Abstract
The type II heat-labile enterotoxins, LT-IIa and LT-IIb, exhibit potent adjuvant properties. However, little is known about their immunomodulatory activities upon interaction with innate immune cells, unlike the widely studied type I enterotoxins that include cholera toxin (CT). We therefore investigated interactions of LT-IIa and LT-IIb with human monocytic THP-1 cells. We found that LT-II enterotoxins were inactive in stimulating cytokine release, whereas CT induced low levels of interleukin-1β (IL-1β) and IL-8. However, all three enterotoxins potently regulated cytokine induction in cells activated by bacterial lipopolysaccharide or fimbriae. Induction of proinflammatory (tumor necrosis factor α [TNF-α]) or chemotactic (IL-8) cytokines was downregulated, whereas induction of cytokines with anti-inflammatory (IL-10) or mucosal adjuvant properties (IL-1β) was upregulated by the enterotoxins. These effects appeared to depend on their A subunits, because isolated B-pentameric subunits lacked regulatory activity. Enterotoxin-mediated inhibition of proinflammatory cytokine induction in activated cells was partially attributable to synergism for endogenous production of IL-10 and to an IL-10-independent inhibition of nuclear factor κB (NF-κB) activation. In sharp contrast to the holotoxins, the B pentamers (LT-IIaB and, to a greater extent, LT-IIbB) stimulated cytokine production, suggesting a link between the absence of the A subunit and increased proinflammatory properties. In this regard, the ability of LT-IIbB to activate NF-κB and induce TNF-α and IL-8 was antagonized by the LT-IIb holotoxin. These findings support distinct immunomodulatory roles for the LT-II holotoxins and their respective B pentamers. Moreover, the anti-inflammatory properties of the holotoxins may serve to suppress innate immunity and promote the survival of the pathogen.
Vibrio cholerae and Escherichia coli produce structurally related heat-labile enterotoxins which cause diarrheal symptoms in humans and animals. Although their molecular structure is similar, consisting of an enzymatically active and toxic A subunit noncovalently linked to a pentameric binding (B) subunit, these AB5-type toxins are serologically distinguished into two groups, termed type I and type II (17). Type I includes cholera toxin (CT) and E. coli heat-labile toxin I (LT-I), whereas type II includes E. coli LT-IIa and LT-IIb (17). LT-IIa, LT-IIb, and nontoxic derivatives thereof have been recently shown to exhibit mucosal adjuvant properties that are quite distinct from those of CT, the prototypical enterotoxin adjuvant (21-23). Differences in adjuvant mechanisms between type I and II enterotoxins (and within each group) may reflect differential binding to ganglioside receptors. Type I toxins bind avidly to ganglioside GM1. LT-IIa binds, in order of decreasing affinity, to GD1b, GD1a, and GM1, whereas LT-IIb lacks affinity for GM1 or GD1b but binds strongly to GD1a (12). Ganglioside binding by AB5 toxins is required for toxicity through adenylate cyclase-dependent elevation of cyclic AMP (cAMP) levels (17). In enterocytes, increase in intracellular cAMP levels causes massive secretion of fluid and electrolytes into the gut lumen (19). However, elevated intracellular cAMP mediates multiple biological effects in a variety of cell types, mainly through activation of protein kinase A (PKA) and the ensuing phosphorylation of transcription factors that act through cAMP-responsive elements in the promoter region of target genes (20).
Several cytokine genes contain cAMP-responsive elements that are important for their transcriptional regulation (5, 18, 28). Thus, elevation of intracellular cAMP caused by heat-labile enterotoxins, such as LT-IIa, LT-IIb, and CT, may modulate expression of those cytokine genes. An investigation of potential interactions between heat-labile enterotoxins and innate immune cells presents a dual interest. First, the molecular mechanisms involved in cytokine induction and regulation are likely to be associated with the potent adjuvant properties of the enterotoxins. Indeed, innate immune responses are important not only in first-line defense but also in initiating adaptive immunity (1). Second, early recognition events between innate immune cells and pathogen components often determine cellular activation or, alternatively, control evasion or diversion of the host response. This latter factor has the potential of increasing the survival capacity of the pathogen and, thus, prolonging an infection.
The type I heat-labile enterotoxins, CT and LT-I, are known to interact with macrophages and other innate immune cells to induce expression of interleukin-1 (IL-1) (4, 11, 36), a cytokine which, in the form of IL-1α or IL-1β, displays potent mucosal adjuvant activity (33). Similar studies to establish the immunomodulatory activities of LT-IIa and LT-IIb on monocytic cells for production of IL-1 or other cytokines have not been reported. The primary objective of this study, therefore, was to determine whether the interaction of LT-IIa or LT-IIb enterotoxins with human monocytic THP-1 cells elicits release of specific cytokines involved in induction or regulation of inflammation or adjuvant stimulation. We found that both LT-IIa and LT-IIb were poor cytokine inducers for THP-1 cells. In contrast, both LT-IIa and LT-IIb strongly inhibited induction of proinflammatory (tumor necrosis factor α [TNF-α]) or chemotactic (IL-8) cytokines by lipopolysaccharide (LPS) or other bacterial stimuli. At the same time, in those same cells LT-IIa and LT-IIb upregulated production of LPS-induced cytokines having anti-inflammatory (IL-10) or mucosal adjuvant properties (IL-1β). Subsequent experiments established that these immunomodulatory properties of the LT-IIa and LT-IIb holotoxins are mediated by their A subunits, because neither LT-IIaB nor LT-IIbB retained these regulatory activities. The LT-II B pentamers, however, were significantly more potent than their respective holotoxins in inducing cytokine release in THP-1 monocytic cells. These observations have direct importance toward understanding the immunomodulatory activities of LT-IIa and LT-IIb and possibly in the pathogenesis of bacteria which express heat-labile enterotoxins such as LT-IIa and LT-IIb.
MATERIALS AND METHODS
Engineering and purification of holotoxins and their B subunits.
To engineer a His-tagged version of LT-IIa, a fragment encoding a portion of the A polypeptide and the B polypeptide was PCR amplified from pTDC400 (7) using the synthetic oligonucleotides 5′-GATGGGATCCTTGGTGTGCATGGAGAAAG-3′ (BamHI site is underlined) and 5′-AAATAAACTAGTTTAGTGGTGGTGGTGGTGGTGTGACTCTCTATCTAATTCCAT-3′ (BcuI site is underlined; His codons are double underlined) as primers. PCR conditions were the following: denaturation at 95°C for 45 s, annealing at 44°C for 45 s, and extension at 72°C for 2 min, 30 cycles. After digestion with SacI and BcuI, the resulting PCR fragment was substituted for the SacI/BcuI fragment of pTDC200ΔS. This plasmid was derived from pTDC200 (7) upon removal of a redundant SacI restriction site by partial digestion with SacI, followed by blunting the digested site with Klenow fragment and religation with T4 DNA ligase. The plasmid encoding the LT-IIa holotoxin with a His-tagged B polypeptide was denoted pHN4.
