Abstract
Borrelia burgdorferi, the Lyme disease spirochete, undergoes dramatic changes in antigenic composition as it cycles between its arthropod and mammalian hosts. A growing body of evidence suggests that these changes reflect, at least in part, the need for spirochetes to adapt to the physiological stresses imposed by abrupt changes in environmental conditions and nutrient availability. In many microorganisms, global responses are mediated by master regulators such as alternative sigma factors, with Escherichia coli RpoS (σS) serving as a prototype. The importance of this transcriptional activator in other bacteria, coupled with the report by Hübner et al. (A. Hübner, X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard, Proc. Natl. Acad. Sci. USA 98:12724-12729, 2001) demonstrating that the borrelial RpoS ortholog controls expression of OspC and decorin-binding protein A (DbpA), prompted us to examine more closely the roles of RpoS-dependent and -independent differential gene expression in physiological adaptation by the Lyme disease spirochete. We observed that B. burgdorferi rpoS (rpoSBb) was induced following temperature shift and transcript levels were further enhanced by reduced pH (pH 6.8). Using quantitative real-time reverse transcription-PCR (RT-PCR), we demonstrated that, in contrast to its ortholog (rpoSEc) in Escherichia coli, rpoSBb was expressed at significant levels in B. burgdorferi throughout all phases of growth following temperature shift. By comparing a B. burgdorferi strain 297 rpoSBb mutant to its wild-type counterpart, we determined that RpoSBb was not required for survival following exposure to a wide range of environmental stresses (i.e., temperature shift, serum starvation, increased osmolality, reactive oxygen intermediates, and increased or reduced oxygen tension), although the mutant was more sensitive to extremes of pH. While B. burgdorferi strains lacking RpoS were able to survive within intraperitoneal dialysis membrane chambers at a level equivalent to that of the wild type, they were avirulent in mice. Lastly, RT-PCR analysis of the ospE-ospF-elp paralogous lipoprotein families complements earlier findings that many temperature-inducible borrelial loci are controlled in an RpoSBb-independent manner. Together, these data point to fundamental differences between the role(s) of RpoS in B. burgdorferi and that in E. coli. Rather than functioning as a master regulator, RpoSBb appears to serve as a stress-responsive activator of a subset of virulence determinants that, together with the RpoS-independent, differentially expressed regulon, encompass the spirochete's genetic programs required for mammalian host adaptation.
Borrelia burgdorferi, the Lyme disease spirochete, is transmitted via the bite of an Ixodes tick and is maintained within nature by small mammalian reservoir hosts, typically wild rodents. To be sustained within this enzootic cycle, B. burgdorferi must be able to adapt rapidly to these two strikingly different host environments. The ability to host adapt, therefore, is presumed to be central to the spirochete's pathogenic programs and likely involves the expression (or repression) of genes encoding factors that prepare the bacterium for growth within the new milieu. Indeed, it has now been well established that tick feeding initiates extensive changes in borrelial transcriptome and protein composition that continue throughout the infectious process (2, 4, 14, 18, 21, 31, 38, 41, 51, 63, 70, 73, 78, 85, 89, 91). The best-studied example of borrelial differential gene expression involves the reciprocal synthesis of outer surface protein A (OspA) and OspC during tick feeding, first described in a pioneering study by Schwan et al. (74), and subsequently confirmed by others (22, 32, 57, 94). Numerous other borrelial proteins, most of which are plasmid encoded, have been studied in the context of arthropod feeding and mammalian host adaptation and, for this reason, are presumed to be relevant to spirochetal virulence and disease pathogenesis (2-4, 6, 14, 15, 21, 22, 27, 29-31, 35, 38, 41, 55, 56, 60, 63, 67, 68, 72, 74, 81-83, 86, 91, 97). Although a number of in vitro studies have demonstrated that temperature and pH are key environmental signals influencing borrelial differential gene expression (2, 4, 7, 14, 16, 17, 25, 41, 51, 61, 66, 69, 79, 86, 89, 90), we and others have obtained compelling evidence that as-yet-undefined environmental cues are also essential for triggering the genetic program(s) underlying mammalian host adaptation (2, 4, 14, 27, 41, 51, 63, 67, 69, 70, 86, 89, 91).
Many microorganisms have evolved master regulators such as alternative sigma factors to coordinate the expression of multiple loci required for adaptation to environmental and/or physiological stress (1, 9, 42, 54, 64, 84, 88, 95). One of the best-studied examples of a global stress response is the induction of the RpoS-dependent regulon in Escherichia coli (44). The studies described herein point to several novel features that distinguish RpoS expression and function in B. burgdorferi from those of its E. coli counterpart. Our data suggest that RpoS is subject to a significant degree of transcriptional control in response to a number of environmental stimuli. In contrast to E. coli, both B. burgdorferi rpoS (rpoSBb) and RpoSBb-dependent loci were expressed early during exponential growth in rich medium. Although associated with environmental stress adaptation in a number of bacterial systems, including E. coli (45), the loss of RpoS in B. burgdorferi did not affect the spirochete's ability to adapt to the majority of stress agents tested, including growth within dialysis membrane chambers (DMCs). The inability of B. burgdorferi rpoSBb mutant isolates to survive in either C3H/HeJ or SCID mice, however, points to a central role for rpoSBb in pathogenesis, likely beginning early in the infectious cycle (e.g., tick feeding). The environmental stimuli driving expression of RpoSBb-dependent loci also appear to influence differential expression of RpoSBb-independent loci, which may be of equal importance to host adaptation during the enzootic cycle. Rather than serving as a master regulator of a global environmental and/or physiological stress response or adaptation to stationary phase, our data have led us to postulate that RpoSBb functions as a stress-responsive activator of a critical, but most likely limited, subset of virulence determinants that, together with the RpoS-independent, differentially expressed regulon, encompass the genetic programs required for mammalian host adaptation and virulence expression by the Lyme disease spirochete.
METHODS AND MATERIALS
Bacterial strains and culture conditions
Virulent wild-type B. burgdorferi strain 297, originally isolated from the cerebrospinal fluid of a Lyme disease patient (77), and strain CE162 (a clonal derivative of wild-type strain 297) were cultivated in BSK-H medium (Sigma-Aldrich Chemical Co., St. Louis, Mo.) supplemented with 6% rabbit serum (Pel-Freeze Biologicals, Rogers, Alaska) and passaged no more than three times before experimental manipulations were performed. The mutants used in these studies, AH200 (297 rpoS::ermC) (47), generously provided by Michael Norgard (University of Texas Southwestern Medical Center, Dallas, Tex.), CE174 (CE162 rpoS::ermC) (see Table 1; also described below), and the complemented rpoSBb mutant (CE467) were maintained under selection in BSK-H medium containing either erythromycin (0.06 μg ml−1; AH200 and CE174) or both erythromycin (0.06 μg ml−1) and kanamycin (400 μg ml−1; CE467). The plasmid content of all strains was monitored as described previously (23). For standard growth experiments, cultures were maintained at 33°C. For temperature shift experiments, organisms were cultivated at 23°C to mid-logarithmic phase (∼1 × 107 to 3 × 107 spirochetes per ml), and 3,000 spirochetes per ml were then transferred to BSK-H medium prewarmed to 37°C; cultures maintained at 23°C were then allowed to continue until late logarithmic phase (∼7 × 107 to 1 × 108 spirochetes per ml) prior to being harvested. For growth curves, cultures were temperature shifted from 23 to 37°C (in triplicate) as described above into BSK-H medium at pH 7.5 or adjusted to pH 6.8. Cells were enumerated over 12 to 16 days by dark-field microscopy with a Petroff-Hausser counting chamber (Hausser Scientific, Horsham, Pa.). Calculations of growth curves were performed for each strain under each condition in triplicate in at least two independent trials. To obtain organisms in a host-adapted state, spirochetes were cultivated in DMCs (Spectra-Por, 8,000 molecular weight cutoff) at a starting dilution of 3,000 spirochetes per ml in BSK-H medium and implanted into the peritoneal cavities of either rats (2) or rabbits (75), as previously described.
TABLE 1.
RpoSBb is required for virulence in B. burgdorferi
| Strain | Description | Virulence in:
|
Reference or source | ||
|---|---|---|---|---|---|
| C3H/HeJa | SCIDb | DMCc | |||
| 297 | Uncloned wild type | 9/9 | ND | Y | 77 |
| AH200 | 297 rpoS::ermr | 0/9 | ND | Y | 47 |
| CE162 | Wild-type 297 clone | 10/10 | 8/8 | Y | This work |
| CE174 | 162 rpoS::ermr | 0/10 | 0/8 | Y | This work |
| CE467 | CE164 + rpoS/pCE320 | 5/5 | 7/8 | ND | This work |
Infectivity determined by immunoblot assay and cultivation of tissue biopsies.
Infectivity determined by cultivation of tissue biopsies. ND, not determined.
Survival of spirochetes within DMCs assessed by enumeration following explantation. ND, not determined.
Generation of B. burgdorferi rpoSBb mutant.
