Abstract
The microsporidia are ubiquitous, obligate intracellular eukaryotic spore-forming parasites infecting a wide range of invertebrates and vertebrates, including humans. The defining structure of microsporidia is the polar tube, which forms a hollow tube through which the sporoplasm is transferred to the host cell. Research on the molecular and cellular biology of the polar tube has resulted in the identification of three polar tube proteins: PTP1, PTP2, and PTP3. The major polar tube protein, PTP1, accounts for at least 70% of the mass of the polar tube. In the present study, PTP1 was found to be posttranslationally modified. Concanavalin A (ConA) bound to PTP1 and to the polar tube of several different microsporidia species. Analysis of the glycosylation of Encephalitozoon hellem PTP1 suggested that it is modified by O-linked mannosylation, and ConA binds to these O-linked mannose residues. Mannose pretreatment of RK13 host cells decreased their infection by E. hellem, consistent with an interaction between the mannosylation of PTP1 and some unknown host cell mannose-binding molecule. A CHO cell line (Lec1) that is unable to synthesize complex-type N-linked oligosaccharides had an increased susceptibility to E. hellem infection compared to wild-type CHO cells. These data suggest that the O-mannosylation of PTP1 may have functional significance for the ability of microsporidia to invade their host cells.
Microsporidia are eukaryotic obligate intracellular parasitic protists that are widely distributed in the world. Many of the microsporidia have emerged as pathogens in immune-compromised hosts, such as those with AIDS or organ transplantation (37, 43). The majority of these infections have presented as gastrointestinal disease, although keratoconjunctivitis, disseminated infection, and encephalitis are also well described with these pathogens (43). Microsporidia have also been observed in immune-competent hosts, where diarrhea and keratoconjunctivitis have been the primary manifestations of infection (37).
The microsporidia form an environmentally resistant spore that is important in the transmission of these infections. These spores contain a highly specialized invasion organelle, consisting of the polar tube (filament), plasmalemma, and anchoring disk (17). During infection, the spore ruptures at its anterior end and the polar tube is extruded, forming a 50- to 100-μm hollow tube through which the nucleus and sporoplasm of the spore are transferred into a host cell in a process referred to as germination (17, 43). This process appears to be driven by an osmotic difference between the spore and the surrounding environment and does not appear to require any specific energy source (e.g., ATP) (17). There have been few studies addressing the interaction of the polar tube and the host cell membrane. Some data suggest that the adhesion of spores to host cells can be decreased by the presence of glycosaminoglycans in cell culture medium (J. R. Hayman and T. Nash, Mol. Parasitol. Meet. XIII, Woods Hole, Mass., abstr. 267C, 2002). It has been hypothesized that the polar tube pierces the host cell, acting like a hypodermic needle in delivering spore contents into the host cell. It is also possible that the polar tube causes an invagination of the host cell membrane and the contents of the spore are delivered into this microenvironment, but that the final penetration of the host cell membrane is due to an interaction of the host cell and the microsporidian sporoplasm (7).
Polar tubes resist dissociation in detergents and acids, but not in thiol reducing agents. It is, therefore, possible to isolate polar tube proteins (PTPs) by washing glass bead-disrupted spores with 1% sodium dodecyl sulfate (SDS) and 9 M urea, followed by solubilization of the polar tube in 2% dithiothreitol (DTT) (12-15, 17, 38, 39). The resultant PTPs can then be separated by reverse-phase high-performance liquid chromatography (HPLC) after blocking the cysteine residues of these proteins to prevent protein aggregation (15). By this method, the major PTP (PTP1) has been purified to homogeneity from several species of microsporidia (13, 15). Three distinct PTPs, PTP1 (4, 5, 15), PTP2 (4), and PTP3 (21), have been identified as components of the microsporidian polar tube. Amino acid analysis and cloning of PTP1 from several microsporidia have demonstrated that it is a proline-rich protein (4, 5, 15). The major PTP of Encephalitozoon hellem (EhPTP1) appears to account for 70% of the polar tube mass and migrates as a broad band at 55 kDa by immunoblotting and SDS-PAGE, while its primary amino acid sequence predicts it to be a 43-kDa protein (15). Analysis of the amino acid sequence revealed several potential N- and O-glycosylation sites. This aberrant electrophoretic migration might be a consequence of the posttranslation modifications of this protein. This study was, therefore, undertaken to characterize the posttranslational modifications of EhPTP1.
MATERIALS AND METHODS
Culture and production of microsporidian spores.
E. hellem, Encephalitozoon intestinalis, and Encephalitozoon cuniculi were cultured in RK13 cells (rabbit kidney cells CCL37; American Type Culture Collection, Rockville, Md.) at 37°C. Infected RK13 cells were maintained in continuous culture in minimum essential medium (MEM) supplemented with 7% heat-inactivated fetal calf serum and 1% penicillin-streptomycin-amphotericin B (Invitrogen, Carlsbad, Calif.). Cultures were subpassaged every 3 weeks. Brachiola algerae spores were used to infect confluent RK13 monolayers at 30°C in a 5% CO2 incubator. Fresh RK13 monolayers that had been grown at 37°C were infected with B. algerae spores every 2 weeks. Supernatants from infected flasks containing microsporidian spores were collected twice weekly and replaced with fresh medium. Spores of Glugea americanus were purified from cysts found in the cranial and spinal ganglia of the angler fish Lophius americanus and were stored in seawater as previously described (14). Spore concentrations were determined by counting spores using a hemocytometer (improved Neubauer).
PTP DTT preparation.
Pooled tissue culture supernatants containing spores were centrifuged at 2,000 × g, resuspended in phosphate-buffered saline (PBS), and then filtered through a 12-μm nucleopore filter followed by filtration through a 3-μm nucleopore filter. Purified spores were then centrifuged at 2,000 × g and resuspended in PBS at a concentration of approximately 109 per ml of PBS. Spores (5 × 108 to 7 × 109) were glass bead disrupted and sequentially extracted with 1% SDS five times and 9 M urea once and then solubilized in 2% DTT as previously published (13).
Purification of native PTP1.
HPLC was used to purify PTP1 as previously published (12, 14, 15). The DTT-solubilized PTPs were subject to reductive alkylation with 4-vinylpyridine followed by reverse-phase chromatography with a Hewlett Packard series II 1090 liquid chromatograph employing a C8 column (Aquapore RP300; 220 by 2.1 mm; Applied Biosystems, Inc., Foster City, Calif.). The column elution was monitored at 214 and 280 nm, and proteins were eluted using a flow rate of 200 μl min−1 and a linear gradient of H2O and acetonitrile (0 to 60% over 30 min) containing 0.1% trifluoroaectic acid. HPLC-purified native EhPTP1 (nEhPTP1) was used to produce a rabbit polyclonal antiserum (anti-nEhPTP1) that reacted with both nEhPTP1 and recombinant EhPTP1 (recEhPTP1) (12, 14, 15).
Production of recEhPTP1-GST.
As previously described, the EhPTP1 gene had been cloned in frame into pGEX-4T1, a glutathione S-transferase (GST) expression vector (Pharmacia Biotech, Piscataway, N.J.) (15). Recombinant protein was purified from isopropyl-β-d-thiogalactopyranoside-induced Escherichia coli cells containing pGEX-EhPTP1 by using glutathione-Sepharose 4B following the protocol provided by the manufacturer (Pharmacia Biotech) (15). This recEhPTP1 was used to produce a rabbit polyclonal antiserum (anti-recEhPTP1) that reacted with both native and recombinant EhPTP1 (15).
