Abstract
Murine vaginal infection with the obligate intracellular bacterium Chlamydia muridarum is commonly used as a model for ascending Chlamydia infections of the human female genital tract. Gamma interferon-producing Th1 cells, in concert with other mononuclear infiltrates, primarily mediate antichlamydial immunity. However, many factors modify this response, including the bacterial load. To investigate the manner in which the inoculating dose of C. muridarum modulates a genital infection, we measured innate and adaptive cell numbers, CD4+ lymphocyte cytokine profile, chemokine expression, course of infection, and pathological sequelae in genital tracts of BALB/c mice infected with doses of C. muridarum ranging from 104 to 107 inclusion-forming units. We found that the influx of both innate and adaptive immune cells responded similarly in the lower genital tract (cervical-vaginal tissues) and upper genital tract (oviduct tissues) to increasing inoculating doses. However, cells expressing the innate markers Gr-1 and CD11c were affected to a greater degree by increasing dose than lymphocytes of the adaptive immune response (Th1, CD4+, CD8+, CD19+), resulting in a change in the balance of innate and adaptive cell numbers to favor innate cells at higher infecting doses. Surprisingly, we detected greater numbers of viable chlamydiae in the oviducts at lower inoculating doses, and the number of organisms appeared to directly correlate with hydrosalpinx formation after both primary infection and repeat infection. Taken together, these data suggest that innate immune cells contribute to control of ascending infection.
Chlamydia trachomatis is the most prevalent cause of sexually transmitted infections in humans and is associated with reproductive dysfunction (9) and increased risk of acquiring human immunodeficiency virus (40) and developing cervical dysplasia (1). Approximately 4 million cases, the majority of which are asymptomatic, occur annually in the United States, and the cost of their management exceeds $2 billion (58). C. trachomatis is a gram-negative, obligate intracellular bacterium characterized by a unique, two-phase developmental cycle of replication: an extracellular infectious form (elementary body), which is endocytosed by eukaryotic cells into a cytoplasmic inclusion, followed by conversion to an intracellular, replicative form (reticulate body) that multiplies within the inclusion by binary fission. As the inclusion fills with reticulate bodies, chlamydiae revert back into metabolically inert elementary bodies, which are released and infect other host cells. Genital tract infection of mice with Chlamydia muridarum, previously named the mouse pneumonitis biovar of C. trachomatis (MoPn), closely mimics acute genital tract infection in women. Mice naturally resolve infection in approximately 4 weeks but develop infections upon subsequent inoculation. However, these infections are of shortened duration and magnitude when the inoculum is given within a few months, indicating development of short-term immunity.
It is well recognized that cell-mediated adaptive immunity contributes to the resolution of Chlamydia genital infection. CD4+ T cells dominate the lymphocytic infiltrate (25, 38) and are necessary for eradication of Chlamydia from the genital mucosa (37). Further, investigations have shown that CD4+ Th1 and not Th2 cells are required for Chlamydia resolution in a gamma interferon (IFN-γ)-dependent manner (17, 20). Consistent with a predominant role of Th1 cells, Th1-cell-associated chemokines CCL5/RANTES and CXCL10/IFN-γ-inducible protein 10 (IP-10) were shown to be primarily induced in infected genital tissue (6, 34). Further, unpublished data from our laboratory demonstrates that the corresponding receptor for CXCL10, CXCR3, plays a role in the recruitment of Th1 cells during chlamydial infection. By contrast, neither B cells, antibodies, nor CD8+ T cells contribute an essential effector function for the resolution of primary infection, but they may contribute to protection following reinfection (36).
Immunity in response to chlamydial infection requires fine-tuned coordination to maintain a balance between tissue protection and potential immunopathology. During Chlamydia infection, the immune response in the upper genital tract appears to shift from a Th1-dominated response to a mixed Th1/non-Th1 response (38, 43). The non-Th1 cytokine interleukin 10 (IL-10) produced from antigen-presenting cells has been shown to inhibit Th1 cell function and interfere with eradication of chlamydiae locally from the genital tract (19, 62). Given the protective role afforded by a cell-mediated Th1 response in the resolution of infection, inflammatory damage within the upper genital tract may be the result of a prematurely terminated Th1 response, resulting in chronic infection (5), or the result of an exaggerated proinflammatory Th1 response (4, 18) that ultimately mediates reproductive dysfunction.
The severity of upper-genital-tract sequelae in mice following infection with C. muridarum can vary with mouse strain, age, hormone levels, and inoculating dose (13, 41, 42). Historically, a variety of inoculative doses of C. muridarum have been used among different laboratories, ranging from 103 to 107 inclusion-forming units (IFU), leading to difficulties in data interpretation and comparisons among research groups. The bacteria load with which mice are challenged has been shown to influence elicited immunity and infection outcome in other microorganism models, such as Leishmania major and Mycobacterium tuberculosis (7, 16). To shed light on the effect of inoculative dose on Chlamydia infection, we challenged mice intravaginally with C. muridarum at doses 1 × 104, 1.5 × 105, or 1 × 107 IFU. We investigated qualitative and quantitative features of the innate and adaptive immune response, the infectious loads within different genital tract regions, and ensuing oviduct dilation following C. muridarum genital infection.
MATERIALS AND METHODS
Antibodies.
The following rat antimouse monoclonal antibodies were purchased from Pharmingen (San Diego, Calif.): CD14 (clone rm-C53), biotin-conjugated CD4 (clone H129.19), CD8 (clone 53-6.7), CD19 (clone 1D3), LY-6G (Gr-1) (clone RB6-8C5), CD11c (clone HL3), (peridinin chlorophyll protein)-conjugated CD4 (clone RM-45), phycoerythrin (PE)-conjugated IFN-γ (clone XMG1.2), and allophycocyanin-conjugated IL-4 (clone 11B11). Hamster anti-mouse CD69-fluorescein isothiocyanate (FITC) (clone H1.2F3) was also purchased from Pharmingen. Isotype control antibodies were purchased from Pharmingen. Streptavidin conjugated with PE (Pharmingen) was used as a secondary reagent for biotin-labeled antibodies, and goat anti-rat immunoglobulin G (IgG) conjugated with FITC (Biosource, Camarillo, Calif.) was used as a secondary reagent for CD14 staining.
Mice.
Female BALB/c mice were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.) and were housed according to the American Association of Accreditation of Laboratory Animal Care guidelines. Experimental procedures were approved by the UCLA Institutional Animal Care and Use Committee. All mice, 5 to 7 weeks of age, were first injected subcutaneously with 2.5 mg of medroxyprogesterone acetate (Upjohn, Kalamazoo, Mich.) in 100 μl of sterile phosphate-buffered saline. Medroxyprogesterone acetate drives mice into a state of anestrous, thus eliminating the variability in the rate and severity of infection due to the estrus cycle. Seven days later, while under sodium pentobarbital anesthesia, mice were inoculated with one of three doses of C. muridarum grown in McCoy cells (50% infective dose = 2.5 × 103 IFU): 1 × 104, 1.5 × 105, or 1 × 107 IFU. Mice were sacrificed on days 7, 14, 21, and 49 after inoculation to assess primary infection and immunity. A group of mice was reinfected on day 50 with the original Chlamydia dose 7 days after medroxyprogesterone acetate injection as described for a primary infection. This group was sacrificed on days 3 and 6 after infection to analyze secondary infection (2°) and immunity. Infection was monitored by obtaining cervical-vaginal swabs (Dacroswab type 1; Spectrum Labs, Houston, Tex.) every 3 days and tissue homogenates from oviducts and cervical-vaginal tissues on days of sacrifice. Swabs were stored in sucrose-phosphate buffer at −70°C until analyzed.
Isolation of chlamydiae from cervical-vaginal swabs and tissue homogenates.