To generate a second B. burgdorferi rpoS mutant in addition to AH200, a 3-kb region of chromosomal DNA containing the rpoS::ermC locus and flanking regions was amplified from AH200 with PCR primers upsRpoS-5′ (5′-AACTTATCTTGGAGGAAATTGATG-3′) and dwnRpoS-3′ (5′-CTTGCAAATGCCTGAGTTATTGCA-3′) and used directly in an electrotransformation reaction mixture. Ten identical 50-μl reaction mixtures, with reactions carried out with TaKaRa ExTaq (Fisher Scientific) high-fidelity polymerase, were combined, purified with the Eppendorf Gel Clean-Up kit (Fisher Scientific), concentrated by ethanol precipitation, and used to electrotransform the virulent strain 297 clone according to the method described by Samuels (71). Transformants were recovered in 4 ml of BSK-H at 33°C overnight, plated in two 96-well plates each in a total of 40 ml of BSK-H containing the appropriate antibiotic, and monitored over a 5-week period for a color change in the medium that would be indicative of growth. Erythromycin-resistant spirochetes were passaged into larger culture volumes and then assessed for the insertion within rpoSBb by PCR amplification with primers PrpoS-5′ (5′-GACTGCAGAACAAATCTTAAAAATAAAGAGGG-3′) and rpoS-3 (5′-CTTGCAAATGCCTGAGTTATTGCA3′). The isolate CE174 retained a full-strain 297 plasmid complement and was chosen for further analyses. The RpoSBb mutant phenotype was also confirmed by analysis of whole-cell lysates by silver staining and Western blotting for the absence of OspC and decorin-binding protein A (DbpA), respectively (47).
Complementation of rpoSBb mutation in B. burgdorferi strain 297.
To generate a complementation construct, a 1.8-kb region of chromosomal DNA containing the rpoSBb locus and upstream flanking region containing the promoter was amplified from wild-type strain 297 genomic DNA in a 50-μl reaction mixture with ExTaq and primers rpoS+prom(extd)-5′SphI (5′-GAGCATGCAACAAATCTTAAAAATAAAGAGGG-3′) and rpoS-3′XbaI (5′-ACTCTAGATTAATTTATTTCTTCTTTTAATTTTTA-3′), containing the indicated restriction enzyme sites (underlined). The resulting PCR amplicon was first cloned into pCR2.1-TOPO (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions, then subsequently digested with SphI and XbaI (New England Biolabs, Beverly, Mass.), and subcloned into similarly digested cp32-based shuttle vector pCE320 (23). Purified recombinant rpoSBb/pCE320 plasmid DNA (10 to 20 μg) was then used to transform the strain 297 rpoSBb mutants AH200 and CE174, as described above. After multiple attempts, no transformants were obtained with AH200. One erythromycin- and kanamycin-resistant CE174 transformant designated CE467, containing the complementing plasmid and all other strain 297 plasmids, was chosen for these studies. RpoSBb complementation in CE467 was demonstrated by the restoration of OspC and DbpA expression in whole-cell lysates following temperature shift.
Nucleotide sequencing and computer analyses.
Nucleotide sequencing was performed by the University of Connecticut Health Center Molecular Core Facility with a model 373A automated DNA sequencer and PRISM ready reaction DyeDeoxy Terminator cycle sequencing kits, according to the manufacturer's instructions (Applied Biosystems, Inc., Foster City, Calif.). Routine and comparative sequence analyses were performed with MacVector version 7.2 software (Accelrys Bioinformatics, San Diego, Calif.).
SDS-PAGE and Western blot analyses.
B. burgdorferi whole-cell lysates were prepared from spirochetes cultivated in BSK-H medium at 23 or 37°C following a temperature shift from 23°C unless otherwise indicated. Cultures were harvested by centrifugation at 8,500 × g for 20 min, and the resulting pellets were washed twice with phosphate-buffered saline. Equivalent amounts of cells were resuspended, boiled in reducing Laemmli sample buffer (Bio-Rad, Hercules, Calif.), and separated through 12.5% separating polyacrylamide minigels. Separated proteins were visualized by silver staining according to the method described by Morrissey (58). Levels of OspC present in samples run on silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) minigels were calculated with the densitometry functions available in the ChemiImager 4400 (Alpha Innotech Corp., software version 5.5). For immunoblotting, proteins were transferred to nylon-supported nitrocellulose membranes (Micro Separations, Inc., Westborough, Mass.) and incubated with 1:1,000 to 1:5,000 dilutions of previously described rat polyclonal antiserum directed against strains carrying DbpA (37), OspCcN40, OspE (2), and OspF (4). Blots were assessed for loading and electrotransfer uniformity by immunoblotting with a monoclonal antibody (1H6-33) directed against FlaB (4). Blots were then probed with a 1:40,000 to 1:60,000 dilution of horseradish peroxidase-conjugated goat anti-rat or goat anti-mouse antiserum (Southern Biotechnology Associates, Birmingham, Ala.) and developed with the SuperSignal West Pico chemiluminescence substrate (Pierce, Rockford, Ill.).
Environmental stress assays.
Serum starvation assays were performed essentially as previously described by Alban et al. (5). Briefly, cultures grown in BSK-H medium at 33°C to late logarithmic phase (∼5 × 107 to 8 × 107 spirochetes per ml) were centrifuged at 9,000 × g for 10 min at 4°C, then resuspended to a density of 1.5 × 107 spirochetes per ml in RPMI 1640 medium (Invitrogen) with or without the addition of supplemental rabbit serum (final concentration, 6%), and incubated at 37°C. Aliquots were removed daily for 7 days and assayed for spirochete viability as described below. For oxygen tension assays, wild-type strain 297 and AH200 were diluted to 3,000 spirochetes per ml in BSK-H medium and incubated in standard, increased, and decreased oxygen environments. For standard (microaerophilic) conditions, 15-ml aliquots of diluted culture were placed in tightly capped 15-ml screw-cap tubes and placed in an ambient air incubator. For increased oxygen, 15-ml aliquots of diluted culture were transferred to loosely capped 50-ml conical tubes and placed at an ∼30° angle in a 20% O2-5% CO2-75% N2 incubator; this growth environment has been shown to result in an approximately twofold-higher concentration of dissolved oxygen than that under standard growth conditions (87). For the anaerobic condition, 15-ml aliquots of diluted culture were transferred to loosely capped 15-ml screw-cap tubes and placed in an anaerobe jar treated with BBL GasPak Plus anaerobic system envelopes with palladium catalysts. Cultures grown under standard, increased oxygen, and anaerobic conditions were incubated for 6 days, and endpoint densities were determined by the enumeration of triplicate cultures with a Petroff-Hausser counting chamber. For high-osmolarity and oxidative stress assays, wild-type 297 and AH200 were grown at 33°C to late logarithmic density in BSK-H medium. For high osmolarity, assays were performed as previously described (26). Briefly, cultures of each strain were divided into 1-ml aliquots containing 3.5 × 107 spirochetes per ml. To increase osmolarity, 0.25 ml of sterile 5 M NaCl was added to one aliquot (final concentration, 1 M), while sterile Milli-Q water was added to a corresponding control. Cultures were returned to 33°C, and aliquots were removed for plating at 30, 60, and 120 min. For oxidative stress assays, cultures identical to those described for the high-osmolarity assays were exposed to 0.5 and 1 mM hydrogen peroxide (Sigma-Aldrich) for 30 min at 33°C, while a corresponding control was left untreated. For acid shock assays, cultures were grown at 33°C to late logarithmic phase in BSK-H medium and diluted to a density of 3.5 × 106 spirochetes per ml into BSK-H medium adjusted to a final pH value of 6.0. Cultures were incubated at 33°C for 2, 6, 12, and 24 h, and spirochete viability was determined as described below. Spirochete viability was assayed by growth endpoint determinations performed by first diluting bacterial samples 1:100 (except for acid shock assays, where a 1:10 dilution was used) into fresh BSK-H medium, corresponding to ∼3.5 × 105 spirochetes per ml. Cultures were then serially diluted 1:2 with BSK-H medium in sterile 96-well microtiter plates. For each environmental condition, duplicate twofold serial dilutions were performed for each experimental sample, and the plates were incubated in a CO2 incubator at 33°C for approximately 21 days. The number of spirochetes surviving exposure to environmental stress was calculated by using the endpoint dilution well containing viable spirochetes as determined by dark-field microscopy. Percent survival was calculated as the number of viable spirochetes after treatment divided by the number of live spirochetes in the untreated control for each assay condition. Wild-type and mutant isolates were compared in at least two independent assays for each experimental condition.
Statistics.
To determine the statistical significance of observed differences, values were compared either with Prism, version 3.00 (GraphPad Software, San Diego, Calif.), or with an unpaired t test with two-tailed P values and a 95% confidence interval. In the data presented in this study, asterisks indicate a level of significance where P values are <0.05; error bars represent the standard error of the mean. In addition, the product limit (Kaplan-Meier) method was used to compare the overall rates of survival for wild-type and mutant isolates following exposure to environmental stress agents (JMP 5.0 software, SAS Institute, Cary, N.C.).
Standard RT-PCR.
Total RNA was isolated from wild-type and AH200 (rpoSBb mutant) spirochetes cultivated in vitro to late logarithmic phase at 23°C and following temperature shift (37°C at either pH 7.5 or 6.8) in BSK-H medium and following implantation within DMCs with Trizol reagent (Invitrogen) according to the manufacturer's instructions. RNAs were resuspended in RNase-free water and treated with RQ1 RNase-free DNase (Promega) to remove contaminating genomic DNA, followed by phenol-chloroform extraction and ethanol precipitation. Primers specific for rpoSBb are as follows: rpoS-RTPCR 5′ (5′-CAGTAAGAGAACACAAGCTAATTACTCAC-3′) and rpoS-RTPCR 3′ (5′-ATCCTCGTATAGATTCAAGAGTGTTG-3′). Primer pairs specific for each of the strain 297 ospE-ospF-elp genes and flaB are the same as those described by Hefty et al. (41). Standard reverse transcription-PCRs (RT-PCRs) were performed with the Titan One-Step RT-PCR kit (Roche) with 50 to 100 ng of total RNA per 25-μl reaction mixture. Negative control reactions were performed by substituting Expand High Fidelity Taq polymerase (Roche) for the Titan RT enzyme mixture. Positive-control reactions were performed with Expand High Fidelity Taq polymerase and either wild-type or AH200 genomic DNA purified with the IsoQuick DNA purification kit (Orca Research, Bothell, Wash.). Reaction conditions for all reactions consisted of a single cycle at 50°C for 30 min and 94°C for 3 min; then 38 cycles of 94°C for 15 s, 54°C for 15 s, and 68°C for 1 min; followed by a final extension at 68°C for 5 min. Five microliters of each amplification reaction mixture was then subjected to agarose gel electrophoresis and subsequently stained with ethidium bromide to visualize amplicons.
