Abstract
Moraxella catarrhalis is a major cause of acute otitis media in young children and has also been implicated as an important cause of exacerbations in adults with underlying pulmonary disease. Due to the considerable level of antibiotic resistance and the high degree of carriage rates in young children, it is likely that the incidence of M. catarrhalis infections will continue to rise. M. catarrhalis is a strict human respiratory pathogen, and this bacterium uses both transferrin and lactoferrin receptors to fulfill the essential iron requirement for survival in vivo. However, these are the only described iron acquisition systems for this organism. In this report we have demonstrated that M. catarrhalis can also utilize hemin as a sole source of iron for growth. In addition, we have identified and characterized an outer membrane protein with homology (26 to 28% similarity) to other known hemin binding and uptake proteins in related gram-negative organisms (i.e., Bordetella and Yersinia spp.). This newly described M. catarrhalis protein, termed HumA, is capable of directly binding to hemin coupled to a solid-phase matrix. M. catarrhalis HumA expressed on the surface of an Escherichia coli hemA-deficient strain (K-12 EB53) is fully capable of complementing the defect and thus restoring the ability of this strain to grow in the presence of hemin. When M. catarrhalis is grown in the presence of hemin, HumA expression is clearly increased as shown by Western blotting with polyclonal antiserum developed against a HumA peptide. In addition, growth analyses revealed that a HumA-deficient mutant of M. catarrhalis (7169::humA) is restricted for growth in the presence of hemin as the sole iron source compared to the wild-type strain. We conclude that HumA is an essential component of a hemin uptake and utilization system previously undescribed for M. catarrhalis, thus providing another mechanism of iron acquisition that may facilitate persistent colonization of the mucosal surface.
Moraxella catarrhalis, a gram-negative diplococcus bacterium, continues to emerge as a leading human mucosal pathogen. The most recent data reveal that M. catarrhalis remains one of the three leading causes of acute otitis media (AOM) in children (21). In addition to AOM, this pathogen is now becoming recognized as a factor in episodes of acute bacterial rhinosinusitis in children (1). M. catarrhalis is also a well-documented cause of exacerbations in adults with chronic obstructive pulmonary disease (2, 16).
Due to the ability of this organism to cause disease in both children and adults, there are substantial health care costs associated with these infections (26). Also of major concern is the expression of β-lactamase by greater than 90% of all M. catarrhalis clinical isolates, resulting in resistance to the commonly prescribed antibacterial drug amoxicillin and certain cephalosporins (12, 21, 27). Clinical studies have revealed the inability to distinguish infection caused by M. catarrhalis from other pathogens based solely on symptomology, subsequently leading to the overuse of antibiotics (21). For these reasons there is clearly a need for further characterization of the virulence factors as well as the basic biology of this organism in order to understand the mechanisms of pathogenesis and the host immune response elicited by this pathogen.
A key factor allowing M. catarrhalis to survive and cause disease on the human respiratory mucosa is the ability to acquire essential iron. The iron-limited environment of the human host requires pathogens to be diverse in their repertoire of iron acquisition systems. Host iron is sequestered and present in complexes with transferrin, lactoferrin, heme, and hemoglobin (Hb). The ability of M. catarrhalis to utilize human transferrin and lactoferrin for growth has been well studied (5, 18, 19, 23, 25). Previous work studying related organisms, such as Neisseria and Haemophilus spp., has extensively characterized both the transferrin and lactoferrin receptor systems for these important human pathogens in addition to identifying multiple systems for hemoprotein utilization that exist among species as well as redundant systems within species (6, 7, 14, 15, 29). However, to date, transferrin and lactoferrin remain the only defined usable sources of iron described for M. catarrhalis.
The studies described in this report were designed to investigate the ability of this organism to utilize hemoproteins for growth. These data have led to the identification of an outer membrane protein (OMP) involved specifically in hemin (Hm) utilization by M. catarrhalis, termed HumA. Expression of M. catarrhalis 7169 HumA in a hemA-deficient Escherichia coli strain restored the ability to grow in the presence of Hm, thus revealing that HumA is targeted to the outer membrane and functions in Hm acquisition. Our studies demonstrate that this protein is an Hm binding protein, and with growth in the presence of Hm, HumA expression is upregulated in the wild-type strain, 7169. These data provide a further understanding of the iron acquisition mechanisms that this pathogen uses to survive and cause disease in the human host.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
M. catarrhalis 7169, a middle-ear isolate from a child with otitis media, was kindly provided by Howard Faden (Children's Hospital, Buffalo, N.Y.). This strain was used to construct the HumA mutant 7169::humA. Routine culture of wild-type 7169 and 7169::humA was done at 35°C in 5% CO2 for agar plate-grown organisms and at 37°C with rotary shaking at 225 rpm for liquid broth growth. Both brain heart infusion (BHI) and GC broth and plates were used, as well as the previously described Chelex 100-treated chemically defined medium (CDM 0) supplemented with either 8 μM Hm (bovine; Sigma, St. Louis, Mo.) or 5 μM Hb (human; Sigma) in the presence of the iron chelator desferoxamine mesylate (10 μM; Sigma) (5). The antibiotic kanamycin was added for culture of the mutant strain 7169::humA at a concentration of 20 μg/ml. E. coli XL1-Blue was cultured using Luria-Bertani (LB) liquid medium or agar plates under the conditions described above with the appropriate antibiotic (ampicillin [100 μg/ml] and/or kanamycin [20 μg/ml]). E. coli K-12 EB53 (aroB tsx malT hemA) (11) was cultured using LB supplemented with 10 μM δ-aminolevulinic acid (ALA; Sigma) for routine growth and with kanamycin (25 μg/ml), ampicillin (100 μg/ml), isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM), and Hm (30 μM) for the complementation assay.