Swabs were prepared as previously described (26). Individual wells of McCoy cell monolayers in 96-well plates were inoculated with 200 μl of the solution from vaginal swabs or homogenized genital tract tissue, followed by centrifugation at 1,900 × g for 1 h at 35°C. The plates were incubated for 2 h at 35°C. At this time, the isolation solutions were removed, fresh cycloheximide medium was added, and the plates were incubated for an additional 32 h. The cultures were then fixed with methanol. Chlamydia inclusions were identified by the addition of monoclonal antibody directed against C. trachomatis lipopolysaccharide (Pharmingen) and anti-mouse IgG conjugated to fluorescein isothiocyanate (ICN Immunobiologicals, Irvine, Calif.). The inclusion bodies within 20 fields (×40) were counted under a fluorescence microscope, and numbers of IFU per milliliter were calculated. Data was adjusted for IFU per milligram of crude homogenized genital tract (GT) tissue (cervical-vaginal tissue, uterine horns, and oviducts).
Tissue homogenates.
Genital tracts were divided into the cervical-vaginal region, uterine horns, and oviducts with the ovaries removed. For isolation of chlamydiae and enzyme-linked immunosorbent assays (ELISAs), tissue sections from individual mice were placed in 1 ml of a protease-inhibitor buffer (1 μg each of antipain, aprotinin, leupeptin, and pepstatin A/ml and 2 mM phenylmethylsulfonyl fluoride in sterile phosphate-buffered saline) (Sigma, St. Louis, Mo.) and homogenized as previously described using a hand-held homogenizer (Omni Intl., Warrenton, Va.). Aliquots of each homogenate were removed for isolation of chlamydiae. The remaining homogenate volumes were sonicated at 4 μm for 1 min and then centrifuged at 900 × g for 15 min at 10°C to remove cellular debris. Supernatants were filtered through 0.2-μm-pore-size Acrodisks (Gelman Sciences, Ann Arbor, Mich.) to remove chlamydiae. Clarified and nonclarified homogenates were stored at −70°C until analysis.
CXCL10 ELISA.
Recombinant protein and antibodies against CXCL10 were purchased from R&D Systems (Minneapolis, Minn.) for use in ELISAs. Clarified oviduct (OD) and cervical-vaginal (CV) homogenates were added to duplicate wells of microtiter enzyme immunoassay plates (Costar/Corning, Acton, Mass.) and assayed according to the manufacturer's protocol. CXCL10 primary and secondary antibody concentrations were 4 and 0.6 μg/ml, respectively. The recommended substrate was replaced with 1-StepTM Turbo TMB-ELISA substrate (Pierce Chemical Co., Rockford, Ill.). The optical densities were read at 450 nm with a microplate reader (model 550; Bio-Rad, Hercules, Calif.). Chemokine values were determined using microplate reader software. Chemokine values were corrected for total protein by using a micro-bicinchoninic acid protein assay kit (Pierce).
Isolation of leukocytes.
Whole genital tracts were harvested and separated into CV and OD regions. Single cell suspensions were prepared from pooled tissues (20 mice each) of like segments that were minced with scissors and subjected to collagenase digestion (type I; 5 mg/ml in Hanks balanced salt solution; Sigma) for 45 min at 37°C. Single cell suspensions were prepared by expressing the digests through a 70-μm-pore-size filter (Falcon, Becton Dickinson, Franklin Lakes, N.J.) in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Gaithersburg, Md.). For intracellular cytokine analysis, cells were cultured (2 × 106 cells/ml) for 48 h at 37°C in RPMI medium containing 10% fetal bovine serum, 200 mM glutamine, 10,000 U of penicillin/ml, 10,000 μg of streptomycin/ml, 1 M nonessential amino acids, 1 M HEPES, 1 M sodium pyruvate, 5 μM 2-mercaptoethanol, and 5 μg of UV-inactivated C. muridarum elementary bodies/ml that were purified by Renografin-60 (Bracco Diagnostics, Princeton, N.J.) gradient centrifugation (8).
Flow cytometry.
Single cell suspensions (3 × 105 to 5 × 105 cells) were stained in DMEM containing 1% bovine serum albumin (Sigma) and 0.1% sodium azide, using the microplate method as previously described (25). For single-color staining, isolated cells were first incubated with 10 μg of rat anti-mouse cell surface markers (see “Antibodies”)/ml for 25 min on ice and then washed twice with DMEM containing 10% bovine serum albumin. The cells were then resuspended in streptavidin conjugated to PE (Pharmingen) at a concentration of 0.2 μg/ml or, for CD14 staining, in 20 μg of FITC-conjugated goat anti-rat IgG (Biosource)/ml for 25 min on ice. Following the washing step described above, the cells were fixed in phosphate-buffered saline containing 1% paraformaldehyde and kept at 4°C until analyzed.
For intracellular cytokine staining, cultured cells were purified by density gradient centrifugation using Lympholyte M (Cedarlane Laboratories, Ontario, Canada) according to the manufacturer's protocol and then pretreated with GolgiStop (Pharmingen) in order to block intracellular protein transport according to the manufacturer's protocol. The cells were stained for mouse cell surface markers using 4 μg of CD4-peridinin chlorophyll protein and CD69-FITC/ml as described above. Cells were permeabilized by resuspension in Cytofix/Cytoperm (Pharmingen) as suggested by the manufacturer. Cells were then resuspended in cytokine rat antimouse antibodies (see “Antibodies”) for 25 min on ice. Following additional washes in Cytofix/Cytoperm, the cells were fixed and stored as described above.
Flow cytometry was performed on a fluorescence activated cell sorting analyzer equipped with a 488-nm argon laser and CellQuest software (FACScan; Becton Dickinson, San Jose, Calif.). The instrument was calibrated with beads (CaliBRITE; Becton Dickinson), using AutoCOMP software. Dead cells were excluded on the basis of forward-angle and 90° light scatter, and 10,000 gated cells were analyzed for each sample.
Histopathology.
For histological analysis of oviduct tissue, mice were sacrificed 6 days post-secondary infection. The upper genital tract including ovary and oviduct was removed, and a latitudinal incision was made. Individual oviducts were submerged in Optimal Cutting Temperature embedding medium (Tissue Tek; Sakura Finetek, Torance, Calif.) and stored at −80°C. Frozen tissue blocks were sectioned by the Human Tissue Research Core facility at University of California, Los Angeles. Tissue blocks were cut transversally from the ovary, and sections were collected at the beginning of the transitional region between ovary and oviduct. Sections were stained with hematoxylin and eosin (H&E), and the diameter of the oviduct lumen was measured using a grid-containing lens (×10) (BH-2; Olympus, Tokyo, Japan). Each value is a mean of four diameter measurements for a single oviduct. For each dose, eight mice were analyzed and diameter measurements for two oviducts per mouse were averaged.
Statistics.
Statistical differences in the ratios of leukocytes in oviduct and cervical-vaginal tissue and in the diameter measurements of oviducts were tested using one-way analysis of variance (ANOVA) on ranks. Two-way ANOVAs were used to determine statistical differences in the number of leukocyte subsets per 106 total cells and CXCL10 protein levels among the three inocula and two genital tract regions. Two-way ANOVAs were also used to assess differences in the level of infection among the three genital tract regions and vaginal swabs (log 10 transformation). The Spearman rank order correlation test was used to determine the strength of association between the scores assigned for hydrosalpinx severity and inocula. This correlation statistic does not assume that the association between two variables is linear. The above-mentioned statistical tests were suggested by and performed by using SigmaStat software based on the distribution of the data and sample size (Jandel Scientific, San Rafael, Calif.). Groups were considered statistically different at P values of <0.05.
RESULTS
Infecting dose of Chlamydia does not affect the distribution of CD4+ or Th1 cells between oviduct and cervical-vaginal tissues.