To construct a recombinant plasmid encoding the His-tagged B polypeptide of LT-IIa, pHN4 was digested with SacI and BcuI. The obtained DNA fragment (corresponding to the B polypeptide) was inserted into pBluescript KSII+ (Stratagene, La Jolla, Calif.) at the SacI/BcuI sites to produce pHN15.
To engineer a His-tagged version of LT-IIb, a fragment carrying the genes for A and B polypeptides was PCR amplified from pTDC100 (8) using the synthetic oligonucleotides 5′-CGGGATCCATGCTCAGGTGAG-3′ (BamHI site is underlined) and 5′-GGAATTCTTAGTGGTGGTGGTGGTGGTGTTCTGCCTCTAACTCGA-3′ (EcoRI site is underlined; His codons are double underlined). PCR conditions were the following: denaturation at 95°C for 45 s, annealing at 44°C for 45 s, and extension for 2 min, 30 cycles. After digestion with BamHI and EcoRI, the PCR fragment was ligated into pBluescript KSII+ at the BamHI/EcoRI sites to produce pHN1, encoding LT-IIb holotoxin with a His-tagged B polypeptide.
Recombinant plasmid pHN16.1, encoding only the His-tagged B polypeptide of LT-IIb, was engineered by ligating the B-polypeptide-encoding XhoI/EcoRI fragment from pHN1 into pBluescript KSII+ at the XhoI and EcoRI sites.
To engineer a His-tagged version of the B subunit of CT (CTB), a fragment encoding a portion of the A polypeptide and the B polypeptide was PCR amplified from pSBR-CTΔA1 (14) using the synthetic oligonucleotides 5′-TAAGAGCTCACTCGAGGCTTGGAGGGAAGAG-3′ (SacI site is underlined) and 5′-TAACTAGTGCTGAGCTTAGTGGTGGTGGTGGTGGTGTATTTGCCATACTAATTGC-3′ (BcuI site is underlined; His codons are double underlined) as primers. PCR conditions were the following: denaturation at 95°C for 45 s, annealing at 44°C for 45 s, and extension at 72°C for 1 min, 30 cycles. After digestion with SacI and BcuI, the PCR fragment (corresponding to the B polypeptide) was inserted into pBluescript KSII+ at the SacI/BcuI sites to produce pHN14. CT was purchased from List Biological Laboratories, Campbell, Calif.
All plasmids were introduced into E. coli DH5αF′Kan (Life Technologies, Inc., Gaithersburg, Md.). Expression of recombinant holotoxin and B pentamers was induced by isopropyl-β-D-thiogalactoside, and the proteins were extracted from the periplasmic space by using polymyxin B treatment as previously described (22). Periplasmic protein extracts were precipitated by addition of ammonium sulfate to 60% saturation (390 g/liter). The precipitate was collected by centrifugation and was dissolved in phosphate-buffered saline (pH 7.4). The dissolved precipitate was dialyzed overnight in phosphate-buffered saline to remove ammonium sulfate, after which the recombinant proteins were purified by means of affinity chromatography using a His · Bind resin column (Novagen, Madison, Wis.) according to a protocol provided by the manufacturer. The eluted fraction was passed through a 0.45-μm-pore-size syringe filter and was further purified by means of gel filtration chromatography (Sephacryl-100; Pharmacia, Piskataway, N.J.) using an ÄKTA-FPLC (Pharmacia). The peak fractions were then concentrated using Vivaspin concentrators (Viva Science, Hanover, Germany). The purity of the recombinant proteins was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. All protein preparations were also analyzed by quantitative Limulus amebocyte lysate (LAL) assays (using kits from BioWhittaker, Walkersville, Md., or from Charles River Endosafe, Charleston, S.C.) to measure incidental endotoxin contamination. All holotoxin and B-pentamer preparations were essentially free of LPS (≤0.0064 ng/μg of protein). This was subsequently verified (see Results) in cytokine induction assays, the results of which were unaffected by the presence of the LPS inhibitor polymyxin B (10 μg/ml). Further evidence against contamination with heat-stable contaminants was obtained upon holotoxin or B-pentamer boiling, which destroyed their biological activity (see Results). The addition of His tag had no effect on the cytokine-inducing ability of the enterotoxins, as shown in preliminary experiments comparing non-His-tagged and His-tagged molecules (data not shown), which were thus subsequently used in the experiments reported in this study.
Other bacterial molecules and antibodies.
The fimbrillin subunit (FimA) of Porphyromonas gingivalis fimbriae was purified by means of size-exclusion and anion-exchange chromatography from E. coli BL21(DE3) transformed with the fimA gene of strain 381 (16). No LPS activity was detected in the FimA preparation by the LAL assay (BioWhittaker) following chromatography through agarose-immobilized polymyxin B (Detoxi-Gel; Pierce, Rockford, Ill.). LPS was purified from P. gingivalis 381 (Pg-LPS) or E. coli K235 (Ec-LPS) as previously described (15), yielding molecules that activate NF-κB exclusively through TLR2 or TLR4, respectively (13). Recombinant human IL-10 and a neutralizing monoclonal antibody (MAb) to IL-10 were obtained from R&D Systems (Minneapolis, Minn.).
THP-1 cell culture and cytokine induction assays.
Human monocytic THP-1 cells (ATCC TIB-202) were differentiated with 10 ng of phorbol myristate acetate/ml for 3 days in 96-well polystyrene culture plates at 37°C in a humidified atmosphere containing 5% CO2. This cell line has been widely used as a model of human monocytes/macrophages (2). The culture medium consisted of RPMI 1640 (Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies), 2 mM l-glutamine, 10 mM HEPES, 100 U of penicillin G/ml, 100 μg of streptomycin/ml, and 0.05 mM 2-mercaptoethanol. Differentiated THP-1 cells (1.5 × 105/well) were washed three times and were used in cytokine induction assays in the absence or presence of bacterial molecules. To determine the effect of toxins on cellular activation by LPS or other stimuli, the cells were pretreated for 1 h with the toxins prior to stimulation. In certain experiments, toxins and LPS were added concomitantly to the cell cultures. Either approach yielded similar data (see Results). All toxins and their B pentamers were predominantly used at 2 μg/ml; preliminary experiments showed that when these molecules were tested at lower concentrations, only LT-IIbB induced substantial levels of cytokine responses. The doses used for Ec-LPS, Pg-LPS, and FimA were chosen on the basis of results from earlier publications (13, 15, 16). None of the bacterial molecules tested was found to affect the viability of the cells in the assays, as determined by trypan blue exclusion. Culture supernatants were collected after overnight incubation (16 h) and were stored at −80°C until assayed. TNF-α, IL-1β, IL-6, IL-8, and IL-10 released into the culture medium were quantitated using enzyme-linked immunosorbent assay (ELISA) kits (purchased from eBioscience, San Diego, Calif., or Cell Sciences, Canton, Mass.) according to protocols recommended by the manufacturers.
