Abstract
We report the first phase I trial to assess the safety and immunogenicity of a malaria vaccine candidate, ICC-1132 (Malarivax), composed of a modified hepatitis B virus core protein (HBc) containing minimal epitopes of the Plasmodium falciparum circumsporozoite (CS) protein. When expressed in Escherichia coli, the recombinant ICC-1132 protein forms virus-like particles that were found to be highly immunogenic in preclinical studies of mice and monkeys. Twenty healthy adult volunteers received a 20- or a 50-μg dose of alum-adsorbed ICC-1132 administered intramuscularly at 0, 2, and 6 months. The majority of volunteers in the group receiving the 50-μg dose developed antibodies to CS repeats as well as to HBc. Malaria-specific T cells that secreted gamma interferon were also detected after a single immunization with ICC-1132-alum. These studies support ICC-1132 as a promising malaria vaccine candidate for further clinical testing using more-potent adjuvant formulations and confirm the potential of modified HBc virus-like particles as a delivery platform for vaccines against other human pathogens.
Vaccination to protect against Plasmodium falciparum, the most deadly species of malaria agents in humans, was first demonstrated in volunteers experimentally immunized with attenuated sporozoites delivered by the bite of irradiated malaria-infected mosquitoes (10). Sporozoite-induced immunity in humans, and in experimental rodent and primate models, is associated with high levels of antibody to a repetitive epitope within a sporozoite surface antigen, the circumsporozoite (CS) protein, and with CS-specific cellular responses (26, 29). Levels of gamma interferon (IFN-γ), produced by CD4+ or CD8+ T cells, also correlate with sporozoite-induced protection in murine studies. A subunit vaccine eliciting high levels of humoral immunity to target the extracellular sporozoite, combined with cellular immunity to target the intracellular liver stages, would effectively block development of the erythrocytic parasite life cycle that is responsible for clinical disease.
Phase I and II trials of the first malaria subunit vaccines, comprising peptide-protein conjugates or recombinant proteins containing CS repeats, demonstrated for a small number of volunteers that sterile immunity could be elicited by CS-based vaccines (2, 18). The early peptide vaccines were limited by low epitope density and suboptimal adjuvant formulations, and considerable effort has been invested in identifying new, more effective vaccine delivery platforms. Recently, virus-like particles have been shown to provide highly immunogenic vaccine candidates for a number of infectious diseases (20, 28). Phase I and II trials of a virus-like particle malaria vaccine, RTS,S, composed of a hepatitis B virus (HBV) surface antigen (HBsAg) containing the repeat region and C terminus of P. falciparum CS protein (amino acids [aa] 207 to 395), demonstrated sterile protection, i.e., complete absence of detectable blood-stage infection, in approximately 41% of immunized volunteers (5, 15, 19, 43). Induction of sterile immunity depended on use of a potent proprietary adjuvant containing monophosphoryl lipid A and QS21, a purified saponin, in an oil-in-water emulsion (43). Resistance to challenge was associated with high levels of CS-specific antibody and IFN-γ-producing T cells (15, 19, 44). However, protection in RTS,S-immunized volunteers was short-lived, decreasing to background levels ∼2 months after immunization (5, 42).
In addition to HBsAg, the HBV genome encodes a core (HBc) protein that also self-assembles into virus-like particles. Recombinant HBc engineered to express viral or bacterial epitopes can induce strong pathogen-specific cellular and humoral immunity in various animal models (34, 46). Mice immunized with HBc particles expressing epitopes of rodent malaria CS protein, produced in recombinant Salmonella enterica serovar Typhimurium, developed high levels of anti-CS repeat antibody and were totally protected against blood-stage infection following challenge with infectious sporozoites (37, 38).
In recent preclinical studies, Escherichia coli expressing modified hybrid core (HBc) particles containing T- and B-cell epitopes of P. falciparum CS protein elicited anti-CS repeat antibody titers of >1 × 106 in mice and monkeys and CS-specific CD4+ T cells in mice (3, 4, 22). This HBc particle candidate vaccine, ICC-1132 (Malarivax), is composed of a protective B-cell epitope from the immunodominant CS repeat region and two T-cell epitopes defined by human CD4+ T-cell clones derived from sporozoite-immunized volunteers (Fig. 1). One of these T-cell epitopes, termed T*, elicits strong Th1-type CD4+ T cells and is presented by a broad range of HLA class II molecules, and it is therefore considered to be “universal” (7, 23). The other epitope, termed T1, is recognized in the context of a limited number of HLA class II molecules (25, 27). Synthetic peptide malaria vaccines containing these CS sequences elicited anti-CS repeat antibodies and T1- and T*-specific CD4+ T cells in small phase I trials (24, 27). These peptide-induced human CD4+ T-cell clones proliferated and produced IFN-γ when stimulated with ICC-1132, indicating that the T1 and T* epitopes are effectively processed and presented in the context of HBc virus-like particles (3).
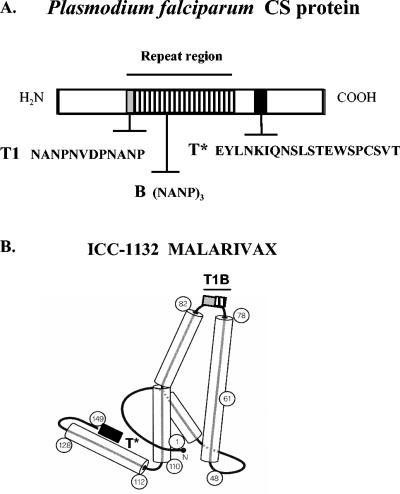
FIG. 1.
(A) Illustration showing the location of immunodominant B-cell epitope and the T1 epitope within the central repeat region and the universal T* epitope in the C terminus of P. falciparum CS protein. (B) Diagram of ICC-1132 monomer showing the CS protein T1 and B repeat epitopes inserted in the loop region (light and hatched boxes) and the T* epitope inserted at the C terminus of the truncated HBc protein (dark box). HBc monomer α-helical regions are represented as cylinders, and HBc amino acid residues are shown in circles. Reprinted and modified from Nature (6) with permission of the author and publisher.
The ability to express these minimal B- and T-cell epitopes of P. falciparum CS at high density in HBc virus-like particles, which are known to elicit high levels of persistent antibody responses following HBV infection and to function as highly immunogenic carriers for foreign epitopes, makes ICC-1132 a promising malaria vaccine candidate. The present report summarizes the results obtained in the first phase I trial to assess the safety and immunogenicity of ICC-1132. The outcome of this study confirms the potential of modified HBc virus-like particles as a delivery platform for human vaccines.
MATERIALS AND METHODS
ICC-1132 (Malarivax) production.