Quantitative RT-PCR.
Total RNA from wild-type and AH200 (rpoSBb mutant) spirochetes were cultivated in vitro to late logarithmic phase at 23°C throughout all phases of growth following temperature shift (37°C at either pH 7.5 or 6.8); following implantation within DMCs, the RNA was isolated with Trizol reagent and subjected to DNase, as described above. Real-time RT-PCR was performed with a Roche LightCycler instrument and the LightCycler RNA Master SYBR Green I kit (Roche Applied Science, Indianapolis, Ind.) according to the manufacturer's instructions. Reactions containing either 50 ng (rpoS) or 0.5 ng (flaB) of total RNA in the reaction mixture were performed with primer pair rpoS-LC 5′ (5′-CAGGACAAATACAAAGAGGCAATG-3′) and rpoS-LC 3′ (5′-CGGGTCATATTTTTCAGCAGCTC-3′) and primer pair flaB-LC 5′ (5′-CTTCTCAAGGCGGAGTTAATTCTC-3′) and flaB-LC 3′ (5′-TTAGTTGTTGCTGCTACAACCTCA-3′) under the following conditions: 1 cycle of 61°C for 20 min and 95°C for 30s, followed by 45 cycles of 95°C for 1 s, 60°C for 5 s, and 72°C for 10 s. RNAs (50 ng per reaction mixture) were assayed for contaminating DNA with the LightCycler FastStart DNA Master SYBR Green I kit (Roche) with the same primers and under the same reaction conditions as described above. For quantitation, rpoS-LC and flaB-LC amplicons were first cloned into the pCR2.1-TOPO cloning vector (Invitrogen), and then purified recombinant plasmid DNAs containing the amplicon of interest were diluted (107 to 102 copies μl−1) and used to generate external standard curves according to the manufacturer's instructions. Each assay run included RT-PCRs containing relevant purified plasmid (106 copies μl−1) DNA as a template to serve as an internal control for the calibration of external standard curves. Quantitative real-time RT-PCRs (qRT-PCRs) containing experimental samples, calibration standards, or negative controls were each performed in duplicate. Copies of rpoSBb and flaB present in each sample were calculated with LightCycler data analysis software (version 3.5.3) based on their respective standard curves, subtracted for background present in the negative control reaction mixtures, and the copies of flaB were corrected for dilution. To determine increase, the ratio of copies of rpoSBb to copies of flaB was calculated for each sample, and then the rpoSBb/flaB ratio of each sample was compared to that of the 23°C sample.
Analysis of fluorescent reporters.
E. coli cultures were maintained in Luria-Bertani (LB) broth (1% tryptone, 0.5% yeast extract, 1% NaCl) or LB agar (LB broth with 1.5% agar) supplemented with kanamycin (50 μg ml−1). Shuttle vectors containing green fluorescent protein (GFP) reporters under the control of either borrelial (PospC and PospE) or E. coli (PosmY) promoter regions transformed into E. coli MC4100 [F− araD139 Δ(argF-lacZYA) U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR] (76) have been described previously (41). Cultures containing the various gfp-PBb shuttle vectors were grown overnight at 25°C, then diluted to an A600 of 0.01 in SOB (2% tryptone, 0.5% yeast extract, 0.05% NaCl, 2.5 mM KCl, 10 mM MgCl2) supplemented with zeocin (50 μg ml−1), and incubated at 37°C with shaking at 250 rpm. For each assay, the optical density at 600 nm was determined at hourly intervals; beginning 2 h after inoculation, 0.5 ml of sample was fixed in 1% paraformaldehyde (final concentration, 0.8%) in Difco FA buffer (Becton Dickinson, Palo Alto, Calif.) and analyzed by flow cytometry as described below. Assays were performed in triplicate for each construct. For flow cytometric analyses, samples containing ≥107 paraformaldehyde-fixed cells were analyzed on a FACScalibur flow cytometer (Becton Dickinson) with a 15-mW 488-nm air-cooled argon laser and an ≈635-nm red diode laser as previously described (23). Data were collected in duplicate for 50,000 events for each sample. Flow cytometry data were analyzed using either CELLQUEST version 3.3 (Becton Dickinson) or WinMDI, version 2.8 (http://facs.scripps.edu/software.html).
Animal infectivity studies.
B. burgdorferi wild-type clone 297 (CE162), rpoSBb mutant AH200 (47), CE174 (a transformable insertionally inactivated rpoSBb mutant in the CE162 background; see above), and CE467 (CE174 complemented in trans with a shuttle vector containing a copy of rpoSBb under the control of its native promoter; see above) were assessed for infectivity using 3- to 5-week-old female C3H/HeJ (Jackson Laboratories) and BALB/c SCID (T.V. Rajan, University of Connecticut Health Center) mice. Low-passage cultures to be used for infections were grown to mid-logarithmic density in BSK-H medium at 33°C, enumerated by dark-field microscopy, and diluted to a density of 2 × 105 spirochetes per ml. Mice (four to five per group, per strain) were inoculated intradermally with 0.05 ml of diluted culture for a dose of 104 organisms per mouse. Mice were assessed for infection at 2 to 4 weeks postinoculation by cultivation of ear punch biopsies in BSK-H medium containing Borrelia Antibiotic Cocktail (Sigma-Aldrich) grown at 33°C. Negative cultures were monitored weekly for at least 6 weeks by dark-field microscopy. Immunocompetent mice were subjected to tail bleeding at 2 and 4 weeks postinoculation, and the collected sera were assayed by Western immunoblot analyses with preparative minigel electrotransfers of whole-cell lysates prepared from B. burgdorferi wild-type 297 grown at 37°C in BSK-H medium.
RESULTS
Transcription of rpoSBb is induced following temperature shift and further enhanced by reduced pH.
Previous studies have demonstrated that the B. burgdorferi RpoS ortholog (BB0771) is required for increased expression of two borrelial lipoproteins, DbpA and OspC, following a shift in growth temperature in vitro from 23 to 37°C (47). This finding, coupled with the importance of RpoS for stress adaptation in other bacterial systems, prompted us to more closely examine its role in mediating differential gene expression and/or physiological adaptation in B. burgdorferi. In contrast to E. coli, where levels of RpoS are controlled primarily through posttranscriptional mechanisms (44), expression of rpoSBb in B. burgdorferi is under the control of a second alternate sigma factor, RpoN (47), which in turn requires an interaction with the response regulator Rrp2 for activity (92). This unique control mechanism suggested that expression of RpoSBb could be partially or completely regulated at the transcriptional level. We therefore began our studies by assessing rpoSBb levels following temperature shift. To do this, total RNAs prepared from wild-type B. burgdorferi strain 297 grown to late logarithmic phase (8 × 107 cells per ml) in BSK-H medium at either 23°C or after temperature shift to 37°C were used to perform qualitative RT-PCR for rpoSBb; control reactions for flaB were used to check RNA integrity and to normalize samples. Results from these studies demonstrated a substantial increase in rpoSBb transcript following temperature shift (data not shown); using densitometry, we estimated at least a twofold increase in rpoSBb transcript in samples after temperature shift at pH 7.5 (data not shown). Expression of RpoSBb and RpoSBb-dependent genes (e.g., OspC) has been shown to be enhanced by a reduction in the pH of BSK-H medium from 7.5 to 6.8, in concert with temperature shift (47). To examine the contribution of pH to rpoSBb expression, we performed RT-PCR on RNAs isolated from late-logarithmic-phase cultures of wild-type B. burgdorferi after temperature shift to 37°C in BSK-H medium at pH 6.8. Results from these studies demonstrated that the level of rpoSBb present following temperature shift at pH 6.8 was further increased over that seen in similarly temperature-shifted samples at pH 7.5 (2.9-fold compared to that at 23°C) (data not shown). These results are consistent with immunoblot data demonstrating that, while undetectable when spirochetes were cultivated to late logarithmic phase in BSK-H medium at 23°C, RpoSBb was readily detectable following temperature shift in standard BSK-H (37°C; pH 7.5) and further enhanced when the pH was adjusted to 6.8 (data not shown).
Induction kinetics of RpoS and RpoS-dependent promoters differ in B. burgdorferi and E. coli.