Mass spectrometry.
A 1.0-μl aliquot of HPLC-purified EhPTP1 was mixed with 1.0 μl of saturated 3,5-dimethoxy-4-hydroxy-cinamic acid solution in 50% acetonitrile solution containing 0.1% trifluoroacetic acid, and 1.0 μl of the resulting solution was deposited on a clean mass spectrometry probe surface. Matrix-assisted laser desorption ionization (MALDI) spectra were recorded on a Voyager-DE STR MALDI time of flight mass spectrometer (PerSeptive Biosystems, Framingham, Mass.) equipped with a 2.0-m flight tube and a 337-nm nitrogen laser.
Lectin overlay procedure.
A lectin overlay technique was used to determine the reactivity of EhPTP1 with lectin BPA (Bauhina pupurea), which reacts to α-GalNAc and β-GalNAc; concanavalin A (ConA) (Canavalia ensiformis), which reacts with α-Man (branched mannoses), α-Glc, and α-GlcNAc; DBA (Doichos biflorus), which reacts with α-GalNAc [GalNAc(α1,3)GalNAc]; GS-II (Griffonia simplicifolia II), which reacts with α-GlcNAc and β-GlcNAc; UEA-I (Ulex europaeus I), which reacts with α-fucose [Fuc(α1,2)Gal(β1,4)-GlcNAc]; MPA (Maclura pomifera), which reacts with α-Gal and α-GalNAc [Gal(β1,3)GalNAc]; WGA (Triticum vulfaris), which reacts with β-GlcNAc [GlcNAc(β1,4)GlcNAc] and sialic acid; SBA (Glycine max), which reacts with α-GalNAc and β-GalNAc [GalNAc(α1,3)Gal]; GS-1 (G. simplicifolia I), which reacts with α-Gal and α-GalNAc; GNA (Galanthus nivalis), which reacts with α-Man [Man(α 1,3)Man]; and PNA (Arachis hypogaea), which reacts with β-Gal Gal(β1,3)GalNAc (EY Laboratories, Inc., San Mateo, Calif.).
For the lectin overlay, purified EhPTP1 (1.4 μg/lane) was run on an SDS-10% polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred to nitrocellulose membrane, and then blocked with PBS containing 0.1% Tween 20 and 5% nonfat dried milk (PBS-M). The membrane was incubated for 1 h with horseradish peroxidase-conjugated lectin (0.5 mg/ml) at a 1:200 dilution in PBS containing 0.1% Tween 20 (PBS-T), followed by washing four times with PBS-T for 5 min and once for 15 min. Lectin binding was then detected by using a chemiluminescent substrate (ECL Western blotting system; Amersham Bioscience UK Limited). As a positive control for the presence of EhPTP1, rabbit anti-nEhPTP1 was used at a 1:5,000 dilution (15) for 1 h, the membrane was washed three times for 5 min each time with PBS-T, incubated with alkaline phosphatase-conjugated anti-rabbit antibody at a 1:5,000 dilution, and washed twice for 5 min each time with PBS-T, and antibody binding was visualized with a chemiluminescent substrate (Western Light; Tropix, Bedford, Mass.).
DTT-solubilized polar tube preparations were produced from E. cuniculi, E. intestinalis, E. hellem, B. algerae, and G. americanus as described above. To assess ConA binding to these microsporidian PTP preparations, samples (20 μg/lane) were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and blocked with PBS-M. ConA-horseradish peroxidase conjugate (0.5 mg/ml) was incubated at a 1:200 dilution in PBS for 1 h with the membrane, the membrane was then washed four times for 5 min and once for 15 min with PBS-T, and ConA binding was visualized by using a chemiluminescent substrate (ECL Western blotting system; Amersham Bioscience UK Limited).
ConA affinity chromatography.
DTT-solubilized PTPs (200 μg) were subjected to ConA affinity chromatography (0.5 ml of ConA gel [EY Laboratories, Inc.]). After loading and washing, specifically bound proteins were eluted with 0.2 M α-methyl-mannopyranoside-0.15 mM NaCl. The collected fractions in the eluate were separated by SDS-PAGE (10% polyacrylamide gel) and transferred to nitrocellulose using standard techniques (12, 14, 15). Rabbit anti-recEhPTP1 (1:5,000 dilution), followed by an alkaline phosphatase anti-rabbit antibody (1:5,000), was used for immunoblotting and was detected with a chemiluminescent substrate (Western Light; Tropix).
Analysis of N-linked glycosylation.
To evaluate glycosylation of PTP1, the Glyko enzymatic deglycosylation kit for enzymatic removal of Asn- and Thr/Ser-linked oligosaccharides (Glyko, Inc., Novato, Calif.) was utilized. Two reaction mixtures were set up, each using 10 μg of HPLC-purified nEhPTP1. In the first reaction, NANase II was incubated with EhPTP1 for 1 h at 37°C. In the second reaction, EhPTP1 was incubated with NANase II and O-glycosidase for 1 h at 37°C followed by the addition of PNGase F and further incubation at 37°C for 24 h. As a control, fetuin (a known N-linked glycoprotein) was subjected to the same reaction conditions. To detect N-linked deglycosylation, size changes in treated and untreated EhPTP1 and fetuin were analyzed by Coomassie blue staining of an SDS-10% PAGE gel. In the case of EhPTP1, a duplicate gel was transferred to nitrocellulose for immunoblot analysis. The membrane was incubated with rabbit polyclonal anti-nEhPTP1 at a 1:5,000 dilution (15) for 1 h, washed three times for 5 min each time with PBS-T, incubated with alkaline phosphatase-conjugated anti-rabbit antibody at a 1:5,000 dilution, and washed three times for 5 min each time with PBS-T, and then antibody binding was determined by using a chemiluminescent substrate (Western Light; Tropix).
Analysis of O-linked glycosylation.
Fifty microliters of 0.1 N NaOH was added to 8 μg of purified EhPTP1 at 50°C. The reaction was terminated at 0, 5, 10, 15, 20, 30, and 40 min by placing the reaction mixture on ice and adding 10 μl of 10% acetic acid. These samples were analyzed using SDS-10% PAGE followed by transfer to nitrocellulose membranes for analysis by immunoblotting with rabbit anti-nEhPTP1 (1:5,000) and lectin overlay with horseradish peroxidase-conjugated ConA (0.5 mg/ml at a 1:1,000 dilution). The intensity of all bands for a given reagent (e.g., ConA or anti-EhPTP1) was determined by densitometry, and each band was normalized to the intensity of the reaction at time zero for each respective reagent (e.g., time zero intensity was equal to 100%).
In vitro assay for effects of carbohydrates on E. hellem infection of RK13 cells.