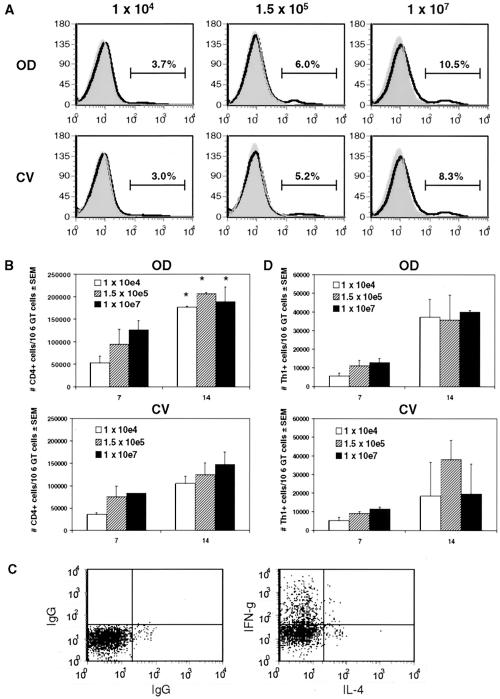
We previously published that there is greater recruitment of CD4+ cells to OD tissue than to CV tissue in mice infected with a high dose (107 IFU/mouse) of chlamydia organisms (27). To investigate whether differential recruitment of CD4+ cells within the genital tract was dependent on inoculative dose, we measured CD4+ cell numbers in OD and CV tissue of mice infected with 1 × 104, 1.5 × 105, or 1 × 107 IFU by flow cytometry (Fig. 1). We found that the ratio of CD4+ cells in OD tissue relative to CV tissue was independent of dose. Percentages of CD4+ cells of total lymphocytes 7 days postinfection, the time of onset of adaptive immunity in the genital tract, showed an initial trend in increased recruitment to OD tissue compared to CV tissue (Fig. 1A). The difference in CD4+ cell recruitment between regions increased by day 10 (data not shown) and was statistically different at day 14, with approximately 1.5- to 2-fold more CD4+ cells in oviducts for all doses measured (Fig. 1B). These data correspond well with our previous report of relative CD4+ cell distribution within the genital tract, using a dose of 107 IFU, during the first 2 weeks of infection, while even greater differences in CD4+ cell distribution were seen 3 to 5 weeks postinfection as total cellular influx increased (27).
FIG. 1.
Effect of dose on the distribution of CD4+ and IFN-γ-producing Th1 cells within the genital tract. (A) FACS profiles of CD4+ cells in CV and OD tissues 7 days postinfection from one representative experiment. Percentages of CD4+ cells were calculated from lymphocytes gated by forward- and side-scatter analysis. (B) Number of CD4+ cells in CV and OD tissues per 106 genital tract cells during the onset of adaptive immunity (days 7 and 14). Data are compiled from two to three independent experiments. There were statistically greater numbers of CD4+ cells on day 14 compared to day 7 for all doses (*, P = 0.01 by two-way ANOVA). (C) Chlamydia-responsive Th1 cells were identified by intracellular cytokines IFN-γ (+) and IL-4 (−) within gated CD4+ CD69+ lymphocytes. (D) Number of Th1 cells in CV and OD tissues per 106 genital tract cells. Data are compiled from two independent experiments.
CD4+ Th1 cells that produce IFN-γ are required for natural Chlamydia eradication from genital tract tissue (20, 46). Our previous characterization of antichlamydial effector cell distribution within the genital tract was limited to CD4+ cell detection. To determine whether differentially recruited CD4+ cells are IFN-γ-producing Th1 cells, we stained lymphocytes from OD and CV tissue for intracellular cytokines IFN-γ and IL-4, CD4, and the activation marker CD69 after ex vivo culture with renografin gradient-purified elementary bodies. Th1 cells were defined as a CD4+ CD69+ population that produced IFN-γ in the absence of IL-4 (Fig. 1C, upper left quadrants). Interestingly, we found that in contrast to CD4+ cells, Th1 cells were recruited in similar numbers to OD and CV tissues (Fig. 1D), suggesting that CD4+ cells that accumulate in the OD represent non-Th1 cells. Th1 cell regional distribution was also independent of dose (days 7 and 14, P > 0.50 by two-way ANOVA). Further, the number of Th1 effector cells but not CD4+ cells was variable from experiment to experiment, particularly in the CV region, at 14 days postinfection, when Chlamydia burden was decreasing (see Fig. 3). These results support the induction of Th1 effector responses in the genital tract following C. muridarum infection and further demonstrate distinct cellular patterning of different T-cell subsets within genital tract regions, independent of inoculating dose.
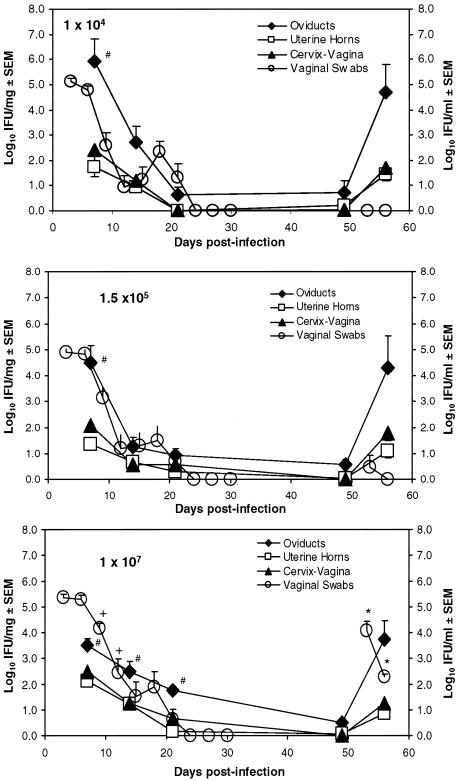
FIG. 3.
Course of infection by vaginal swabs and tissue homogenates following genital inoculation with increasing Chlamydia dose. Vaginal swabs and tissue homogenates of cervical-vaginal regions, uterine horns, and oviducts were collected following primary and secondary inoculations of mice with increasing Chlamydia doses for recovery of viable organisms. Swab data are the means and standard errors of the means of log10 IFU ml−1 for n = 9 mice from two independent experiments (y axis, right); homogenate data are the means and standard errors of the means of log10 IFU/mg of tissue for 5 to 10 mice from two or three independent experiments (y axis, left). Shedding of chlamydiae from genital tracts of mice infected with the highest dose (107 IFU) was significantly greater during the resolution phase of primary and secondary infections than that with lower doses. +, P < 0.05 compared to results for doses of 104 IFU (days 9 and 12) and 1.5 × 105 IFU (day 12) by two-way repeated-measure ANOVAs; *, P < 0.001 compared to results for doses of 104 and 1.5 × 105 IFU (days 53 and 56) by two-way repeated-measure ANOVAs. In contrast, when chlamydiae were cultured from homogenized tissues, there was no difference in the magnitude of infection within a given region (two-way ANOVA), though there was an effect between regions. #, P < 0.05 for results with OD tissue compared to those with CV and uterine tissue by two-way ANOVA. 107 IFU, days 7, 14, and 21; 1.5 × 105 IFU and 104 IFU, day 7.
The magnitude of innate but not adaptive immunity is influenced by infectious dose within the genital tract.
While there was not an effect of dose on the distribution of CD4+ or Th1 cells within the genital tract, we observed a small trend in increasing dose and magnitude of CD4+ and Th1 cell influx in OD and CV tissues (Fig. 1). To determine whether innate cell numbers correlated with differences in adaptive immune cells and dose during the priming phase of antichlamydial immunity, we measured polymorphonuclear (PMN) cells (Gr-1+), dendritic cells (DC) (CD11c+), and monocytes (CD14+) by flow cytometry from genital tract tissues 7 days postinfection (Table 1). As previously reported (12), we detected large infiltrates of PMN in genital tracts within the first week of infection, as well as DC, while very few CD14+ cells were present (data not shown). Increasing Chlamydia inocula resulted in larger numbers of both PMN and DC in OD and CV tissue (Table 1), and the effect was much greater than that for Th1 cells (Table 2), suggesting that initial Chlamydia burden has a direct effect on the magnitude of innate cell infiltrates but that this does not correlate with increased recruitment of an adaptive effector response. Therefore, the activities of PMN and DC appear to partially regulate subsequent induction of Th1 immunity, while negative regulatory mechanisms may specifically limit the degree to which inflammation may occur. Finally, as we saw for adaptive immune cells, the inoculative dose did not affect the anatomical distribution of PMN and DC within the genital tract (Table 1).