To more accurately assess the level of increase in rpoSBb due to decreased pH and/or increased temperature, we performed qRT-PCR using primers specific for rpoSBb on RNAs isolated from spirochetes following temperature shift at pHs 7.5 and 6.8 and compared these transcript levels to that found in spirochetes grown to late logarithmic phase in BSK-H (pH 7.5) at 23°C. To correlate the effect of growth phase with rpoSBb transcript levels under these growth conditions, total RNA was isolated daily beginning with the mid-log phase of growth (the earliest time point at which spirochetes could be accurately enumerated and at which sufficient material for SDS-PAGE analysis could be obtained) until cultures had entered stationary phase (Fig. 1A). Results from qRT-PCR studies with primers specific for rpoSBb indicate an average relative ratio of 0.0005 copies of rpoSBb per copy of flaB at 23°C, whereas temperature-shifted cultures grown in BSK-H at pH 7.5 had significantly higher levels of rpoSBb (6.30-fold increase in the rpoSBb/flaB ratio) at the earliest time point assayed (Fig. 1B, lane T1). Moreover, the levels of rpoSBb in B. burgdorferi were essentially unchanged throughout all phases of logarithmic growth, although we did observe an increase (6.36-fold; pH 7.5) (Fig. 1B, lane T6 versus T1) during entry into stationary phase. This increase, however, may be due to the increased acidity of the culture medium as a consequence of spirochetal growth, as indicated by color change of the phenol red indicator dye present in BSK-H. This explanation is supported by the consistently higher levels of rpoSBb observed when spirochetes were temperature shifted in BSK-H at pH 6.8 (Fig. 1B), where levels of rpoSBb transcript at pH 6.8 were higher than those at pH 7.5 throughout all phases of growth. The enhancement observed during entry into stationary phase of pH 7.5 cultures was also observed in temperature-shifted cultures at pH 6.8 (Fig. 1B, lane T4). Analysis of total RNA isolated from DMC-cultivated spirochetes found that the level of rpoSBb within the mammalian host is equivalent to the highest levels detected in vitro in BSK-H at pH 7.5 (Fig. 1B, lanes DMC and T6) and approached the highest levels observed at pH 6.8 (Fig. 1B). We next analyzed whole-cell lysates of wild-type strain 297 cultures grown to late logarithmic phase at 23°C and at sequential time points following temperature shift to determine whether sufficient levels of RpoSBb were present to mediate the induction of RpoSBb-dependent genes. Not surprisingly, significant levels of OspC were observed during mid-logarithmic-phase growth, well before entry into stationary phase (Fig. 1C). Indeed, if one uses densitometry to correct for the unavoidable underloading at this sample point (Fig. 1C, lane 1), then the level of OspC expression during logarithmic growth is approximately 70% of levels achieved during stationary phase; expression of DbpA also showed similar induction kinetics (data not shown).
FIG. 1.
Expression kinetics of rpoSBb and RpoS-dependent loci in B. burgdorferi. (A) Growth curve indicating time points at which samples were taken from wild-type strain 297, following temperature shift from 23 to 37°C in BSK-H medium at either pH 7.5 (solid squares) or 6.8 (open squares), for subsequent RNA and protein analyses. (B) qRT-PCR analysis of rpoSBb. RNAs were isolated from spirochetes cultivated at 23°C and after temperature shift to 37°C in BSK-H medium at either pH 7.5 or 6.8 and after cultivation within DMCs. (C) Whole-cell lysates of wild-type B. burgdorferi strain 297 (∼107 per lane) cultivated at 23°C and following temperature shift to 37°C in BSK-H medium at pH 7.5 were separated by SDS-PAGE and then silver stained. Molecular mass markers (in kilodaltons) are indicated. Sample numbering in panels B and C corresponds to similarly numbered points in panel A. wt, wild type.
To determine whether these findings reflect a functional distinction between RpoS in B. burgdorferi and E. coli or, alternatively, a unique feature of the RpoSBb-dependent promoters elements themselves, we used a newly developed GFP reporter system for analyzing borrelial promoters in E. coli by flow cytometry (24). For these studies, we transformed both wild-type (ZK126) (19) and rpoS mutant (ZK1000) (12) E. coli with a PospC-gfp reporter contained on a cp32-based shuttle vector (pCE320) (23) and measured GFP expression over time by flow cytometry. As shown in Fig. 2, expression of GFP from the borrelial ospC promoter (PospC-gfp) in E. coli did not become readily detectable until cultures reached late logarithmic phase (6 h), with maximal expression not observed until cultures entered stationary phase (10 h). This expression pattern closely mirrored that of a reporter under the control of the promoter for osmY (PosmY-gfp), a gene known to be induced during stationary phase in an RpoS-dependent manner in E. coli (52, 96) (Fig. 2). The low-level expression of the PospC-gfp reporter observed in the rpoSEc mutant background is likely due to the unmasking of σ70 promoter recognition sequences in the absence of RpoS or, alternatively, could reflect differences in promoter recognition by the corresponding transcriptional factors orthologs in B. burgdorferi and E. coli (24). Taken together, these findings further support the existence of differences between the kinetics of RpoS expression and RpoS-dependent genes in B. burgdorferi and E. coli.
FIG. 2.
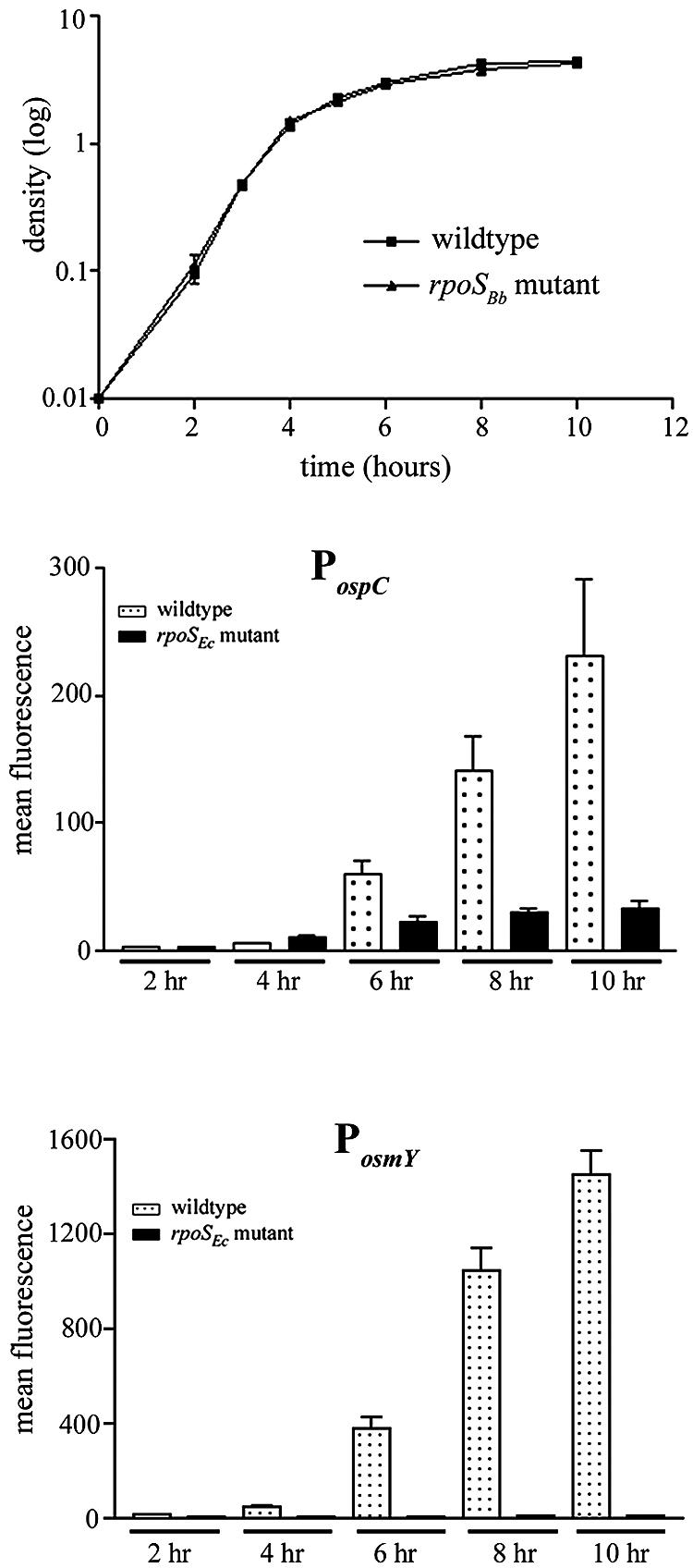
Induction kinetics of RpoS-dependent promoters differ in B. burgdorferi and E. coli. Analysis of RpoS-dependent B. burgdorferi (PospC) and E. coli (PosmY) promoters with a GFP reporter system in E. coli. Samples from wild-type (ZK126; squares) and rpoSEc mutant (ZK1000; triangles) E. coli transformed with the indicated reporter constructs analyzed by flow cytometry. The top panel depicts a representative growth curve for both wild-type and rpoSEc mutant isolates. Mean fluorescence intensities for PospC-gfp (middle panel) and PosmY-gfp (bottom panel) reporter constructs were plotted against time on the representative graphs. Each graph represents the average of three trials.
Survival of B. burgdorferi wild-type and RpoS mutant AH200 following exposure to environmental stress.
