthesia, and sera were frozen at −80°C. Cells and debris were spun out of the first wash of BALF, and the fluid was frozen for use later. A crude sonicate of Pneumocystis (10 μg/ml) was coated onto microtiter plates for 2 h, and coated wells were blocked with 5% dry milk in HBSS supplemented with 0.05% Tween 20 for 1 h. Test sera were diluted 1:100, and BALF was used neat and incubated in plates overnight (4°C). Plates were washed extensively, and bound antibody was detected by using appropriate dilutions of alkaline phosphatase-conjugated specific antibodies (anti-IgM, anti-IgG, and anti-IgA). After 4 h at 37°C, plates were washed and developed by using p-nitrophenylphosphate (1 mg/ml) in diethanolamine buffer. The optical density at 405 nm is reported.
Analysis of cytokine levels in BALF with CBA.
The quantitation of multiple cytokines in each sample of BALF was performed by using flow cytometry-based cytokine bead array (CBA) kits purchased from PharMingen. An inflammation CBA kit was used to quantitate murine interleukin-6 (IL-6), IL-10, monocyte chemoattractant protein 1 (MCP-1), gamma interferon (IFNγ), tumor necrosis factor alpha (TNF-α), and IL-12p70. Assays were performed according to the manufacturer's instructions.
Statistical analysis.
Data are expressed as the means ± standard deviations (SD) for three to five mice per group. Differences between experimental groups were determined by using Student's t tests or analysis of variance followed by Student Neuman Kuels post-hoc test where appropriate. Differences were considered statistically significant when P was <0.05. SigmaStat statistical software (SPSS, Inc., Chicago, Ill.) was used for all analyses.
RESULTS
Activation of alveolar macrophages is delayed in neonatal mice compared to adults.
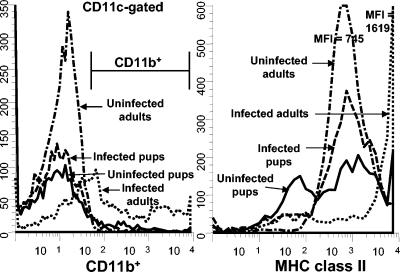
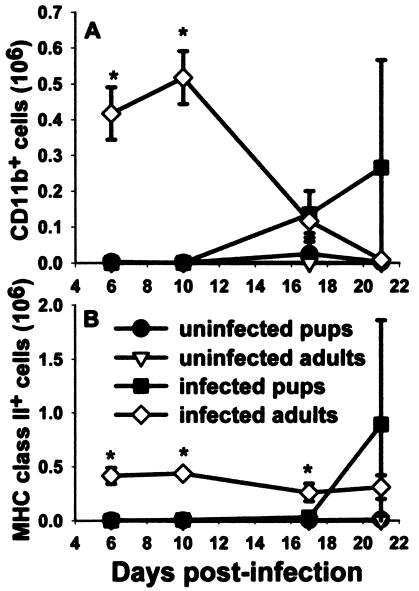
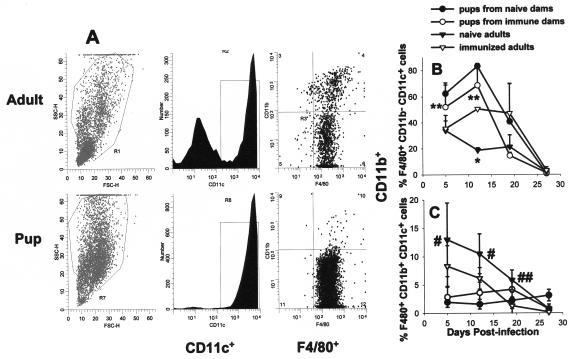
It has previously been published that there is a 3-week delay in the host response of mice infected with Pneumocystis as neonates compared to the response of mice infected as adults (8, 9, 33). To determine whether alveolar macrophage activation is also delayed in neonatal mice in response to Pneumocystis, we used flow cytometry to examine macrophage markers at various times postinfection. Mice were infected with Pneumocystis within 72 h of birth or as adults, and lungs were lavaged over time to examine macrophage activation with antibodies specific for CD11b, MHC class II, CD40, and CD80. Cells were gated on large nonlymphocytes, and expression data for CD11b are shown in Fig. 1. Interestingly, CD11b expression was very low for the nonlymphocyte population of cells isolated from BALF of Pneumocystis-infected neonates at day 10 postinfection compared to that of adults (Fig. 1). The number of nonlymphocytes that expressed CD11b and MHC class II was nearly undetectable in the alveolar spaces of Pneumocystis-infected neonates through 2 weeks postinfection, whereas these cells were found in adult lungs by day 6 (Fig. 2). Expression levels of costimulatory molecules CD80 and CD40 were also higher on adult macrophages than on neonatal macrophages (data not shown).
FIG. 1.
Expression levels of large nonlymphocytes of CD11b and MHC class II from alveolar spaces of Pneumocystis-infected animals were very low at day 10 postinfection in neonatal mice compared to those for adult mice. Mice were infected with Pneumocystis as neonates (24 to 72 h after birth) or adults, and lungs were lavaged at day 10 postinfection to examine macrophage levels. Cells were stained with antibodies specific for CD11c and CD11b or MHC class II, and phenotypes were examined by using flow cytometry. Representative histograms of CD11b-positive cells (gated on CD11c+ cells) and MHC class II-positive cells (gated on large nonlymphocytes) are shown. Cells were gated for large nonlymphoid cells by using forward and side light scatter. Controls included BALF cells from uninfected neonates or adults. Data are representative of results for three to five mice per group.
FIG. 2.