TABLE 1.
Numbers of PMN and DC recruited to OD and CV tissues with differing inoculative doses
| Dose | No. of PMNa
|
Ratioc | No. of DCb
|
Ratioc | ||
|---|---|---|---|---|---|---|
| OD | CV | OD | CV | |||
| 1 × 107 | 370 (57) | 220 (52) | 1.7 | 230 (22) | 210 (38) | 1.1 |
| 1.5 × 105 | 320 (63) | 240 (35) | 1.3 | 180 (9.6) | 180 (22) | 1.0 |
| 1 × 104 | 190 (45) | 140 (27) | 1.4 | 100 (16) | 98 (11) | 1.0 |
Number of PMN (103) per 106 GT cells (± standard errors of the means) 7 days postinfection. PMN were identified in a pool of genital tract cells by flow cytometry, using LY6G monoclonal antibody. Data are compiled from three independent experiments.
Number of DC (103) per 106 GT cells (± standard errors of the means) 7 days postinfection. DC were identified in a pool of genital tract cells by flow cytometry using CD11c monoclonal antibody. Data are compiled from three independent experiments.
No statistical difference in ratios of PMN or DC in oviduct/cervical-vaginal tissue was observed between doses by a one-way ANOVA on ranks.
TABLE 2.
Comparison of innate and adaptive cell infiltrates to OD and CV tissues with differing inoculative doses
| Dose | OD
|
Ratio | CV
|
Ratio | ||
|---|---|---|---|---|---|---|
| No. of Innate cellsa | No. of Th1 cellsb | No. of Innate cellsa | No. of Th1 cellsb | |||
| 1 × 107 | 600 (74) | 40 (0.8) | 15 | 390 (83) | 20 (16) | 20 |
| 1.5 × 105 | 500 (73) | 36 (13) | 14 | 400 (23) | 39 (10) | 10 |
| 1 × 104 | 300 (61) | 37 (9.7) | 8.1 | 230 (38) | 18 (18) | 13 |
Number of PMN (103) and DC (103) per 106 GT cells (± standard errors of the means) 7 days postinfection. PMN and DC were identified in a pool of genital tract cells by flow cytometry, using LY6G and CD11c monoclonal antibodies, respectively. Data were compiled from three independent experiments.
Number of Th1 cells (103) per 106 GT cells (± standard errors of the means) 14 days postinfection. Chlamydia-responsive Th1 cells were identified in a pool of genital tract cells using IFN-γ and IL-4 intracellular cytokine staining following a 48-h stimulation with Chlamydia antigen. Data were compiled from two independent experiments.
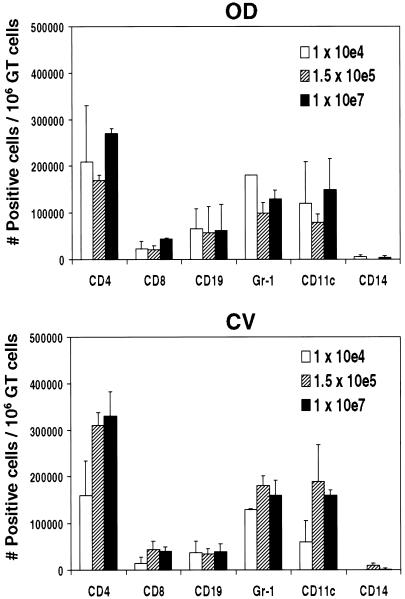
To consider the dose-dependent effect of innate cell recruitment on the magnitude of memory-T-cell responses, we reinoculated mice after resolution of primary Chlamydia infections with an inoculum equal to that originally given on day 50 and analyzed cellular infiltrates 6 days later (Fig. 2). CD4+ cell numbers were approximately fivefold greater in CV tissues during secondary infection than 7 days after primary infection, consistent with a memory cell response, for all doses. Also consistent with memory induction, we detected fewer innate cell infiltrates. Interestingly, there was a correlative trend in infecting dose, the number of CD4+ and CD11c+ cells in CV tissue, and shedding of chlamydiae from vaginal swabs. These findings suggest that CD11c+ and CD4+ memory cell recruitment reflects organisms residing in the epithelium and not within the tissues. In addition, total CD4 cell numbers were approximately fivefold greater compared to Th1 cells, and may include a non-T-cell population, since the CD4 molecule is also detected on non-T-cell populations. We also did not see an effect of dose on other adaptive immune cells during the memory response, including CD8+ and CD19+ cells, while we did observe a trend in dose and CD8+ cell infiltrates during primary infection (data not shown). CD8+ cells have been shown to mediate IFN-γ-dependent antichlamydial effector responses during primary infections (30, 33) but do not confer significant protection against reinfection (39). Our data furthersuggest that CD8+ cell function may make a greater contribution to effector responses during primary infections than during secondary infections. Further, B cells were demonstrated to play a role in protection during secondary infection with C. trachomatis (35, 39), though the necessity of this response for protection is not evident (22, 55). Our data suggest that the recruitment of B cells to the genital mucosa may be a limiting factor in antibody-mediated protection.
FIG. 2.
Effect of dose on innate and adaptive immunity following secondary challenge with C. muridarum. Leukocytes were isolated from pools of collagenase-digested genital tracts of 20 mice, infected with increasing doses of C. muridarum, and reinfected 50 days later with the original dose, 6 days post-secondary infection. We used immunofluorescence and flow cytometry to identify polymorphonuclear cells (Gr-1+), dendritic cells (CD11c+), monocytes/macrophages (CD14+), and CD4+, CD8+, and CD19+ cells in two separate experiments. Data are the means and standard errors of the means for the number of positive cells per 106 genital tract cells. There was no statistical difference in the magnitude or distribution of any cell type in the genital tract with dose upon secondary infection.
Shedding of Chlamydia organisms from cervical vaginal tissue decreases during the resolution phase of infection with decreasing doses.
Natural immunity against Chlamydia muridarum infection results in eradication of chlamydial organisms from genital tract tissue within 3 to 5 weeks postinoculation. To determine whether there was an effect of Chlamydia dose on the magnitude or duration of infection, we measured the number of viable chlamydiae present on vaginal swabs collected every 3 days postinfection (Fig. 3). All mice were infected regardless of the dose used. We also found that there was no difference in the number of chlamydiae recovered from vaginal swabs after initial infection with increasing doses. However, a significantly greater number of chlamydiae were shed from the GTs of mice infected with a higher infecting dose (107) during the resolution phase of infection (day 9: 107 mean equals 4.16 ± 0.20 log10 IFU ml−1; 1.5 × 105 mean equals 3.13 ± 0.41 log10 IFU ml−1; 104 mean equals 2.57 ± 0.53 log10 IFU ml−1; day 12: 107 mean equals 2.45 ± 0.53 log10 IFU ml−1; 1.5 × 105 mean equals 1.19 ± 0.52 log10 IFU ml−1; 104 mean equals 0.913 ± 0.46 log10 IFU ml−1). These data suggest that the numbers of viable chlamydiae measured in epithelial cells decreased at a more rapid rate with lower inoculating doses.