General DNA manipulations.
All molecular biology reagents including restriction endonucleases were used according to standard protocols and were purchased from New England Biolabs, Inc. (Beverly, Mass.), or Promega (Madison, Wis.). Chromosomal DNA from M. catarrhalis was prepared as previously described (22). PCR amplifications were performed using Platinum Taq High Fidelity Polymerase (Invitrogen Life Technologies Corp., Carlsbad, Calif.). All PCR products and plasmid constructs were purified using the Qiagen MinElute kit and QIAprep spin kit, respectively (Qiagen, Santa Clarita, Calif.). Automated sequence analysis of PCR products was performed at the RPCI Biopolymer Facility (Roswell Park Cancer Institute, Buffalo, N.Y.) and analyzed using MacVector software (version 7.2; Genetics Computer Group, Madison, Wis.). Threading analysis and protein modeling of HumA were performed at the Center of Excellence in Bioinformatics, University at Buffalo (Buffalo, N.Y.) (17, 24).
Cloning and mutagenesis of M. catarrhalis 7169 humA.
M. catarrhalis humA was identified through BLAST searches based on its homology to other hemoprotein receptors, often TonB dependent, in the National Center for Biotechnology Information data bank, such as those described in a recent review by Wandersman and Stojiljkovic (29). Those regions of homology in the patent database for M. catarrhalis were then analyzed for potential open reading frames (ORFs) (MacVector 7.2), which were then resubmitted through BLAST. The ORF encoding HumA returned the highest homologies to those proteins shown in the ClustalW alignment in Fig. 1. PCR primers were designed for cloning humA based on the nucleotide sequence submitted under Incyte Genomics sequence 34, patent WO0078968, accession number AX067459. PCR amplification of 380 bp upstream of the predicted 5′ transcription start site and 245 bp downstream of the 3′ predicted stop site was performed using primers 474 (5′-CGGCGTAAATGAACAGG-3′) (sense) and 475 (5′-CAAGACACTATCTATCAGAGTCG-3′) (antisense). Primers 474 and 475 resulted in a 3,082-bp product that was ligated into pGEM-T Easy (Promega), resulting in pTB34-3KF. E. coli XL1-Blue was transformed with pTB34-3KF by using electroporation. PCR analysis, restriction digestion, and sequence analysis were performed using this plasmid to confirm the nucleotide organization of 7169 humA.
FIG. 1.
Amino acid homologies demonstrated that M. catarrhalis HumA contains conserved regions required for transporting Hm into the periplasm. Conserved amino acids are shown with asterisks.
A deletion-insertion isogenic mutant of 7169 humA was constructed by inverse PCR with pTB34-3KF as a template. Primers 610 (5′-ATATCTCGAGTGGGATTATCGTCTTGGGGG-3′) (sense) and 609 (5′-CGCGAGATCTTTTGAAACTGTGATGCCACTG-3′) (antisense), with engineered XhoI and BglII sites, respectively (underlined), were designed to create a 1,425-bp deletion internal to the ORF of humA. Amplification of aphA-3, the nonpolar kanamycin resistance cassette, from pUC18K was performed using primers 417 (5′-TATAAGATCTGGGTGACTAACTAGGAGGAATAAATGGCTA-3′) (sense) and 491 (5′-TATACTCGAGGTCGACTCTAGAGGATCCCCGGGTCATTA-3′) (antisense) with complementing restriction sites for directional cloning (20). Restriction digestion of the PCR products and ligation resulted in pTB34K-KF. E. coli XL1-Blue was transformed with pTB34K-KF by using electroporation. Sequence analysis confirmed proper insertion of the kanamycin resistance cassette.
Primers 474 and 475 were used to amplify a 2.5-kb product from pTB34K-KF (humA plus aphA-3) that was used to naturally transform M. catarrhalis 7169 as previously described (13). Sequence analysis of 7169::humA chromosomal DNA was performed to confirm that the inactivated humA gene had recombined into the chromosome.
Restoration of HumA expression in 7169::humA through reversion to wild type.