The ICC-1132 recombinant protein was expressed in E. coli (strain BLR) transfected with the expression plasmid V17.Pf3.1, which is a kanamycin resistance version of the expression plasmid described previously (3). The plasmid encodes a truncated HBc gene (aa 1 to 149) containing the CS protein T* epitope fused to the C terminus following Val149 and CS repeat epitopes, T1 and B, inserted into the HBc immunodominant loop between amino acid residues Asp78 and Pro79 (Fig. 1). Native and recombinant HBc proteins self-assemble into an icosahedral virus-like particle approximately 30 nm in diameter and composed of 240 monomers (3, 6). Based on HBV core protein structure (6, 12), the CS repeats contained in ICC-1132 are localized at the tip of surface spikes on the particle formed by dimerization of HBc monomers (Fig. 1). The T* epitope replaces the HBc protamine domain (residues 150 to 183) responsible for binding the viral nucleic acid and is therefore most likely oriented to the inner surface of the core particle.
ICC-1132 was purified by ammonium sulfate precipitation, followed by size exclusion, hydrophobic interaction, and hydroxyapatite chromatography, and sterilized by filtration through 0.2-μm-pore-size filters. Endotoxin levels in the final purified product are less than 3 U of endotoxin/μg of ICC-1132, as determined by a Limulus Amoebocyte Lysate test (USP <85>). ICC-1132 was adsorbed to aluminum hydroxide (Alhydrogel; Superfos, Frederikssund, Denmark), with >95% adsorption as determined by measurement of residual unbound protein. Each 1.0 ml of vaccine contained 1 mg of aluminum as Al(OH)3.
Study design.
Twenty-four healthy adult volunteers aged 18 to 45 were recruited in Cardiff, Wales, United Kingdom. Informed consent was obtained from all volunteers, and the phase I trial was approved by the Ethics Committee (SIMBEC) in Cardiff, United Kingdom and the Institutional Review Board at New York University School of Medicine.
The volunteers were screened by physical examination and by laboratory analysis of hematology, serum biochemistry, serology, and urine. Exclusion criteria included medical history or serologic indications of malaria, hepatitis or human immunodeficiency virus infection, or any significant cardiovascular, hepatic, or renal function abnormalities.
The phase I trial was conducted as a double-blind, placebo-controlled, dose-escalating study to assess the safety and immunogenicity of 20- and 50-μg doses of ICC-1132-alum. In each dose cohort, 10 volunteers received vaccine and 2 volunteers received a saline placebo, administered as a 0.5-ml injection in the deltoid muscle on days 0, 56, and 168. Immunizations were staggered so that the reactogenicity of the 20-μg dose of vaccine could be assessed by an independent medical monitor prior to injection of the 50-μg dose. Booster injections were administered after review of 2-week follow-up safety data from the prior injection.
Local and systemic reactions were assessed for 60 min after each injection. Clinical examinations were carried out at 1, 7, and 14 days postinjection. Follow-up was monitored by telephone interview on days 2 and 4 and by self-report in personal diaries. Variables evaluated included fever, chills, malaise, local pain, and headache and associated physical findings of erythema, induration, or lymphadenopathy. Urinalysis and blood sample analysis for hematology, biochemistry, and serology were carried out at the time of immunization and 14 days after each injection.
Serologic assays.
Serum samples obtained at various time points during immunization were analyzed by an enzyme-linked immunosorbent assay (ELISA) using peroxidase-labeled antibodies specific for human immunoglobulin G (IgG), IgM (Cappel, Durham, N.C.), or IgG1 to IgG4 subtypes (Southern Biotechnology, Birmingham, Ala.) (24, 27). Wells were coated with ICC-1132, HBc recombinant protein, or malaria tetrabranched synthetic peptides containing the CS repeat sequence (T1B)4 or the T* sequence (T*)4 (24). The endpoint titer was the reciprocal serum dilution giving an optical density (OD) greater than the mean plus 2 standard deviations of results obtained with preimmune sera. Differences of ≥4-fold in titers were considered significant.
An indirect immunofluorescence assay (IFA) was carried out using air-dried P. falciparum (NF54) sporozoites and fluorescein isothiocyanate-labeled anti-human IgG (Kirkegaard and Perry, Gaithersburg, Md.) (24, 27). Reactivity of immune sera with viable sporozoites was determined by measuring circumsporozoite precipitin (CSP) reactions by using transgenic rodent Plasmodium berghei sporozoites expressing P. falciparum CS repeats (31). CSP is a precipitate that forms on viable sporozoites when surface CS protein is cross-linked by anti-repeat antibodies (11, 33, 47).
Cellular assays.
Peripheral blood mononuclear cells (PBMC) were obtained prior to and 14 days after the second dose of 50 μg of ICC-1132. The cells were purified with a citrate CPT Vacutainer (BD Biosciences, San Diego, Calif.) and assayed with IFN-γ enzyme-linked immunospot (ELISPOT) kits (BD Biosciences). PBMC at a concentration of 1 × 106 to 2 × 106/ml were cultured for 7 days in medium containing 5 μg of ICC-1132/ml and recombinant human interleukin-2 (20 U/ml; Sigma). Cells were incubated for 24 h in medium alone prior to incubation in anti-IFN-γ-coated nitrocellulose wells in the presence or absence of 10-μg/ml concentrations of ICC-1132, HBc, or recombinant CS (rCS) protein or synthetic peptide containing the CS T* or repeat sequences. The rCS protein was nearly full-length, minus the first 26 aa in the N terminus and the final 13 aa of the C terminus. Following incubation for 16 to 18 h, washed wells were reacted with biotinylated anti-IFN-γ antibody and avidin-horseradish peroxidase followed by addition of 3-amino-9-ethylcarazole substrate. Spots, representing individual IFN-γ-secreting cells, were counted by using an ELISPOT reader (Zeiss, Hallbergmoos, Germany). Volunteers' cells showed significant variation in the number of spot-forming cells (SFC) in unstimulated wells (day 56 mean, 175 ± 137 SFC/106 cells; day 70 mean, 112 ± 186 SFC/106 cells), and results therefore are expressed as the increase (n-fold), calculated by dividing the number of SFC in antigen-stimulated wells by the number of SFC in wells without antigen. An increase in SFC of ≥5-fold was defined as positive. Cells from the group receiving the 20-μg dose could not be evaluated due to high backgrounds.
RESULTS
Safety.
Immunization with three doses of ICC-1132 was well tolerated, with no serious adverse events or abnormal blood chemistries associated with the vaccine (Table 1). Two volunteers in the 50-μg dose group were removed from the trial, one due to noncompliance and one due to cellulitis of the lip that developed 35 days after the first injection and was considered unlikely to be vaccine associated.
TABLE 1.