RK13 cells were plated into a 24-well tissue culture plate and incubated at 37°C until the monolayers were at least 70% confluent. These cells were divided into the following treatment groups for each carbohydrate tested (mannose, glucose, and sucrose): no sugar control, 0.05, 0.1, 0.15, and 0.2 M carbohydrate in the culture medium. A total of 104 E. hellem spores were added into each well, and the medium was then changed every 3 days for 9 days. For pretreatment with carbohydrate, the RK13 cells were plated onto 24-well plates and incubated with medium containing 0.1 M carbohydrate for 3 days prior to infection with 104 E. hellem spores per well. The medium was then changed to medium without carbohydrate and changed every 3 days for 9 days (mannose and sucrose) or for 6 days (glucose). Plates were then fixed with 2.5% glutaraldehyde and stained for at least 5 min with 0.5% Calcoflour white M3R-0.01 N NaOH. All conditions were performed in quadruplicate, and each experiment was repeated three times. Infection was quantified as the number of infected foci seen under a fluorescence microscope in twenty 40× fields per well. Comparison of the numbers of infective foci was performed using Student's t test (SigmaStat; Jandel Scientific).
In vivo assay for the ability of E. hellem to infect different CHO cell lines.
Chinese hamster ovary (CHO) cell lines W5 and Lec1 were grown in α-MEM (Invitrogen) supplemented with RNA, DNA precursors, and 10% fetal calf serum in 24-well plates, with a change of medium every 3 days. When the cells were >70% confluent, they were infected with 105 E. hellem spores per well and incubated for 3 days; thereafter, the medium was changed every 3 days for 9 days. The plates were then fixed with 2.5% glutaraldehyde and stained for 5 min with 0.5% Calcoflour white M3R-0.01 N NaOH. All conditions were performed in quadruplicate, and the experiment was repeated three times. The infection was quantified as the number of infected foci seen under a fluorescence microscope in 20 40× fields per well. Comparisons of infective foci were made using Student's t test (SigmaStat; Jandel Scientific).
Electron microscopy.
To determine the time necessary for spore activation, 5-μl aliquots of B. algerae spores were placed on several microscope slide coverslips and allowed to air dry. After drying, 5 μl of germinating buffer (140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 0.05 M Tris-HCl buffer [pH 8.6]) (19) was added to each of the coverslips, and spore activation was monitored by phase-contrast microscopy to determine optimum extrusion times. The same procedure was used for E. hellem spore activation, with the substitution of 3% hydrogen peroxide for germination buffer.
For lectin staining, 10-μl aliquots of each spore type were placed in two microcentrifuge tubes. Twenty microliters of germination buffer was added to each of the two tubes (one experimental and one control) containing B. algerae spores, and 20 μl of 3% hydrogen peroxide was added to the two tubes (one experimental and one control) of E. hellem spores. After 3 min, 20 μl of ConA labeled with 15-nm colloidal gold (EY Laboratories), diluted 1:20 in PBS containing 0.002 M magnesium chloride and 0.002 M calcium chloride, was added to the respective experimental tubes. In the control tubes, 10 μl of 0.2 M α-mannopyranoside (EY Laboratories) was added to the germinating spores just prior to addition of the ConA. The germinated spores were allowed to react with the ConA for 30 min at room temperature. At the end of the incubation period, 40 μl of PBS was added to each tube and the contents were gently mixed by inversion. The tubes were then centrifuged until a soft pellet formed, and the supernatant was then carefully removed. This centrifugation and removal of supernatant was carried out five times, using PBS as a wash. The PBS washes used for the controls contained 0.2 M α-mannopyranoside. After the last centrifugation, each of the four pellets was resuspended in 40 μl of PBS, and 3 μl of the respective suspension was pipetted onto formvar-coated grids and allowed to settle for 1 min. After settling, an equal amount (3 μl) of Nanovan (Nanoprobes, Inc., Yaphank, N.Y.) was added to each grid, and after 5 min the liquid was carefully wicked away with filter paper. The grids were placed on filter paper, stored in petri dishes, and allowed to air dry overnight. The samples on the grids were observed and photographed with a Phillips Tecnai 12 operated at 80 kV at the Rutgers University at Newark electron microscopy facility.
RESULTS
HPLC and mass spectrometry of purified nEhPTP1.
E. hellem PTPs were isolated by washing glass bead-disrupted spores with 1% SDS and 9 M urea, followed by solubilization of 2% DTT as previously published (13, 15). After pyridylethylation and reverse-phase HPLC, a single major UV absorbing peak was observed (Fig. 1). The identity of this peak as EhPTP1 (Fig. 1, insert, lane 1) was confirmed by its immunoblotting reactivity with rabbit anti-recEhPTP1 (Fig. 1, insert, lane 2) and anti-nEhPTP1 (data not shown). Based on its primary amino acid sequence, EhPTP1 is predicted to have a molecular weight of 43,107 after removal of the signal peptide. However, by SDS-PAGE (and immunoblotting), it migrated as a broad band at 55 kDa (13, 15). Some of this aberrant electrophoretic mobility may be a consequence of the primary structure of the protein, such as its high proline content. For example, bacterially expressed recombinant EhPTP1-GST ran as a sharp band, whereas when the GST was removed with thrombin, the released recEhPTP1 ran aberrantly as a broad band (data not shown). Posttranslational modifications may also account for some of the aberrant migration of the native protein. On HPLC, a decrease in the slope of the acetonitrile gradient to 0.5% per min from 1.0% per min resulted in EhPTP1 peak broadening and the emergence of “saw toothing” of the peak, consistent with the presence of complex posttranslational modifications. By mass spectrometry, we found that the recEhPTP1-GST fusion protein had the expected mass predicted by the primary amino acid sequence. In contrast, nEhPTP1 did not ionize easily and had a measured mass of 48,240 Da, which was larger than the predicted mass from the primary amino acid sequence. This difference in mass from that predicted from the primary amino acid sequence was most likely due to the presence of posttranslational glycosylation. Examination of the EhPTP1 amino acid sequence by using O-GlycBase (www.cbs.dtu.dk) and PROSITE (www.cbs.dtu.dk) revealed nine putative N-glycosylation sites (NXT/S) and multiple potential O-glycosylation sites in this protein.
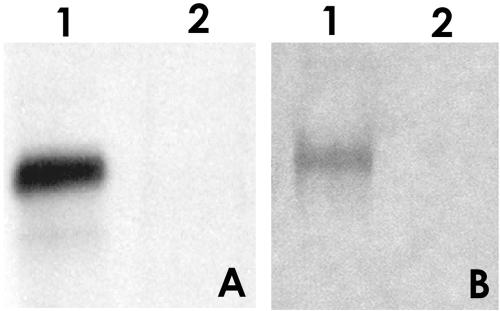
FIG. 1.
Analysis and purification of the major PTP of E. hellem (EhPTP1) by HPLC. This figure demonstrates the typical major PTP peak (EhPTP1) seen with HPLC purification of DTT-solubilized E. hellem PTPs (12-15). The major peak at 35 min in the figure is EhPTP1. (Insert) Lane 1, SDS-PAGE of peak at 35 min stained with SYPRO Ruby; lane 2; immunoblot of 35-min peak using rabbit antibody to recEhPTP1.
Binding of ConA to PTP1.
An overlay procedure was used to examine the ability of EhPTP1 to bind to the lectins DBA, BPA, GS-II, UEA-I, MPA, PNA, SBA, GNA, WGA, GS-1, and ConA. Only ConA was found to bind to EhPTP1 (Fig. 2A, lane 1). This binding could be competed by α-methyl-mannopyranoside (Fig. 2A, lane 2) or α-d-mannose (data not shown).
FIG. 2.