Primers 474 and 475 (described above) were used to amplify the native humA gene from M. catarrhalis 7169 chromosomal DNA. The resulting PCR product was purified using the MinElute kit (Qiagen) and was used to naturally transform 7169::humA by the method previously described (13). Potential revertant clones were tested for the loss of kanamycin resistance through replicate plating on both BHI and BHI plus kanamycin (20 μg/ml) agar plates. One clone that demonstrated the loss of kanamycin resistance was chosen for further study. Chromosomal DNA was isolated from this strain and subjected to PCR analysis with primers 474 and 475. OMPs were isolated from this revertant strain and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with anti-HumA antiserum (as described below).
HumA peptide design and antiserum production.
The 10-amino-acid HumA peptide, EPDIRFKRLC (native amino acid sequence underlined; the terminal C was added in order to couple this peptide to keyhole limpet hemocyanin by using maleimidobenzoyl-N-hydroxysuccinimide ester), was chosen for production (Sigma-Genosys, The Woodlands, Tex.) based on multiple factors outlined by the manufacturer. This peptide was used to generate anti-HumA polyclonal antiserum with New Zealand White rabbits. Affinity-purified anti-HumA antiserum was diluted using phosphate-buffered saline (pH 7.0) to a concentration of 0.28 mg/ml and stored at −20°C. Western blotting (anti-HumA antiserum concentration of 28 μg/ml) and colony lift analyses (anti-HumA antiserum concentration of 2.8 μg/ml) were performed as described previously (4, 5).
Hm-agarose binding of HumA.
Hm-agarose binding was performed essentially as described by Dashper et al. (10) with the following modifications. Briefly, 50 μl of Hm-agarose (Sigma) was washed with 100 mM NaCl-25 mM Tris-HCl (pH 7.4). Washes were performed three times by resuspending the agarose in 200 μl of buffer and centrifuging (7,000 rpm for 5 min in an Eppendorf model 5415C centrifuge). Zwittergent 3-14-extracted OMP preparations (10 μl) plus phosphate-buffered saline (40 μl) from either CDM Hm-grown 7169 or 7169::humA were incubated with the Hm-agarose for 3 h at 20°C with mixing. The samples were centrifuged, and the supernatants were removed. The Hm-agarose was washed three times as before, and wash fractions were concentrated using Centricon filters (Millipore, Bedford, Mass.). Bound proteins were eluted for 5 min with 3 M NaCl or 2 M guanidine-HCl (30 μl). Concentrated wash samples, Hm-agarose bound, and eluted proteins were subjected to SDS-7% PAGE and Western blotting (anti-HumA antiserum concentration of 28 μg/ml) analyses.
E. coli K-12 EB53 complementation analysis.
E. coli K-12 EB53 (aroB tsx malT hemA) (11) was transformed with pREP4 (Qiagen; lacI; kanamycin resistance) to provide regulation of the pQE-70 (Qiagen; ampicillin resistance) protein expression plasmid designed for complementation. M. catarrhalis 7169 humA was cloned into pQE-70 by using wild-type 7169 chromosomal DNA and PCR primers 739 (5′-GCGCAGATCTTCATATATGAAGGTTACCAT-3′) (sense) and 740 (5′-ATATAAGCTTCAAGACACTATCTATCAGAGTCG-3′) (antisense) with the engineered restriction sites BglII and HindIII, respectively (underlined), and the native humA transcriptional start site (ATG) as noted by italics. As a negative control, the deletion-insertion mutant copy of M. catarrhalis humA was also cloned into the pQE-70 background by using 7169::humA chromosomal DNA and PCR primers 739 and 740. The resulting PCR products were purified, digested with the appropriate restriction endonucleases, and then ligated to pQE-70 (also digested using appropriate enzymes). The plasmid responsible for expressing full-length M. catarrhalis 7169 HumA, pQE-P91E, as well as the negative control for HumA expression, pQE-P91EMut, was electroporated into E. coli XL1-Blue and subjected to sequence analysis. E. coli K-12 EB53(pREP4) was transformed with either pQE-P91E or pQE-P91EMut. For the complementation assay, LB agar plates were supplemented with kanamycin (25 μg/ml), ampicillin (100 μg/ml), IPTG (1 mM), and Hm (30 μM) and were inoculated for single colonies.
Growth and OMP analyses.
CDM 0 (iron-depleted) and CDM Hm (8 μM Hm) 10-ml cultures were inoculated to a starting optical density at 600 nm (OD600) of 0.1 from overnight GC agar plate-grown M. catarrhalis 7169 or 7169::humA. These strains were cultured at 37°C with rotary shaking at 225 rpm overnight (16 h). Both wild-type 7169 and 7169::humA cultures were centrifuged at 2,700 × g for 10 min, and fresh 10-ml cultures including desferoxamine mesylate were inoculated to a starting OD600 of 0.06 (CDM 0-0, no added iron source in preculture or experimental culture; CDM 0-Hm, no added iron source in preculture and Hm added for experimental culture; CDM Hm-Hm, Hm added in both preculture and experimental culture). CDM Hb (5 μM Hb) was inoculated to a starting OD600 of 0.1 from overnight GC agar plate-grown M. catarrhalis 7169 or 7169::humA without prior overnight culture. Growth, as described above, was measured for 7 h. OMPs were prepared as previously described (4, 5) following overnight (16-h) growth in CDM Hm that was inoculated from a 4-h culture in CDM Hm. OMPs were also prepared from M. catarrhalis 7169 and 7169::humA grown in CDM 0, CDM 100 [100 μM Fe(NO3)3], and CDM Hb without prior growth in CDM conditions. OMPs were subjected to SDS-7% PAGE and Western blot analyses (anti-HumA antiserum concentration of 28 μg/ml).