Frequency of local and systemic reactions
| Reaction | No. (%) of vaccinees reporting reaction after indicated injection with:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 μg of ICC-1132
|
50 μg of ICC-1132
|
Placeboa
|
|||||||
| 1st (n = 10) | 2nd (n = 10) | 3rd (n = 10) | 1st (n = 10) | 2nd (n = 8) | 3rd (n = 8) | 1st (n = 4) | 2nd (n = 4) | 3rd (n = 4) | |
| Local | |||||||||
| Tenderness | 0 | 0 | 0 | 40 | 75 | 0 | 0 | 0 | 0 |
| Pain at injection site | 0 | 0 | 20 | 30 | 25 | 25 | 0 | 0 | 0 |
| Erythema | 0 | 0 | 10 | 10 | 38 | 0 | 0 | 0 | 0 |
| Arm limitation | 0 | 0 | 10 | 10 | 38 | 13 | 0 | 0 | 0 |
| Systemic | |||||||||
| Headache | 40 | 30 | 10 | 30 | 0 | 25 | 75 | 50 | 0 |
| Painful arm | 0 | 20 | 0 | 0 | 13 | 13 | 0 | 25 | 0 |
Four volunteers in the placebo group received saline injections.
Of the 18 vaccinees who received all three doses of vaccine, the most frequent complaints noted were mainly local and included tenderness and pain at the injection site (Table 1). There was a trend toward increased reactogenicity with increasing dose of vaccine, with the majority of reactions reported after the first and second immunizations with 50 μg of ICC-1132. Systemic reactions, consisting predominantly of headache and painful arm, were similar for volunteers receiving vaccine and placebo.
Immunogenicity. (i) Humoral immune responses induced by ICC-1132.
All of the volunteers seroconverted to ICC-1132 following two to three immunizations with the 20- or 50-μg dose, indicating that the response to the vaccine was not genetically restricted (Table 2). The vaccine was highly immunogenic, with the majority of volunteers (5 of 8) in the 50-μg dose group having antibodies to ICC-1132 detectable 14 to 28 days after a single immunization (data not shown). Peak anti-ICC-1132 titers from seropositive volunteers were not significantly different in the 20-μg dose group (geometric mean titer [GMT] of 2,389) and the 50-μg dose group (GMT of 2,153) (Table 2).
TABLE 2.
IgG antibody titers in seropositive volunteers immunized with ICC-1132-alum
| Dose | ICC-1132
|
HBc
|
CS repeats
|
|||
|---|---|---|---|---|---|---|
| No. positive/no. tested (%)a | GMTb | No. positive/no. tested (%) | GMT | No. positive/no. tested (%) | GMT | |
| 20 μg | 10/10 (100) | 2,389 (320-40,960) | 9/10 (90) | 1,613 (320-20,480) | 4/10 (40) | 538 (160-2,560) |
| 50 μg | 8/8 (100) | 2,153 (320-40,960) | 7/8 (88) | 2,100 (320-40,960) | 6/8 (75) | 508 (80-2,560) |
Seroconversion rate is shown as the number of volunteers in each cohort with positive antibody responses as detected by an ELISA using ICC-1132, HBc, or CS repeats as antigen.
GMTs of seropositive volunteers measured 14 days after the third immunization with ICC-1132-alum (day 182). The range of antibody titers in each dosage group is shown in parentheses.
The development of HBc-specific antibodies was delayed relative to the response to ICC-1132, with only a single individual in each dosage group seroconverting after the first injection (data not shown). The delay in development of anti-HBc antibodies (Fig. 2) is consistent with disruption of an immunodominant HBc B-cell epitope located at aa 78 to 82 of the loop region by insertion of the CS repeat epitopes. Following booster injections, the majority (88 to 90%) of volunteers developed anti-HBc antibodies that reached peak GMTs similar to peak anti-ICC-1132 titers. HBc GMTs in seropositive individuals in the 20-μg dose group were lower than, but not significantly different from, those in individuals in the 50-μg dose group.
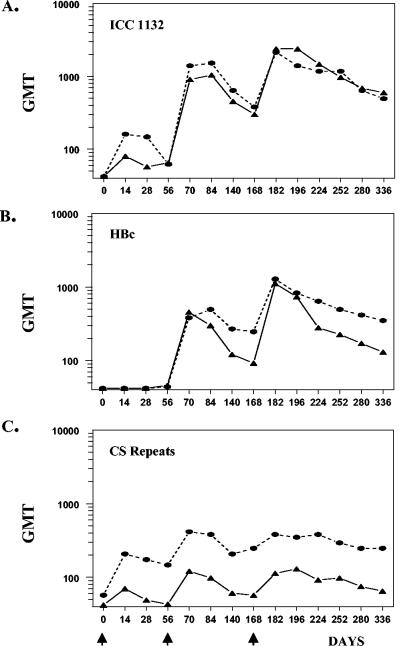
FIG. 2.
Kinetics of the IgG antibody response measured by ELISA using ICC-1132 (A), HBc protein without CS inserts (B), and P. falciparum CS repeat peptide (C). Results are shown as GMTs for all vaccinees in the 50-μg dose (circles) and 20-μg dose (triangles) cohorts at various time points postvaccination (vaccinations are indicated by arrows).
Consistent with localization of the CS repeat epitopes in the HBc immundominant loop region, anti-CS repeat antibodies could be detected after a single immunization with either 20 or 50 μg of ICC-1132 (data not shown). Following booster immunizations, 75% of volunteers in the 50-μg dose group developed anti-CS repeat antibodies, although the peak GMT in seropositive volunteers was three- to fourfold lower than titers to ICC-1132 and HBc (Table 2). The anti-CS repeat seroconversion rate was dose dependent, with 40% seropositivity in the 20-μg dose group compared to 75% in the 50-μg dose group. As noted in the response to ICC-1132 and HBc, there was no significant difference in anti-CS repeat GMTs in seropositive individuals in the 20- and 50-μg dose groups. In contrast to the antibody response to the malaria CS repeat epitope, little or no antibody to the T* epitope was detected in the vaccinees (data not shown).
Although of lower magnitude, the kinetics of the anti-CS repeat antibody response were similar to that of ICC-1132 (Fig. 2A and C). While the primary response level to HBc was low, booster immunizations induced secondary anti-HBc antibody responses with kinetics similar to those of ICC-1132 and CS repeat responses (Fig. 2B). Titers for all three antigens decreased during the 4 months following the second immunization, with decreases in HBc and CS greater in the 20-μg dose group than in the 50-μg dose group. Following the third dose of vaccine, antibody titers increased, reaching peak titers that were not significantly different from peak titers obtained after the second dose.
The antibody response elicited by ICC-1132 persisted to day 336, approximately 6 months after the third and final immunization, when the trial was concluded. In the six volunteers seropositive for CS repeats in the 50-μg dose group, the anti-CS repeat titers at day 336 did not decrease significantly from the peak GMT at day 182, and IFA remained positive in three of six volunteers. Persistence of the anti-CS repeat response was dose dependent, as only two of four seropositive volunteers in the 20-μg dose group still had positive anti-CS repeat ELISA and IFA titers on day 336.
(a) Antibody reactivity with Plasmodium sporozoites.