Interaction of EhPTP1 with the lectin ConA. (A) ConA overlay. A DTT-solubilized polar tube preparation was used for SDS-PAGE, transferred to nitrocellulose, and incubated with labeled ConA in the presence and absence of α-methyl-mannopyranoside. ConA bound to a single band (lane 1), and this binding was inhibited by α-methyl-mannopyranoside (lane 2). (B) ConA affinity chromatography. Soluble E. hellem PTPs were loaded onto a ConA-Sepharose column, and specifically retained proteins were eluted with 0.2 M α-methyl-mannopyranoside-0.15 mM NaCl. The flowthrough of the column (e.g., soluble PTPs after loading on the column) and the specific eluate were analyzed by SDS-PAGE followed by immunoblot analysis with rabbit antibody to recEhPTP1. The specific eluate contained EhPTP1 (lane 1), while the flowthrough no longer contained EhPTP1 (lane 2).
Based on the lectin overlay procedure, we evaluated the ability of immobilized ConA to affinity purify EhPTP1. Either HPLC-purified EhPTP1 or DTT-solubilized PTPs were applied to a ConA-Sepharose column in PBS, and the column was washed with 20 bed volumes of PBS. Specifically bound proteins were then eluted from the column with 0.2 M α-methyl-mannopyranoside. A 55-kDa protein reactive with rabbit anti-recEhPTP1 was eluted from the ConA-Sepharose column by α-methyl-mannopyranoside with either purified EhPTP1 or DTT-solubilized PTPs (Fig. 2B, lane 1). This protein was also reactive with rabbit anti-nEhPTP1 (data not shown).
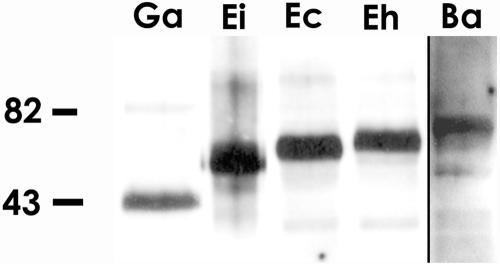
A similar overlay procedure demonstrated that ConA was also able to bind to a single band (presumptive PTP1) from a wide range of microsporidia, including E. intestinalis, E. cuniculi, G. americanus, and B. algerae (Fig. 3). The masses of the ConA-reactive proteins were consistent with what we have reported previously for the PTP1 in E. intestinalis, E. cuniculi, and G. americanus (13, 15). The B. algerae protein was similar in size to what we have seen with HPLC-purified PTP1 from this organism (unpublished data).
FIG. 3.
PTP1 reactivity with ConA is seen in multiple microsporidia. Solubilized (2% DTT) polar tube preparations of five microsporidia species, G. americanus (Ga), E. hellem (Eh), E. cuniculi (Ec), E. intestinalis (Ei), and B. algerae (Ba), were used for a lectin overlay with labeled ConA. ConA reacted with a band in each polar tube preparation that had a size consistent with that seen on SDS-PAGE and immunoblot analysis with antibody to PTP1 (12-15).
N-glycosylation is probably not present on nEhPTP1.
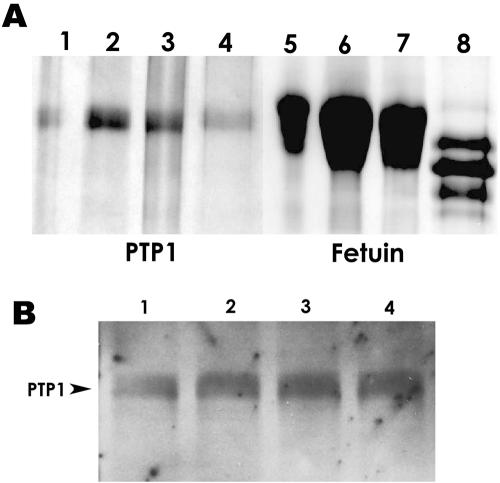
HPLC-purified EhPTP1 was treated with NANase II, NANase II-O-glycosidase, or NANase II-O-glycosidase-PNGase F. No alteration in the electrophoretic mobility of EhPTP1 was observed with any of these enzymatic treatments (Fig. 4A). In contrast, the control reaction with fetuin demonstrated the expected decrease in mass due to removal of N-linked oligosaccharides (Fig. 4A). Immunoblotting with anti-nEhPTP1 and ConA lectin overlay demonstrated that there was no change in mass or loss of reactivity of EhPTP1 with these enzymatic treatments, suggesting that N-glycosylation is probably not present on nEhPTP1 (Fig. 4B).
FIG. 4.
Analysis of N-glycosylation of the major PTP of E. hellem (EhPTP1). (A) N-deglycosylation assay results. Coomassie-stained SDS-PAGE gels of EhPTP1 (lanes 1 to 4) and fetuin (lanes 5 to 8) were treated with NANase II, O-glycosidase, and/or PHGase F. Lanes 1 and 5, no enzymes; lanes 2 and 6, treatment with NANase II; lanes 3 and 7, treatment with NANase II and O-glycosidase; lanes 4 and 8, treatment with NANase II, O-glycosidase, and PNGase F. There was no apparent molecular weight change of EhPTP1 with treatment. Fetuin was used as control and demonstrated the expected changes in molecular weight following enzymatic treatment. (B) ConA reactivity of EhPTP1 following N-deglycosylation. Shown are the results of ConA lectin overlay of EhPTP1 without treatment (lane 1), EhPTP1 treated with NANase II (lane 2), EhPTP1 treated with NANase II and O-glycosidase (lane 3), and EhPTP1 treated with NANase II, O-glycosidase, and PNGase F (lane 4). There was no significant change in the molecular weight of EhPTP1 with treatment. ConA still bound to EhPTP1 after treatment with NANase II, O-glycosidase, and PNGase F.
O-glycosylation is present on nEhPTP1.
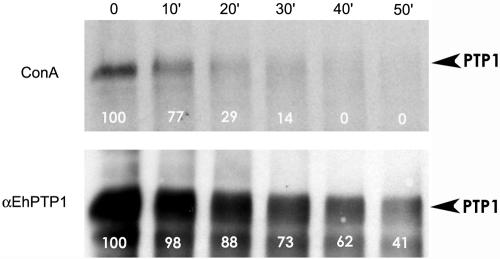
To evaluate the possibility of O-glycosylation occurring in EhPTP1, we examined the effect of 0.1 N NaOH treatment on the ability of nEhPTP1 to bind ConA. O-linked sugars are labile under these conditions. Treatment with NaOH resulted in the elimination of ConA binding (Fig. 5A). At the point at which ConA binding was eliminated (by densitometry, compared to binding at time zero), immunoblot analysis with anti-nEhPTP1 demonstrated that there was still significant remaining immunoreactive EhPTP1 present (by densitometry, compared to the binding at time zero) (Fig. 5B). Similar results were obtained with anti-recEhPTP1 (data not shown). Thus, while degradation of EhPTP1 occurred due to NaOH treatment, the elimination of ConA binding was faster, suggesting that EhPTP1 is O-glycosylated and that ConA is reacting with an O-linked sugar. By immunoblot analysis, nEhPTP1 did not react with anti-OGlcNA (gift of G. W. Hart, Johns Hopkins University, Baltimore, Md.), suggesting that O-GlcNA is not present on EhPTP1 (data not shown).
FIG. 5.