Nucleotide sequence accession number.
The M. catarrhalis 7169 humA sequence has been deposited in GenBank under accession no. AY623791.
RESULTS AND DISCUSSION
Identification, cloning, and sequence analysis of M. catarrhalis 7169 humA.
Searches of the National Center for Biotechnology Information patent sequence data bank for potential Hm receptor proteins revealed an M. catarrhalis ORF of 2.4 kb. This ORF was cloned and sequenced, and the encoded protein (815 amino acids; 91.2-kDa predicted molecular mass) was homologous to the class of heme iron utilization systems that utilize a single-component outer-membrane TonB-dependent receptor composed of plug-barrel architecture to acquire heme (28, 30). Gram-negative organisms expressing proteins with the highest homology to this previously undefined M. catarrhalis protein include Bordetella pertussis (BhuR), Yersinia pestis (HmuR), and Shigella dysenteriae (ShuA) (26 to 28% similar) (Fig. 1). Based on these similarities the protein was termed HumA (Hm utilization protein of M. catarrhalis).
Conserved domains of HumA correlated with the previously defined regions of TonB-dependent OMPs (8, 9), as well as the FRAP/H/NPNL amino acid domains characteristic of receptors known to transport heme into the periplasm (highlighted with asterisks in Fig. 1) (3). In addition, computer modeling and threading analyses of HumA revealed predicted structural homology to the outer membrane ferric citrate transporter of E. coli, FecA (17, 24, 30). These analyses also suggested an N-terminal globular domain thought to contain a region important for TonB interaction as well as C-terminal extracellular loops that are predicted to be the site(s) of substrate recognition (17, 24). These data suggested that HumA traverses the outer membrane by a series of β-strands forming a typical β-barrel architecture. This structure generated two solvent-exposed surfaces, one located in the periplasm potentially available for interaction with the energy-transducing TonB/ExbB/ExbD complex and the other exposed to the extracellular milieu and therefore expected to be the site of Hm binding.
Construction and characterization of the M. catarrhalis humA isogenic mutant, 7169::humA.
To study the ability of M. catarrhalis 7169 to utilize Hm for growth as well as to determine the role of HumA in this process, an isogenic humA mutant was constructed, 7169::humA. A substantial 1.4-kb internal deletion was created in the humA ORF in order to ensure loss of function. A highly histidine-rich region central to HumA was included in this deletion, as we hypothesize that the approximately 22-amino-acid stretch of alternating acidic and basic charged amino acids may play a role in transporting Hm.
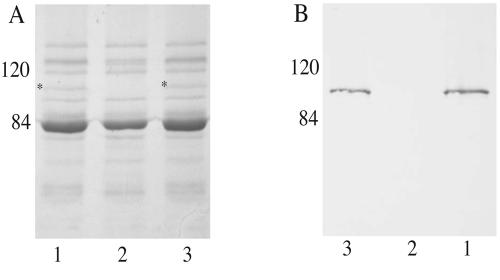
In order to begin to characterize HumA expression, polyclonal antibodies were developed to a HumA-specific peptide. OMPs were isolated from the wild type and 7169::humA and analyzed by SDS-PAGE (Fig. 2A) and Western blotting (Fig. 2B). Comparison of the OMP profiles revealed that 7169::humA (Fig. 2A, lane 2) was defective in the expression of a protein with a predicted molecular mass of 91.2 kDa that was expressed by the wild-type strain (Fig. 2A, lane 1, asterisk). Western blot analysis, using the HumA-specific peptide antiserum, demonstrated that this 91.2-kDa protein, expressed by strain 7169, was HumA (Fig. 2B, lane 1), and this immunoblot also confirmed that 7169::humA had lost expression of this protein (Fig. 2B, lane 2).
FIG. 2.
Analysis of the wild-type strain, 7169::humA, and the revertant strain with antiserum specific to HumA. OMP analyses by SDS-7% PAGE (A) and Western blotting with anti-HumA antiserum (B) reveal the expression of HumA by the wild-type and revertant strain (HumA, *). Molecular masses are shown in kilodaltons.