NF-κB activation assay.
NF-κB activation in THP-1 cells was determined by means of an NF-κB p65 ELISA-based transcription factor assay kit (Active Motif, Carlsbad, Calif.) (13, 15). The detecting antibody used in this ELISA recognizes an epitope on the p65 subunit of NF-κB that is accessible only when NF-κB is activated and bound to its target DNA (containing the NF-κB consensus binding site 5′-GGGACTTTCC-3′) attached to 96-well plates. The assay was used to determine LT-IIbB-induced NF-κB activation and its regulation by holotoxins. Specifically, differentiated THP-1 cells were preincubated at 37°C for 1 h with culture medium or in the presence of holotoxins as potential downregulators of NF-κB activation. Cells were subsequently stimulated for 90 min with LT-IIbB. IL-10 was used as a positive control for downregulation of NF-κB activation while FimA was utilized as a positive control for NF-κB activation. Extract preparation and ELISA to detect NF-κB p65 were performed according to the manufacturer's protocols. The optimal time of stimulation and amount of total protein (7.5 μg) used in the ELISA were determined empirically in preliminary experiments.
Statistical analysis.
Data were evaluated by analysis of variance and the Dunnett multiple-comparison test using the InStat program (GraphPad Software, San Diego, Calif.). Statistical differences were considered significant at the level of P < 0.05. Where appropriate (Table 1), two-tailed t tests were also performed. Experiments were performed with triplicate samples and were performed twice or more to verify the results.
TABLE 1.
Effect of anti-IL-10 on the ability of holotoxins to inhibit cytokine release in LT-IIbB-activated THP-1 cellsa
| Pretreatment | Amt (pg/ml) of cytokine released (mean ± SD; n = 3)
|
|
|---|---|---|
| TNF-α | IL-8 | |
| None | 908 ± 132 | 12,873 ± 1,347 |
| LT-IIa | 162 ± 47* | 4,912 ± 581* |
| LT-IIa + anti-IL-10 | 286 ± 61** | 6,502 ± 675** |
| LT-IIb | 124 ± 35* | 5,208 ± 740* |
| LT-IIb + anti-IL-10 | 261 ± 67** | 7,009 ± 803** |
| CT | 102 ± 41* | 4,623 ± 419* |
| CT + anti-IL-10 | 232 ± 34** | 6,149 ± 849** |
THP-1 cells were pretreated for 1 h with holotoxins (either LT-IIa, LT-IIb, or CT; all at 2μg/ml) in the absence or presence of anti-IL-10 MAb (10 μg/ml). The cells were then stimulated with LT-IIbB (2 μg/ml). After 16 h, culture supernatants were analyzed by ELISA for TNF-α and IL- 8 release. *, Statistically significant (P < 0.05) inhibition of LT-IIbB-induced cytokine release by holotoxin. **, Statistically significant (P < 0.05) counteraction of the holotoxin inhibitory effect on LT-IIbB-induced cytokine release. Substitution of isotype-matched control for anti-IL- 10 was not statistically different from pretreatment with holotoxin alone (data not shown).
RESULTS
Cytokine induction by the LT-II holotoxins.
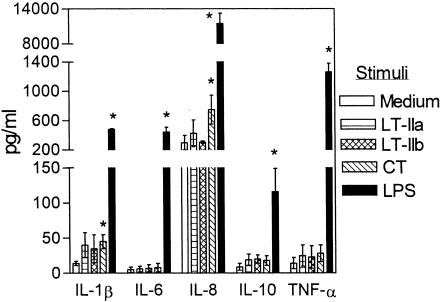
Unlike CT or LT-I, LT-II toxins have not been previously examined for their capacity to induce cytokine release in monocytes/macrophages. This possibility was addressed in experiments using human monocytic THP-1 cells, which display a macrophage-like phenotype upon differentiation with phorbol myristate acetate (2, 16). We examined induction of IL-1β, which possesses mucosal adjuvant properties, as well as cytokines that display proinflammatory (TNF-α), chemotactic (IL-8), immunoenhancing (IL-6), or anti-inflammatory (IL-10) properties. LT-IIa and LT-IIb were tested at 2 μg/ml in comparison with an equal concentration of CT and with 10 ng of Ec-LPS/ml, a potent cytokine-inducing agonist. We found that LT-IIa and LT-IIb did not induce significant release of any of the cytokines tested (Fig. 1). In contrast, CT significantly (P < 0.05) yet modestly elevated IL-1β and IL-8 release, whereas Ec-LPS induced high levels of all five cytokines (Fig. 1). LT-IIa and LT-IIb did not induce significant cytokine release even when the dose was increased to 5 μg/ml (data not shown). It should be noted that the enterotoxin preparations were essentially free of LPS (see Materials and Methods) and the IL-1β- and IL-8-inducing ability of CT was not affected by the presence of polymyxin B (10 μg/ml), a strong inhibitor of LPS (data not shown).
FIG. 1.
Cytokine induction by the LT-II toxins and CT. THP-1 cells were incubated for 16 h in the absence or presence of heat-labile enterotoxins (LT-IIa, LT-IIb, and CT; all at 2 μg/ml) or with E. coli LPS (10 ng/ml; positive control). Culture supernatants were assayed for cytokine content by ELISA. Results are presented as means ± standard deviations of triplicate determinations. Values that are statistically significantly different (P < 0.05) from those of controls treated only with medium are indicated by an asterisk.
Anti-inflammatory activity of the LT-II and CT holotoxins.