Analysis of O-glycosylation of EhPTP1. HPLC-purified EhPTP1 was treated with 0.1 N NaOH at 50°C for 0, 10, 20, 30, 40, and 50 min. After treatment, samples were analyzed by SDS-PAGE followed by either labeled ConA overlay (top panel) or immunoblotting with polyclonal rabbit antibody to HPLC-purified nEhPTP1 (bottom panel). The intensities of all bands were determined by densitometry. The numbers on each lane reflect the band intensity compared to the amount of binding present at time zero (100%). At 40 min, EhPTP1 was still present by immunoblotting, but there was no ConA binding.
Demonstration of ConA reactivity on the polar tube by electron microscopy.
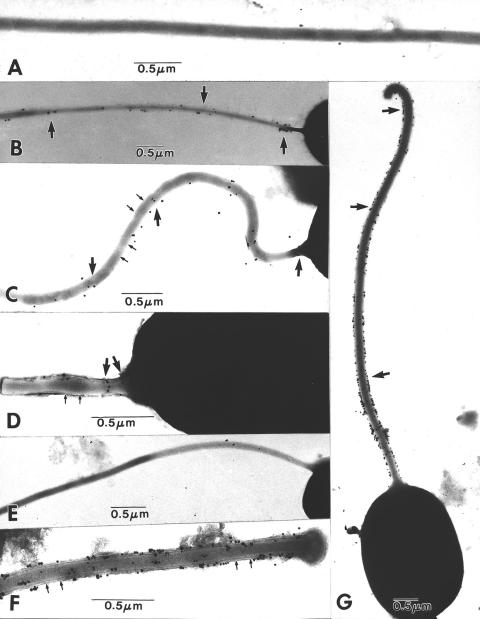
Electron microscopy was utilized to investigate if intact polar tubes could bind ConA. Spores of B. algerae or E. hellem were extruded in microwell plates with germination buffer and incubated with ConA-gold and then negatively stained with Nanovan as described in Materials and Methods. The specificity of ConA binding was evaluated by the addition of 0.2 M α-methyl-mannopyranoside during the ConA-gold incubation step. As demonstrated in Fig. 6, ConA bound to the polar tube of both E. hellem (Fig. 6B, C, and D) and B. algerae (Fig. 6F and G), and this binding could be inhibited by α-methyl-mannopyranoside (Fig. 6A and E).
FIG. 6.
Electron micrographs of the reaction of extruded microsporidian polar tubes of E. hellem (A to D) and B. algerae (E to G) with colloidal gold-labeled ConA. (A) Fully extruded E. hellem polar tube demonstrating no reaction with colloidal gold-labeled ConA in the presence of 0.2 M α-mannopyranoside. A few unbound gold particles are present in the background. (B) Fully extruded E. hellem polar tube decorated with small clusters of gold particles (arrows). Note the intense clustering on the portion of the tube near the spore. (C) Fully extruded E. hellem polar tube decorated with numerous individual particles of gold (arrows). The periodicity of the polar tube substructure is evident (small arrows). (D) Partially extruded E. hellem polar tube decorated with a few individual particles of gold (arrows). The localization appears to be more intense in the area close to the spore. At this relatively early stage of extrusion, the polar tube substructure is evident (small arrows). (E) Fully extruded B. algerae polar tube demonstrating no reaction with colloidal gold-labeled ConA in the presence of 0.2 M α-mannopyranoside. (F) Partially extruded B. algerae polar tube, uniformly decorated with many particles of gold along its surface (arrows). The periodicity of the polar tube substructure is evident (small arrows). (G) Fully extruded B. algerae polar tube intensely decorated with individual and clusters of gold particles along its entire length (arrows). While ConA binding was present on polar tubes of both E. hellem and B. algerae, the binding appeared to be significantly more abundant on Brachiola polar tubes.
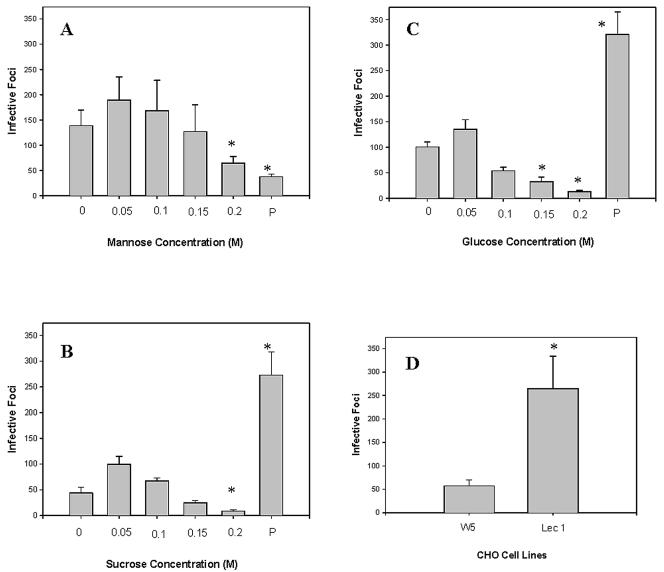
Effects of carbohydrates on the ability of E. hellem to infect RK13 cells.
The addition of 0.05 or 0.1 M α-mannose (Fig. 7A), glucose (Fig. 7B), or sucrose (Fig. 7C) to RK13 cells at the time of infection with E. hellem spores resulted in an increase in infective foci (although this effect was not statistically significant). At a concentration of 0.15 or 0.2 M α-mannose, glucose, or sucrose decreased infective foci (P < 0.05). This is consistent with known inhibitory effects of elevated osmotic environments on spore germination (8, 33, 34). Pretreatment of RK13 cells with 0.1 M α-mannose for 3 days prior to infection resulted in a significant decrease in infective foci (Fig. 7A); however, pretreatment with either sucrose or glucose significantly increased the number of infective foci (Fig. 7B and C).
FIG. 7.
Studies on the effects of carbohydrates and host cell glycosylation on the infection of host cells by E. hellem. The ability of E. hellem to infect RK13 cells in the presence of different concentrations of carbohydrates and the effects of pretreatment of host cells with carbohydrates for 3 days preceding infection were assessed. Each well was infected with 104 E. hellem spores, and infection was quantified as the number of infected foci seen under a fluorescence microscope in 20 40× fields per well. Comparisons of infective foci counts were done with Student's t test. (A) Mannose results. RK13 cells were cultured with various concentrations of α-mannose (0, 0.05, 0.1, 0.15, and 0.2 M) at the time of infection. In addition, RK13 cells were pretreated (P) with 0.1 M mannose for 3 days prior to infection and then infected and cultured in the absence of mannose. Significant inhibition of infection was seen with either 0.2 M α-mannose in the medium during infection or pretreatment of cells with 0.1 M α-mannose prior to infection (P < 0.05). (B) Sucrose results. RK13 cells were cultured with various concentrations of sucrose (0, 0.05, 0.1, 0.15, and 0.2 M) at the time of infection. In addition, RK13 cells were pretreated (P) with 0.1 M sucrose for 3 days prior to infection and then infected and cultured in the absence of sucrose. Significant inhibition of infection was seen with 0.2 M sucrose in the medium during infection. A significant increase in infection was seen with pretreatment of cells with 0.1 M sucrose prior to infection (P < 0.05). Infection was also increased by the presence of 0.05 M sucrose in the medium. (C) Glucose results. RK13 cells were cultured with various concentrations of glucose (0, 0.05, 0.1, 0.15, and 0.2 M) at the time of infection. In addition, RK13 cells were pretreated (P) with 0.1 M glucose for 3 days prior to infection and then infected and cultured in the absence of glucose. Significant inhibition of infection was seen with 0.15 and 0.2 M glucose in the medium during infection. A significant increase in infection was seen with pretreatment of cells with 0.1 M glucose prior to infection (P < 0.05). Infection was also increased by the presence of 0.05 M glucose in the medium. (D) CHO cell results. The infectivity of E. hellem for wild-type CHO cells (W5) and mutant CHO cells (Lec1), which could bind more ConA than wild type, was assessed. Using the same infective dose of E. hellem, the number of infective foci was significantly higher in Lec1 cells than in W5 (wild-type) CHO cells (P < 0.05).