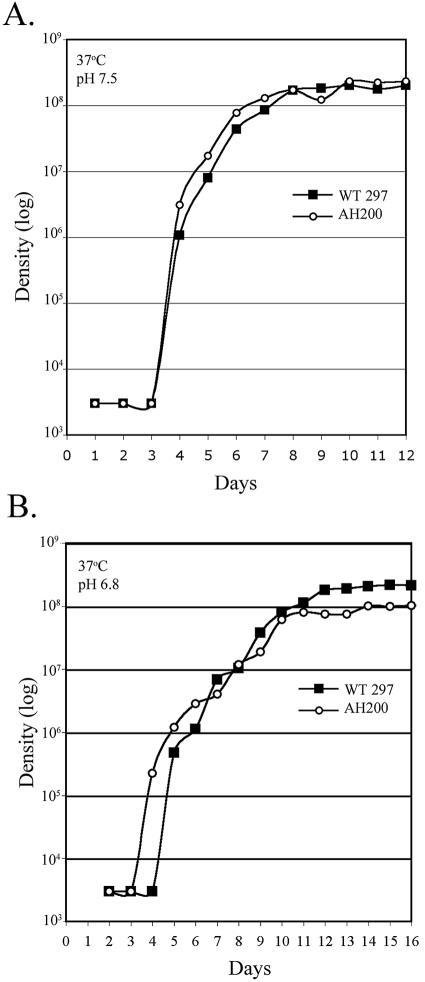
The temperature-inducible expression of rpoSBb in B. burgdorferi suggests that spirochetes may be experiencing some level of stress under this condition. This notion is supported by in vitro studies by Hubalek et al. (46) demonstrating that 37°C approaches the upper limit of permissible growth temperatures (22 to 39°C) for B. burgdorferi sensu stricto isolates. We therefore examined whether loss of rpoSBb rendered spirochetes more sensitive to increased temperatures. At the outset, we performed routine temperature shift assays to reproduce the phenotype associated with AH200 (a strain 297 rpoSBb mutant) observed by Hübner et al. (47); as expected, AH200 failed to express either OspC or DbpA following a shift in growth temperature from 23 to 37°C as assessed by both silver staining (OspC) and Western blot (DbpA) analysis (see below). To assess whether the loss of RpoSBb results in altered growth following temperature shift, we performed growth curve assays comparing wild-type strain 297 to the rpoSBb mutant AH200 following temperature shift from 23 to 37°C in BSK-H at pHs 7.5 and 6.8. As shown in Fig. 3, wild-type strain 297 and AH200 grew with nearly identical kinetics under both in vitro growth conditions. Thus, although rpoSBb and RpoS-dependent genes are significantly induced under these conditions, RpoSBb itself does not appear to be required for adaptation to increased temperature at either pH 7.5 or 6.8.
FIG. 3.
Growth phenotypes of wild-type and rpoSBb mutant bacteria at increased temperatures and reduced pH. Growth curve analyses of wild-type (WT; solid squares) and rpoSBb mutant (AH200; open circles) B. burgdorferi strain 297 cultivated in BSK-H medium following temperature shift to 37°C at pH 7.5 (A) and 6.8 (B). The graph depicting the growth of the wild-type isolates is the same as that shown in Fig. 1A.
We next assessed whether RpoSBb was required for survival following exposure to conditions that have a measurably detrimental effect on growth of wild-type virulent strain 297. For this, we chose a number of well-established classical environmental stresses that have been associated with the induction of an RpoS-dependent general stress response in other organisms: (i) nutrient deprivation (growth in RPMI medium with or without the addition of serum) (Fig. 4A), (ii) increased or decreased dissolved oxygen (Fig. 4B), (iii) high osmolarity (30, 60, and 120 min of exposure to 1 M NaCl) (Fig. 4C), (iv) exogenous peroxide (30 min of exposure to 0.5 or 1 mM H2O2) (Fig. 4D), and (v) acidic pH (pH 6.0) (Fig. 4E). In the first four cases, the rpoSBb mutant AH200 survived exposure to the stress agent to the same extent as the wild-type isolate. In contrast, the loss of RpoSBb resulted in increased sensitivity to acidic pH (Fig. 4E); Kaplan-Meier analysis revealed that survival of the rpoSBb mutant was diminished in comparison to the wild type following exposure to acidic pH (log rank χ2 = 3.809; P = 0.051). Exposure to pH values below 6.0 (pHs 5.0 and 4.0) resulted in no survival of either wild-type or rpoSBb mutant isolates at any time point (data not shown).
FIG. 4.
RpoS is not required for survival following exposure to environmental stress. Survival of B. burgdorferi wild-type strain 297 and rpoSBb mutant AH200 following cultivation in RPMI with (+) or without (−) 6% normal rabbit serum (A), under various oxygen tensions (standard microaerophilic, increased oxygen [CO2], and anaerobic) (B), or following exposure to high osmolarity (1 M NaCl) (C), exogenous peroxide (0.5 or 1 mM H2O2) (D), or acidic pH (pH 6.0) (E). Percent survival was calculated based on no-exposure controls for each sample. Percent survival values represent the averages of two independent trials. The log rank and P values for the results shown in panel E, determined by the Kaplan-Meier method, were χ2 = 3.809 and P = 0.051. wt, wild type.
RpoS mutants grow within DMCs but are avirulent in mice.
The above in vitro studies do not preclude the possibility that RpoSBb controls the expression of one or more loci essential for adaptation to growth within the mammalian host. To examine this possibility, we grew wild-type and rpoSBb mutant isolates within DMCs implanted into the peritoneal cavities of rats and rabbits. In addition to reflecting the differential gene expression patterns observed during mammalian host adaptation (2, 14, 41, 63, 70), this animal model is presumably representative of the physiological and nutritional stresses to which spirochetes must adapt in vivo (27, 67). The use of low starting numbers of inocula (103 spirochetes/ml) in these studies ensures that spirochetes harvested following cultivation within DMCs have undergone numerous rounds of replication in vivo. Following explantation, both the wild-type and rpoSBb mutants consistently grew to the same approximate density (6 × 106 to 2 × 107 spirochetes per ml) within DMCs (data not shown). It has previously been demonstrated by us and others that expression of OspC and DbpA increases during growth in DMCs (2, 14, 69). Consistent with these earlier studies, qRT-PCR performed on total RNAs isolated from wild-type strain 297 cultivated within DMCs demonstrated that rpoSBb was expressed at higher levels during DMC cultivation than after temperature shift alone (Fig. 1B). Thus, while rpoSBb and RpoSBb-dependent genes were strongly induced during DMC cultivation, the corresponding RpoS-dependent regulon does not appear to be required for the physiological adaptation(s) mediated by this growth environment.
By design, cultivation within DMCs sequesters spirochetes from the external cellular milieu; as a consequence, this methodology does not allow for identification of phenotypes associated with virulence-related functions such as dissemination, cell adhesion, and/or immune evasion. Growth of rpoSBb mutants within DMCs, therefore, does not rule out a requirement for one or more RpoSBb-dependent loci in colonization of various mammalian tissues and/or infectivity. To further examine the role of RpoSBb in vivo, we performed virulence studies with C3H/HeJ mice with wild-type strain 297 and the rpoSBb mutant AH200. Prior to infection, isolates were tested to ensure that each possessed a full complement of strain 297 plasmids, thereby ruling out the likelihood that any loss of virulence was due to the absence of the virulence-associated plasmids lp25 and lp28-1 (27, 55, 68). Results from these studies are summarized in Table 1. With the combined results from two independent infection studies, we found that the rpoSBb mutant AH200 was avirulent (0 of 9 mutant strain samples were avirulent versus 9 of 9 of the wild type). To confirm that this phenotype was due to loss of rpoSBb and not a secondary mutation, we constructed a complementation plasmid encoding a wild-type copy of rpoSBb under the control of its native promoter contained on pCE320, a cp32-based shuttle vector (23). Unfortunately, we were unable to electrotransform AH200 with this or other vectors. To overcome this technical hurdle, we constructed a second strain 297 rpoS mutant, CE174, in the wild-type transformable strain 297 clone CE162. Combined data from two independent mouse infection studies using this second rpoSBb mutant yielded results identical to those obtained with AH200 (0 of 10 samples were avirulent for CE174 versus 10 of 10 samples for CE162), while studies performed using CE467, the CE174-derived rpoSBb mutant transformed with rpoSBb/pCE320, demonstrated that infectivity could be restored (5 of 5 samples) when RpoSBb was supplied in trans (Table 1). Essentially identical results were obtained with these same isolates with SCID mice (Table 1), a background that should allow for the growth of spirochetes defective in RpoS-dependent loci potentially associated with immune evasion functions. Ear punch biopsies taken from SCID mice infected with either CE162 (8 of 8 mice) or CE467 (7 of 8 mice) were culture positive 2 weeks postinfection, while all mice infected with CE174 were culture negative (0 of 8 mice) (Table 1). To our knowledge, this represents the first report of complementation of an rpoSBb mutant with a wild-type copy of rpoSBb.
Analysis of ospE-ospF-elp expression in wild-type and rpoSBb mutant B. burgdorferi.
Recent studies have demonstrated that a number of borrelial cp32-encoded loci (Bdr and Mlp paralogous gene families) that have previously been shown to exhibit increased expression following temperature shift and during host adaptation (2, 65, 89, 91, 98) are expressed in an RpoSBb-independent manner (70, 93). Here, we extended these studies by examining the RpoSBb dependence of the cp32-encoded ospE-ospF-elp lipoprotein genes (2-4, 15, 40, 41, 41) following temperature shift in vitro. Immunoblot analyses revealed that expression of OspF, but not OspE, was eliminated in AH200 (Fig. 5A); identical results were obtained with CE174 (24). Although the high degree of amino acid similarity makes it difficult to track expression of the more closely related alleles by immunoblotting, we were able to perform RT-PCR with primers specific for each of the remaining ospE-ospF-elp genes in wild-type and AH200 isolates following temperature shift. As shown in Fig. 5B, only ospF and its nearly identical paralog bbk2.11 are expressed in an RpoSBb-dependent manner. In contrast, the third ospF allele in strain 297, bbk2.10, was expressed in an RpoSBb-independent manner. This result is interesting and also not completely unexpected, given that the mature Bbk2.10 polypeptide is quite distantly related to OspF/Bbk2.11 (3, 15) and therefore potentially serves a separate functional role during infection.