Numbers of nonlymphoid cells expressing CD11b and MHC class II in the alveolar spaces of Pneumocystis-infected neonates were significantly lower than those in adult lungs through 2 weeks postinfection. Mice were infected with Pneumocystis as neonates (24 to 72 h after birth) or adults. Control mice received HBSS vehicle. Lungs were lavaged at the indicated time points, cells were stained with antibodies specific for CD11b and MHC class II, and cells were examined by using flow cytometry. Cells were gated on large nonlymphocytes, and the numbers of cells expressing (A) CD11b and (B) MHC class II were counted. Data represent the means ± SD for three to five mice per group. *, P < 0.05 compared to all other groups at the same time point.
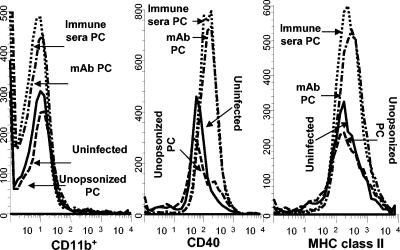
To determine whether opsonization of Pneumocystis by specific antibody contributes to the activation of alveolar macrophages, organisms were incubated with either immune serum obtained from Pneumocystis-infected adult mice or with a MAb (IgG1) specific for Pneumocystis KEX1 (11). As shown in Table 1, opsonization of Pneumocystis with polyclonal immune serum prior to i.n. inoculation into neonatal mice resulted in reduced lung burden at day 10 postinfection compared to that of mice infected either with untreated Pneumocystis or with organisms treated with MAb. The MAb we used for opsonization has been shown to inhibit lung Pneumocystis growth in adult SCID mice exposed to an airborne challenge of Pneumocystis by cohousing with other Pneumocystis-infected mice (11). These antibodies were unable to control organism growth in neonatal mice when used as opsonins prior to infection. However, when infused directly into the lungs of Pneumocystis-infected infant mice, the anti-KEX1 MAb was able to control the lung burden compared to mice given control antibody even though use of the MAb did not result in complete clearance of the organisms (data not shown). Even though serum opsonization of Pneumocystis organisms prior to inoculation resulted in reduced lung burden, there did not appear to be a difference in the expression levels of CD11b and MHC class II on alveolar nonlymphocytes isolated 10 days postinfection (Fig. 3).
TABLE 1.
Effects of opsonization of Pneumocystis prior to inoculation on clearance of organisms from neonatal lungsa
| Treatment group | Log10Pneumocystis nuclei/lungsb |
|---|---|
| Uninfected (control) | <3.23, <3.23, <3.23c |
| Unopsonized Pneumocystis | 5.80, 5.64, 5.82 |
| Immune serum-treated Pneumocystis | 5.07, 5.23, 5.18, 5.21c |
| MAb 4F11(G1)-treated Pneumocystis | 5.89, 5.80, 5.40 |
Neonatal mice were given i.n. inoculations of PBS or Pneumocystis organisms that were preincubated with polyclonal immune serum or KEX1-specific MAb. The numbers of organisms per lungs were detected microscopically at day 10 postinfection.
Organism burden (expressed as log10 Pneumocystis nuclei per lungs) for individual mice from a single experiment are shown. Note that the limit of detection is 3.23 log10 Pneumocystis nuclei.
P < 0.05 compared to all other groups.
FIG. 3.
No difference was observed in the expression of MHC class II, CD40, and CD11b on alveolar lymphocytes isolated 10 days postinfection in mice treated with opsonized Pneumocystis (PC). Neonatal mice were infected with unopsonized Pneumocystis or organisms were pretreated with polyclonal antiserum or MAb 4F11(G1). A control group was uninfected. Lung lavage cells were stained with antibodies specific for MHC class II, CD40, or CD11b, and the phenotypes of large nonlymphoid cells were determined by flow cytometry. Data are histograms of nonlymphoid gated cells from individual mice at day 10 postinfection and are representative of results from three to four mice per group.
Maternal antibody penetrates to the lungs and facilitates clearance of Pneumocystis.
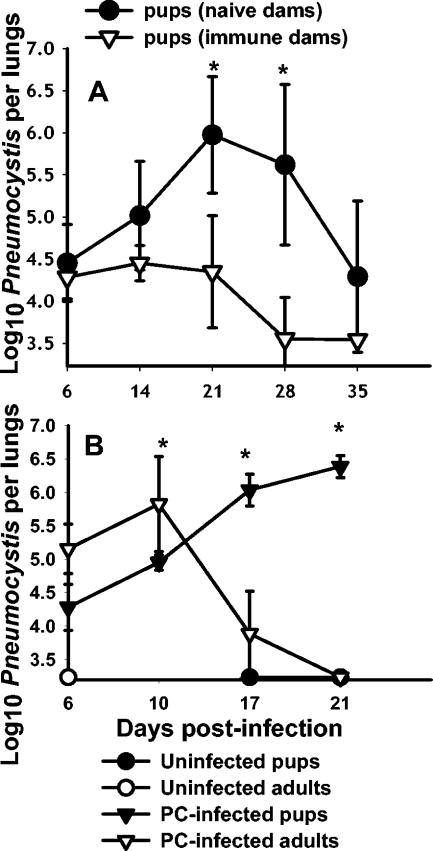
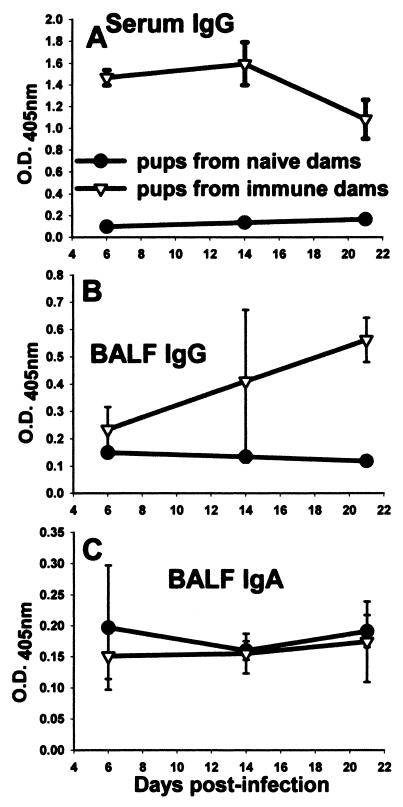
It was previously shown that immunization of dams resulted in enhanced clearance kinetics of Pneumocystis in infant mice (8, 9) (Fig. 4A). Mice born to naïve dams do not control infection until after 4 weeks, whereas mice from immunized dams are able to control growth of Pneumocystis for 3 weeks and clear much of the lung burden by 4 weeks (Fig. 4A). However, as seen in Fig. 4, the kinetics of clearance of infant mice born to immunized dams (Fig. 4A) were not significantly different, and were even slower, than those of adult mice undergoing a primary response (Fig. 4B). To determine when maternal antibody appeared in the lungs of infant mice, Pneumocystis-specific ELISAs were performed with BALF isolated from mice born to immunized or naïve dams. Pneumocystis-specific IgG in both BALF and serum was at the limit of detection at days 6, 14, and 21 postinfection in pups born to naïve dams, whereas significant amounts of specific IgG were found in the lungs of mice born to immunized dams between days 6 and 14 postinfection (Fig. 5). Moreover, these levels were increased at day 21 postinfection (Fig. 5) and at later time points when CD4 T cells had infiltrated the lungs (reference 8 and data not shown). Together, these data indicate that the presence of Pneumocystis-specific IgG in the lungs of infected neonatal mice expedites clearance of infection such that the kinetics are similar to those of the primary response in adult mice.