The anti-CS repeat antibodies elicited by immunization with ICC-1132 recognized native CS on the surface of P. falciparum sporozoites. There was a positive correlation between IFA titers and anti-CS repeat peptide ELISA titers for the majority of vaccinees (r = 0.95) (Fig. 3). Important for vaccine efficacy, the anti-CS repeat antibodies elicited by ICC-1132 also reacted with viable Plasmodium sporozoites and elicited positive CSP reactions when incubated with viable transgenic sporozoites expressing P. falciparum CS repeats. Only sera from volunteers with high titers of anti-repeat antibodies were able to cross-link the CS protein on the surface of viable sporozoites and elicit CSP reactions.
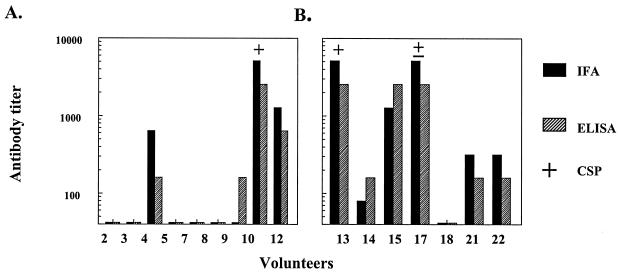
FIG. 3.
CS-specific antibody responses in sera obtained 2 weeks after the third immunization with 20-μg (A) or 50-μg (B) doses, as measured by IFA (dark bars) or CS repeat peptide ELISA (hatched bars). All sera were tested in a CSP assay using viable transgenic sporozoites expressing P. falciparum CS repeats. +, sera with strong CSP reactions (greater than one-half the length of the sporozoite); ±, weak positive reactions.
It was noteworthy that the ability to elicit CSP reactions and the quality of the reactions varied in sera with similar high ELISA and IFA titers (Fig. 3). Serum from volunteer 15 was CSP negative, despite having antibody titers comparable to those of serum from volunteers 10 and 13. Similarly, sera from volunteers 10 and 13 elicited strong CSP, frequently consisting of long terminal precipitates greater than two to four times the length of the sporozoite, while serum from volunteer 17 elicited shorter and weaker reactions. Qualitative differences were also noted; for 20 sporozoites counted in each assay, sera from volunteers 10 and 13 averaged 16 and 7 CSP-positive sporozoites, respectively, while serum from volunteer 17 averaged only 2 CSP-positive sporozoites (data not shown). Moreover, CSP reactivity persisted at a 1:4 dilution of sera from volunteers 10 and 13 but not 17 (data not shown).
(b) Antibody isotypes.
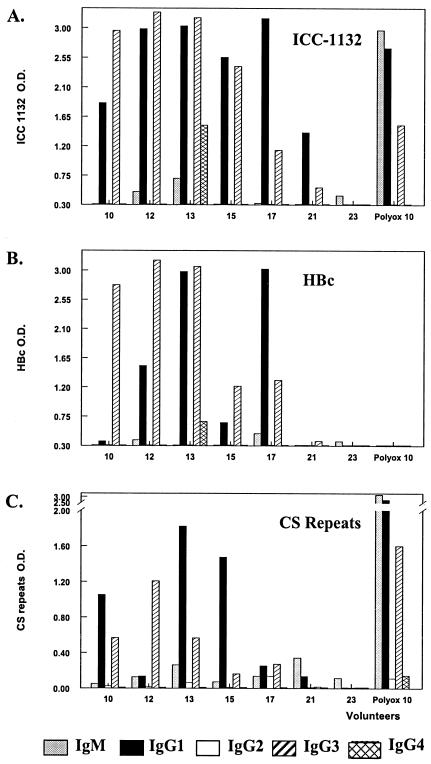
Immunization with ICC-1132 elicited IgG1 and IgG3 antibodies specific for CS repeats, as well as for the immunogen and HBc (Fig. 4). The ratio of IgG1 to IgG3 differed in each serum, depending on donor and antigen. For example, volunteers 10 and 15 had a strong IgG1 response to CS repeats but not to HBc, and conversely, volunteer 12 had a strong IgG1 response to HBc but not to CS repeats. Frequency of Th2-type antibody subtypes was low. Volunteer 13 had IgG4 antibodies specific for both ICC-1132 and HBc. Volunteer 17 had detectable IgG2 antibodies to CS repeats but only low levels of IgG1 and IgG3.
FIG. 4.
IgM and IgG subtypes measured using ICC-1132 (A), HBc (B), or CS repeats (C) as the ELISA antigen. Results shown are the ΔOD (the OD of antigen-stimulated wells minus the OD of wells coated with bovine serum albumin) obtained with a 1:100 dilution of sera from vaccinees in the 20-μg (volunteers 10 and 12) and 50-μg (volunteers 13, 15, 17, and 21) dose groups. Volunteer 23 was a saline control. A positive control serum, Polyox 10, was obtained from a volunteer in a previous phase I trial of a polyoxime peptide vaccine containing the same CS epitopes (24).
IgM responses to all antigens were low (ELISA titer: range, 160 to 320) in all of the ICC-1132 vaccinees (data not shown). This result is in contrast to the strong IgM anti-CS repeat antibody response noted in volunteers immunized with synthetic peptide malaria vaccines (24, 27). This high IgM response is illustrated by polyox 10 serum (Fig. 4A and C) obtained from a volunteer immunized with a (T1BT*) polyoxime malaria peptide vaccine in a previous phase I study (24).
(ii) Cellular immunity induced by ICC-1132.
The production of IFN-γ by Th1-type T helper cells stimulates differentiation of IgG1- and IgG3-producing B cells. Consistent with the Th1 bias of the antibody response in the vaccinees, antigen-specific IFN-γ-secreting T cells were detected by ELISPOT on days 56 and 70. A single immunization induced a pronounced ICC-1132-specific IFN-γ response in three of eight volunteers in the 50-μg dose group (Fig. 5A). Cells from two of these volunteers, 14 and 18, also responded to stimulation with rCS protein, with 7- to 11-fold increases in the number of SFC (mean, 641 ± 288 SFC/106 cells). In addition, cells of these two volunteers had an 11- to 20-fold-higher response to the malaria T* peptide (mean, 1,181 ± 511 SFC/106 cells). The CS repeat peptide and HBc protein did not stimulate positive IFN-γ ELISPOT (increase, ≤3-fold), supporting the specificity of the responses to P. falciparum epitopes. Cells from placebo controls, volunteers 16 and 23, did not have increased responses to any of the antigens (mean, ≤4-fold), indicating that T-cell responses detected in the ELISPOT assay were not due to in vitro antigen priming.