FIG. 5.
ospE-ospF-elp paralogous loci utilize predominantly RpoS-independent pathways. (A) Analysis of wild-type (WT) and rpoSBb mutant (AH200) B. burgdorferi strain 297 samples cultivated at 23°C and following temperature shift to 37°C in BSK-H medium at pH 7.5 or 6.8. Whole-cell lysates (∼107 lysates per lane) were separated by SDS-PAGE and either silver stained (OspC) or immunoblotted with sera directed against DbpA, OspF, OspE, or FlaB. (B) RT-PCR of wild-type (wt) strain 297 and AH200 following temperature shift to 37°C with primers specific for individual ospE-ospF-elp loci and flaB with (+) or without (−) reverse transcriptase. DNA controls were also performed with 297 genomic DNA (not shown). Molecular weight markers in kilobases are indicated to the left.
DISCUSSION
During growth in rich medium, E. coli RpoS is primarily responsible for directly or indirectly coordinating the expression of at least 100 genes during entry into stationary phase (45, 48, 53). During logarithmic growth, RpoS is also required for survival following exposure to diverse environmental stresses, including acidic pH, UV irradiation, various forms of starvation, high osmolality, and reactive oxygen species (44). Studies by Hübner et al. (47) demonstrating that expression of at least two borrelial lipoprotein-encoding loci (OspC and DbpA) is controlled by the putative borrelial RpoS ortholog in response to increased temperature and decreased pH, combined with the importance of this alternative sigma factor in other bacterial systems, prompted us to undertake a more detailed examination of RpoSBb to determine its role in mediating differential gene expression and physiological adaptation in B. burgdorferi. We began our studies by more closely examining the effect of temperature, a key environmental signal involved in borrelial differential gene expression (2, 4, 8, 13, 14, 25, 41, 47, 61, 65, 69, 79, 86, 89, 91), on rpoSBb expression. Using qRT-PCR, we demonstrated that rpoSBb is induced at least sixfold following a temperature shift from 23 to 37°C in vitro beginning early in mid-logarithmic-phase growth. While expression of rpoSBb was further increased (∼3- to 4-fold) in response to reduced pH in combination with increased temperature, pH does not appear to exert its effects independent of temperature, since growth at 23°C at reduced pH does not induce expression of RpoSBb-dependent genes (data not shown). Our findings are discordant, however, with two previously published microarray studies which failed to detect a significant increase in rpoSBb (61, 69); Ojaimi et al. (61) did observe a 1.6-fold increase in rpoSBb following a temperature shift to 35°C, but this change did not exceed their statistical threshold. The disparity between these studies is most likely due to an underestimation of induction ratios as a result of the relatively limited dynamic range of microarrays compared to qRT-PCR (20). Our qRT-PCR data were also supported by similar increases in RpoSBb protein levels. While results from the RT-PCR studies presented herein suggest that reduced pH positively influences rpoSBb expression in vitro, there is some cause to question whether reduced pH is necessary for host adaptation, since DMC-cultivated organisms express increased rpoSBb (∼38-fold higher in DMCs versus conditions at 23°C) and exhibit an antigenic profile characteristic of mammalian infection (2) within a physiological pH range; the average measured pH of DMC fluid following explantation is ∼7.6 (data not shown). Although recent studies suggest that some degree of posttranscriptional control may be mediated by DsrA, a small regulatory RNA (M. C. Lybecker and D. S. Samuels, personal communication), RpoSBb appears to be unequivocally regulated at the level of transcription.
In many bacterial systems, including E. coli (43) and Pseudomonas aeruginosa (49), the role of RpoS is twofold. During growth in rich medium, RpoS directly (or indirectly) controls the expression of loci involved in growth adaptation during stationary phase (45). This alternate sigma factor also controls the expression of loci involved in survival following exposure to environmental stresses during logarithmic-phase growth (10, 11, 28, 33, 43, 49, 80, 95). The studies described in this report, however, provide compelling evidence to suggest that RpoS in B. burgdorferi is functionally distinct on both accounts from its orthologs in other bacteria, most notably that of E. coli. First, the substantial levels of rpoSBb and RpoS-dependent gene expression observed in exponentially growing borrelial cultures clearly distinguish B. burgdorferi from E. coli. Although a modest increase was observed upon entry of spirochetes into stationary phase, more than ample levels of RpoSBb were present during mid-logarithmic growth to achieve near maximal expression levels of at least two RpoSBb-dependent loci, ospC and dbpA. These expression kinetics are fully consistent with studies demonstrating that OspC is expressed early during arthropod feeding (36, 62, 72) and further point to an early, perhaps pivotal, role for RpoS in host adaptation, a process that presumably initiates with tick feeding. The second notable difference between RpoSBb and its orthologs in many other bacteria is exemplified by in vitro environmental stress assays demonstrating that loss of RpoSBb did not significantly alter the ability of spirochetes to survive exposure to a wide range of stresses, acidic pH adaptation notwithstanding. These findings are not entirely consistent with an earlier report by Elias et al. (26) which found that a B31 mutant (A74 rpoS::gyrBr) was more sensitive to high-salt conditions than its wild-type parent when cultures were examined late in stationary phase. This discrepancy may be due to strain differences rather than RpoS itself; the parental isolate used to make the B31 mutant (B31-A74) is an avirulent high-passage isolate that may contain secondary mutations in addition to rpoSBb. Results from our nutrient deprivation assay, on the other hand, are consistent with studies by Murgia et al. (59) demonstrating that an rpoSBb mutant B. burgdorferi formed nonmotile cysts under unfavorable growth conditions (i.e., serum starvation and exposure to antibiotics) at a rate equal to that of wild-type isolates. The ability of B. burgdorferi rpoSBb mutants to grow to wild-type levels within DMCs provides further compelling evidence that RpoSBb is not central to the physiological (i.e., nutritional) adaptation associated with growth within the mammalian host.
At first glance, our findings were surprising in light of the role of RpoS in stress adaptation in other bacteria, most notably E. coli, Salmonella (33), and P. aeruginosa (49), but these organisms have evolved to adapt to truly diverse growth environments. B. burgdorferi, an organism with only limited biosynthetic capabilities (34), does not exist in nature as a free-living organism and therefore is confined to a narrow host range with only limited exposure to environmental stresses. Accordingly, BLAST searches of the borrelial genome yielded, at best, putative orthologs for only 22 of the 118 loci believed to be controlled by RpoS in E. coli and Salmonella (45, 48, 53). Our finding that loss of RpoSBb resulted in increased sensitivity to acidic pH, on the other hand, is consistent with a more typical role for RpoS in stress adaptation (33), but this phenotype was only observed in vitro at a pH well below that to which spirochetes would be presumably exposed to during the enzootic cycle. Indeed, the acid tolerance of B. burgdorferi appears to be within a very narrow range with little to no survival observed after exposure to pH values below 6.0 (data not shown), in contrast to the ability of other organisms, such as E. coli, to tolerate a much wider range of pH levels (33). Taken together, results from our qRT-PCR and acid exposure studies are suggestive of a role for RpoSBb in differential gene expression in response to acidic pH and/or during entry into stationary phase; a better understanding of the RpoSBb-dependent regulon will be necessary to delineate those loci responsive to reduced pH versus those sensitive to growth phase-mediated RpoSBb-dependent regulation.
While RpoSBb appears to serve only a limited role in environmental stress adaptation, the failure of rpoSBb mutants to infect mice implies that this transcriptional activator controls the expression of one or more essential virulence determinants. Although the ability of rpoSBb mutants to grow within DMC but not infect mice might seem contradictory, we believe that this phenotypic distinction actually provides considerable insight into the role of the RpoSBb-dependent regulon in vivo by helping to distinguish between the physiological and nonphysiological demands placed on spirochetes during growth within the mammalian host. The failure of rpoSBb mutants to establish infection in both C3H/HeJ and SCID mice strongly suggests that the function(s) of RpoSBb-dependent virulence-associated loci is not involved exclusively in immunoevasion and/or antigenic variation and instead suggests that RpoSBb-dependent loci are involved in other virulence-related functions such as adhesion, dissemination, and/or survival within mammalian tissues. Although there is still some debate as to the precise role of OspC during the enzootic cycle (36, 62), recent studies by Grimm et al. (36) demonstrated that insertional inactivation of ospC results in a loss of virulence. We, therefore, cannot rule out the possibility that the avirulence of rpoSBb mutants is due to the loss of ospC alone. One way to test this hypothesis would be to compare the infectivity of RpoSBb- and OspC-deficient backgrounds supplied with ospC in trans; the requirement for use of a heterologous RpoSBb-independent promoter element (e.g., PflaB) to drive expression of ospC in an rpoSBb mutant, however, might in fact confound the interpretation of results from such studies by producing constitutively high levels of OspC and/or inappropriate expression kinetics. The use of a temperature-inducible RpoSBb-independent promoter such as PospE with an expression pattern that more closely mirrors that of PospC (24) should help to minimize the likelihood that one of these scenarios influences the results of ospC complementation studies. More importantly, such studies will enable us to examine whether any of the other known (e.g., DbpA-, DbpB-, or OspF-dependent) or as-yet-unidentified RpoSBb-dependent loci contribute to either spirochetal growth in vivo or immune evasion. A more detailed characterization of the RpoSBb-dependent regulon expressed in vivo combined with targeted mutagenesis should help to address this question. The requirement for RpoSBb-dependent loci during infection is consistent with a well-established role for RpoS in virulence of a number of pathogens, including Salmonella (28, 50), Legionella pneumophila (9, 39), and P. aeruginosa (80); in most of these organisms, however, RpoS also contributes to environmental stress adaptation. While our finding that RpoSBb was required for spirochetal virulence but not environmental stress adaptation was somewhat unexpected, it is not without precedent. In L. pneumophila, RpoS is required for survival within both macrophages (9) and protozoan hosts (39) but does not appear to be required for growth phase-dependent responses to low pH and peroxide (39).