FIG. 4.
Control of Pneumocystis infection in neonates is expedited in the presence of maternal antibody; however, it still lags behind clearance in adult mice. (A) Adult female mice were challenged with i.t. inoculations of Pneumocystis (PC) on the day they were placed into cages with males for breeding. They were given a boost at day 16 of gestation. Control dams were inoculated with PBS. Neonatal mice were infected with Pneumocystis within 72 h of birth, and lung burden was determined at the indicated time points. Data are expressed as the means ± SD for four to five mice per group. *, P < 0.05 compared to pups from immunized dams. (B) Adult and neonatal mice were given i.n. inoculations of Pneumocystis (PC), and the numbers of organisms were detected microscopically over time. Control mice were given inoculations of PBS. Data represent the means ± SD for four to five mice per group. *, P < 0.05 compared to infected pups.
FIG. 5.
Pups born to immunized dams had significant levels of Pneumocystis-specific IgG in the serum and BALF. Dams were given i.t. inoculations of Pneumocystis on the day of breeding and boosted with i.n. inoculums on day 16 of gestation. Control dams received inoculations of PBS. Pups in both groups were given i.n. inoculations of Pneumocystis within 72 h of birth. ELISAs were performed on BALF and serum collected from pups in each group to determine the level Pneumocystis-specific (A, B) IgG or (C) IgA present. Data are expressed as the optical density at 405 nm. BALF samples were undiluted and serum samples were diluted 1:100 for the ELISA assay. Data represent the means ± SD for four to five mice per group. *, P < 0.05 compared to pups from naïve dams.
To determine whether antibody acquired from dams leaked into the lungs as a result of lung injury, we measured albumin in BALF. There was very little albumin found in the lungs of mice from any of the experimental groups (data not shown). Mice born to immunized dams consistently had reduced levels of albumin in the lungs compared to those of pups born to naïve dams; however, all groups maintained between 11 and 15 μg of albumin/ml through day 27 postinfection. Notably, we found that the levels of albumin in the lungs of uninfected pups or adults were on average around 12 μg/ml of BALF (data not shown). It was previously shown that BALF albumin levels can be 100 μg/ml or higher in Pneumocystis-infected mice that were depleted of CD4+ T cells and that had significant respiratory impairment as evidenced by significant changes in levels of arterial partial O2 and partial CO2 pressure (34). These data indicate that there was no significant vascular leak into the lungs of Pneumocystis-infected infant mice.
Control of Pneumocystis infection occurred in mice from immunized dams in the absence of significant inflammation in the lungs.
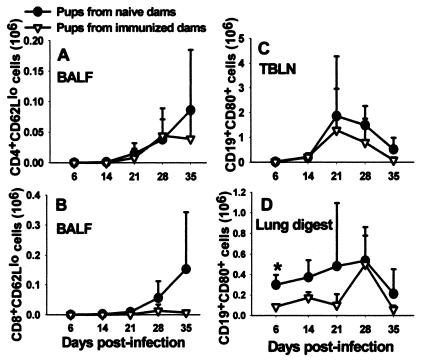
It was previously found that the delay in Pneumocystis clearance in mice infected as neonates was associated with an inflammatory response that developed a full 2 weeks later than did the response in adult mice challenged with Pneumocystis (8, 9). To determine whether the presence of specific antibody in the lungs resulted in enhanced inflammation, we examined the cellular responses in mice born to either immunized or naïve dams and infected with Pneumocystis as neonates. The total number of cells isolated by bronchoalveolar lavage was either the same or, at day 28, less in the pups born to immune dams than that of pups born to naïve dams (data not shown). Similar results were found for the total number of CD4+ and CD8+ cells infiltrating the alveolar space (data not shown). Moreover, the number of CD4+ and CD8+ cells with an activated phenotype (CD44hi CD62Llo) followed the same trend as did total cells and total T cells (Fig. 6). Likewise, the numbers of CD19+ B cells found in lung tissue were either similar in the two groups of mice or were lower in the lungs of mice born to immune dams than those of pups born to naïve dams. The kinetics of infiltration of CD4+ T cells into the alveolar spaces lagged behind clearance of Pneumocystis in the mice born to immune dams (Fig. 4 and 6). Together, these data indicate that control of Pneumocystis infection did not correspond to infiltration of lymphocytes into the lungs of mice born to immune dams but rather corresponded to the presence of specific antibody.