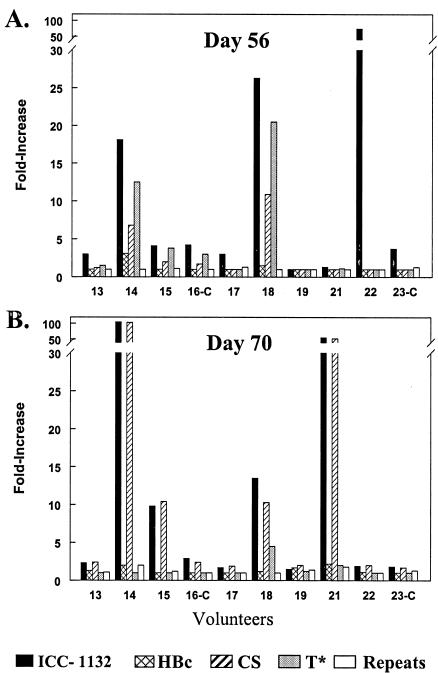
FIG. 5.
IFN-γ ELISPOT carried out using cultured PBMC obtained from volunteers in the 50-μg dose group 56 days after the first immunization (A) and 14 days after the second immunization (day 70) (B). IFN-γ SFC were measured following overnight incubation with recombinant proteins (ICC-1132, HBc, or rCS) or malaria CS peptides (T* or repeats). Due to the wide range of backgrounds in unstimulated wells obtained with cells from different volunteers (day 56 range, 24 to 384 SFC/106 cells in unstimulated wells; day 70 range, 7 to 563 SFC/106 cells in unstimulated wells), the results are shown as the increase (n-fold) in the number of SFC (mean SFC in triplicate wells with antigen divided by mean SFC in wells without antigen). Volunteers 16-C and 23-C were saline placebo controls.
Following the second dose of the vaccine, four of eight volunteers in the 50-μg dose group showed IFN-γ secretion recalled by the immunogen, with a mean 46-fold increase in the number of SFC (mean, 606 ± 264 SFC/106 cells) versus unstimulated wells (Fig. 5B). All four vaccinees also developed a similar 44-fold increase in SFC numbers following stimulation with the malaria rCS protein (mean, 586 ± 278 SFC/106 cells) (Fig. 5B). Two of the volunteers with positive ICC-1132 and rCS ELISPOTs on day 70 were also positive on day 56 (volunteers 14 and 18), and two volunteers (15 and 21) became positive after the booster inoculation. Volunteer 18 remained positive for T* peptide, although at a low level.
The positive IFN-γ ELISPOT results did not correlate with anti-ICC-1132 or anti-CS repeat antibody responses in individual vaccinees. Volunteer 13 had high antibody titers to both antigens but did not have increased IFN-γ ELISPOTs, while volunteer 18, with positive ELISPOTs, had antibody titers of 640 and <80 to immunogen and CS repeats, respectively.
DISCUSSION
The present study represents the first phase I testing of a parentally administered purified HBc virus-like particle as a vaccine carrier in volunteers. The alum-adsorbed ICC-1132 was well tolerated and elicited malaria-specific antibody, as well as CS-specific T cells that produced IFN-γ, in the majority of vaccinees receiving the 50-μg dose. All volunteers seroconverted to ICC-1132 at both doses. The anti-CS repeat seroconversion rate was dose dependent, with 40 and 75% of responders in the 20- and 50-μg dose groups, respectively, undergoing seroconversion (Table 2).
There was a strong positive correlation between ELISA and IFA titers, indicating that the anti-CS repeat antibodies elicited by ICC-1132 reacted with native CS protein on the surface of P. falciparum sporozoites. These vaccine-induced antibody responses were biologically relevant, as sera with high levels of anti-CS repeat antibodies efficiently cross-linked surface CS, producing a CSP reaction on viable transgenic sporozoites expressing P. falciparum CS repeats. The difference in the presence and quality of CSP reactions noted in sera with comparable ELISA and IFA titers (Fig. 3) suggests that analysis of CSP reactivity with viable sporozoites may provide additional information on the affinity and/or avidity of vaccine-induced anti-CS repeat antibodies. These differences may be functionally important, as binding of anti-CS repeat antibodies to surface CS immobilizes the sporozoite and blocks parasite invasion of host cells (29, 41).
The anti-malaria antibody response elicited in the ICC-1132 vaccinees was directed against the CS repeats, with little antibody to the T* epitope. This result is consistent with the location of the CS repeats in the HBc immunodominant loop region, while the T* epitope at the hydrophobic C terminus of the HBc was located in a position that frequently elicits poor antibody responses (36, 46). These malaria antibody specificities are comparable to those of P. falciparum sporozoite-immunized volunteers which were predominantly anti-CS repeat, with few or no antibodies to nonrepeat regions of the CS protein (8). The insertion of the T- and B-cell epitopes in strategic positions within HBc virus-like particles may more accurately mimic presentation of epitopes in the native CS protein on the sporozoite surface. Insertion of larger CS fragments in HBsAg virus-like particles, as in RTS,S, elicited significant antibody response to nonrepeat epitopes in the C terminus of the CS protein (15, 19, 43). Thus far, only antibodies to CS repeats have been shown to be protective in vivo (16, 19, 29). The inclusion of only well-defined minimal CS epitopes in ICC-1132 may focus the immune response on relevant P. falciparum epitopes and elicit antibody specificities comparable to those observed following immunization with irradiated sporozoites, which remains the “gold standard” for vaccine development.
The vaccinees developed IgG1 and IgG3 antibodies to CS repeats, with little or no Th2-type antibody. In contrast, in murine studies all IgG subtypes were elicited following immunization with ICC-1132 (3). This strongly biased Th1-type response in humans was obtained despite the use of alum adjuvant, which enhances Th2-type antibodies. Whether differences in antibody subtypes have functional consequences in vivo following P. falciparum sporozoite challenge is unknown. Human IgG1 and IgG3 are opsonizing antibodies that can synergistically interact with IFN-γ-activated macrophages to enhance phagocytosis and destruction of opsonized sporozoites (13). Recent in vitro studies, based on uptake of fluorescein isothiocyanate-labeled CS by a human macrophage cell line, demonstrated a positive correlation with opsonizing antibodies in sera from RTS,S-immunized volunteers and resistance to P. falciparum sporozoite challenge (40).
While T* is a poor B-cell epitope, it is a strong T-cell epitope. Volunteers immunized with irradiated sporozoites, or synthetic peptide vaccines containing this epitope, develop T*-specific CD4+ T cells that produce IFN-γ when stimulated with rCS protein (23, 24). IFN-γ is important for vaccine efficacy, as it not only provides helper factor for antibody production but also directly inhibits parasite exoerythrocytic forms in the liver, thereby blocking initiation of the parasite erythrocytic cycle responsible for clinical disease (14, 39). Th1-type CD4+ T cells can protect against sporozoite challenge in rodent malaria models (9, 45, 48). Recent studies of RTS,S-immunized volunteers and naturally infected individuals have correlated resistance to P. falciparum sporozoites with IFN-γ-producing CD4+, as well as CD8+, T cells (35, 44).