Ability of E. hellem to infect CHO cells with altered lectin binding.
Wild-type (W5) CHO and Lec1 CHO cells, which bind much more ConA than wild-type CHO, were infected with E. hellem spores. There was a significant increase in infection in the Lec1 CHO cells (300 ± 18) versus the wild-type CHO cells (60 ± 15).
DISCUSSION
It has been over 100 years since the observations made by Thelohan on the formation of the polar tube (32). Since that time it has been demonstrated that the polar tube is a key structure in the transmission of the microsporidian sporoplasm from the spore into their host cells. On exposure to the appropriate environmental stimulation, the polar tube is rapidly discharged out of the spore, forming a hollow tube through which the sporoplasm travels (2). Ultrastructural studies have demonstrated that the polar tube can pierce host cell membranes and that invagination of the host cell membrane occurs as the polar tube pushes into the host cell (8, 39). The molecular mechanism(s) of the polar tube and host cell membrane interaction has not yet been elucidated. Our group had previously cloned the major PTP (PTP1) from E. hellem. Analysis of DTT-solubilized polar tubes suggested that PTP1 represented at least 70% of the protein mass of the polar tube. In the present report, our findings are consistent with posttranslational modifications being present in PTP1. These modifications may be important in the interaction of PTP1 with the host cell membrane, thus facilitating infection of the host cell during germination of the microsporidian spore.
Our investigators have previously demonstrated that EhPTP1, and also the PTP1 of other microsporidia species, migrates aberrantly on SDS-PAGE (11-17). An HPLC technique can be used to purify PTP1 to homogeneity from solubilized polar tube preparations (13-15). Purified native PTP1 demonstrates a saw tooth pattern when the peak is spread out with a shallow acetonitrile graduation. This type of pattern is often a consequence of posttranslational modifications in proteins. Mass spectrometry confirmed that nEhPTP1 was larger than predicted from the primary amino acid sequence, which is consistent with the presence of posttranslational modifications. Well-defined modifications, e.g., phosphorylation, sulfation, myristylation, etc., can be inferred simply by the increase in the mass of a peptide, as particular modifications increase the predicted size of the protein by a characteristic mass. The observed mass increase in EhPTP1 was consistent with glycosylation being present, as it did not fit any of the other defined modifications.
A previous observation on staining of the polar tube by the periodic acid Schiff reaction suggested that the polar tube contained glycoprotein(s) (35). Microsporidia have been demonstrated to have Golgi which are involved in the formation of the polar tube during development, consistent with the presence of glycosylation on the polar tube (30, 31). Analysis of the EhPTP1 primary amino acid sequence revealed several N- and O-glycosylation sites. Using a lectin overlay procedure, we were able to demonstrate that ConA bound to EhPTP1 and that binding was competed by α-mannose. Binding of ConA was not limited to E. hellem PTP1, and in fact ConA binding to protein bands consistent with PTP1 was present in DTT-solubilized polar tube preparations from E. cuniculi, E. intestinalis, B. algerae, and G. americanus. ConA affinity chromatography could also be used to purify PTP1 directly from solubilized polar tubes. As no other proteins were purified with PTP1 from the DTT-solubilized polar tube mixture, we believe that only PTP1 has posttranslational modifications that bind ConA. It had been previously suggested that ConA-ferritin bound to the polar tube of Ameson michaelis; however, the specificity of this interaction was not confirmed (40). In this paper we have provided direct demonstration that the polar tubes of both E. hellem and B. algerae are glycosylated and bind ConA-gold and that this binding is inhibited by α-methyl-mannopyranoside, a competitive sugar for this lectin.
Other lectins that share some of the same binding sites as ConA did not bind to EhPTP1. GNA, which binds to terminal α(1, 3)-mannose residues, and GS-II, which binds to β-N-acetyl-d-galactosamine residues, did not react with EhPTP1. GNA has, however, recently been reported to bind to a PTP (presumably PTP1) from Nosema grylli (V. V. Dolgikh and Y. Y. Sokolova, Emerg. Pathogens 21st Cent.: 1st United Workshop Microsporidia Invertebrate Vertebrate Hosts, České Budìjovice, abstr. 12, 2004). This suggests that the binding of ConA to EhPTP1 is either to branched mannose residues or possibly to α-glucose residues. No evidence for N-glycosylation of EhPTP1 was found by using standard deglycosylation procedures, and ConA binding was not eliminated by these enzymatic treatments. Antibody to O-GlcNA did not react with EhPTP1. In addition, O-glycosidase treatment that removes O-linked glucose but not O-linked mannose residues did not affect ConA binding to EhPTP1. In contrast, ConA binding was eliminated by treatment of EhPTP1 with NaOH, suggesting that the ConA binding of EhPT1 was dependent on the O-glycosylation of this protein. ConA binding to O-mannosylated proteins has been described in fungi (9, 28, 42). Based on these data and our observations, we believe that PTP1 is modified by O-mannosylation.