FIG. 6.
No differences in the number of activated CD4+ and CD8+ or B cells in the lungs or draining lymph nodes were observed between pups born to either immunized or naïve dams. Dams were given i.t. inoculations of Pneumocystis on the day of breeding and boosted with i.n. inoculums on day 16 of gestation. Control dams received inoculations of PBS. Pups in both groups were then given i.n. inoculations of Pneumocystis within 72 h of birth. Activated T cells in the BALF (A, B) and B cells in the TBLN (C) and digested lung tissue (D) were determined by using fluorochrome-labeled antibodies specific for CD4, CD8, CD62L, CD19, and CD80 and flow cytometry at days 6, 14, 21, 28, and 35. Data represent the means ± SD for three to five mice per group.
Since opsonization of Pneumocystis and phagocytosis could lead to activation of macrophages, we used flow cytometry to examine the phenotype of alveolar macrophages of pups born to immune dams compared to that of immunized adults. The phenotype of alveolar macrophages is unique in that they all express CD11c, a molecule usually associated with dendritic cells (14). To examine the presence of macrophages in the BALF of pups and adults infected with Pneumocystis, cells were gated on large, granular cells by forward and side light scatter and then on CD11c+ cells as shown in Fig. 7A. Pneumocystis-infected naïve adult mice had both CD11c-positive and -negative populations in the BALF (Fig. 7A). The CD11c− cells were likely neutrophils since they were also mostly CD11b+ and F4/80− (reference 14 and data not shown). Differential counts of BALF cells confirmed a large proportion of neutrophils in pup lungs between 2 and 4 weeks postinfection. Interestingly, pups from either naïve or immune dams had a single CD11c+ population of large cells in the BALF at days 5 and 12 postinfection, whereas naïve adults had this CD11c− CD11b+ F4/80− population in BALF as early as day 5 postinfection. In contrast, these cells were not found in any appreciable amount in the BALF of immunized adults over the course of the experiment (data not shown).
FIG. 7.
The phenotype of alveolar macrophages was not changed in pups born to immune dams versus naïve dams over time. Dams were given i.t. inoculations of Pneumocystis on the day of breeding and boosted with i.n. inoculums on day 16 of gestation. Control dams received inoculations of PBS. Pups in both groups were then given i.n. inoculations of Pneumocystis within 72 h of birth. BALF was collected at the indicated time points postinfection, and cells were stained for macrophage and activation markers including F4/80, CD11b, CD11c, and MHC class II and analyzed by flow cytometry. (A) BALF flow cytometry data from an individual Pneumocystis-infected naïve adult and a pup from a naïve dam at day 12 postinfection. Forward and side scatters are shown in the left panels. The center panels show CD11c histograms of cells in the large cell region of the scatter profiles. The dot plots on the right side are F4/80 and CD11b staining of the CD11c-positive cells. Proportions of F4/80+ CD11b− CD11c+ (B) and F4/80+ CD11b+ CD11c+ (C) alveolar macrophages present in the BALF of Pneumocystis-infected adult mice compared to neonates are shown. Data represent the means ± SD for four to five mice per group. *, P < 0.05 compared to all other groups at the same time point; **, P < 0.05 compared to adult groups at the same time point; #, P < 0.05 compared to pup groups at the same time point; ##, P < 0.05 compared to immunized adults at the same time point.
When the expression levels of CD11b and F4/80 were examined (both putative macrophage markers) on the CD11c+ cells, we found that almost all of the cells were also positive for F4/80; however, very few of these cells isolated from BALF of pups were positive for CD11b at early time points postinfection (Fig. 7A). In the adult mice, there was a significant population of cells that were CD11c+ F4/80+ CD11b+, whereas this cell population was very small in the lungs of mice infected as neonates at any of the time points examined (Fig. 7A and C). There were significantly more F4/80+ CD11c+ alveolar macrophages in the lungs of Pneumocystis-infected adult mice than in neonates (data not shown); however, the proportion of cells that were F4/80+ CD11c+ was greater in the pups over the first 3 weeks postinfection due to the lack of a lymphocytic response to infection. Interestingly, previous immunization status did not significantly alter the numbers or proportions of these cells isolated from BALF of pups; however, a greater proportion of BALF cells were F4/80+ CD11c+ CD11b− in the immunized adult lungs than in naïve adult lungs (Fig. 7B). Interestingly, the presence of maternal antibody did not result in changes in CD11b expression on neonatal alveolar macrophages (Fig. 7C). In contrast, in previously immunized adult lungs, there were reduced numbers of CD11b+ alveolar macrophages compared to those in Pneumocystis-infected naïve adults (Fig. 7C).
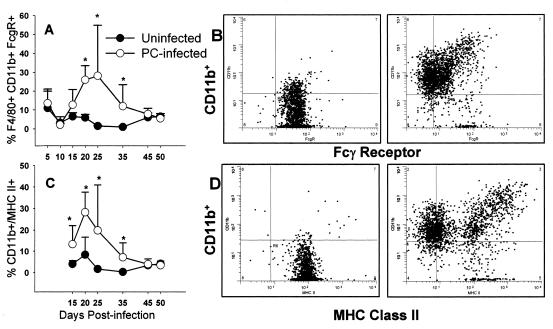
The clearance of organisms by opsonophagocytosis is dependent on receptors for opsonins. As reported above, we found that the αM integrin chain for complement receptor 3, CD11b, is expressed on a reduced proportion of pup alveolar macrophages compared to adults. Since opsonization of Pneumocystis organisms by specific antibody resulted in the control of organism growth, we examined Fcγ receptor expression in mice infected as neonates compared to that in uninfected mice. As shown in Fig. 8B, all F4/80+ alveolar macrophages isolated from pups express FcγR on their surface. However, this pattern changes after day 14 of infection with Pneumocystis. Interestingly, alveolar macrophages expressing FcγR both downregulate FcγR expression and upregulate CD11b expression by day 21 postinfection (Fig. 8B). The proportion of cells in the BALF that express F4/80, CD11b, and FcγR increases significantly at day 21 postinfection in pups (Fig. 8A), whereas there is a significant increase in this population between days 5 and 10 postinfection in adult mice (data not shown). A similar pattern of expression was seen with MHC class II expression in that same experiment (Fig. 8C and D).