Consistent with predominantly Th1-type antibody response in the ICC-1132 vaccinees, IFN-γ producing T cells were detected by ICC-1132 ELISPOT in PBMC from three of eight volunteers after primary immunization and in four of eight volunteers following secondary immunization. The fine specificity of these IFN-γ-producing T cells is critical for vaccine efficacy, as only malaria-specific, not HBc-specific, Th cells can function in anamnestic antibody responses and provide IFN-γ to inhibit hepatic parasites following sporozoite challenge. Importantly, IFN-γ-producing T cells that recognized P. falciparum rCS protein were detected in the ICC-1132-immunized volunteers. In the four volunteers positive by ELISPOT on day 70, equivalent increases (n-fold) in SFC were elicited by rCS protein and ICC-1132, with little or no response to HBc, suggesting that the majority of vaccine-induced IFN-γ-producing T cells were malaria specific. Similar results were obtained in a previous murine study, in which spleen cells of ICC-1132-immunized mice produced similar amounts of IFN-γ following stimulation with ICC-1132 or rCS protein (3).
P. falciparum specificity was also supported by the ability of the malaria T* peptide to stimulate IFN-γ-producing T cells in two of the four volunteers with positive rCS ELISPOT reactions on day 56. The (NANP)n peptide did not elicit positive ELISPOT reactions, consistent with the limited HLA allele specificity of CS repeats (7, 24, 27). A predominance of T* cellular responses, with minimal responses to repeats, is consistent with the fine specificity of CD4+ T-cell clones derived from sporozoite- and peptide-immunized volunteers (23, 24, 26; J. M. Calvo-Calle, unpublished data). Although a direct comparison of responses in different vaccine trials is difficult, it is encouraging that the magnitude of the response to T* peptide elicited in volunteers immunized with ICC-1132-alum is comparable to that observed following immunization with RTS,S formulated in a more potent adjuvant, AS02A (21, 32, 44).
In the present phase I study, it was not possible to determine whether CD4+ and/or CD8+ T cells were producing IFN-γ due to the limited number of PBMC available. More extensive analysis of T-cell responses are being carried out in a second phase I and II trial of ICC-1132-alum currently in progress at the Center for Vaccine Development, University of Maryland (A. Gregson, unpublished data).
In addition to malaria-specific responses, the vaccinees also developed anti-HBc antibodies, which potentially could indirectly modulate immune responses to the CS inserts. However, HBc particles containing rodent CS repeats elicited comparable anti-CS repeat titers in mice with or without preexisting anti-HBc antibodies (38). Similarly, in RTS,S phase I trials, the presence of preexisting anti-HBsAg antibody, due to prior HBsAg vaccination or HBV infection, did not inhibit development of antibodies to CS repeats (5, 15). These findings suggest that negative immunomodulation by hepatitis-specific antibodies may not be a limiting factor for HBV virus-like particle malaria vaccines.
ICC-1132 provides a number of structural and immunological advantages over recombinant or peptide malaria vaccines, in particular the ability to express a high density of malaria epitopes. ICC-1132 core particles are composed of 240 hybrid monomers, all of which contain the CS repeat epitopes T1 and B and the T* epitope (Fig. 1). The CS repeats inserted in the loop region are localized at the tips of spikes on the virus-like particle surfaces, effectively mimicking the high-density presentation of CS repeats on the sporozoite surface. The unique structural properties of recombinant core proteins will facilitate construction of high-density multiepitope malaria vaccines composed of hybrid HBc expressing, in addition to CS, epitopes of other preerythrocytic or blood-stage antigens.
It is encouraging that the immunogenicity of ICC-1132-alum exceeded by four- to fivefold the IFA and/or ELISA titers obtained with other alum-formulated CS subunit vaccines based on peptide-protein conjugates, recombinant repeat proteins, synthetic peptides, or RTS,S (1, 15, 18, 27). However, the immune responses in the ICC-1132-alum vaccinees were not optimal, as anti-CS repeat titers that were an order of magnitude higher have been obtained in volunteers immunized with irradiated P. falciparum sporozoites or CS vaccines formulated in more-potent adjuvants (17, 27, 43). The expectation that better adjuvants will further increase immune responses to ICC-1132 is supported by a recent phase I trial using ICC-1132 administered in Montanide ISA 720 (Seppic Inc., Paris, France), an oil-in-water adjuvant (30). A single immunization with 50 μg of ICC-1132-ISA 720 elicited anti-CS antibodies in all volunteers, with GMTs comparable to those obtained following two to three injections of the ICC-1132-alum formulation. These results encourage optimization of adjuvant formulations for HBc-based vaccines to elicit high levels of immunity to target extracellular and intracellular malaria parasites and confirm the potential of HBc virus-like particles as a vaccine carrier for human infectious diseases.
Acknowledgments
We thank the volunteers for their participation in the trial. P. falciparum-infected mosquitoes were generously provided by the Naval Medical Research Institute. We thank Ruth Nussenzweig and Victor Nussenzweig for review of the manuscript and Diana Barrios Rodriquez and Rita Altszuler for their expert technical assistance.
Studies at New York University were supported by NIAID RO1 AI045138 and research funding from Apovia, Inc. Studies at Cardiff were sponsored by Apovia, Inc.