We also measured numbers of viable chlamydiae in tissue homogenates of distinct genital tract regions, including the CV, uterine horn, and OD. The use of homogenized tissue allows the additional detection of organisms in submucosal layers. Chlamydiae were cultured from individual mouse tissue homogenates 7, 14, 21, and 49 days postinfection (Fig. 3). Surprisingly, we found a trend in the ability of chlamydiae to ascend to OD tissue with decreasing dose (oviduct day 7: 107 mean equals 3.50 ± 0.27 log10 IFU mg−1; 1.5 × 105 mean equals 4.50 ± 0.65 log10 IFU mg−1; 104 mean equals 5.91 ± 0.91 log10 IFU mg−1), which suggests that lower initial bacterial cell numbers in CV tissue may provide an advantage for bacterial ascension. The number of organisms detected in OD tissue was significantly greater than that detected in uterine horn or CV tissues on day 7 for all doses. Interestingly, this significant difference in bacterial burden in OD compared to that in other genital tract regions was maintained only in mice infected with a high Chlamydia dose, suggesting that lower levels of chlamydiae result in less-sufficient bacterial eradication from the OD.
Primary genital tract infection with Chlamydia has been shown to result in protective immunity against reinfection with the same serovar (48). To determine whether the infecting dose of Chlamydia had a similar effect on the number of viable organisms recovered upon repeat infection, mice were infected with one of three Chlamydia doses and reinoculated with the same dose 50 days later. At this time, note that all groups of mice had resolved a genital tract infection as defined by vaginal swabs. However, we were able to recover viable organism from the upper tract tissue, albeit at similar numbers from all groups of mice. Six days later, we measured the number of cumulative chlamydiae in genital tract tissues. Data from vaginal swabs indicated that a statistically greater number of organisms were detected from mice infected with a high dose of Chlamydia 3 and 6 days post-secondary infection than with other doses and that clearance of organisms from these mice required more time, since all mice infected with 1 × 104 or 1.5 × 105 IFU but not 1 × 107 IFU were culture negative by day 56. This is similar to what we observed during resolution of primary infection using vaginal swabs. Chlamydia shedding 3 days post-secondary infection was less than that detected following primary infection, which likely reflects differences in the kinetics of primary and memory immunity. However, Chlamydia tissue burden as determined by homogenates was similar during secondary and primary infections, suggesting that the organisms can infect submucosal layers equally during both a primary and secondary infection and at all inoculating doses tested and potentially evade immunity. Finally, the trend in the ability of Chlamydia to ascend to OD tissue with decreasing dose was less apparent in the homogenates upon secondary infection but occurs at all inoculating doses. However, infection of surface epithelial cells is detected only at higher inoculating doses. This suggests that in contrast to primary infections, adaptive cells play a significant role in restricting bacterial infection of surface epithelial cells but not infected tissue cells in the submucosal layers.
Increasing the infecting dose of Chlamydia does not affect the relative expression pattern or magnitude of the Th1 chemokine CXCL10 in the genital tract.
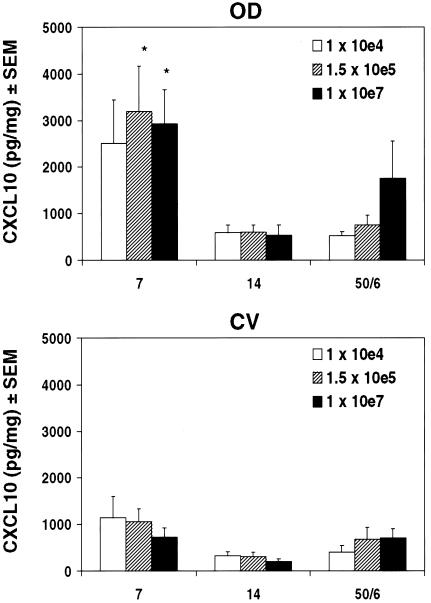
We previously reported that chemokines associated with Th1 cell recruitment are induced in the genital tract within 3 to 7 days after Chlamydia infection and are preferentially expressed in OD tissue compared to CV tissue (34), and we have recently demonstrated a functional role of these chemokines in the chemotaxis of Chlamydia-specific lymphocytes (unpublished data). To determine whether the inoculative dose of Chlamydia affected the distribution or magnitude of induction of Th1-associated chemokines, we measured the CXCR3 ligand CXCL10/IP-10 by ELISA in clarified OD and CV tissue homogenates (Fig. 4). The kinetics of CXCL10 induction, characterized by a peak in protein levels within 1 week of infection and return to baseline by 14 days, were independent of dose and were similar to levels previously reported (34). Additionally, higher levels of CXCL10 were detected in OD tissue than in CV tissue 7 days postinfection for all doses, which may reflect greater numbers of organisms. Interestingly, CXCL10 induction was between twofold and fivefold less upon secondary infection than that in primary (day 7) infection in OD, despite a four- to eightfold greater number of Th1 cells (data not shown). This suggests that reduced CXCL10 levels and/or possibly other chemokines recruit Th1 during a second infection. Therefore, the inoculating dose did not affect CXCL10 induction or distribution within distinct genital tract regions.
FIG. 4.
Inducible protein expression of the CXCR3 ligand CXCL10/IP-10 in the genital tract with increasing Chlamydia dose. CXCL10/IP-10 protein was detected by ELISA in OD and CV tissue homogenates of individual mice infected with increasing Chlamydia inocula, 7 and 14 days post-primary infection and 6 days post-secondary infection. Data are expressed relative to total homogenate protein and are the means and standard errors of the means for two independent experiments with five mice per dose per experiment. There was no effect of Chlamydia dose on increased CXCL10/IP-10 in OD tissues compared to CV tissues after primary infection or on the magnitude or kinetics of CXCL10/IP-10 induction following primary and secondary infections. However, at higher doses there was a statistical increase in the distribution between OD and CV tissues 7 days after primary infection (1 × 107 and 1.5 × 105 IFU; *, P < 0.05 by two-way ANOVA).
Increasing Chlamydia dose correlates with decreased severity of oviduct sequelae.
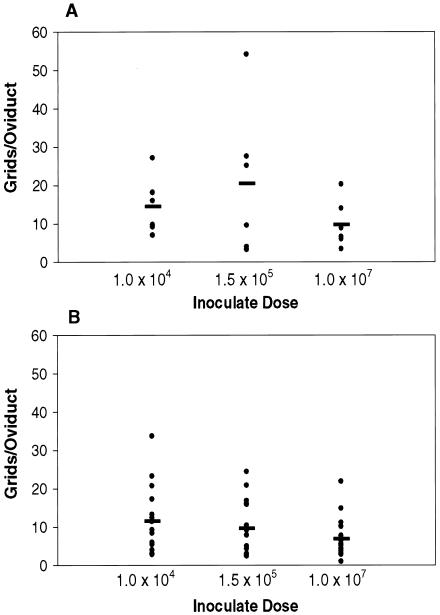
Multiple infections with C. muridarum result in tubal obstruction, increased inflammation, and OD dilation (43, 44), similar to what is observed in women with chronic C. trachomatis infections. To determine whether there was an effect of dose on oviduct pathology in mice following both primary and a repeat infection with C. muridarum, we assessed gross tissue damage or hydrosalpinx and measured OD diameters of H&E-stained tissues (Table 3 and Fig. 5). Gross hydrosalpinx of two OD per mouse were scored on a scale of 0 to 4 following removal of genital tracts from groups of mice sacrificed 49 days post-primary infection and 6 days after reinfection. There was no difference in the frequency with which mice developed hydrosalpinx between doses during a primary or repeat infection. Studies have suggested that increasing dose correlates with increasing frequency of hydrosalpinx (42); however, statistical differences were not examined among infected mice. To assess by a more sensitive means whether oviduct dilation differed by dose, we measured diameters of oviducts from H&E-stained sections that were cut transversally from ovaries and collected at the ovary-to-oviduct transition (Fig. 5). We found that while there was a large degree of overlap in diameter measurements for all three doses, there was also a distinct trend of decreasing oviduct diameter with increasing dose evident following repeated infection. This suggests that the ability of Chlamydia to ascend to oviduct tissue, which was increased with smaller C. muridarum inocula, directly correlates with oviduct dilation.
TABLE 3.