FIG. 8.
The pattern of expression of FcγR changed in mice infected with Pneumocystis as neonates. Neonatal mice (48 to 72 h old) were given i.n. inoculations of Pneumocystis or given a sham inoculation of buffer. BALF was collected at the indicated time points postinfection, and cells were stained for macrophage and activation markers including F4/80, CD11b, CD11c, and either FcγR or MHC class II and analyzed by flow cytometry. The proportions of F4/80+ CD11b+ FcγR+ cells (A) and F4/80+ CD11b+ MHC class II-positive cells (C) in BALF over time postinfection are shown. Dot plots showing CD11b versus either FcγR (B) or MHC class II (D) expression in individual mice at day 21 postinfection are shown. Data were gated on nonlymphocytes and F4/80+ cells. Panels on the left show data from a representative uninfected mouse, and panels on the right show data from a Pneumocystis-infected mouse. Data represent the means ± SD for four to five mice per group. *, P < 0.05 compared to pups at the same time point.
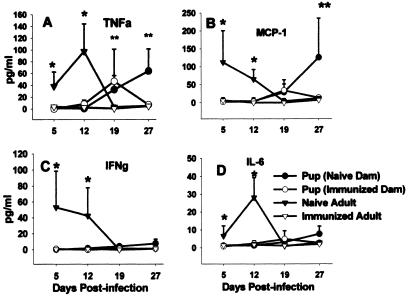
It was previously reported that the delayed clearance of Pneumocystis infection seen in mice infected as neonates corresponds to delayed upregulation of proinflammatory cytokines and chemokines (9, 33). Surprisingly, there was no difference in the concentrations of TNF-α, IFNγ, MCP-1, and IL-6 in the BALF of Pneumocystis-infected pups from immunized dams compared to those of pups born to naïve dams through day 19 postinfection (Fig. 9). By day 27, TNF-α and MCP-1 levels in the BALF of pups from naïve dams had increased significantly above those of BALF of pups from immunized dams (Fig. 9A and B). Consistent with mRNA expression in the lungs (references 9 and 33 and data not shown), there was a significant delay in the appearance of TNF-α, IFNγ, MCP-1, and IL-6 protein in the BALF of Pneumocystis-infected pups born to naïve dams compared to infected naïve adults (Fig. 9). Interestingly, cytokine levels remained very low in the BALF of previously immunized adults even though Pneumocystis lung burden was approximately 30-fold lower in the immune adults (4.0 ± 0.5) than in the naïve adults (log10 Pneumocystis nuclei, 5.6 ± 0.5) at day 12 postinfection. Together, these data indicate that Pneumocystis clearance from either neonatal or adult lungs in the presence of specific antibody takes place without stimulating significant cytokine or chemokine production in the alveolar spaces.
FIG. 9.
No differences in the concentrations of inflammatory cytokines were seen in BALF of Pneumocystis-infected pups born to naïve dams compared to those seen in pups born to immune dams through day 19 postinfection. Dams were given i.t. inoculations of Pneumocystis on the day of breeding and boosted with i.n. inoculums on day 16 of gestation. Control dams received inoculations of PBS. Pups in both groups were then given i.n. inoculations of P. carinii within 72 h of birth. BALF was collected from pups from immunized and naïve dams and from immunized and naïve adult mice. TNF-α, IFNγ, MCP-1, and IL-6 (panels A, B, C, and D, respectively) concentrations were determined by flow cytometry using cytometric bead arrays. Data represent the means ± SD for four to five mice per group. *, P < 0.05 compared to all other groups at the same time point, **, P < 0.05 compared to adult groups at the same time point.
DISCUSSION
Clearance of Pneumocystis is reliant on CD4+ T cells as well as B cells, (16, 24); however, the effector cells required for killing and clearance of the organisms are likely alveolar macrophages (23). It was previously shown that infiltration of CD4+ T cells into the lungs of Pneumocystis-infected neonates is considerably delayed compared to adults (8). Likewise, it was shown that Pneumocystis-specific serum immunoglobulin does not appear until 4 to 5 weeks postinfection in mice infected as neonates (8). Here, we have shown that alveolar macrophage activation is also delayed in Pneumocystis-infected neonates compared to adults. Additionally, we found that rather than stimulating activation of alveolar macrophages, the opsonization of organisms by specific antibody resulted in decreased expression of activation markers on neonatal alveolar macrophages as well as reduced levels of cytokines associated with macrophage activation. These data suggest that neonatal alveolar macrophages are unresponsive to Pneumocystis as measured by activation markers and cytokines; however, opsonization did result in faster clearance kinetics of the organisms.