Editor: J. D. Clements
REFERENCES
- 1.Ballou, W. R., S. L. Hoffman, J. A. Sherwood, M. R. Hollingdale, F. A. Neva, W. T. Hockmeyer, D. M. Gordon, I. Schneider, R. A. Wirtz, J. F. Young, et al. 1987. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet 1:1277-1281. [DOI] [PubMed] [Google Scholar]
- 2.Ballou, W. R., J. Rothbard, R. A. Wirtz, D. M. Gordon, J. S. Williams, R. W. Gore, I. Schneider, M. R. Hollingdale, R. L. Beaudoin, W. L. Maloy, et al. 1985. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science 228:996-999. [DOI] [PubMed] [Google Scholar]
- 3.Birkett, A., K. Lyons, A. Schmidt, D. Boyd, G. A. Oliveira, A. Siddique, R. Nussenzweig, J. M. Calvo-Calle, and E. Nardin. 2002. A modified hepatitis B virus core particle containing multiple epitopes of the Plasmodium falciparum circumsporozoite protein provides a highly immunogenic malaria vaccine in preclinical analyses in rodent and primate hosts. Infect. Immun. 70:6860-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkett, A., B. Thornton, D. Milich, G. A. Oliveira, A. Siddique, R. Nussenzweig, J. M. Calvo-Calle, and E. H. Nardin. 2001. Hepatitis B virus core antigen particles containing minimal T and B cell epitopes of Plasmodium falciparum CS protein elicit high levels of malaria specific immune responses in mice and non-human primates. Am. J. Trop. Med. Hyg. 65:258. [Google Scholar]
- 5.Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358:1927-1934. [DOI] [PubMed] [Google Scholar]
- 6.Bottcher, B., S. A. Wynne, and R. A. Crowther. 1997. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature 386:88-91. [DOI] [PubMed] [Google Scholar]
- 7.Calvo-Calle, J. M., J. Hammer, F. Sinigaglia, P. Clavijo, Z. R. Moya-Castro, and E. H. Nardin. 1997. Binding of malaria T cell epitopes to DR and DQ molecules in vitro correlates with immunogenicity in vivo: identification of a universal T cell epitope in the Plasmodium falciparum circumsporozoite protein. J. Immunol. 159:1362-1373. [PubMed] [Google Scholar]
- 8.Calvo-Calle, J. M., E. H. Nardin, P. Clavijo, C. Boudin, D. Stuber, B. Takacs, R. S. Nussenzweig, and A. H. Cochrane. 1992. Recognition of different domains of the Plasmodium falciparum CS protein by the sera of naturally infected individuals compared with those of sporozoite-immunized volunteers. J. Immunol. 149:2695-2701. [PubMed] [Google Scholar]
- 9.Charoenvit, Y., V. F. Majam, G. Corradin, J. B. Sacci, Jr., R. Wang, D. L. Doolan, T. R. Jones, E. Abot, M. E. Patarroyo, F. Guzman, and S. L. Hoffman. 1999. CD4+ T-cell- and gamma interferon-dependent protection against murine malaria by immunization with linear synthetic peptides from a Plasmodium yoelii 17-kilodalton hepatocyte erythrocyte protein. Infect. Immun. 67:5604-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clyde, D. F. 1990. Immunity to falciparum and vivax malaria induced by irradiated sporozoites: a review of the University of Maryland studies, 1971-75. Bull. W. H. O. 68:9-12. [PMC free article] [PubMed] [Google Scholar]
- 11.Cochrane, A. H., M. Aikawa, M. Jeng, and R. S. Nussenzweig. 1976. Antibody-induced ultrastructural changes of malarial sporozoites. J. Immunol. 116:859-867. [PubMed] [Google Scholar]
- 12.Conway, J. F., N. Cheng, A. Zlotnick, P. T. Wingfield, S. J. Stahl, and A. C. Steven. 1997. Visualization of a 4-helix bundle in the hepatitis B virus capsid by cryo-electron microscopy. Nature 386:91-94. [DOI] [PubMed] [Google Scholar]
- 13.Danforth, H. D., M. Aikawa, A. H. Cochrane, and R. S. Nussenzweig. 1980. Sporozoites of mammalian malaria: attachment to, interiorization and fate within macrophages. J. Protozool. 27:193-202. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira, A., L. Schofield, V. Enea, H. Schellekens, P. van der Meide, W. E. Collins, R. S. Nussenzweig, and V. Nussenzweig. 1986. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science 232:881-884. [DOI] [PubMed] [Google Scholar]
- 15.Gordon, D. M., T. W. McGovern, U. Krzych, J. C. Cohen, I. Schneider, R. LaChance, D. G. Heppner, G. Yuan, M. Hollingdale, M. Slaoui, et al. 1995. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J. Infect. Dis. 171:1576-1585. [DOI] [PubMed] [Google Scholar]
- 16.Heppner, D. G., D. M. Gordon, M. Gross, B. Wellde, W. Leitner, U. Krzych, I. Schneider, R. A. Wirtz, R. L. Richards, A. Trofa, T. Hall, J. C. Sadoff, P. Boerger, C. R. Alving, D. R. Sylvester, T. G. Porter, and W. R. Ballou. 1996. Safety, immunogenicity, and efficacy of Plasmodium falciparum repeatless circumsporozoite protein vaccine encapsulated in liposomes. J. Infect. Dis. 174:361-366. [DOI] [PubMed] [Google Scholar]
- 17.Herrington, D., J. Davis, E. Nardin, M. Beier, J. Cortese, H. Eddy, G. Losonsky, M. Hollingdale, M. Sztein, M. Levine, et al. 1991. Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am. J. Trop. Med. Hyg. 45:539-547. [DOI] [PubMed] [Google Scholar]
- 18.Herrington, D. A., D. F. Clyde, G. Losonsky, M. Cortesia, J. R. Murphy, J. Davis, S. Baqar, A. M. Felix, E. P. Heimer, D. Gillessen, et al. 1987. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature 328:257-259. [DOI] [PubMed] [Google Scholar]
- 19.Kester, K. E., D. A. McKinney, N. Tornieporth, C. F. Ockenhouse, D. G. Heppner, T. Hall, U. Krzych, M. Delchambre, G. Voss, M. G. Dowler, J. Palensky, J. Wittes, J. Cohen, and W. R. Ballou. 2001. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J. Infect. Dis. 183:640-647. [DOI] [PubMed] [Google Scholar]
- 20.Koutsky, L. A., K. A. Ault, C. M. Wheeler, D. R. Brown, E. Barr, F. B. Alvarez, L. M. Chiacchierini, and K. U. Jansen. 2002. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 347:1645-1651. [DOI] [PubMed] [Google Scholar]
- 21.Lalvani, A., P. Moris, G. Voss, A. A. Pathan, K. E. Kester, R. Brookes, E. Lee, M. Koutsoukos, M. Plebanski, M. Delchambre, K. L. Flanagan, C. Carton, M. Slaoui, C. Van Hoecke, W. R. Ballou, A. V. Hill, and J. Cohen. 1999. Potent induction of focused Th1-type cellular and humoral immune responses by RTS,S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J. Infect. Dis. 180:1656-1664. [DOI] [PubMed] [Google Scholar]
- 22.Milich, D. R., J. Hughes, J. Jones, M. Sallberg, and T. R. Phillips. 2001. Conversion of poorly immunogenic malaria repeat sequences into a highly immunogenic vaccine candidate. Vaccine 20:771-788. [DOI] [PubMed] [Google Scholar]
- 23.Moreno, A., P. Clavijo, R. Edelman, J. Davis, M. Sztein, F. Sinigaglia, and E. Nardin. 1993. CD4+ T cell clones obtained from Plasmodium falciparum sporozoite-immunized volunteers recognize polymorphic sequences of the circumsporozoite protein. J. Immunol. 151:489-499. [PubMed] [Google Scholar]