Gross hydrosalpinx scores following primary and secondary infections with differing doses
| Infectiona | Doseb | No. of OD with score ofc:
|
|||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| 1° | 1 × 107 | 17 | 6 | 9 | 2 |
| 1.5 × 105 | 12 | 8 | 5 | 7 | |
| 1 × 104 | 10 | 13 | 9 | 2 | |
| 2° | 1 × 107 | 6 | 9 | 14 | 3 |
| 1.5 × 105 | 9 | 11 | 8 | 4 | |
| 1 × 104 | 11 | 11 | 3 | 7 | |
1°, primary infection; 2°, repeat infection.
There was no statistical difference by dose in the number of oviducts with respective hydrosalpinx scores by Spearman rank order correlation (P > 0.05).
Number of oviducts per dose assigned respective hydrosalpinx scores based on gross diameter following removal of genital tracts from mice 49 days post-primary infection (1°) or 6 days after a repeat infection (2°). Data are representative of results from four independent experiments with four to seven mice per experiment. Hydrosalpinx scores are as follows: 0, none; 1, increase; 2, 2.5 mm in diameter; and 3, ≥ 3.5 mm in diameter.
FIG. 5.
Oviduct dilation in mice following infection with increasing Chlamydia dose. Oviduct diameters were measured from H&E-stained sections collected transversally at the ovary-to-oviduct transition of three to eight mice from one or two independent experiments during (A) primary infection (day 49) and (B) 6 days post-repeat infection. Data are expressed as the average number of grids (one grid equals approximately 1 μm) of two diameter measurements per oviduct section of four sections per oviduct. There was no statistical difference in oviduct diameter for increasing dose during primary or secondary infection by one-way ANOVA.
DISCUSSION
T-cell-mediated immunity is an important determinant of natural Chlamydia eradication (49, 51), and IFN-γ has been shown to be a critical effector molecule in this response (11, 46). Our present data support a role of IFN-γ-producing Th1 cells in Chlamydia clearance by showing, first, infiltration of endogenous Th1 cells to the genital tract following Chlamydia inoculation and, second, an increase in variability of cervical-vaginal Th1 cell numbers at times when the Chlamydia burden was decreasing in this region. Interestingly, Th1 cell distribution within the OD and CV regions did not correlate with CD4+ cells or expression of the Th1 chemokine CXCL10, suggesting that Th1 cells are only a subset of effector cells that are recruited during Chlamydia genital infection. Our data also identify an important role of innate cells in controlling Chlamydia infection. We found that the magnitude of the innate cell response directly depended on the inoculative dose of Chlamydia, which further revealed a critical relationship between the strength of the innate response elicited in the lower genital tract and the ability of chlamydiae to ascend to upper genital tract tissues. Ascension of Chlamydia correlated with increased oviduct damage. Interestingly, a dose effect was not seen for induction of Th1 responses, suggesting that development of adaptive immunity is regulated apart from the magnitude of innate immunity. Taken together, these data emphasize the significance of early cellular events in the genital mucosa for limiting Chlamydia dissemination and ensuing tissue pathology and a subsequent role of IFN-γ-dependent adaptive immunity in the control of infection in lower and upper genital tract regions.
Our data demonstrate clearly that there are regional differences within the female reproductive tract that determine immune effector responses. We have confirmed previous results from our laboratory that indicated enhanced recruitment and accumulation of CD4+ cells in OD tissue (27) as well as induction of IFN-γ-inducible chemokines following Chlamydia infection (34). Other evidence for regional immune specificity of genital tract tissue include differences in adhesion molecules expression (25), cytokine production (60), and cellular localization of chemokines (34). The biological effects of regional distribution of immune-modulating factors in the genital mucosa, particularly in the context of invading pathogens, have recently come to light. Based on available findings, we and others (54) hypothesize that the immune hyporesponsiveness of CV tissue, which may result from high titers of complex microflora (up to 109 per milliliter in normal women), creates a permissive environment for ascending infections, while the availability of distinct subsets of immune cells in different regions as well as differences in mucosal epithelia may serve to either limit or potentiate infection (recently reviewed in reference 47). In further support of this, preliminary data from our laboratory suggest the recruitment of unique innate cell populations with preferential homing within the genital mucosa that modulate C. muridarum infection. Additional characterization of cellular subsets within distinct anatomical regions of the genital tract will be a critical determinant in our understanding of normal genital tract homeostasis, response to invading pathogens, and our subsequent ability to prevent ascending disease.
The adaptive immune component of upper-genital-tract tissue damage is poorly understood. Immunopathology may be mediated by the proinflammatory Th1 response that is elicited in oviduct tissue or, alternatively, by early termination of the Th1 response and onset of IL-10-dependent T-cell responses that favor bacterial persistence, such as the Th2 or type 1 T-regulatory response. Our data show that there is no correlation between Th1 cell numbers in oviducts and the severity of oviduct dilation and, further, that CD4+ but not Th1 cells accumulate in oviducts over time, suggesting that a non-Th1 cell type contributes to oviduct pathology. Additionally, when we measured intracellular cytokine production of lymphocytes isolated from mice 49 days post-primary infection, we found that mice inoculated with 104 IFU, the dose at which oviduct damage was most severe, had the greatest number of CD4+ CD69+ IL-10+ cells as determined by fluorescence-activated cell sorting in oviducts (data not shown). Experiments are under way to identify whether T-regulatory cells in fact develop during Chlamydia genital tract infection and infiltrate oviduct tissue and what factors these cells may depend on for recruitment to genital tract tissue.
Progesterone has been shown to influence Chlamydia infection via multiple mechanisms that are not well defined and would be predicted to modulate a genital infection in vivo. Progesterone has been shown to act on the uterus to increase susceptibility to infections (32), including C. trachomatis infection (21, 50). Further, progesterone also enhances progression of the organism to the upper GT (41). In addition, progesterone has also been reported to modulate immune function during infection with C. muridarum (24). In this report, we have found that the inoculating dose alters representation of different immune cell types present in the genital tract during infection. Progesterone mediates its action via specific receptors which quantitatively differ on epithelial, stromal, and leukocyte populations (57); therefore, its effect would be difficult to predict in this study. Thus, to isolate the effect of dosage from the effect of progesterone on Chlamydia genital infection, we administered a consistent amount of hormone to all groups of mice and varied only the inoculating dose.
Our data indicate that there is a direct relationship between the dose of C. muridarum and the magnitude of innate cells in cervical-vaginal tissue. Further high numbers of innate cells (Gr-1+ and CD11c+) correlated with reduced chlamydial ascension to oviducts and severity of oviduct dilation. Previous studies have suggested an important role of innate cells in controlling early stages of Chlamydia infection, though ours is the first to demonstrate a role for innate cells in limiting Chlamydia dissemination from cervical-vaginal tissue. Monoclonal antibody depletion of neutrophils in mice infected intravaginally with C. muridarum resulted in increased shedding of chlamydiae and a delay in the resolution of infection (3), and Darville et al. demonstrated by mouse strain comparisons that increased susceptibility to infection correlates with a significant delay in neutrophil infiltration to the lower genital tract (12). Also, NK cells (56) and macrophages (52, 53) have been shown to participate in chlamydial resistance through their ability to produce IFN-γ, and interferons secreted by dendritic cells may likewise play a critical antimicrobial role. Although some innate cell subsets have been well defined and their role during Chlamydia infection has been investigated, there are also data to suggest the presence of yet-unidentified innate cells that infiltrate the genital mucosa and control infection. It is of interest to further characterize these cells, which may comprise the Gr-1+ and CD11c+ subsets presently identified, in order to understand the protection they afford in preventing upper-genital-tract infection.