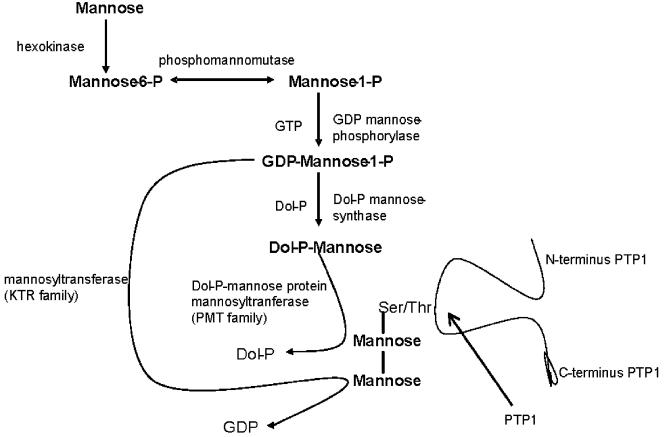
O-mannosylation in eukaryotes was first described in Saccharomyces cerevisiae (22) and is essential for viability (42). It was considered a fungus-specific protein modification for many years (9), but it is now recognized that this modification occurs in some mammalian proteins. Current phylogenetic evidence suggests that microsporidia are related to the fungi (41, 43). In fungi, O-mannosyl glycan synthesis is initiated in the endoplasmic reticulum (ER) by the transfer of a mannosyl residue from dolichyl phosphate-activated mannose (Dol-P-Man) to a specific serine/threonine residue on proteins entering the secretory pathway, with further modifications occurring in the Golgi apparatus. Dol-P-Man:protein-O-mannosyltransfereases (PMTs) are an evolutionarily conserved family of enzymes that initiate protein O-mannosylation at the ER (9, 28, 42). Analysis of the genome of E. cuniculi demonstrated the presence of Dol-P-Man synthase, which is probably the key mannosyl donor for the synthesis of O-linked oligosaccharides (10, 36). The genome, however, lacks an oligosaccharyltransferase complex that can catalyze transfer of a preassembled high-mannose oligosaccharide onto asparagine residues, nor are there gene candidates for specific glycosidases that would remove glucose and mannose residues to produce N-linked core structures. Dol-P-Man also functions in the formation of O-linked oligosaccharides, and two potential Dol-P-mannose protein mannosyltransferases of the PMT family are present in the E. cuniculi genome. This predicts that O-glycosylation of E. cuniculi proteins would begin with Dol-P-Man in the ER. Consistent with this, PTP1 has a signal sequence predicted by PSORT to result in targeting through the ER. In the E. cuniculi genome, there is also a GDP-mannose transporter similar to Candida or Saccharomyces Vrg4 (also called Gog5) and a mannosyltransferase (KTR family) that uses GDP-mannose as substrate and forms α-1,2 linkages (9, 28, 36, 42). As is true of many microsporidia pathways, the organism has single genes for most of these enzymes, rather than multiple copies as seen in fungi. Vrg4 has been demonstrated to be an essential gene in Candida albicans (20) and may also be a critical gene in the microsporidia. Thus, the machinery for O-mannosylation is present in this organism (Fig. 8); however, that for N-glycosylation appears to be absent (or could be atypical) (10, 36).
FIG. 8.
A proposed O-mannosylation pathway for E. cuniculi. Analysis of the published E. cuniculi genome (10, 36) demonstrated the presence of the enzymes required for the O-mannosylation of proteins.
It is likely that O-glycosylation of PTP1 plays a role in the function of the polar tube, as this type of glycosylation can increase the stability of proteins protecting them from degradation. Glycosylation may, therefore, be important in protecting the microsporidian polar tube from degradation in the gastrointestinal tract of their hosts. Carbohydrate interactions may also be important in creating a “sticky” polar tube that adheres to as-yet-unidentified cell membrane receptors, such as mannose receptors, facilitating the penetration of the polar tube into its host cell. Pretreatment of host cells with mannose decreased invasion, whereas treatment with glucose or sucrose increased infectivity. It is possible this is due to the down regulation of a mannose binding protein or lectin on the cell surface that binds to PTP1. The addition of exogenous sugars is known to block the adhesion of E. coli, preventing infection (1), as well as of various other species of bacteria in a variety of animals, including rats, guinea pigs, monkey, calves, and mice (23). In many intestinal protozoa, adherence to the mucosal cells has also been demonstrated to be dependent on the carbohydrate modification of their surface proteins (3, 44).
Alterations in host cell glycosylation may also affect the adhesion of the microsporidian polar tube to these cells, e.g., Lec1 cells have a higher infection rate than control cells. The Lec1 CHO cell line lacks N-acetylglucosaminyltransferase I, the enzyme responsible for the committed step in the reelaboration and diversification of carbohydrates in the glycosylation pathway (18, 26, 27). Therefore, Lec1 cells are unable to synthesize complex-type N-linked oligosaccharides. As a result of this mutation, these cells produce mannose-rich, homogeneous (GlcNAc)2(Man)5 glycoforms (25). It was also reported that O-linked fucose is present in Lec1 surface glycoproteins (29). With this altered pattern of surface glycoproteins, Lec1 binds more ConA than the wild type. The observations in this paper suggest that such changes in host cell surface glycoproteins can also alter E. hellem infection efficiency.
Overall, these observations suggest that the posttranslational modification of PTP1 to an O-mannosylated glycoprotein has functional significance for the invasion process. In C. albicans, defects in O-mannosylation result in altered morphogenesis, diminished adherence to host cells, and marked attenuation of virulence in murine models of infection (6). In Aspergillus nidulans, a filamentous fungus, defects in O-mannosylation result in problems with the maintenance of polarity (24). Inhibition of O-mannosylation and interference with polar tube-host cell interactions may prove to be useful therapeutic approaches for the treatment of microsporidiosis.
Acknowledgments
This work was supported by National Institutes of Health grants AI31788, GM60067, and NCRR 1S10-RR13959.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aronson, M., O. Medalia, L. Schori, D. Mirelman, N. Sharon, and I. Ofek. 1979. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J. Infect. Dis. 139:329-332. [DOI] [PubMed] [Google Scholar]
- 2.Cali, A., L. M. Weiss, and P. M. Takvorian. 2002. Brachiola algerae spore membrane systems, their activity during extrusion, and a new structural entity, the multilayered interlaced network, associated with the polar tube and the sporoplasm. J. Eukaryot. Microbiol. 49:164-174. [DOI] [PubMed] [Google Scholar]
- 3.Chen, X. M., and N. F. LaRusso. 2000. Mechanisms of attachment and internalization of Cryptosporidium parvum to biliary and intestinal epithelial cells. Gastroenterology 118:368-379. [DOI] [PubMed] [Google Scholar]
- 4.Delbac, F., I. Peuvel, G. Metenier, E. Peyretaillade, and C. P. Vivares. 2001. Microsporidian invasion apparatus: identification of a novel polar tube protein and evidence for clustering of ptp1 and ptp2 genes in three Encephalitozoon species. Infect. Immun. 69:1016-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delbac, F., P. Peyret, G. Metenier, D. David, A. Danchin, and C. P. Vivares. 1998. On proteins of the microsporidian invasive apparatus: complete sequence of a polar tube protein of Encephalitozoon cuniculi. Mol. Microbiol. 29:825-834. [DOI] [PubMed] [Google Scholar]
- 6.Ernst, J. F., and S. K. Prill. 2001. O-glycosylation. Med. Mycol. 39(Suppl. 1):67-74. [PubMed] [Google Scholar]
- 7.Foucault, C., and M. Drancourt. 2000. Actin mediates Encephalitozoon intestinalis entry into the human enterocyte-like cell line, Caco-2. Microb. Pathog. 28:51-58. [DOI] [PubMed] [Google Scholar]
- 8.Frixione, E., L. Ruiz, M. Santillan, L. V. de Vargas, J. M. Tejero, and A. H. Undeen. 1992. Dynamics of polar filament discharge and sporoplasm expusion by microsporidian spores. Cell Motil. Cytoskel. 22:38-50. [Google Scholar]