To demonstrate that the loss of HumA expression was due to the internal deletion described above, the humA wild-type gene alone was reintroduced into the 7169::humA chromosome by natural transformation and confirmed by PCR (data not shown). OMPs were isolated from one revertant strain after culture in Hm and analyzed by SDS-PAGE (Fig. 2A) and Western blotting (Fig. 2B). These studies demonstrated that the introduction of humA alone into the mutant restored the expression of HumA (Fig. 2A, lane 3) and that the level detected in the immunoblot (Fig. 2B, lane 3) was consistent with the wild-type strain (Fig. 2B, lane 1). Although these studies are not ideal, there is currently no reliable method to complement M. catarrhalis in trans, and these data confirm that the expected phenotype was restored in 7169::humA with the wild-type gene alone.
HumA binding of Hm-agarose.
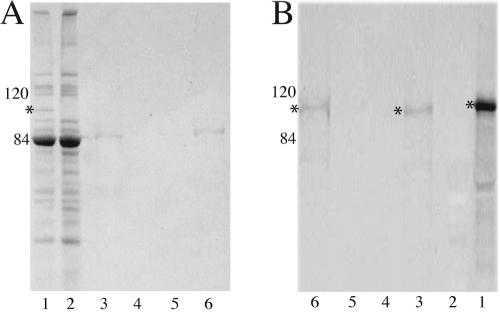
Although M. catarrhalis HumA showed relatively low homologies to known hemoprotein utilization proteins, many of the regions required for Hm binding and internalization were conserved. These data suggested that HumA was capable of direct interaction with Hm. OMPs from 7169 were incubated with Hm-agarose to determine the ability of HumA to bind Hm. SDS-PAGE analysis (Fig. 3A) of bound proteins (lane 3), washes (lanes 4), and eluted proteins (lanes 5 and 6) demonstrated very little nonspecific binding to the Hm-agarose matrix.
FIG. 3.
SDS-7% PAGE analysis (A) and Western blotting with anti-HumA antibodies (B) revealed binding of HumA to Hm-agarose. Lanes: 1, OMPs of CDM Hm-grown 7169; 2, OMPs of CDM Hm-grown 7169::humA; 3, Hm-agarose-bound fraction; 4, concentrated washes; 5, 3 M NaCl elution; 6, 2 M guanidine-HCl elution. Molecular masses are shown in kilodaltons. *, HumA.
Subsequent Western blot analysis with anti-HumA antibodies (Fig. 3B) demonstrated that HumA was capable of binding to Hm-agarose and was removed from this matrix only with the stringent 2 M guanidine-HCl elution, a strong chaotropic agent (lanes 3 and 6, respectively). HumA was not released from the matrix with a high-salt elution (lane 5), indicating that binding of HumA to Hm is not due simply to nonspecific charge-charge interactions. As expected, HumA was not detected under any condition when 7169::humA OMPs were incubated with Hm-agarose (data not shown).
Outer membrane surface expression of M. catarrhalis HumA complements the hemA-deficient mutant E. coli K-12 EB53.
The E. coli K-12 EB53 (aroB tsx malT hemA) outer membrane is impermeable to Hm; this strain lacks the ability to synthesize its own siderophore enterochelin (aroB) and is unable to synthesize porphyrin due to the hemA mutation (11). Previous studies have demonstrated complementation of this strain with potential Hm binding and uptake proteins (31). To determine if M. catarrhalis HumA functioned as a Hm utilization protein, the full-length HumA protein was expressed in E. coli K-12 EB53 by cloning humA in frame under the control of an IPTG-inducible promoter on an expression plasmid.
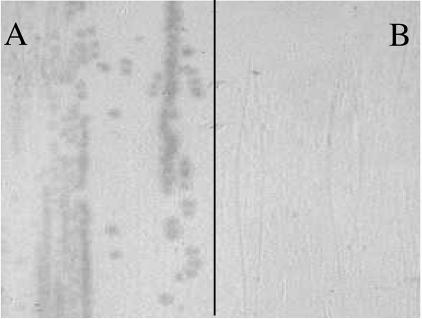
First, to confirm HumA outer membrane surface expression, colony lift analyses from LB agar plates supplemented with ALA and IPTG, as well as the respective antibiotic, were performed using the purified polyclonal antiserum. Figure 4A demonstrates that HumA was detected on the surface in E. coli K-12 EB53(pREP4) plus pQE-P91E (containing humA). As expected, the negative control strain, E. coli K-12 EB53(pREP4) plus pQE-P91EMut, revealed no expression of HumA (Fig. 4B). These data not only confirmed that HumA was expressed in this organism but demonstrated that HumA was transported to the surface of the outer membrane.
FIG. 4.
M. catarrhalis HumA was expressed on the surface of the complemented E. coli strain [K-12 EB53(pREP4) plus (pQE-P91E)] as shown by colony lift analysis with anti-HumA antibodies (A). E. coli K-12 EB53(pREP4) plus pQE-P91EMut was a negative control (B). Growth conditions were LB medium with kanamycin, ampicillin, IPTG, and ALA.