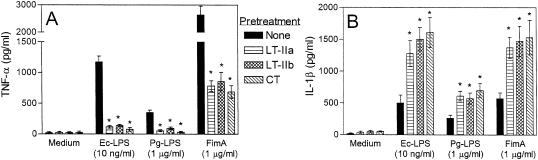
The apparent noninflammatory nature of LT-IIa and LT-IIb prompted us to investigate whether these molecules actively interfere with the proinflammatory activity of Ec-LPS, a strong TLR4 agonist (1). Thus, induction of proinflammatory cytokines by Ec-LPS was examined in THP-1 cells pretreated for 1 h with LT-IIa or LT-IIb enterotoxin or with CT. Other proinflammatory virulence factors that activate additional TLRs were also examined to determine whether inhibitory effects by the holotoxins could be extended to those molecules. Specifically, the effect of LPS from P. gingivalis, which activates TLR2, and of recombinant P. gingivalis FimA, which activates TLR2 and TLR4 (13, 15), were also determined. Strikingly, all three holotoxins significantly (P < 0.05) inhibited TNF-α induction by all three proinflammatory molecules, especially that by Ec-LPS (≥88% inhibition) (Fig. 2A). In stark contrast, the holotoxins significantly upregulated (P < 0.05) IL-1β induction by Ec-LPS, Pg-LPS, or FimA (Fig. 2B). IL-6 induction in activated THP-1 cells was not significantly influenced by any of the holotoxins (data not shown). Cytokine results from this and following experiments were unaffected when the enterotoxins were added to the cells concomitantly with the bacterial stimulants (data not shown) or when the enterotoxins were added to the cells 1 h earlier.
FIG. 2.
LT-II toxins and CT regulate cytokine induction in activated cells. THP-1 cells were pretreated for 1 h with medium only or with 2-μg/ml concentrations of LT-IIa, LT-IIb, or CT. The cells were subsequently incubated for an additional 16 h with medium only, E. coli LPS (Ec-LPS), P. gingivalis LPS (Pg-LPS), or FimA. Culture supernatants were assayed for TNF-α (A) or IL-1β (B) responses by ELISA. Results are shown as means ± standard deviations of triplicate determinations. Asterisks indicate statistically significant (P < 0.05) inhibition of TNF-α (A) or enhancement of IL-1β (B) responses in LPS- or FimA-activated cells by the toxins.
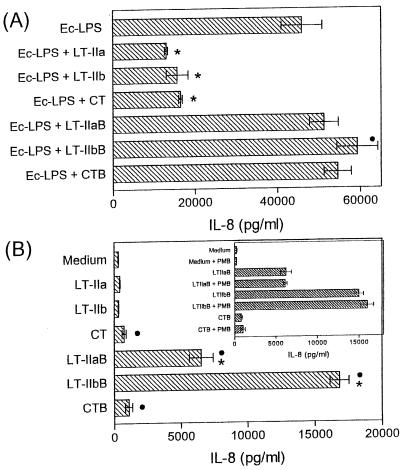
To provide further evidence that the LT-II enterotoxins and CT interfere with inflammatory responses, we examined whether the enterotoxins also inhibit IL-8 induction by Ec-LPS. To determine concomitantly whether inhibition depended upon the catalytically active A subunits of the enterotoxins rather than solely on the ganglioside-binding activities, the B pentamers of each enterotoxin were examined in parallel with their respective holotoxins. The LT-II and CT holotoxins significantly (P < 0.05) and potently inhibited IL-8 induced in response to a high concentration (1 μg/ml) of Ec-LPS (Fig. 3A), thus confirming their anti-inflammatory potential. In contrast, none of the B pentamers inhibited Ec-LPS-induced IL-8 (Fig. 3A). Instead, the B pentamers appeared to additively augment the Ec-LPS-induced IL-8 response (see also Fig. 3B), although this effect reached statistical significance (P < 0.05) for LT-IIbB only (Fig. 3A). The holotoxins, but not the B pentamers, also inhibited IL-8 induced in response to Pg-LPS (10 μg/ml). The IL-8 response induced by Pg-LPS alone (44,385 ± 2,206 pg/ml) was reduced to 17,894 ± 1,638, 18,004 ± 1,106, or 13,758 ± 611 pg/ml in the presence of LT-IIa, LT-IIb, or CT, respectively. None of the B pentamers could inhibit Ec-LPS-induced TNF-α release (data not shown), in contrast to findings from treatment with the holotoxins (Fig. 2A).
FIG. 3.
AB5 toxins inhibit, whereas their B pentamers promote, IL-8 induction. THP-1 cells were pretreated for 1 h with medium only or with 2-μg/ml concentrations of LT-IIa, LT-IIb, CT, or their respective B pentamers. The cells were subsequently incubated for an additional 16 h with 1 μg of Ec-LPS/ml (A) or were left without further treatment (B). The insert summarizes the results of an independent experiment in which THP-1 cells were incubated for 16 h with medium only or with B pentamers in the absence or presence of 10 μg of polymyxin B (PMB)/ml. Induction of IL-8 release in culture supernatants was assayed by ELISA, and data shown are means ± standard deviations of triplicate determinations. (A) Statistically significant (P < 0.05) inhibition or enhancement of LPS-induced IL-8 release is indicated by an asterisk or a black circle, respectively. (B) B-pentamer-induced IL-8 responses that are statistically significantly (P < 0.05) higher than those corresponding to their respective holotoxins are indicated by asterisks, while IL-8 responses that are statistically significantly (P < 0.05) elevated over medium-only-treated controls are indicated by black circles.
In the experiment described above, the holotoxins and their B pentamers were also tested alone for their ability to induce IL-8 (Fig. 3B). The holotoxins exhibited either little (CT) or no (LT-IIa and LT-IIb) IL-8-inducing activity, in accordance with earlier results (Fig. 1). Interestingly, however, LT-IIaB and especially LT-IIbB induced substantial levels of IL-8 release that were significantly higher (P < 0.05) than those induced by their respective holotoxins. Compared to the medium-only control treatment, CTB stimulated a significant (P < 0.05) IL-8 release, but this was not significantly higher than the IL-8 response induced by CT (Fig. 3B). Although the purity of the B pentamers with regard to LPS contamination was verified in the LAL assay, to further rule out any stimulatory effects by incidental LPS contamination we repeated the assay of B-pentamer-induced IL-8 in the presence or absence of 10 μg of polymyxin B/ml. Polymyxin B had no effect on the ability of any of the B pentamers to stimulate IL-8 production (Fig. 3B insert), whereas it almost completely inhibited IL-8 induction by Ec-LPS (data not shown).
Cytokine induction by the B subunits of LT-IIa and LT-IIb.