- 9.Gentzsch, M., and W. Tanner. 1997. Protein-O-glycosylation in yeast: protein-specific mannosyltransferases. Glycobiology 7:481-486. [DOI] [PubMed] [Google Scholar]
- 10.Katinka, M. D., S. Duprat, E. Cornillot, G. Metenier, F. Thomarat, G. Prensier, V. Barbe, E. Peyretaillade, P. Brottier, P. Wincker, F. Delbac, H. El Alaoui, P. Peyret, W. Saurin, M. Gouy, J. Weissenbach, and C. P. Vivares. 2001. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414:450-453. [DOI] [PubMed] [Google Scholar]
- 11.Keohane, E., P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1994. The identification and characterization of a polar tube reactive monoclonal antibody. J. Eukaryot. Microbiol. 41:48S. [PubMed] [Google Scholar]
- 12.Keohane, E. M., G. A. Orr, P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1999. Analysis of the major microsporidian polar tube proteins. J. Eukaryot. Microbiol. 46:29S-30S. [PubMed] [Google Scholar]
- 13.Keohane, E. M., G. A. Orr, P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1999. Polar tube proteins of microsporidia of the family Encephalitozoonidae. J. Eukaryot. Microbiol. 46:1-5. [DOI] [PubMed] [Google Scholar]
- 14.Keohane, E. M., G. A. Orr, P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1996. Purification and characterization of a microsporidian polar tube protein. Mol. Biochem. Parasitol. 79:255-259. [DOI] [PubMed] [Google Scholar]
- 15.Keohane, E. M., G. A. Orr, H. S. Zhang, P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1998. The molecular characterization of the major polar tube protein gene from Encephalitozoon hellem, a microsporidian parasite of humans. Mol. Biochem. Parasitol. 94:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keohane, E. M., P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1996. Identification of a microsporidian polar tube protein reactive monoclonal antibody. J. Eukaryot. Microbiol. 43:26-31. [DOI] [PubMed] [Google Scholar]
- 17.Keohane, E. M., and L. M. Weiss. 1998. Characterization and function of the microsporidian polar tube: a review. Folia Parasitol. 45:117-127. [PubMed] [Google Scholar]
- 18.Kornfeld, R., and S. Kornfeld. 1985. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 54:631-664. [DOI] [PubMed] [Google Scholar]
- 19.Leitch, G. J., Q. He, S. Wallace, and G. S. Visvesvara. 1993. Inhibition of the spore polar filament extrusion of the microsporidium, Encephalitozoon hellem, isolated from an AIDS patient. J. Eukaryot. Microbiol. 40:711-717. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa, A., J. B. Poster, Y. Jigami, and N. Dean. 2002. Molecular and phenotypic analysis of CaVRG4, encoding an essential Golgi apparatus GDP-mannose transporter. J. Bacteriol. 184:29-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peuvel, I., P. Peyret, G. Metenier, C. P. Vivares, and F. Delbac. 2002. The microsporidian polar tube: evidence for a third polar tube protein (PTP3) in Encephalitozoon cuniculi. Mol. Biochem. Parasitol. 122:69-80. [DOI] [PubMed] [Google Scholar]
- 22.Sentrandreu, R., and D. H. Northcote. 1969. The characterization of oligosaccharides attached to threonine and serine in a mannan glycopeptide obtained from the cell wall of yeast. Carbohydr. Res. 10:584-585. [Google Scholar]
- 23.Sharon, N., and I. Ofek. 2000. Safe as mother's milk: carbohydrates as future anti-adhesion drugs for bacterial diseases. Glycoconj. J. 17:659-664. [DOI] [PubMed] [Google Scholar]
- 24.Shaw, B. D., and M. Momany. 2002. Aspergillus nidulans polarity mutant swoA is complemented by protein O-mannosyltransferase pmtA. Fungal Genet. Biol. 37:263-270. [DOI] [PubMed] [Google Scholar]
- 25.Stanley, P. 1989. Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol. Cell. Biol. 9:377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanley, P. 1985. Membrane mutants of animal cells: rapid identification of those with a primary defect in glycosylation. Mol. Cell. Biol. 5:923-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanley, P., and W. Chaney. 1985. Control of carbohydrate processing: the lec1A CHO mutation results in partial loss of N-acetylglucosaminyltransferase I activity. Mol. Cell. Biol. 5:1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strahl-Bolsinger, S., M. Gentzsch, and W. Tanner. 1999. Protein O-mannosylation. Biochim. Biophys. Acta 1426:297-307. [DOI] [PubMed] [Google Scholar]
- 29.Stults, N. L., and R. D. Cummings. 1993. O-linked fucose in glycoproteins from Chinese hamster ovary cells. Glycobiology 3:589-596. [DOI] [PubMed] [Google Scholar]
- 30.Takvorian, P. M., and A. Cali. 1994. Enzyme histochemical identification of the Golgi apparatus in the microsporidian, Glugea stephani. J. Eukaryot. Microbiol. 41:63S-64S. [PubMed] [Google Scholar]
- 31.Takvorian, P. M., and A. Cali. 1996. Polar tube formation and nucleoside diphosphatase activity in the microsporidian, Glugea stephani. J. Eukaryot. Microbiol. 43:102S-103S. [DOI] [PubMed] [Google Scholar]
- 32.Thelohan, P. 1894. Sur la presence d'une capsule a filament dans les spores des microsporidies. C. R. Acad. Sci. 118:1425-1427. [Google Scholar]
- 33.Undeen, A. H. 1990. A proposed mechanism for the germination of microsporidian (Protozoa, Microspora) spores. J. Theor. Biol. 142:223-235. [Google Scholar]
- 34.Undeen, A. H., and E. Frixione. 1990. The role of osmotic pressure in the germination of Nosema algerae spores. J. Protozool. 37:561-567. [DOI] [PubMed] [Google Scholar]
- 35.Vavra, J. 1976. Structure of the Microsporidia, p. 1-85. In L. A. Bulla, Jr., and T. C. Cheng (ed.), Comparative pathobiology, vol. 1. Plenum Press, New York, N.Y. [Google Scholar]
- 36.Vivares, C. P., and G. Metenier. 2004. The Microsporidia genome: living with minimal genes as an intracellular eukaryote, p. 215-242. In D. S. Lindsay and L. M. Weiss (ed.), Opportunistic infections: Toxoplasma, Sarcocystis, and Microsporidia, vol. 8. Kluwer Academic Press, Norwell, Mass. [Google Scholar]
- 37.Weber, R., and R. T. Bryan. 1994. Microsporidial infections in immunodeficient and immunocompetent patients. Clin. Infect. Dis. 19:517-521. [DOI] [PubMed] [Google Scholar]
- 38.Weidner, E. 1982. The microsporidian spore invasion tube. III. Tube extrusion and assembly. J. Cell Biol. 93:976-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weidner, E. 1976. The microsporidian spore invasion tube. The ultrastructure, isolation, and characterization of the protein comprising the tube. J. Cell Biol. 71:23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weidner, E. 1972. Ultrastructural study of microsporidian invasion into cells. Z. Parasitenkd. 40:227-242. [DOI] [PubMed] [Google Scholar]
- 41.Weiss, L. M., T. D. Edlind, C. R. Vossbrinck, and T. Hashimoto. 1999. Microsporidian molecular phylogeny: the fungal connection. J. Eukaryot. Microbiol. 46:17S-18S. [PubMed] [Google Scholar]
- 42.Willer, T., M. C. Valero, W. Tanner, J. Cruces, and S. Strahl. 2003. O-mannosyl glycans: from yeast to novel associations with human disease. Curr. Opin. Struct. Biol. 13:621-630. [DOI] [PubMed] [Google Scholar]
- 43.Wittner, M., and L. M. Weiss. 1999. The microsporidia and microsporidiosis. ASM Press, Washington, D.C.
- 44.Zhang, Z., M. Duchene, and S. L. Stanley, Jr. 2002. A monoclonal antibody to the amebic lipophosphoglycan-proteophosphoglycan antigens can prevent disease in human intestinal xenografts infected with Entamoeba histolytica. Infect. Immun. 70:5873-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]