We also extended our analysis of the role of RpoSBb in differential gene expression by examining the RpoSBb dependence of the strain 297 ospE-ospF-elp loci whose expression parallels that of ospC and dbpA. Previous studies have demonstrated that expression of these lipoprotein loci is temperature inducible (2, 4, 41, 61, 78, 79) and further enhanced by mammalian host signals (2, 4, 41). The high degree of sequence identity within the respective ospE-ospF-elp promoter regions, combined with their similar expression patterns, suggests that these loci should be regulated by a common mechanism. The RT-PCR studies presented here, however, demonstrated that these loci are, in fact, regulated by both RpoSBb-dependent and RpoSBb-independent pathways. These results have also been confirmed with a gfp reporter system in both B. burgdorferi and E. coli (24). Our observation that the majority of ospE-ospF-elp loci are expressed in an RpoSBb-independent manner is consistent with reports examining the expression of two other borrelial paralogous gene families. Yang et al. (93) demonstrated that although the expression of class I and II Mlp family members is influenced by temperature, pH, and cell density through regulatory pathways involving RpoN/RpoS, no members exhibited the strict dependence on RpoSBb observed with OspC, DbpA, and OspF/Bbk2.11. An analysis of the Bdr family similarly revealed that while expression of several members of this large paralogous family was increased in response to many of the same signals as RpoSBb-dependent loci, including cultivation within DMCs, no detectable differences in their expression were observed with an rpoSBb mutant (70).
In summary, these studies have enabled us to refine our understanding of the role of the alternate sigma factor RpoS in B. burgdorferi. While expression of rpoSBb and RpoSBb-dependent genes is responsive to multiple environmental stimuli, RpoSBb itself does not appear to function as a master regulator of physiological and/or environmental stress adaptation in vitro or in vivo. RpoSBb does, however, control the expression of one or more essential virulence determinants that, together with the RpoSBb-independent differentially expressed loci, encompass the spirochetal regulon responsible for host adaptation. Analysis of differential gene expression within DMC-cultivated spirochetes and B. burgdorferi-infected ticks and tissues by genome-wide methodologies, such as microarrays, should provide a meaningful framework for in vitro experiments aimed at defining the degree to which these two regulatory pathways are influenced by environmental signals and, ultimately, provide rational targets for future mutagenesis studies.
Acknowledgments
We acknowledge the exceptional technical contribution of Cynthia Gonzales to these studies. We extend our thanks to Frank Yang and Michael Norgard (University of Texas Southwestern Medical Center) for providing us with the AH200 isolate and helpful discussions. We also thank David Nelson (University of Rhode Island) for helpful discussions regarding our environmental stress assays. We are indebted to Juan Salazar and Georgine Burke (Connecticut Children's Hospital) for their statistical expertise and assistance. We thank Megan Lybecker and Scott Samuels (University of Montana) for allowing us to refer to their DsrA studies prior to publication.
This work was supported by grants AI-29735 (M.J.C., C.H.E., and J.D.R.) from the Lyme disease program and AI-10573 (K.R.O.H.) awarded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Editor: J. B. Bliska
REFERENCES
- 1.Akbar, S., T. A. Gaidenko, C. M. Kang, M. O'Reilly, K. M. Devine, and C. W. Price. 2001. New family of regulators in the environmental signaling pathway which activates the general stress transcription factor σB of Bacillus subtilis. J. Bacteriol. 183:1329-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins, D. R., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 101:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akins, D. R., M. J. Caimano, X. Yang, F. Cerna, M. V. Norgard, and J. D. Radolf. 1999. Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect. Immun. 67:1526-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akins, D. R., S. F. Porcella, T. G. Popova, D. Shevchenko, S. I. Baker, M. Li, M. V. Norgard, and J. D. Radolf. 1995. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homolog. Mol. Microbiol. 18:507-520. [DOI] [PubMed] [Google Scholar]
- 5.Alban, P. S., P. W. Johnson, and D. R. Nelson. 2000. Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology 146:119-127. [DOI] [PubMed] [Google Scholar]
- 6.Anguita, J., S. Samanta, B. Revilla, K. Suk, S. Das, S. W. Barthold, and E. Fikrig. 2000. Borrelia burgdorferi gene expression in vivo and spirochete pathogenicity. Infect. Immun. 68:1222-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babb, K., N. El Hage, J. C. Miller, J. A. Carroll, and B. Stevenson. 2001. Distinct regulatory pathways control expression of Borrelia burgdorferi infection-associated OspC and Erp surface proteins. Infect. Immun. 69:4146-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babb, K., J. D. McAlister, J. C. Miller, and B. Stevenson. 2004. Molecular characterization of Borrelia burgdorferi erp promoter/operator elements. J. Bacteriol. 186:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachman, M. A., and M. S. Swanson. 2001. RpoSBb co-operates with other factors to induce Legionella pneumophila virulence in the stationary phase. Mol. Microbiol. 40:1201-1214. [DOI] [PubMed] [Google Scholar]
- 10.Badger, J. L., and V. L. Miller. 1995. Role of RpoSBb in survival of Yersinia enterocolitica to a variety of environmental stresses. J. Bacteriol. 177:5370-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bearson, S., B. Bearson, and J. W. Foster. 1997. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 147:173-180. [DOI] [PubMed] [Google Scholar]
- 12.Bohannon, D. E., N. Connell, J. Keener, A. Tormo, M. Espinosa-Urgel, M. M. Zambrano, and R. Kolter. 1991. Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and role of σ70. J. Bacteriol. 173:4482-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bono, J. L., K. Tilly, B. Stevenson, D. Hogan, and P. Rosa. 1998. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology 144:1033-1044. [DOI] [PubMed] [Google Scholar]
- 14.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caimano, M. J., X. Yang, T. G. Popova, M. L. Clawson, D. R. Akins, M. V. Norgard, and J. D. Radolf. 2000. Molecular and evolutionary characterization of the cp32/18 family of supercoiled plasmids in Borrelia burgdorferi 297. Infect. Immun. 68:1574-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll, J. A., R. M. Cordova, and C. F. Garon. 2000. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect. Immun. 68:6677-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Champion, C. I., D. R. Blanco, J. T. Skare, D. A. Haake, M. Giladi, D. Foley, J. N. Miller, and M. A. Lovett. 1994. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect. Immun. 62:2653-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connell, N., Z. Han, F. Moreno, and R. Kolter. 1987. An E. coli promoter induced by the cessation of growth. Mol. Microbiol. 1:195-201. [DOI] [PubMed] [Google Scholar]
- 20.Conway, T., and G. K. Schoolnik. 2003. Microarray expression profiling: capturing a genome-wide portrait of the transcriptome. Mol. Microbiol. 47:879-889. [DOI] [PubMed] [Google Scholar]
- 21.Das, S., S. W. Barthold, S. S. Giles, R. R. Montgomery, S. R. Telford III, and E. Fikrig. 1997. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and mammalian host. J. Clin. Investig. 99:987-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Silva, A. M., S. R. Telford, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43:281-295. [DOI] [PubMed] [Google Scholar]
- 24.Eggers, C. H., M. J. Caimano, and J. D. Radolf. 2004. Analysis of promoter elements involved in the transcriptional initiation of RpoSBb-dependent Borrelia burgdorferi genes. J. Bacteriol. 186:7390-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Hage, N., and B. Stevenson. 2002. Simultaneous coexpression of Borrelia burgdorferi Erp proteins occurs through a specific, erp locus-directed regulatory mechanism. J. Bacteriol. 184:4536-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias, A. F., J. L. Bono, J. A. Carroll, P. Stewart, K. Tilly, and P. Rosa. 2000. Altered stationary-phase response in a Borrelia burgdorferi rpoS mutant. J. Bacteriol. 182:2909-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang, F. C., S. J. Libby, N. A. Buchmeier, P. C. Loewen, J. Switala, J. Harwood, and D. G. Guiney. 1992. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11978-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fikrig, E., S. W. Barthold, W. Sun, W. Feng, S. R. Telford, and R. A. Flavell. 1997. Borrelia burgdorferi p35 and p37 proteins, expressed in vivo, elicit protective immunity. Immunity 6:531-539. [DOI] [PubMed] [Google Scholar]
- 30.Fikrig, E., M. Chen, S. W. Barthold, J. Anguita, W. Feng, S. R. Telford III, and R. A. Flavell. 1999. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol. Microbiol. 31:281-290. [DOI] [PubMed] [Google Scholar]
- 31.Fikrig, E., W. Feng, S. W. Barthold, S. R. Telford III, and R. A. Flavell. 2000. Arthropod- and host-specific Borrelia burgdorferi bbk32 expression and the inhibition of spirochete transmission. J. Immunol. 164:5344-5351. [DOI] [PubMed] [Google Scholar]