Second, the E. coli constructs described above were then evaluated for the ability to grow in the presence of Hm. E. coli K-12 EB53(pREP4) complemented with pQE-P91E expressing HumA allowed for single colony formation when inoculated onto selective LB agar plates (Fig. 5A). The negative control for expression of HumA, pQE-P91EMut, did not restore growth in this strain (Fig. 5B). E. coli K-12 EB53 and E. coli K-12 EB53(pREP4) were tested under these same conditions and were unable to grow (data not shown). Control conditions for growth of these strains included LB plus ALA with or without IPTG and/or Hm, all of which promoted growth as expected (data not shown).
FIG. 5.
E. coli K-12 EB53 complementation with HumA of M. catarrhalis restored the ability to grow with Hm. (A) E. coli K-12 EB53(pREP4) with pQE-P91E; (B) E. coli K-12 EB53(pREP4) with pQE-P91EMut. Growth conditions were LB medium with kanamycin, ampicillin, IPTG, and Hm.
Function of HumA in vivo was demonstrated through the complementation of this E. coli strain deficient in the ability to utilize Hm for growth. Restoration of the growth phenotype with Hm when HumA is expressed supports the hypothesis that HumA is targeted to the outer membrane and is able to bind to and internalize Hm. The fact that HumA expression alone promotes growth with Hm further suggests that this protein functions in a single-component receptor fashion as suggested above.
Growth analyses of 7169 versus 7169::humA in the presence of Hm and Hb.
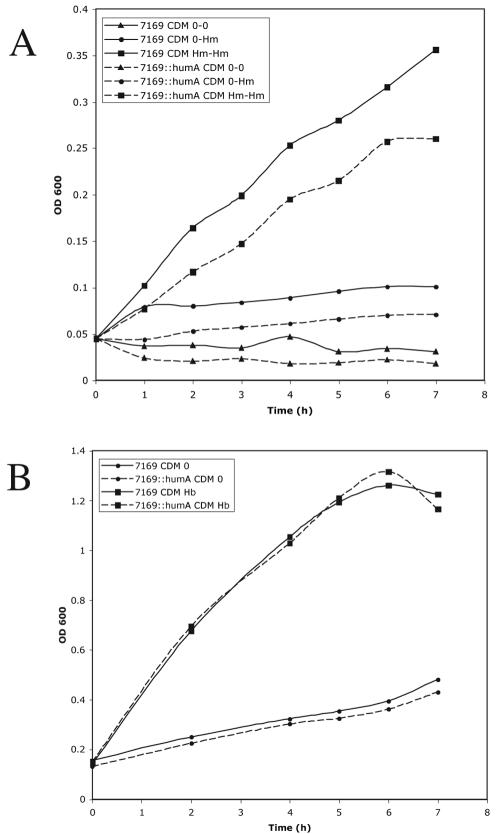
To determine the role and specificity of HumA in growth of M. catarrhalis 7169 with hemoproteins as the sole iron source, analyses were performed using both Hm and Hb. Conditions for which 7169 revealed a consistent growth pattern in the presence of Hm required that the organism be exposed to a medium containing 8 μM Hm (higher concentrations proved toxic) for a period of 16 h prior to growth studies in fresh medium (CDM Hm-Hm). As shown in Fig. 6A, wild-type 7169 was able to utilize Hm, while 7169::humA was clearly restricted in the ability to use this iron source, never able to reach wild-type levels of growth. These results are similar to those defined for Bordetella spp. (28). Those cultures that were not exposed to Hm prior to the studies (CDM 0-Hm) failed to grow and mimicked negative controls (Fig. 6A). In Fig. 6B, it is clear that both 7169 and the mutant 7169::humA were equally capable of utilizing Hb as a sole source of iron, thus attesting to the specificity of HumA for Hm.
FIG. 6.
Growth curve analyses comparing the abilities of 7169 and 7169::humA to grow with Hm (A) and Hb (B) as sole iron sources. All cultures contained 10 μM desferoxamine mesylate, an iron chelator.
While the overall growth rate in Hm was not comparable to that seen with other iron sources, i.e., transferrin and lactoferrin, there is an obvious difference between these isogenic strains, further supporting the role of HumA in Hm utilization by M. catarrhalis. We further hypothesize that, although 7169::humA shows a noticeable growth defect, the ability to grow with Hm (Fig. 6A) suggests the presence of a secondary Hm uptake system yet to be defined. As stated earlier, many gram-negative bacteria express numerous redundant systems involved in iron acquisition.
HumA expression is increased in the presence of Hm.
Based on data obtained from the analyses described above, equivalent amounts of OMPs were isolated from M. catarrhalis grown with various iron sources to determine the optimal condition for HumA expression. SDS-PAGE analysis of M. catarrhalis 7169 OMP profiles revealed a clear upregulation of HumA in cultures exposed to Hm (CDM Hm; Fig. 7A, lane 7, asterisk). In contrast, those organisms grown in the presence of no added iron source (CDM 0), Fe(NO3)3 (CDM 100), or Hb (CDM Hb) alone exhibited undetectable levels of HumA (Fig. 7A, lanes 1, 3, and 5, respectively). As expected, HumA was not expressed by 7169::humA under any condition tested (Fig. 7A, lanes 2, 4, 6, and 8).