To determine whether the B pentamers of LT-IIa and LT-IIb induced release of cytokines other than IL-8, THP-1 cells were treated with each B pentamer and the levels of TNF-α, IL-1β, and IL-6 were measured in the culture supernatants. All three cytokines were elicited by treatment with LT-IIbB. In the case of TNF-α and IL-1β the level of induction was nearly comparable to that induced by application of 10 ng of Ec-LPS/ml (Fig. 4). LT-IIaB induced a low but detectable amount of IL-1β which was significantly (P < 0.05) elevated over that of control cells (Fig. 4). Boiling of the B pentamers for 20 min destroyed their ability to induce cytokines above the levels released by cells treated with medium only (data not shown). This further demonstrated that their effects were not mediated by incidental contamination with LPS in the preparations of purified B pentamers. Treatment of THP-1 cells with CTB did not elicit production of TNF-α, IL-1β, and IL-6 at either 2 μg/ml (Fig. 4) or at 5 μg/ml (data not shown). Collectively, the data shown on Fig. 1, 3, and 4 suggest that the absence of the A subunit from the LT-II B pentamers facilitates their capacity for cytokine induction in monocytic THP-1 cells.
FIG. 4.
LT-IIbB is a more potent cytokine inducer than LT-IIaB or CTB. THP-1 cells were incubated for 16 h in the absence or presence of LT-IIaB, LT-IIbB, or CTB (all at 2 μg/ml) or with E. coli LPS (10 ng/ml; positive control). Induction of TNF-α, IL-1β, or IL-6 release in culture supernatants was assayed by ELISA. Results are presented as means ± standard deviations of triplicate determinations. Values that are statistically significantly different (P < 0.05) from those of medium-only-treated controls are indicated by an asterisk.
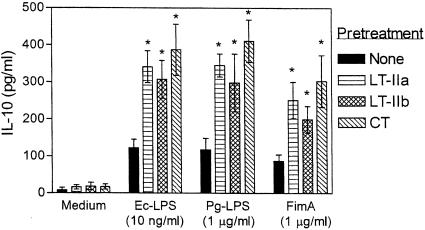
Synergism for IL-10 induction and correlated activities.
A plausible mechanism whereby the LT-II holotoxins and CT may inhibit proinflammatory cytokine induction by Ec-LPS or other bacterial stimuli, such as Pg-LPS and FimA (Fig. 2A and Fig. 3A), may involve IL-10-associated effects. This cytokine is a strong inhibitor of macrophage proinflammatory cytokines (10). Because none of the holotoxins induced significant IL-10 responses in our experimental system (Fig. 1), we determined their ability to augment IL-10 induction by Ec-LPS, Pg-LPS, or FimA. We found that all three toxins significantly (P < 0.05) upregulated IL-10 induction by all three bacterial stimuli (Fig. 5). As the enterotoxins had no detectable capacity to induce IL-10 when used alone (Fig. 1 and 5), it is likely that the observed effects of the enterotoxins in the comixture experiments were synergistic. In contrast, a synergistic effect was not observed when the B pentamers were substituted for the holotoxins in these experiments (data not shown).
FIG. 5.
The LT-II toxins and CT synergize with proinflammatory stimuli in IL-10 induction. THP-1 cells were pretreated for 1 h with medium only or with 2-μg/ml concentrations of LT-IIa, LT-IIb, or CT. The cells were subsequently incubated for an additional 16 h with medium only, Ec-LPS, Pg-LPS, or FimA. Induction of IL-10 release in culture supernatants was assayed by ELISA. Results are presented as means ± standard deviations of triplicate determinations. Asterisks indicate statistically significant (P < 0.05) enhancement of IL-10 induction compared to treatment with proinflammatory stimuli in the absence of LT-II toxins or CT.
Careful study of the data indicated that there was a correlation between the ability of the holotoxins to upregulate IL-10 (Fig. 5) and their ability to downregulate TNF-α (Fig. 2A) or IL-8 (Fig. 3A). To confirm this correlation in a single experiment, the effect of LT-IIb holotoxin on LT-IIb B-pentamer-induced IL-10, TNF-α, and IL-8 production (Fig. 6) was determined. Treatment of THP-1 cells with LT-IIb resulted in significant (P < 0.05) elevation of IL-10 levels in LT-IIbB-activated cells which correlated with a decrease in IL-8 and TNF-α levels (Fig. 6). LT-IIb was also found to enhance LT-IIbB-induced IL-1β release (Fig. 6), which was consistent with observations in cells activated with Ec-LPS, Pg-LPS, or FimA (Fig. 2A).
FIG. 6.
Cytokine induction by the LT-IIb B pentamer is regulated by LT-IIb holotoxin. THP-1 cells were incubated for 16 h with medium only, LT-IIbB alone, LT-IIbB plus LT-IIb, or LT-IIb alone (all at 2 μg/ml). Culture supernatants were assayed for cytokine content by ELISA. Results are presented as means ± standard deviations of triplicate determinations. Cytokine responses in cells treated with both LT-IIbB and LT-IIb holotoxin that are statistically significantly different (P < 0.05) from those for treatment with LT-IIbB alone are indicated by asterisks.
Involvement of IL-10 in holotoxin-mediated TNF-α and IL-8 downregulation in activated cells.
To determine whether the downregulatory effects of the holotoxins on TNF-α and IL-8 induction in activated cells were mediated via induction of IL-10, experiments were conducted using a neutralizing MAb to IL-10 (10 μg/ml). If, indeed, the effects were caused by IL-10, then addition of the anti-IL-10 MAb to the cell cultures would be expected to reverse the inhibitory effects of LT-IIa, LT-IIb, or CT on production of these proinflammatory cytokines by cells activated with LT-IIbB. Although anti-IL-10 significantly (P < 0.05) counteracted holotoxin-mediated inhibition of TNF-α or IL-8 induction by LT-IIbB, the reversal was only partial (Table 1). The use of a higher concentration of anti-IL-10 (20 μg/ml) did not further enhance the reversal effect (data not shown). Similarly, anti-IL-10 only partially reversed holotoxin-mediated inhibition of FimA-induced TNF-α (data not shown). These data suggested that endogenous production of IL-10 cannot adequately account for the ability of the holotoxins to downregulate proinflammatory cytokine induction. The conclusion from these experiments was that an additional molecular mechanism(s) may be involved in the immunomodulatory effects of the holotoxins.
Effect of LT-II and CT holotoxins on NF-κB activation.