- 32.Fingerle, V., U. Hauser, G. Liegl, B. Petko, V. Preac-Mursic, and B. Wilske. 1995. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus. J. Clin. Microbiol. 33:1867-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster, J. W. 2000. Microbial responses to acid stress, p. 99-115. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 34.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 35.Gorbacheva, V. Y., H. P. Godfrey, and F. C. Cabello. 2000. Analysis of the bmp gene family in Borrelia burgdorferi sensu lato. J. Bacteriol. 182:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagman, K. E., P. Lahdenne, T. G. Popova, S. F. Porcella, D. R. Akins, J. D. Radolf, and M. V. Norgard. 1998. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect. Immun. 66:2674-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagman, K. E., X. Yang, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 2000. Decorin-binding protein (Dbp) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect. Immun. 68:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hales, L. M., and H. A. Shuman. 1999. The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J. Bacteriol. 181:4879-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, and D. R. Akins. 2002. Changes in temporal and spatial patterns of outer surface lipoprotein expression generate population heterogeneity and antigenic diversity in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 70:3468-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, J. D. Radolf, and D. R. Akins. 2001. Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect. Immun. 69:3618-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helmann, J. D., M. F. Wu, A. Gaballa, P. A. Kobel, M. M. Morshedi, P. Fawcett, and C. Paddon. 2003. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J. Bacteriol. 185:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hengge-Aronis, R. 2000. The general stress response in Escherichia coli, p. 161-178. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 44.Hengge-Aronis, R. 2002. Signal transduction and regulatory mechanisms involved in control of the σs (RpoSBb) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hengge-Aronis, R. 1996. Regulation of gene expression during entry into stationary phase, p. 1497-1512. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 46.Hubalek, Z., J. Halouzka, and M. Heroldova. 1998. Growth temperature ranges of Borrelia burgdorferi sensu lato strains. J. Med. Microbiol. 47:929-932. [DOI] [PubMed] [Google Scholar]
- 47.Hübner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoSBb regulatory pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishihama, A. 2000. Functional modulation of Escherichia coli RNA polymerase. Annu. Rev. Microbiol. 54:499-518. [DOI] [PubMed] [Google Scholar]
- 49.Jorgensen, F., M. Bally, V. Chapon-Herve, G. Michel, A. Lazdunski, P. Williams, and G. S. Stewart. 1999. RpoSBb-dependent stress tolerance in Pseudomonas aeruginosa. Microbiology 145:835-844. [DOI] [PubMed] [Google Scholar]
- 50.Kowarz, L., C. Coynault, V. Robbe-Saule, and F. Norel. 1994. The Salmonella typhimurium katF (rpoS) gene: cloning, nucleotide sequence, and regulation of spvR and spvABCD virulence plasmid genes. J. Bacteriol. 176:6852-6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lahdenne, P., S. F. Porcella, K. E. Hagman, D. R. Akins, T. G. Popova, D. L. Cox, L. I. Katona, J. D. Radolf, and M. V. Norgard. 1997. Molecular characterization of a 6.6-kilodalton Borrelia burgdorferi outer membrane-associated lipoprotein (lp6.6) which appears to be downregulated during mammalian infection. Infect. Immun. 65:412-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lange, R., M. Barth, and R. Hengge-Aronis. 1993. Complex transcriptional control of the σS-dependent stationary-phase-induced and osmotically regulated osmY (csi-5) gene suggests novel roles for Lrp, cyclic AMP (cAMP) receptor protein-cAMP complex, and integration host factor in the stationary-phase response of Escherichia coli. J. Bacteriol. 175:7910-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loewen, P. C., B. Hu, J. Strutinsky, and R. Sparling. 1998. Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 44:707-717. [DOI] [PubMed] [Google Scholar]
- 54.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, and I. Smith. 2001. The Mycobacterium tuberculosis ECF sigma factor σE: role in global gene expression and survival in macrophages. Mol. Microbiol. 41:423-437. [DOI] [PubMed] [Google Scholar]
- 55.McDowell, J. V., S. Y. Sung, M. Labandeira-Rey, J. T. Skare, and R. T. Marconi. 2001. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect. Immun. 69:3670-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller, J. C., K. von Lackum, K. Babb, J. D. McAlister, and B. Stevenson. 2003. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect. Immun. 71:6943-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montgomery, R. R., S. E. Malawista, K. J. M. Feen, and L. K. Bockenstedt. 1996. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J. Exp. Med. 183:261-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrissey, J. H. 1981. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal. Biochem. 117:307-310. [DOI] [PubMed] [Google Scholar]
- 59.Murgia, R., C. Piazzetta, and M. Cinco. 2002. Cystic forms of Borrelia burgdorferi sensu lato: induction, development, and the role of RpoSBb. Wien. Klin. Wochenschr. 114:574-579. [PubMed] [Google Scholar]
- 60.Narasimhan, S., F. Santiago, R. A. Koski, B. Brei, J. F. Anderson, D. Fish, and E. Fikrig. 2002. Examination of the Borrelia burgdorferi transcriptome in Ixodes scapularis during feeding. J. Bacteriol. 184:3122-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parveen, N., M. Caimano, J. D. Radolf, and J. M. Leong. 2003. Adaptation of the Lyme disease spirochaete to the mammalian host environment results in enhanced glycosaminoglycan and host cell binding. Mol. Microbiol. 47:1433-1444. [DOI] [PubMed] [Google Scholar]
- 64.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Volker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Porcella, S. F., C. A. Fitzpatrick, and J. L. Bono. 2000. Expression and immunological analysis of the plasmid-borne mlp genes of Borrelia burgdorferi B31. Infect. Immun. 68:4992-5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porcella, S. F., T. G. Popova, D. R. Akins, M. Li, J. D. Radolf, and M. V. Norgard. 1996. Borrelia burgdorferi supercoiled plasmids encode multicopy tandem open reading frames and a lipoprotein gene family. J. Bacteriol. 178:3293-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Purser, J. E., M. B. Lawrenz, M. J. Caimano, J. K. Howell, J. D. Radolf, and S. J. Norris. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753-764. [DOI] [PubMed] [Google Scholar]
- 68.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roberts, D. M., M. Caimano, J. McDowell, M. Theisen, A. Holm, E. Orff, D. Nelson, S. Wikel, J. Radolf, and R. T. Marconi. 2002. Environmental regulation and differential production of members of the Bdr protein family of Borrelia burgdorferi. Infect. Immun. 70:7033-7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Electrotransformation protocols for microorgansims. Methods Mol. Biol. 47:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwan, T. G., and J. Piesman. 2002. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 8:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sellati, T. J., D. A. Bouis, M. J. Caimano, J. A. Feulner, C. Ayers, E. Lien, and J. D. Radolf. 1999. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J. Immunol. 163:2049-2056. [PubMed] [Google Scholar]
- 76.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions, p. 107-112. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 77.Steere, A. C., R. L. Grodzicki, J. E. Craft, M. Shrestha, A. N. Kornblatt, and S. E. Malawista. 1984. Recovery of Lyme disease spirochetes from patients. Yale J. Biol. Med. 57:557-560. [PMC free article] [PubMed] [Google Scholar]
- 78.Stevenson, B., J. L. Bono, T. G. Schwan, and P. A. Rosa. 1998. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect. Immun. 66:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suh, S. J., L. Silo-Suh, D. E. Woods, D. J. Hassett, S. E. West, and D. E. Ohman. 1999. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J. Bacteriol. 181:3890-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suk, K., S. Das, W. Sun, B. Jwang, S. W. Barthold, R. A. Flavell, and E. Fikrig. 1995. Borrelia burgdorferi genes selectively expressed in the infected host. Proc. Natl. Acad. Sci. USA 92:4269-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sung, S. Y., J. V. McDowell, and R. T. Marconi. 2001. Evidence for the contribution of point mutations to vlsE variation and for apparent constraints on the net accumulation of sequence changes in vlsE during infection with Lyme disease spirochetes. J. Bacteriol. 183:5855-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Templeton, T. J. 2004. Borrelia outer membrane surface proteins and transmission through the tick. J. Exp. Med. 199:603-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Testerman, T. L., A. Vazquez-Torres, Y. Xu, J. Jones-Carson, S. J. Libby, and F. C. Fang. 2002. The alternative sigma factor σE controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Mol. Microbiol. 43:771-782. [DOI] [PubMed] [Google Scholar]
- 85.Wallich, R., C. Brenner, M. D. Kramer, and M. M. Simon. 1995. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect. Immun. 63:3327-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang, X.-G., B. Lin, J. M. Kidder, S. Telford, and L. T. Hu. 2002. Effects of environmental changes on expression of the oligopeptide permease (opp) genes of Borrelia burgdorferi. J. Bacteriol. 184:6198-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitehouse, C. A., L. R. Williams, and F. E. Austin. 1997. Identification of superoxide dismutase activity in Borrelia burgdorferi. Infect. Immun. 65:4865-4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wiedmann, M., T. J. Arvik, R. J. Hurley, and K. J. Boor. 1998. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 180:3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]
- 90.Yang, X., T. G. Popova, M. S. Goldberg, and M. V. Norgard. 2001. Influence of cultivation media on genetic regulatory patterns in Borrelia burgdorferi. Infect. Immun. 69:4159-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang, X., T. G. Popova, K. E. Hagman, S. K. Wikel, G. G. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 1999. Identification, character-ization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect. Immun. 67:6008-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang, X. F., S. M. Alani, and M. V. Norgard. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 100:1100-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang, X. F., A. Hübner, T. G. Popova, K. E. Hagman, and M. V. Norgard. 2003. Regulation of expression of the paralogous Mlp family in Borrelia burgdorferi. Infect. Immun. 71:5012-5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yildiz, F. H., and G. K. Schoolnik. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yim, H. H., R. L. Brems, and M. Villarejo. 1994. Molecular characterization of the promoter of osmY, an RpoSBb-dependent gene. J. Bacteriol. 176:100-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]
- 98.Zückert, W. R., J. Meyer, and A. G. Barbour. 1999. Comparative analysis and immunological characterization of the Borrelia Bdr protein family. Infect. Immun. 67:3257-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]