FIG. 7.
SDS-7% PAGE OMP analysis of 7169 and 7169::humA when cultured under iron limitation (CDM 0) or in the presence of Fe(NO3)3 (CDM 100), Hb (CDM Hb), or Hm (CDM Hm) (A). Western blot analysis with anti-HumA antibodies revealed upregulation of HumA (*) (A) when strains were cultured with Hm (B). Lanes: 1, CDM 0, 7169; 2, CDM 0, 7169::humA; 3, CDM 100, 7169; 4, CDM 100, 7169::humA; 5, CDM Hb, 7169; 6, CDM Hb, 7169::humA; 7, CDM Hm, 7169; 8, CDM Hm, 7169::humA. Molecular masses are shown in kilodaltons.
Antiserum specific to M. catarrhalis 7169 HumA was used to confirm the differential expression levels of HumA under the varied growth conditions. Figure 7B is a Western blot probed with anti-HumA antibodies, demonstrating that M. catarrhalis HumA expression was visibly increased in OMPs isolated from bacteria cultured in the presence of Hm (Fig. 7B, lane 7) compared to OMPs isolated from bacteria cultured in the absence of an additional iron source or in the presence of Fe(NO3)3 or Hb (Fig. 7B, lanes 1, 3, and 5, respectively). These results, along with the growth studies, suggest the ability of M. catarrhalis 7169 to sense Hm levels in the environment and regulate transcription of humA.
These studies demonstrate the ability of this pathogen to adapt to its environment and utilize a range of iron sources. During pronounced inflammation, particularly conditions associated with AOM in the middle ear, it is likely that the local tissue damage results in the release of various iron sources including Hb and Hm. Therefore, having a diverse repertoire of iron acquisition mechanisms contributes to the ability of M. catarrhalis to cause disease and persist on the human respiratory mucosa. Future studies will focus on further defining HumA as well as its potential regulation by Fur and its interaction with TonB. Although we have previously characterized the M. catarrhalis Fur, a consensus Fur box sequence has not yet been experimentally defined (13). It will also be important to characterize the human antibody response to HumA and to determine the merit of HumA as a potential vaccine component.
Acknowledgments
We thank Klaus Hantke for kindly providing the strain E. coli K-12 EB53 for our complementation analyses and also Jeffrey Skolnick and Iain Hay for their assistance with the computer modeling of HumA.
This research was supported by grant AI46469 from the National Institutes of Health (NIAID) to A.A.C.
Editor: D. L. Burns
REFERENCES
- 1.Anon, J. B. 2003. Acute bacterial rhinosinusitis in pediatric medicine: current issues in diagnosis and management. Paediatr. Drugs 5:25-33. [PubMed] [Google Scholar]
- 2.Bakri, F., A. L. Brauer, S. Sethi, and T. F. Murphy. 2002. Systemic and mucosal antibody response to Moraxella catarrhalis after exacerbations of chronic obstructive pulmonary disease. J. Infect. Dis. 185:632-640. [DOI] [PubMed] [Google Scholar]
- 3.Bracken, C. S., M. T. Baer, A. Abdur-Rashid, W. Helms, and I. Stojiljkovic. 1999. Use of heme-protein complexes by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J. Bacteriol. 181:6063-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campagnari, A. A., T. F. Ducey, and C. A. Rebmann. 1996. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect. Immun. 64:3920-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campagnari, A. A., K. L. Shanks, and D. W. Dyer. 1994. Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production. Infect. Immun. 62:4909-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cope, L. D., S. E. Thomas, Z. Hrkal, and E. J. Hansen. 1998. Binding of heme-hemopexin complexes by soluble HxuA protein allows utilization of this complexed heme by Haemophilus influenzae. Infect. Immun. 66:4511-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cope, L. D., R. Yogev, U. Muller-Eberhard, and E. J. Hansen. 1995. A gene cluster involved in the utilization of both free heme and heme:hemopexin by Haemophilus influenzae type b. J. Bacteriol. 177:2644-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelissen, C. N., G. D. Biswas, J. Tsai, D. K. Paruchuri, S. A. Thompson, and P. F. Sparling. 1992. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J. Bacteriol. 174:5788-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, A. J., M. L. Hunt, J. D. Boyce, and B. Adler. 2003. Functional characterization of HgbB, a new hemoglobin binding protein of Pasteurella multocida. Microb. Pathog. 34:287-296. [DOI] [PubMed] [Google Scholar]