Our data are the first to show a direct relationship between Chlamydia burden in oviduct tissue and the magnitude of oviduct dilation, suggesting that bacterial ascension is a critical determinant of upper-genital-tract damage. In support of this, many studies have shown associations between circulating levels of antibody to chlamydial heat shock protein 60 and diagnoses of pelvic inflammatory disease (PID) (15, 45) and tubal factor infertility (2). Furthermore, LaVerda et al. (31) have shown that antibody to chlamydial heat shock protein 10 correlates with the diagnoses of tubal factor infertility in patients developing an antibody response to Chlamydia and may serve as a marker of ongoing infection. Chlamydial heat shock proteins are capable of eliciting intense leukocyte and stromal cell (macrophages, fibroblasts, and smooth muscle cells) infiltration and proliferation (28), resulting in metalloproteinase induction (29) and oxidation of low-density lipoprotein (23), which may result in fallopian tube scarring. As such, a higher Chlamydia burden in oviducts of mice infected with a low dose of Chlamydia may result in the generation of a more potent monocytic response with increased metalloproteinase production, collagen deposition, and tubal scaring. Modalities designed to reduce increased numbers of organisms in the upper GT may reduce reproductive dysfunction in humans.
Induction of natural antichlamydial immunity in the human female reproductive tract is not sufficient for bacterial eradication or lasting memory. Studies in the United States have reported rates of recurrent infections between 20 and 40% of women in target populations (14, 61). This is also evidenced by murine models of infection where organisms were culturable only after treatment with the immunosuppressant cyclophosphamide or cortisone acetate (10). Further, development of PID is estimated to occur in approximately 20% of women with primary Chlamydia infections, with risk for tubal factor infertility increasing twofold with repeated PID episodes (59). Interestingly, our present data strongly suggest that a low inoculative dose of Chlamydia facilitates ascending infection to upper- genital-tract tissues and increased oviduct dilation, following secondary infection with C. muridarum. Although there are few clinical data to suggest the minimal infectious dose of C. trachomatis in women, it is estimated that as few as 300 to 1,000 organisms are sufficient to establish lower-genital-tract infections, suggesting that upper-genital-tract sequelae may specifically result from repeated exposure to low doses of chlamydial organisms. This should be a consideration in the design of vaccination strategies and further development of animal models for studying the requirements of protective antichlamydial immunity. Further, the contribution of inflammatory responses elicited by C. trachomatis to chronic tissue pathology has not yet been delineated from those that contribute to benign resolution of infection. Therefore, further investigation of the roles of cellular mediators of natural immunity is essential for determining the requirements of an efficacious vaccine.
Acknowledgments
We thank Ann M. Chan for excellent technical assistance and Natalia Mataan for data generated from enzyme-linked immunosorbent assays.
This work was supported by PHS grant R01 AI26328-13 from NIH. H.K.M. was supported by the Microbial Pathogenesis Training Grant (5-T32-AI-07323-16).
Editor: J. D. Clements
REFERENCES
- 1.Anttila, T., P. Saikku, P. Koskela, A. Bloigu, J. Dillner, I. Ikaheimo, E. Jellum, M. Lehtinen, P. Lenner, T. Hakulinen, A. Narvanen, E. Pukkala, S. Thoresen, L. Youngman, and J. Paavonen. 2001. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA 285:47-51. [DOI] [PubMed] [Google Scholar]
- 2.Ault, K. A., B. D. Statland, M. M. King, D. I. Dozier, M. L. Joachims, and J. Gunter. 1998. Antibodies to the chlamydial 60 kilodalton heat shock protein in women with tubal factor infertility. Infect. Dis. Obstet. Gynecol. 6:163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barteneva, N., I. Theodor, E. M. Peterson, and L. M. de la Maza. 1996. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect. Immun. 64:4830-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty, W. L., G. I. Byrne, and R. P. Morrison. 1994. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 2:94-98. [DOI] [PubMed] [Google Scholar]
- 5.Beatty, W. L., R. P. Morrison, and G. I. Byrne. 1994. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol. Rev. 58:686-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belay, T., F. O. Eko, G. A. Ananaba, S. Bowers, T. Moore, D. Lyn, and J. U. Igietseme. 2002. Chemokine and chemokine receptor dynamics during genital chlamydial infection. Infect. Immun. 70:844-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-977. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cates, W., Jr., and J. N. Wasserheit. 1991. Genital chlamydial infections: epidemiology and reproductive sequelae. Am. J. Obstet. Gynecol. 164 Suppl.:1771-1781. [DOI] [PubMed] [Google Scholar]
- 10.Cotter, T. W., G. S. Miranpuri, K. H. Ramsey, C. E. Poulsen, and G. I. Byrne. 1997. Reactivation of chlamydial genital tract infection in mice. Infect. Immun. 65:2067-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotter, T. W., K. H. Ramsey, G. S. Miranpuri, C. E. Poulsen, and G. I. Byrne. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect. Immun. 65:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darville, T., C. W. Andrews, Jr., J. D. Sikes, P. L. Fraley, and R. G. Rank. 2001. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect. Immun. 69:3556-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darville, T., C. W. Andrews, K. K. Lafoon, W. Shymasani, L. R. Kishen, R. G. Rank, C. W. Andrews, Jr., and K. K. Laffoon. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect. Immun. 65:3065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean, D., R. J. Suchland, and W. E. Stamm. 2000. Evidence for long-term cervical persistence of Chlamydia trachomatis by omp1 genotyping. J. Infect. Dis. 182:909-916. [DOI] [PubMed] [Google Scholar]
- 15.Dieterle, S., and J. Wollenhaupt. 1996. Humoral immune response to the chlamydial heat shock proteins hsp60 and hsp70 in Chlamydia-associated chronic salpingitis with tubal occlusion. Hum. Reprod. 11:1352-1356. [DOI] [PubMed] [Google Scholar]
- 16.D'Souza, C. D., A. M. Cooper, A. A. Frank, R. J. Mazzaccaro, B. R. Bloom, and I. M. Orme. 1997. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J. Immunol. 158:1217-1221. [PubMed] [Google Scholar]
- 17.Hawkins, R. A., R. G. Rank, and K. A. Kelly. 2002. A Chlamydia trachomatis-specific Th2 clone does not provide protection against a genital infection and displays reduced trafficking to the infected genital mucosa. Infect. Immun. 70:5132-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland, M. J., R. L. Bailey, D. J. Conway, F. Culley, G. Miranpuri, G. I. Byrne, H. C. Whittle, and D. C. W. Mabey. 1996. T helper type-1 (Th1)/Th2 profiles of peripheral blood mononuclear cells (PBMC); responses to antigens of Chlamydia trachomatis in subjects with severe trachomatous scarring. Clin. Exp. Immunol. 105:429-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igietseme, J. U., G. A. Ananaba, J. Bolier, S. Bowers, T. Moore, T. Belay, F. O. Eko, D. Lyn, and C. M. Black. 2000. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: potential for cellular vaccine development. J. Immunol. 164:4212-4219. [DOI] [PubMed] [Google Scholar]
- 20.Igietseme, J. U., K. H. Ramsey, D. M. Magee, D. M. Williams, T. J. Kincy, and R. G. Rank. 1993. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific TH1 lymphocyte clone. Reg. Immunol. 5:317-324. [PubMed] [Google Scholar]
- 21.Ito, J. I., H. R. Harrison, R. E. Alexander, and L. J. Billings. 1984. Establishment of genital tract infection in the CF-1 mouse by intravaginal inoculation of a human oculogenital isolate of Chlamydia trachomatis. J. Infect. Dis. 150:577-582. [DOI] [PubMed] [Google Scholar]