- 24.Nardin, E. H., J. M. Calvo-Calle, G. A. Oliveira, R. S. Nussenzweig, M. Schneider, J. M. Tiercy, L. Loutan, D. Hochstrasser, and K. Rose. 2001. A totally synthetic polyoxime malaria vaccine containing Plasmodium falciparum B cell and universal T cell epitopes elicits immune responses in volunteers of diverse HLA types. J. Immunol. 166:481-489. [DOI] [PubMed] [Google Scholar]
- 25.Nardin, E. H., D. A. Herrington, J. Davis, M. Levine, D. Stuber, B. Takacs, P. Caspers, P. Barr, R. Altszuler, P. Clavijo, et al. 1989. Conserved repetitive epitope recognized by CD4+ clones from a malaria-immunized volunteer. Science 246:1603-1606. [DOI] [PubMed] [Google Scholar]
- 26.Nardin, E. H., and R. S. Nussenzweig. 1993. T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu. Rev. Immunol. 11:687-727. [DOI] [PubMed] [Google Scholar]
- 27.Nardin, E. H., G. A. Oliveira, J. M. Calvo-Calle, Z. R. Castro, R. S. Nussenzweig, B. Schmeckpeper, B. F. Hall, C. Diggs, S. Bodison, and R. Edelman. 2000. Synthetic peptide malaria vaccine elicits high levels of antibodies in vaccinees of defined HLA genotypes. J. Infect. Dis. 182:1486-1496. [DOI] [PubMed] [Google Scholar]
- 28.Noad, R., and P. Roy. 2003. Virus-like particles as immunogens. Trends Microbiol. 11:438-444. [DOI] [PubMed] [Google Scholar]
- 29.Nussenzweig, V., and R. S. Nussenzweig. 1989. Rationale for the development of an engineered sporozoite malaria vaccine. Adv. Immunol. 45:283-334. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira, G. A. W. K., J. M. Calvo-Calle, A. Schmidt, A. Birkett, C. H. Gleiter, G. M. Boehmer, P. Kremsner, V. Nussenzweig, R. S. Nussenzweig, and E. Nardin. 2003. Phase I trial to assess safety and immunogenicity of Malarivax, a recombinant hepatitis virus core particle containing repeat and T cell epitopes of Plasmodium falciparum circumsporozoite protein. Am. J. Trop. Med. Hyg. 69:544. [Google Scholar]
- 31.Persson, C., G. A. Oliveira, A. A. Sultan, P. Bhanot, V. Nussenzweig, and E. Nardin. 2002. Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J. Immunol. 169:6681-6685. [DOI] [PubMed] [Google Scholar]
- 32.Pinder, M., W. H. Reece, M. Plebanski, P. Akinwunmi, K. L. Flanagan, E. A. Lee, T. Doherty, P. Milligan, A. Jaye, N. Tornieporth, R. Ballou, K. P. McAdam, J. Cohen, and A. V. S. Hill. 2004. Cellular immunity induced by the recombinant Plasmodium falciparum malaria vaccine, RTS,S/AS02, in semi-immune adults in The Gambia. Clin. Exp. Immunol. 135:286-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potocnjak, P., N. Yoshida, R. S. Nussenzweig, and V. Nussenzweig. 1980. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J. Exp. Med. 151:1504-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pumpens, P., G. P. Borisova, R. A. Crowther, and E. Grens. 1995. Hepatitis B virus core particles as epitope carriers. Intervirology 38:63-74. [DOI] [PubMed] [Google Scholar]
- 35.Reece, W. H., M. Pinder, P. K. Gothard, P. Milligan, K. Bojang, T. Doherty, M. Plebanski, P. Akinwunmi, S. Everaere, K. R. Watkins, G. Voss, N. Tornieporth, A. Alloueche, B. M. Greenwood, K. E. Kester, K. P. McAdam, J. Cohen, and A. V. Hill. 2004. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat. Med. 10:406-410. [DOI] [PubMed] [Google Scholar]
- 36.Schodel, F., A. M. Moriarty, D. L. Peterson, J. A. Zheng, J. L. Hughes, H. Will, D. J. Leturcq, J. S. McGee, and D. R. Milich. 1992. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J. Virol. 66:106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schodel, F., D. Peterson, D. R. Milich, Y. Charoenvit, J. Sadoff, and R. Wirtz. 1997. Immunization with hybrid hepatitis B virus core particles carrying circumsporozoite antigen epitopes protects mice against Plasmodium yoelii challenge. Behring Inst. Mitt. 1997:114-119. [PubMed] [Google Scholar]
- 38.Schodel, F., R. Wirtz, D. Peterson, J. Hughes, R. Warren, J. Sadoff, and D. Milich. 1994. Immunity to malaria elicited by hybrid hepatitis B virus core particles carrying circumsporozoite protein epitopes. J. Exp. Med. 180:1037-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schofield, L., A. Ferreira, R. Altszuler, V. Nussenzweig, and R. S. Nussenzweig. 1987. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J. Immunol. 139:2020-2025. [PubMed] [Google Scholar]
- 40.Schwenk, R., L. V. Asher, I. Chalom, D. Lanar, P. Sun, K. White, D. Keil, K. E. Kester, J. Stoute, D. G. Heppner, and U. Krzych. 2003. Opsonization by antigen-specific antibodies as a mechanism of protective immunity induced by Plasmodium falciparum circumsporozoite protein-based vaccine. Parasite Immunol. 25:17-25. [DOI] [PubMed] [Google Scholar]
- 41.Stewart, M. J., R. J. Nawrot, S. Schulman, and J. P. Vanderberg. 1986. Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect. Immun. 51:859-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoute, J. A., K. E. Kester, U. Krzych, B. T. Wellde, T. Hall, K. White, G. Glenn, C. F. Ockenhouse, N. Garcon, R. Schwenk, D. E. Lanar, P. Sun, P. Momin, R. A. Wirtz, C. Golenda, M. Slaoui, G. Wortmann, C. Holland, M. Dowler, J. Cohen, and W. R. Ballou. 1998. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J. Infect. Dis. 178:1139-1144. [DOI] [PubMed] [Google Scholar]
- 43.Stoute, J. A., M. Slaoui, D. G. Heppner, P. Momin, K. E. Kester, P. Desmons, B. T. Wellde, N. Garcon, U. Krzych, M. Marchand, W. R. Ballou, and J. D. Cohen for the RTS,S Malaria Vaccine Evaluation Group. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N. Engl. J. Med. 336:86-91. [DOI] [PubMed] [Google Scholar]
- 44.Sun, P., R. Schwenk, K. White, J. A. Stoute, J. Cohen, W. R. Ballou, G. Voss, K. E. Kester, D. G. Heppner, and U. Krzych. 2003. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4(+) and CD8(+) T cells producing IFN-gamma. J. Immunol. 171:6961-6967. [DOI] [PubMed] [Google Scholar]
- 45.Tsuji, M., P. Romero, R. S. Nussenzweig, and F. Zavala. 1990. CD4+ cytolytic T cell clone confers protection against murine malaria. J. Exp. Med. 172:1353-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulrich, R., M. Nassal, H. Meisel, and D. H. Kruger. 1998. Core particles of hepatitis B virus as carrier for foreign epitopes. Adv. Virus Res. 50:141-182. [DOI] [PubMed] [Google Scholar]
- 47.Vanderberg, J., R. Nussenzweig, and H. Most. 1969. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. V. In vitro effects of immune serum on sporozoites. Mil. Med. 134:1183-1190. [PubMed] [Google Scholar]
- 48.Wang, R., Y. Charoenvit, G. Corradin, P. De La Vega, E. D. Franke, and S. L. Hoffman. 1996. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J. Immunol. 157:4061-4067. [PubMed] [Google Scholar]