Specific immunoglobulin can facilitate clearance of Pneumocystis, presumably by opsonizing the organisms and targeting them for phagocytosis and killing. However, we show here that neonatal mice fail to mount B-cell responses to Pneumocystis before the age of weaning. It was previously reported that passive transfer of maternal antibody can facilitate clearance of Pneumocystis (8). Clearance from neonatal mice born to immunized dams occurred prior to significant infiltration of CD4+ T cells into the lungs. This clearance is surprising in that the kinetics are very similar to those of naïve adult mice challenged with Pneumocystis. It has been reported that in some animal models, alveolar macrophage function, including phagocytosis, is impaired in neonates (19, 27). However, other studies have shown that neonatal alveolar macrophages are even more phagocytic than adult cells but that the production of reactive nitrogen and oxygen intermediates by neonatal alveolar macrophages is impaired (21, 22, 37). This contradictory data may have to do with the infectious agent or assays used. Although there are very little data regarding the function of neonatal macrophages in the defense of fungal pathogens, it has been reported that cord blood-derived macrophages were as capable of phagocytosing and killing serum-opsonized Candida albicans isolates as were macrophages derived from adult peripheral blood (25). Our data suggest that there is a developmental lag in the function of alveolar macrophages in regard to the phagocytosing and killing of Pneumocystis as well as in stimulating an inflammatory response. This lag occurs in regard to unopsonized phagocytosis as well as antibody-opsonized phagocytosis.
One possible reason for the lag in killing of opsonized Pneumocystis by neonatal alveolar macrophages is that they are deficient in the expression of Fcγ receptors, as has been reported for human infants during the first week of life (18). In this regard, we have found that adult mice that are deficient in Fcγ receptors I and III have delayed Pneumocystis clearance kinetics compared to wild-type mice (24). However, we found that expression levels of FcγR were similar in alveolar macrophages isolated from adult and neonatal mice. Interestingly, it has recently been suggested that granulocyte-macrophage colony-stimulating factor is important in regulating Fcγ receptor-mediated opsonophagocytosis by stimulating IL-18 or IL-12 production by pathogen-stimulated murine alveolar macrophages (5). There are preliminary data that indicate that there is reduced granulocyte-macrophage colony-stimulating factor mRNA expression as well as reduced protein levels in the lungs of Pneumocystis-infected neonates compared to those in the lungs of adults, which may contribute to the inability of neonatal mice to clear opsonized Pneumocystis more quickly (M. H. Qureshi and B. A. Garvy, unpublished data). Another possibility is that neonatal alveolar macrophages require strong costimulation which is missing in the neonatal lung environment. Consistent with this notion, it was previously found that there is a significant delay in the production of TNF-α and IFNγ in the lungs of Pneumocystis-infected neonates compared to that in lungs of adults (9).
Interestingly, we found that the enhanced clearance seen in neonates born to immunized dams compared to that seen in pups born to naïve dams was not a result of the activation of alveolar macrophages resulting in inflammation. We found that control of Pneumocystis occurred without causing increased production of proinflammatory cytokines and without causing significantly increased expression of macrophage activation markers, particularly CD11b (CR3) and MHC class II. This finding is in contrast to the studies that indicate that the cross-linking of Fcγ receptors on macrophages results in signaling through mitogen-activated protein kinase and TNF-α production (17, 35). However, the lack of inflammation in mice with passive antibody is consistent with the notion that antibody responses can either enhance or reduce inflammation depending on the interactions with cell-mediated immunity and with the organisms (6). Passive antibody has been shown to decrease airway inflammation in a murine model of respiratory syncytial virus infection (28) and prolong survival of mice infected with Cryptococcus neoformans by changing the characteristics and intensity of the pulmonary cellular and cytokine response (7). One explanation for the lack of generation of an inflammatory response to Pneumocystis in neonatal mice might be that there is likely transforming growth factor β present in the postnatally developing lungs (33), which has been shown to inhibit Fcγ receptor signaling in murine macrophages (35). However, a direct connection in this regard has not been proven.
Opsonization of Pneumocystis by polyclonal antiserum prior to inoculation into neonates resulted in a significant reduction in the number of organisms in neonatal lungs compared to the number of organisms in mice that were infected with either unopsonized organisms or organisms opsonized with a MAb specific for KEX1. The 4F11(G1) MAb has been previously shown to be protective against an airborne challenge of Pneumocystis infection in mice when infused i.n. into the lungs of adult SCID mice (11, 20). Using a modified protocol, we found that i.n. inoculations of MAb three times a week into neonatal mice controlled the organism burden compared to mice receiving isotype-matched control antibody (data not shown) but did not result in complete clearance. This was a different strategy than that used for adult SCID mice in which the MAb was used as prophylaxis against naturally acquired infection when infused i.n. (11, 20). However, we were surprised that opsonization of organisms with MAb did not result in control of infection, whereas a polyclonal antiserum caused a fourfold reduction in the number of organisms as early as day 10 postinfection (Table 1). One possible explanation for this finding is that murine IgG1, the isotype of the MAb, does not bind complement as efficiently as the other isotypes in polyclonal serum. Although C3-deficient mice are not susceptible to Pneumocystis infection (24), polyclonal antiserum likely bound complement, which may have contributed to the efficiency of clearance by macrophages. Another possible explanation is that the amount of antibody that coated the surface of the organism was significantly reduced in the 4F11(G1) opsonized group, resulting in reduced cross-linking of Fcγ receptors. This could occur if KEX1 is expressed at low levels compared to gpA, the immunodominant glycoprotein, expressed on Pneumocystis. It is likely that much of the specificity in the polyclonal antiserum was to gpA and several other known epitopes making opsonization more efficient. If the polyclonal antisera were more efficient at stimulating phagocytosis, we could not detect this by examining the phenotype of alveolar macrophages. There was no difference in expression levels of MHC class II, CD11b, and CD40 on alveolar macrophages in neonates infected with Pneumocystis opsonized with polyclonal antiserum compared with those of neonates infected with either unopsonized organisms or organisms opsonized with MAb 4F11(G1). This finding could be due to direct effects of polyclonal antibody on the organisms, as has been shown for other organisms including C. neoformans (38), a possibility that cannot be ruled out by our data.