- 22.Johansson, M., M. Ward, and N. Lycke. 1997. B-cell-deficient mice develop complete immune protection against genital tract infection with Chlamydia trachomatis. Immunology 92:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalayoglu, M. V., B. Hoerneman, D. LaVerda, S. G. Morrison, R. P. Morrison, and G. I. Byrne. 1999. Cellular oxidation of low-density lipoprotein by Chlamydia pneumoniae. J. Infect. Dis. 180:780-790. [DOI] [PubMed] [Google Scholar]
- 24.Kaushic, C., F. Zhou, A. D. Murdin, and C. R. Wira. 2000. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect. Immun. 68:4207-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly, K. A., and R. G. Rank. 1997. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect. Immun. 65:5198-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly, K. A., E. A. Robinson, and R. G. Rank. 1996. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect. Immun. 64:4976-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly, K. A., J. C. Walker, S. H. Jameel, H. L. Gray, and R. G. Rank. 2000. Differential regulation of CD4 lymphocyte recruitment between the upper and lower regions of the genital tract during Chlamydia infection. Infect. Immun. 68:1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kol, A., T. Bourcier, A. H. Lichtman, and P. Libby. 1999. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J. Clin. Investig. 103:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kol, A., G. K. Sukhova, A. H. Lichtman, and P. Libby. 1998. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation 98:300-307. [DOI] [PubMed] [Google Scholar]
- 30.Lampe, M. F., C. B. Wilson, M. J. Bevan, and M. N. Starnbach. 1998. Gamma interferon production by cytotoxic T lymphocytes is required for resolution of Chlamydia trachomatis infection. Infect. Immun. 66:5457-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaVerda, D., L. N. Albanese, P. E. Ruther, S. G. Morrison, R. P. Morrison, K. A. Ault, and G. I. Byrne. 2000. Seroreactivity to Chlamydia trachomatis Hsp10 correlates with severity of human genital tract disease. Infect. Immun. 68:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis, G. S. 2004. Steroidal regulation of uterine immune defenses. Anim. Reprod. Sci. 82-83:281-294. [DOI] [PubMed] [Google Scholar]
- 33.Magee, D. M., D. M. Williams, J. G. Smith, C. A. Bleicker, B. G. Grubbs, J. Schachter, and R. G. Rank. 1995. Role of CD8 T cells in primary Chlamydia infection. Infect. Immun. 63:516-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maxion, H. K., and K. A. Kelly. 2002. Differential chemokine expression in distinct regions of the murine genital tract during Chlamydia trachomatis infection. Infect. Immun. 70:1538-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore, T., G. A. Ananaba, J. Bolier, S. Bowers, T. Belay, F. O. Eko, and J. U. Igietseme. 2002. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology 105:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison, R. P., and H. D. Caldwell. 2002. Immunity to murine chlamydial genital infection. Infect. Immun. 70:2741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison, R. P., K. Feilzer, and D. B. Tumas. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison, S. G., and R. P. Morrison. 2000. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infect. Immun. 68:2870-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohshige, K., S. Morio, S. Mizushima, K. Kitamura, K. Tajima, A. Suyama, S. Usuku, P. Tia, L. Hor, S. Heng, V. Saphonn, O. Tochikubo, and K. Soda. 2000. Behavioral and serological human immunodeficiency virus risk factors among female commercial sex workers in Cambodia. Int. J. Epidemiol. 29:344-354. [DOI] [PubMed] [Google Scholar]
- 41.Pal, S., W. Hui, E. M. Peterson, and L. M. de la Maza. 1998. Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital tract infection. J. Med. Microbiol. 47:599-605. [DOI] [PubMed] [Google Scholar]
- 42.Pal, S., E. M. Peterson, and L. M. de la Maza. 2001. Susceptibility of mice to vaginal infection with Chlamydia trachomatis mouse pneumonitis is dependent on the age of the animal. Infect. Immun. 69:5203-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patton, D. L., and C.-C. Kuo. 1989. Histopathology of Chlamydia trachomatis salpingitis after primary and repeated reinfections in the monkey subcutaneous pocket model. J. Reprod. Fertil. 85:647-656. [DOI] [PubMed] [Google Scholar]
- 44.Patton, D. L., C.-C. Kuo, S.-P. Wang, and S. A. Halbert. 1987. Distal tubal obstruction induced by repeated Chlamydia trachomatis salpingeal infection in pig-tailed macaques. J. Infect. Dis. 155:1292-1299. [DOI] [PubMed] [Google Scholar]
- 45.Peeling, R. W., J. Kimani, F. Plummer, I. Maclean, M. Cheang, J. Bwayo, and R. C. Brunham. 1997. Antibody to chlamydial hsp60 predicts an increased risk for chlamydial pelvic inflammatory disease. J. Infect. Dis. 175:1153-1158. [DOI] [PubMed] [Google Scholar]
- 46.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 47.Quayle, A. J. 2002. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J. Reprod. Immunol. 57:61-79. [DOI] [PubMed] [Google Scholar]
- 48.Ramsey, K. H., T. W. Cotter, R. D. Salyer, G. S. Miranpuri, M. A. Yanez, C. E. Poulsen, J. L. DeWolfe, and G. I. Byrne. 1999. Prior genital tract infection with a murine or human biovar of Chlamydia trachomatis protects mice against heterotypic challenge infection. Infect. Immun 67:3019-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramsey, K. H., L. S. F. Soderberg, and R. G. Rank. 1988. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect. Immun. 56:1320-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rank, R. 1999. Models of immunity. ASM Press, Washington, D.C.
- 51.Rank, R. G., L. S. F. Soderberg, and A. L. Barron. 1985. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect. Immun. 48:847-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothfuchs, A. G., D. Gigliotti, K. Palmblad, U. Andersson, H. Wigzell, and M. E. Rottenberg. 2001. IFN-alpha beta-dependent, IFN-gamma secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J. Immunol. 167:6453-6461. [DOI] [PubMed] [Google Scholar]
- 53.Rothfuchs, A. G., M. R. Kreuger, H. Wigzell, and M. E. Rottenberg. 2004. Macrophages, CD4+ or CD8+ cells are each sufficient for protection against Chlamydia pneumoniae infection through their ability to secrete IFN-gamma. J. Immunol. 172:2407-2415. [DOI] [PubMed] [Google Scholar]
- 54.Simhan, H. N., S. N. Caritis, M. A. Krohn, B. Martinez de Tejada, D. V. Landers, and S. L. Hillier. 2003. Decreased cervical proinflammatory cytokines permit subsequent upper genital tract infection during pregnancy. Am. J. Obstet. Gynecol. 189:560-567. [DOI] [PubMed] [Google Scholar]
- 55.Su, H., K. Feilzer, H. D. Caldwell, and R. P. Morrison. 1997. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect. Immun. 65:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tseng, C. K., and R. G. Rank. 1998. Role of NK cells in the early host response to chlamydial genital infection. Infect. Immun. 66:5867-5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uotinen, N., R. Puustinen, S. Pasanen, T. Manninen, M. Kivineva, H. Syvala, P. Tuohimaa, and T. Ylikomi. 1999. Distribution of progesterone receptor in female mouse tissues. Gen. Comp. Endocrinol. 115:429-441. [DOI] [PubMed] [Google Scholar]
- 58.U.S. Department of Health and Human Services, Division of STD Prevention. 2000. Sexually transmitted disease surveillance, 1999. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, Atlanta, Ga.
- 59.Westrom, L. V. 1994. Sexually transmitted diseases and infertility. Sex. Transm. Dis. 21:S32-S37. [PubMed] [Google Scholar]
- 60.Wyrick, P. B., S. T. Knight, T. R. Paul, R. G. Rank, and C. S. Barbier. 1999. Persistent chlamydial envelope antigens in antibiotic-exposed infected cells trigger neutrophil chemotaxis. J. Infect. Dis. 179:954-966. [DOI] [PubMed] [Google Scholar]
- 61.Xu, F., J. A. Schillinger, L. E. Markowitz, M. R. Sternberg, M. R. Aubin, and M. E. St. Louis. 2000. Repeat Chlamydia trachomatis infection in women: analysis through a surveillance case registry in Washington State, 1993-1998. Am. J. Epidemiol. 152:1164-1170. [DOI] [PubMed] [Google Scholar]
- 62.Yang, X., J. Gartner, L. Zhu, S. Wang, and R. C. Brunham. 1999. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J. Immunol. 257:1010-1017. [PubMed] [Google Scholar]