Because NF-κB plays a central role in the activation of genes encoding proinflammatory cytokines (1), it was hypothesized that LT-II enterotoxins and CT downregulate cytokine induction in LT-IIbB-stimulated cells by interfering with NF-κB activation. Although both p50 and p65 subunits of NF-κB bind target DNA upon NF-κB activation, the p65 subunit was selected for examination in this study because p65 is the transactivating subunit of heterodimeric (p50/p65) NF-κB. In fact, p50/p50 homodimers may act as transcriptional repressors (31). THP-1 cells were treated with LT-IIbB, and the level of activation of NF-κB was measured. FimA was used in a parallel experiment as a positive control for NF-κB p65 activation (13), and IL-10 (10 ng/ml) was used as a positive control for inhibition of NF-κB activation (30, 32). Results indicated that LT-IIbB did indeed activate NF-κB p65 (Table 2), thus suggesting a plausible mechanism for proinflammatory cytokine induction by LT-IIbB. Boiling of LT-IIbB at a relatively dilute concentration (<10 μg/ml) to facilitate disassembly of the unusually stable pentameric structure was correlated with a loss in the molecule's ability to activate NF-κB (Table 2). This result excludes the possibility that the activation effect was mediated by incidental heat-stable contaminants in the preparation of purified LT-IIbB. IL-10 significantly (P < 0.05) inhibited both LT-IIbB-mediated activation of NF-κB and the release of TNF-α and IL-1β (Table 2). LT-IIa, LT-IIb, and CT also partially inhibited LT-IIbB-mediated activation of NF-κB (P < 0.05), although the effect was lost when the holotoxins were denatured by boiling (Table 2). It is most likely that the inhibitory effect of the holotoxins on NF-κB activation is IL-10-independent; indeed, inhibition of NF-κB p65 activation occurred within 90 min of cellular activation (Table 2), i.e., earlier than release of IL-10 in our experimental system (IL-10 was undetectable after only 2 h of cellular stimulation with LT-IIbB in the presence or absence of the holotoxins; data not shown). As observed with LT-IIbB, we found that the holotoxins and IL-10 also regulated FimA-mediated NF-κB activation and cytokine release (Table 2).
TABLE 2.
Differential effect of IL-10 or holotoxin pretreatment on LT-IIbB-induced cellular activationa
| Stimulus | Pretreatment | Cellular activation assay with:
|
||
|---|---|---|---|---|
| NF-κB p65 (OD450) | TNF-α (pg/ml) | IL-1β (pg/ml) | ||
| Medium | None | 0.059 ± 0.032 | 9 ± 6 | 7 ± 4 |
| LT-IIbB | None | 1.132 ± 0.145 | 779 ± 113 | 312 ± 61 |
| IL-10 | 0.337 ± 0.054* | 103 ± 24* | 71 ± 33* | |
| LT-IIa | 0.848 ± 0.088* | 144 ± 42* | 603 ± 87* | |
| Boiled LT-IIa | 1.278 ± 0.132 | 723 ± 133 | 299 ± 81 | |
| LT-IIb | 0.778 ± 0.074* | 101 ± 65* | 584 ± 103* | |
| Boiled LT-IIb | 1.084 ± 0.077 | 696 ± 157 | 287 ± 88 | |
| CT | 0.812 ± 0.123* | 156 ± 72* | 650 ± 99* | |
| Boiled CT | 1.098 ± 0.101 | 687 ± 183 | 323 ± 45 | |
| Boiled LT-IIbB | None | 0.102 ± 0.047 | 21 ± 10 | 18 ± 9 |
| FimA (positive control) | None | 1.798 ± 0.286 | 2,474 ± 465 | 343 ± 78 |
| IL-10 | 0.457 ± 0.098* | 482 ± 76* | 92 ± 27* | |
| LT-IIa | 1.352 ± 0.167* | 536 ± 97* | 1,352 ± 282* | |
| Boiled LT-IIa | 1.702 ± 0.208 | 2,547 ± 512 | 387 ± 78 | |
| LT-IIb | 1.211 ± 0.102* | 687 ± 128* | 1,408 ± 335* | |
| Boiled LT-IIb | 1.694 ± 0.187 | 2,163 ± 334 | 362 ± 90 | |
| CT | 1.287 ± 0.129* | 612 ± 110* | 1,208 ± 198* | |
| Boiled CT | 1.762 ± 0.225 | 2,348 ± 292 | 404 ± 98 | |
THP-1 cells were preincubated for 1 h with IL-10 (10 ng/ml) or holotoxins (either LT-IIa, LT-IIb, or CT; all at 2 μg/ml) prior to stimulation with LT-IIbB (2 μg/ml) or FimA (1 μg/ml), which was used as a positive control for NF-κB activation. Boiled LT-IIbB served as a negative control for stimulus, whereas boiled LT-IIb served as a negative control for pretreatment. After 90 min of stimulation, cellular extracts were analyzed for NF-κB p65 activation by using an ELISA-based kit (Active Motif). After 16 h, culture supernatants were analyzed by ELISA for TNF-α and IL-1β release. Data shown are means ± standard deviations, n = 3. *, Statistically significant (P < 0.05) differences between non-pretreated controls and groups pretreated with IL-10 or holotoxin. OD450, optical density at 450 mm.
DISCUSSION
The results of this study show that isolated B pentamers from LT-IIa or LT-IIb possess quite distinct immunomodulatory properties from those of their respective holotoxins upon interaction with monocytic THP-1 cells. Whereas the B pentamers of LT-IIa and LT-IIb readily induced cytokine release (Fig. 3 and 4), the respective holotoxins were essentially inactive in this regard (Fig. 1 and 3). Conversely, the LT-II holotoxins, but not their B pentamers, were potent regulators of cytokine release in activated THP-1 cells, as they downregulated proinflammatory cytokines (TNF-α) or chemokines (IL-8) and upregulated cytokines with anti-inflammatory (IL-10) or mucosal adjuvant properties (IL-1β) (Fig. 2, 3, and 5). These findings regarding the interactions of LT-II holotoxins and their B pentamers with THP-1 cells have just been confirmed with primary mouse macrophages (our unpublished observations). Although LT-IIa and LT-IIb do not induce significant IL-1β release on their own, they may upregulate IL-1β induction and associated adjuvant effects when they are coadministered with certain immunogens. This difference in proinflammatory properties was not quite as pronounced between CTB and CT, because CTB was weaker than the LT-II B pentamers while CT was stronger than the LT-II holotoxins in cytokine induction. However, similar to the LT-II molecules, only the holotoxin structure of CT displayed regulatory activity in cytokine release.
The differences in immunomodulatory activities of the holotoxins and their B pentamers have obvious implications with regard to the adjuvant properties of these immunomodulators for monocytic cells. The LT-IIa B pentamers and the LT-IIb B pentamers may promote adaptive immune responses through promotion of inflammatory activity (25) and/or other activities which are associated with ganglioside binding (29). On the other hand, the LT-IIa and LT-IIb holotoxins may exert their complex adjuvant effects (29) in a relatively noninflammatory manner. Indeed, CT is noninflammatory in an animal model (35). This is in stark contrast to the proinflammatory Clostridium difficile toxin A, another toxin which causes intestinal fluid secretion (35). On the basis of the in vitro data (Fig. 1), it can be surmised that LT-IIa and LT-IIb are similarly noninflammatory. Moreover, their ability to downregulate proinflammatory cytokine induction by LPS (Fig. 2 and 3) may possibly serve as a survival strategy of the pathogens aimed at suppressing the innate immune response against them. The development of an in vivo model may be necessary to test this hypothesis.