- 10.Dashper, S. G., A. Hendtlass, N. Slakeski, C. Jackson, K. J. Cross, L. Brownfield, R. Hamilton, I. Barr, and E. C. Reynolds. 2000. Characterization of a novel outer membrane hemin-binding protein of Porphyromonas gingivalis. J. Bacteriol. 182:6456-6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberspacher, B., and V. Braun. 1980. The involvement of cytochromes in the uptake of ferrichrome by Escherichia coli K-12. FEMS Microbiol. Lett. 7:61-64. [Google Scholar]
- 12.Felmingham, D., D. J. Farrell, R. R. Reinert, and I. Morrissey. 2004. Antibacterial resistance among children with community-acquired respiratory tract infections (PROTEKT 1999-2000). J. Infect. 48:39-55. [DOI] [PubMed] [Google Scholar]
- 13.Furano, K., and A. A. Campagnari. 2003. Inactivation of the Moraxella catarrhalis 7169 ferric uptake regulator increases susceptibility to the bactericidal activity of normal human sera. Infect. Immun. 71:1843-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genco, C. A., and D. W. Dixon. 2001. Emerging strategies in microbial haem capture. Mol. Microbiol. 39:1-11. [DOI] [PubMed] [Google Scholar]
- 15.Lewis, L. A., M. Gipson, K. Hartman, T. Ownbey, J. Vaughn, and D. W. Dyer. 1999. Phase variation of HpuAB and HmbR, two distinct haemoglobin receptors of Neisseria meningitidis DNM2. Mol. Microbiol. 32:977-989. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman, D. 2003. Pseudomonal infections in patients with COPD: epidemiology and management. Am. J. Respir. Med. 2:459-468. [DOI] [PubMed] [Google Scholar]
- 17.Lu, L., H. Lu, and J. Skolnick. 2002. MULTIPROSPECTOR: an algorithm for the prediction of protein-protein interactions by multimeric threading. Proteins Struct. Funct. Genet. 49:350-364. [DOI] [PubMed] [Google Scholar]
- 18.Luke, N. R., and A. A. Campagnari. 1999. Construction and characterization of Moraxella catarrhalis mutants defective in expression of transferrin receptors. Infect. Immun. 67:5815-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luke, N. R., T. A. Russo, N. Luther, and A. A. Campagnari. 1999. Use of an isogenic mutant constructed in Moraxella catarrhalis to identify a protective epitope of outer membrane protein B1 defined by monoclonal antibody 11C6. Infect. Immun. 67:681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmu, A. A., E. Herva, H. Savolainen, P. Karma, P. H. Mèakelèa, and T. M. Kilpi. 2004. Association of clinical signs and symptoms with bacterial findings in acute otitis media. Clin. Infect. Dis. 38:234-242. [DOI] [PubMed] [Google Scholar]
- 22.Russo, T. A., J. E. Guenther, S. Wenderoth, and M. M. Frannk. 1993. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated gene disruption. Mol. Microbiol. 9:357-364. [DOI] [PubMed] [Google Scholar]
- 23.Schryvers, A. B., R. Bonnah, R. H. Yu, H. Wong, and M. Retzer. 1998. Bacterial lactoferrin receptors. Adv. Exp. Med. Biol. 443:123-133. [DOI] [PubMed] [Google Scholar]
- 24.Skolnick, J., and D. Kihara. 2001. Defrosting the frozen approximation: PROSPECTOR—a new approach to threading. Proteins Struct. Funct. Genet. 42:319-331. [PubMed] [Google Scholar]
- 25.Stojiljkovic, I., J. Larson, V. Hwa, S. Anic, and M. So. 1996. HmbR outer membrane receptors of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J. Bacteriol. 178:4670-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teele, D. W., J. O. Klein, B. M. Word, B. A. Rosner, S. Starobin, R. Earle Jr., C. S. Ertel, G. Fisch, R. Michales, R. Heppen, and N. P. Strause. 2001. Antimicrobial prophylaxis for infants at risk for recurrent acute otitis media. Vaccine 19:S140-S143. [DOI] [PubMed] [Google Scholar]
- 27.Thornsberry, C., P. T. Ogilvie, H. P. Holley, Jr., and D. F. Sahm. 1999. Survey of susceptibilities of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis isolates to 26 antimicrobial agents: a prospective U.S. study. Antimicrob. Agents Chemother. 43:2612-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanderpool, C. K., and S. K. Armstrong. 2001. The Bordetella bhu locus is required for heme iron utilization. J. Bacteriol. 183:4278-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wandersman, C., and I. Stojiljkovic. 2000. Bacterial heme sources: the role of heme, hemoprotein receptors and hemophores. Curr. Opin. Microbiol. 3:215-220. [DOI] [PubMed] [Google Scholar]
- 30.Yue, W. W., S. Grizot, and S. K. Buchanan. 2003. Structural evidence for iron-free citrate and ferric citrate binding to the TonB-dependent outer membrane transporter FecA. J. Mol. Biol. 332:353-368. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann, R., V. A. Kempf, E. Schiltz, K. Oberle, and A. Sander. 2003. Hemin binding, functional expression, and complementation analysis of Pap 31 from Bartonella henselae. J. Bacteriol. 185:1739-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]