Together, the data presented here indicate that specific antibody has an important role in the clearance of Pneumocystis from the lungs of mice infected as neonates. Passive transfer of specific antibody might be a reasonable strategy for treating infants with P. carinii pneumonia. However, it appears that alveolar macrophage function develops over time in newborn mice and that it takes several weeks until alveolar macrophages efficiently phagocytose and kill opsonized Pneumocystis organisms. Interestingly, alveolar macrophages in young mice that do phagocytose Pneumocystis do not appear to become fully activated, in that they do not express activation markers or produce proinflammatory cytokines to the same degree as adult alveolar macrophages do. We speculate that this ability to quietly control Pneumocystis infection in the neonatal lung may be an important factor in protecting the postnatally developing lungs from immune-mediated damage.
Acknowledgments
This work was supported by Public Health Service grants HL62053 and HL64524 from the National Heart, Lung, and Blood Institute to B.A.G. and grant AI23302 from the National Institute of Allergy and Infectious Diseases to F.G.
Editor: T. R. Kozel
REFERENCES
- 1.Adkins, B. 1999. T-cell function in newborn mice and humans. Immunol. Today 20:330-335. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B., and R.-Q. Du. 1998. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J. Immunol. 160:4217-4224. [PubMed] [Google Scholar]
- 3.Adkins, B., and K. Hamilton. 1992. Freshly isolated, murine neonatal T cells produce IL-4 in response to anti-CD3 stimulation. J. Immunol. 149:3448-3455. [PubMed] [Google Scholar]
- 4.Bartlett, M. S., S. H. Vermund, R. Jacobs, P. J. Durant, M. M. Shaw, J. W. Smith, X. Tang, J. J. Lu, B. Li, S. Jin, and C.-J. Lee. 1997. Detection of Pneumocystis carinii DNA in air samples: likely environmental risk to susceptible persons. J. Clin. Microbiol. 35:2511-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berclaz, P. Y., Y. Shibata, J. A. Whitsett, and B. C. Trapnell. 2002. GM-CSF, via PU.1, regulates alveolar macrophage FcγR-mediated phagocytosis and the IL-18/IFN-γ-mediated molecular connection between innate and adaptive immunity in the lung. Blood 100:4193-4200. [DOI] [PubMed] [Google Scholar]
- 6.Casadevall, A., and L.-A. Pirofski. 2003. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 24:474-478. [DOI] [PubMed] [Google Scholar]
- 7.Feldmesser, M., A. Mednick, and A. Casadevall. 2002. Antibody-mediated protection in murine Cryptococcus neoformans infection is associated with pleotrophic effects on cytokine and leukocyte responses. Infect. Immun. 70:1571-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garvy, B. A., and A. G. Harmsen. 1996. Susceptibility to Pneumocystis carinii infection: host responses of neonatal mice from immune or naive mothers and of immune or naive adults. Infect. Immun. 64:3987-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garvy, B. A., and M. Qureshi. 2000. Delayed inflammatory response to Pneumocystis carinii infection in neonatal mice is due to an inadequate lung environment. J. Immunol. 165:6480-6486. [DOI] [PubMed] [Google Scholar]
- 10.Gigliotti, F., B. A. Garvy, and A. G. Harmsen. 1996. Antibody-mediated shift in the profile of glycoprotein A phenotypes observed in a mouse model of Pneumocystis carinii pneumonia. Infect. Immun. 64:1892-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gigliotti, F., C. G. Haidaris, T. W. Wright, and A. G. Harmsen. 2002. Passive intranasal monoclonal antibody prophylaxis against murine Pneumocystis carinii pneumonia. Infect. Immun. 70:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gigliotti, F., A. G. Harmsen, and T. W. Wright. 2003. Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect. Immun. 71:3852-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gigliotti, F., and W. T. Hughes. 1988. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J. Clin. Investig. 81:1666-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Juarrero, M., T. S. Shim, A. Kipnis, A. Junqueira-Kipnis, and I. M. Orme. 2003. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J. Immunol. 171:3128-3135. [DOI] [PubMed] [Google Scholar]
- 15.Harmsen, A. G., W. Chen, and F. Gigliotti. 1995. Active immunity to Pneumocystis carinii reinfection in T-cell-depleted mice. Infect. Immun. 63:2391-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harmsen, A. G., and M. Stankiewicz. 1990. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J. Exp. Med. 172:937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, J. W., W. G. Wierda, and Y. B. Kim. 1991. Immobilized IgG immune complex induces secretion of tumor necrosis factor-alpha by porcine alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 5:249-255. [DOI] [PubMed] [Google Scholar]
- 18.Kradin, R. L., K. M. McCarthy, and E. E. Schneeberger. 1986. Opsonic receptor function is reduced on the surface of newborn alveolar macrophages. Am. Rev. Respir. Dis. 133:238-244. [DOI] [PubMed] [Google Scholar]
- 19.Kurland, G., A. T. Cheung, M. E. Miller, S. A. Ayin, M. M. Cho, and E. W. Ford. 1988. The ontogeny of pulmonary defenses: alveolar macrophage function in neonatal and juvenile rhesus monkeys. Pediatr. Res. 23:293-297. [DOI] [PubMed] [Google Scholar]
- 20.Lee, L. H., F. Gigliotti, T. W. Wright, P. J. Simpson-Haidaris, G. A. Weinberg, and C. G. Haidaris. 2000. Molecular characterization of KEX1, a kexin-like protease in mouse Pneumocystis carinii. Gene 242:141-150. [DOI] [PubMed] [Google Scholar]