The capacity of the holotoxins to synergistically enhance IL-10 production in activated monocytic cells suggested the possibility that their anti-inflammatory activities may be induced through IL-10. Endogenously produced IL-10, however, may exert a minor effect in this regard, because a neutralizing antibody to IL-10 only partially reversed (16 to 23%; Table 1) the inhibitory effect of holotoxins on TNF-α or IL-8 release in activated THP-1 cells. This minor effect could be due to insufficient induction levels or delayed kinetics of production of this anti-inflammatory cytokine. In this respect, IL-10 is known to be produced at later time points than TNF-α or other proinflammatory cytokines (9). It is also unlikely that endogenously produced IL-10 contributed to holotoxin-mediated inhibition of the DNA-binding activity of NF-κB p65 (Table 2). Indeed, the activation of this transcription factor in our experimental system was assayed at 90 min following cellular activation, whereas endogenously produced IL-10 was undetectable even at 120 min. A high concentration of exogenous IL-10 (10 ng/ml; i.e., at least 20 times higher than endogenously produced IL-10 in our system) added early in the experiment potently inhibited NF-κB activation and cytokine induction (Table 2). This observation further supports the notion that endogenous IL-10 was apparently too little, too late, to contribute substantially to the immunomodulatory effects of the holotoxins. Moreover, certain holotoxin-mediated effects were diametrically opposite to those of IL-10; specifically, the holotoxins upregulated, whereas IL-10 downregulated, IL-1β release in activated THP-1 cells (Table 2). Thus, the regulatory effects of the holotoxins on cytokine induction seem to be largely independent of IL-10 and may include inhibitory effects on NF-κB activation.
The dramatic differences in proinflammatory activity between the LT-II holotoxins and their B pentamers are likely attributable to what distinguishes them structurally, i.e., the presence or absence of the enzymatically active A subunit. The absence of the A subunit from the LT-II B pentamers was highly correlated with increased proinflammatory properties. It is presently uncertain whether these differences in proinflammatory activities are linked to the property of the A subunit to cause elevation of intracellular cAMP levels (6) or whether some other property of the A subunits is involved. To directly address this question, similar experiments to those performed herein using mutagenically detoxified holotoxins are planned. It is, nonetheless, possible that the cAMP-elevating property of the A subunit is important in inducing the observed anti-inflammatory effects. In this respect, the ability of the holotoxins to inhibit the DNA-binding activity of NF-κB (Table 2) is consistent with a cAMP-dependent mechanism leading to decreased proinflammatory cytokine production. Indeed, elevation of cAMP by prostaglandin E2 (PGE2) has been shown to inhibit the DNA-binding activity of NF-κB (24). Moreover, cAMP downregulates NF-κB activity at a post-DNA-binding step. Specifically, the cAMP-dependent PKA phosphorylates the cAMP response element binding-protein (CREB), which can thereby effectively compete with NF-κB p65 for limiting amounts of a common transcriptional coactivator, the CREB-binding protein (CBP) (27). The property of the LT-II toxins and of CT to elevate intracellular cAMP levels may also be linked to their complex regulatory effects on cytokine induction (i.e., downregulation of TNF-α and IL-8 and upregulation of IL-1β and IL-10) by cells activated with LPS or other proinflammatory factors. Indeed, cAMP not only inhibits TNF-α expression in monocytic cells at the transcriptional level (34) through inhibition of NF-κB activation (24, 27), but it is also involved in positive regulation of IL-1β (5) and IL-10 (3) gene transcription in monocytic cells. Similar to TNF-α, a cAMP-dependent inhibitory mechanism may also apply to IL-8 induction, which is also strongly dependent on NF-κB (26). It is interesting that regulation of cytokine induction by cAMP parallels the immunomodulatory effects observed here in THP-1 cells treated with the holotoxins.
In summary, the B pentamers of the LT-II enterotoxins exhibit proinflammatory properties in human monocytic cells, whereas their respective holotoxins are essentially noninflammatory. However, only the holotoxins are potent regulators of cytokine induction in activated cells. These findings further differentiate the immunomodulatory activities of LT-IIa and LT-IIb holotoxins and their respective B pentamers. Elucidating the mechanisms of immunomodulation by the LT-IIa and LT-IIb holotoxins and their nontoxic derivatives will likely contribute to the rational design of more effective and safer systemic and mucosal adjuvants.
Acknowledgments
This work was supported by U.S. Public Health Service grants DE015254, AI052344, DE06746, and DE13833.