- 21.Lee, P. T., P. G. Holt, and A. S. McWilliam. 2001. Ontogeny of rat pulmonary alveolar macrophage function: evidence for a selective deficiency in IL-10 and nitric oxide production by newborn alveolar macrophages. Cytokine 15:53-57. [DOI] [PubMed] [Google Scholar]
- 22.Lee, P. T., P. G. Holt, and A. S. McWilliam. 2000. Role of alveolar macrophages in innate immunity in neonates. Evidence for selective lipopolysaccharide binding protein production by rat neonatal alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 23:652-661. [DOI] [PubMed] [Google Scholar]
- 23.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Investig. 99:2100-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lund, F. E., K. Schuer, M. Hollifield, T. D. Randall, and B. A. Garvy. 2003. Clearance of Pneumocystis carinii in mice is dependent on B cells but not on P. carinii-specific antibody. J. Immunol. 171:1423-1430. [DOI] [PubMed] [Google Scholar]
- 25.Marodi, L., R. Kaposzta, D. E. Campbell, R. A. Polin, J. Csongor, and R. B. Johnston, Jr. 1994. Candidacidal mechanisms in the human neonate: impaired IFN-γ activation of macrophages in newborn infants. J. Immunol. 153:5643-5649. [PubMed] [Google Scholar]
- 26.Marshall-Clarke, S., D. Reen, L. Tasker, and J. Hassan. 2000. Neonatal immunity: how well has it grown up? Immunol. Today 21:35-41. [DOI] [PubMed] [Google Scholar]
- 27.Martin, T. R., J. T. Ruzinski, C. E. Rubens, E. Y. Chi, and C. B. Wilson. 1992. The effect of type-specific polysaccharide capsule on the clearance of group B streptococci from the lungs of infant and adult rats. J. Infect. Dis. 165:306-314. [DOI] [PubMed] [Google Scholar]
- 28.Mejias, A., S. Chávez-Bueno, A. M. Ríos, J. Saavedra-Lozano, M. Fonseca-Aten, J. Hatfield, P. Kapur, A. M. Gómez, H. S. Jafri, and O. Ramilo. 2004. Anti-respiratory syncytial virus (RSV) neutralizing antibody decreases lung inflammation, airway obstruction, and airway hyperresponsiveness in a murine RSV model. Antimicrob. Agents Chemother. 48:1811-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson, D. J., and P. G. Holt. 1995. Defective regional immunity in the respiratory tract of neonates is attributable to hyporesponsiveness of local dendritic cells to activation signals. J. Immunol. 155:3517-3524. [PubMed] [Google Scholar]
- 30.Nelson, D. J., C. McMenamin, A. S. McWilliam, M. Brenan, and P. G. Holt. 1994. Development of the airway intraepithelial dendritic cell network in the rat from class II major histocompatibility (Ia)-negative precursors: differential regulation of Ia expression at different levels of the respiratory tract. J. Exp. Med. 179:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peglow, S. L., A. G. Smulian, M. J. Linke, C. L. Pogue, S. Nurre, J. Crisler, J. Phair, J. W. Gold, D. Armstrong, and P. D. Walzer. 1990. Serologic responses to Pneumocystis carinii antigens in health and disease. J. Infect. Dis. 161:296-306. [DOI] [PubMed] [Google Scholar]
- 32.Pihlgren, M., C. Tougne, P. Bozzotti, A. Fulurija, M. A. Duchosal, P.-H. Lambert, and C.-A. Siegrist. 2003. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J. Immunol. 170:2824-2832. [DOI] [PubMed] [Google Scholar]
- 33.Qureshi, M. H., and B. A. Garvy. 2001. Neonatal T cells in an adult lung environment are competent to resolve Pneumocystis carinii pneumonia. J. Immunol. 166:5704-5711. [DOI] [PubMed] [Google Scholar]
- 34.Qureshi, M. H., A. G. Harmsen, and B. A. Garvy. 2003. IL-10 modulates host responses and lung damage induced by Pneumocystis carinii infection. J. Immunol. 170:1002-1009. [DOI] [PubMed] [Google Scholar]
- 35.Rose, D. M., B. W. Winston, E. D. Chan, D. W. H. Riches, and P. M. Henson. 1997. Interferon-γ and transforming growth factor-β modulate the activation of mitogen-activated protein kinases and tumor necrosis factor-α production induced by Fcγ-receptor stimulation in murine macrophages. Biochem. Biophys. Res. Commun. 238:256-260. [DOI] [PubMed] [Google Scholar]
- 36.Roths, J. B., and C. L. Sidman. 1993. Single and combined humoral and cell-mediated immunotherapy of Pneumocystis carinii pneumonia in immunodeficient scid mice. Infect. Immun. 61:1641-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherman, M. P., and R. I. Lehrer. 1985. Oxidative metabolism of neonatal and adult rabbit lung macrophages stimulated with opsonized group B streptococci. Infect. Immun. 47:26-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taborda, C. P., and A. Casadevall. 2002. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16:791-802. [DOI] [PubMed] [Google Scholar]
- 39.Totet, A., N. Respaldiza, J.-C. Pautard, C. Raccurt, and G. Nevez. 2003. Pneumocystis jiroveci genotypes and primary infection. Clin. Infect. Dis. 36:1340-1342. [DOI] [PubMed] [Google Scholar]
- 40.Vargas, S. L., W. T. Hughes, M. E. Santolaya, A. V. Ulloa, C. A. Ponce, C. E. Cabrera, F. Cumsille, and F. Gigliotti. 2001. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin. Infect. Dis. 32:855-861. [DOI] [PubMed] [Google Scholar]
- 41.Vargas, S. L., C. A. Ponce, W. T. Hughes, A. E. Wakefield, J. C. Weitz, S. Donoso, A. V. Ulloa, P. Madrid, S. Gould, J. J. Latorre, R. Avila, S. Benveniste, M. Gallo, J. Belletti, and R. Lopez. 1999. Association of primary Pneumocystis carinii infection and sudden infant death syndrome. Clin. Infect. Dis. 29:1489-1493. [DOI] [PubMed] [Google Scholar]
- 42.Wakefield, A. E. 1996. DNA sequences identical to Pneumocystis carinii f. sp. carinii and Pneumocystis carinii f. sp. hominis in samples of air spora. J. Clin. Microbiol. 34:1754-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]