Editor: J. D. Clements
REFERENCES
- 1.Akira, S. 2001. Toll-like receptors and innate immunity. Adv. Immunol. 78:1-56. [DOI] [PubMed] [Google Scholar]
- 2.Auwerx, J. 1991. The human leukemia cell line, THP-1: a multifaceted model for the study of monocyte-macrophage differentiation. Experientia 47:22-31. [DOI] [PubMed] [Google Scholar]
- 3.Brenner, S., S. Prösch, K. Schenke-Layland, U. Riese, U. Gausmann, and C. Platzer. 2003. cAMP-induced interleukin-10 promoter activation depends on CCAAT/enhancer-binding protein expression and monocytic differentiation. J. Biol. Chem. 278:5597-5604. [DOI] [PubMed] [Google Scholar]
- 4.Bromander, A., J. Holmgren, and N. Lycke. 1991. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J. Immunol. 146:2908-2914. [PubMed] [Google Scholar]
- 5.Chandra, G., J. Cogswell, L. Miller, M. Godlevski, S. Stinnett, S. Noel, S. Kadwell, T. Kost, and J. Gray. 1995. Cyclic AMP signaling pathways are important in IL-1 beta transcriptional regulation. J. Immunol. 155:4535-4543. [PubMed] [Google Scholar]
- 6.Chang, P. P., J. Moss, E. M. Twiddy, and R. K. Holmes. 1987. Type II heat-labile enterotoxin of Escherichia coli activates adenylate cyclase in human fibroblasts by ADP ribosylation. Infect. Immun. 55:1854-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell, T., and R. Holmes. 1992. Molecular genetic analysis of ganglioside GD1b-binding activity of Escherichia coli type IIa heat-labile enterotoxin by use of random and site-directed mutagenesis. Infect. Immun. 60:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connell, T. D., and R. K. Holmes. 1995. Mutational analysis of the ganglioside-binding activity of the type II Escherichia coli heat-labile enterotoxin LT-IIb. Mol. Microbiol. 16:21-31. [DOI] [PubMed] [Google Scholar]
- 9.de Waal Malefyt, R., J. Abrams, B. Bennett, C. Figdor, and J. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorentino, D., A. Zlotnik, T. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815-3822. [PubMed] [Google Scholar]
- 11.Foss, D. L., and M. P. Murtaugh. 1999. Role of macrophage cytokines in mucosal adjuvanticity. Adv. Vet. Med. 41:83-104. [DOI] [PubMed] [Google Scholar]
- 12.Fukuta, S., J. L. Magnani, E. M. Twiddy, R. K. Holmes, and V. Ginsburg. 1988. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LTIIa, and LTIIb. Infect. Immun. 56:1748-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajishengallis, G., and R. J. Genco. 2004. Downregulation of the DNA-binding activity of nuclear factor-κB p65 subunit in Porphyromonas gingivalis fimbria-induced tolerance. Infect. Immun. 72:1188-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajishengallis, G., S. K. Hollingshead, T. Koga, and M. W. Russell. 1995. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immunol. 154:4322-4332. [PubMed] [Google Scholar]
- 15.Hajishengallis, G., M. Martin, R. E. Schifferle, and R. J. Genco. 2002. Counteracting interactions between lipopolysaccharide molecules with differential activation of Toll-like receptors. Infect. Immun. 70:6658-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajishengallis, G., M. Martin, H. T. Sojar, A. Sharma, R. E. Schifferle, E. DeNardin, M. W. Russell, and R. J. Genco. 2002. Dependence of bacterial protein adhesins on Toll-like receptors for proinflammatory cytokine induction. Clin. Diagn. Lab. Immunol. 9:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes, R., M. G. Jobling, and T. Connell. 1995. Cholera toxin and related enterotoxins of gram-negative bacteria, p. 225-255. In J. Moss, B. Iglewski, M. Vaughn, and A. T. Tu (ed.), Bacterial toxins and virulence factors in disease. Handbook of natural toxins, vol. 8. Marcel Dekker, Inc., New York, N.Y. [Google Scholar]
- 18.Krueger, J., A. Ray, I. Tamm, and P. B. Sehgal. 1991. Expression and function of interleukin-6 in epithelial cells. J. Cell. Biochem. 45:327-334. [DOI] [PubMed] [Google Scholar]
- 19.Kunzelmann, K., and M. Mall. 2002. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol. Rev. 82:245-289. [DOI] [PubMed] [Google Scholar]
- 20.Lalli, E., and P. Sassone-Corsi. 1994. Signal transduction and gene regulation: the nuclear response to cAMP. J. Biol. Chem. 269:17359-17362. [PubMed] [Google Scholar]
- 21.Martin, M., G. Hajishengallis, D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2001. Recombinant antigen-enterotoxin A2/B chimeric mucosal immunogens differentially enhance antibody responses and B7-dependent costimulation of CD4+ T cells. Infect. Immun. 69:252-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin, M., D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2000. Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins LT-IIa and LT-IIb. Infect. Immun. 68:281-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin, M., D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2001. Distinct cytokine regulation by cholera toxin and type II heat-labile toxins involves differential regulation of CD40 ligand on CD4+ T cells. Infect. Immun. 69:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min, S. Y., W. U. Kim, M. L. Cho, S. Y. Hwang, S. H. Park, C. S. Cho, J. M. Kim, and H. Y. Kim. 2002. Prostaglandin E2 suppresses nuclear factor-κB mediated interleukin 15 production in rheumatoid synoviocytes. J. Rheumatol. 29:1366-1376. [PubMed] [Google Scholar]
- 25.Mondino, A., A. Khoruts, and M. K. Jenkins. 1996. The anatomy of T-cell activation and tolerance. Proc. Natl. Acad. Sci. USA 93:2245-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukaida, N., S. Okamoto, Y. Ishikawa, and K. Matsushima. 1994. Molecular mechanisms of interleukin-8 gene expression. J. Leukoc. Biol. 56:554-558. [PubMed] [Google Scholar]
- 27.Parry, G., and N. Mackman. 1997. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-κB-mediated transcription. J. Immunol. 159:5450-5456. [PubMed] [Google Scholar]
- 28.Platzer, C., E. Fritsch, T. Elsner, M. H. Lehmann, H.-D. Volk, and S. Prösch. 1999. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur. J. Immunol. 29:3098-3104. [DOI] [PubMed] [Google Scholar]
- 29.Rappuoli, R., M. Pizza, G. Douce, and G. Dougan. 1999. Structural and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol. Today 20:493-500. [DOI] [PubMed] [Google Scholar]
- 30.Raychaudhuri, B., C. J. Fisher, C. F. Farver, A. Malur, J. Drazba, M. S. Kavuru, and M. J. Thomassen. 2000. Interleukin 10 (IL-10)-mediated inhibition of inflammatory cytokine production by human alveolar macrophages. Cytokine 12:1348-1355. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz, M. L., and P. A. Baeuerle. 1991. The p65 subunit is responsible for the strong transcription activating potential of NF-κB. EMBO J. 10:3805-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schottelius, A. J. G., M. W. Mayo, R. B. Sartor, and A. S. Baldwin, Jr. 1999. Interleukin-10 signaling blocks inhibitor of κB kinase activity and nuclear factor κB DNA Binding. J. Biol. Chem. 274:31868-31874. [DOI] [PubMed] [Google Scholar]
- 33.Staats, H. F., and F. A. Ennis, Jr. 1999. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J. Immunol. 162:6141-6147. [PubMed] [Google Scholar]
- 34.Taffet, S. M., K. J. Singhel, J. F. Overholtzer, and S. A. Shurtleff. 1989. Regulation of tumor necrosis factor expression in a macrophage-like cell line by lipopolysaccharide and cyclic AMP. Cell. Immunol. 120:291-300. [DOI] [PubMed] [Google Scholar]
- 35.Triadafilopoulos, G., C. Pothoulakis, R. Weiss, C. Giampaolo, and J. T. Lamont. 1989. Comparative study of Clostridium difficile toxin A and cholera toxin in rabbit ileum. Gastroenterology 97:1186-1192. [DOI] [PubMed] [Google Scholar]
- 36.Williams, N. A., T. R. Hirst, and T. O. Nashar. 1999. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol. Today 20:95-101. [DOI] [PubMed] [Google Scholar]