Abstract
Helicobacter pylori exists in two distinct forms, rod shaped or coccoid, in stomachs of infected patients. Based on in vitro proteome comparisons, there are no detectable coccoid-specific proteins, which argues against the specific adaptation of coccoid Helicobacter to distinct biological functions, such as enhanced persistence or transmission to other hosts.
The gram-negative bacterium Helicobacter pylori is an important human pathogen that infects half of the world's population and can cause gastritis, gastric and duodenal ulcers, and gastric cancer (26). Electron micrographs of gastric biopsy samples reveal Helicobacter cells with elongated rod-shaped or coccoid morphologies in variable proportions (4, 17). The functional relevance of this dimorphism is unclear, but the predominance of rods in exponentially growing in vitro cultures suggest that this form represents proliferating Helicobacter cells.
Coccoid forms dominate aging poststationary cultures and are difficult to recultivate, which could suggest that this morphology merely represents degrading dead Helicobacter cells. Indeed, various physiological parameters and lack of infectivity support the passive decay of coccoid cells (6, 11, 23). Moreover, similar changes in morphology also occur in Helicobacter cells after killing by bacteriophage φX174 protein E-mediated lysis (18). On the other hand, coccoid Helicobacter cells could also represent a viable-but-not-culturable state that is more resistant to environmental stresses than actively proliferating cells, and could thereby facilitate transmission to new hosts, or might mediate relapsing infection after incomplete eradication (21, 27). Data on various cellular activities seem to support this view (5, 13-15). Interestingly, mutations in cdrA, a cell division-related gene, prevent the transition of rods to coccoid cells, potentially suggesting an active process (24). In addition, proteome studies previously demonstrated the appearance of several distinct protein species in coccoid Helicobacter total lysates (7) as well as in extracts of surface-associated proteins (16), but these coccoid-specific protein species have not yet been identified. Recent reports about the heterogeneity of coccoid Helicobacter with a viable A form and a nonviable B form might partially resolve this long controversy (19, 20). However, even if some coccoid Helicobacter cells remain viable and potentially infectious, it is unclear if such cells have distinct properties that could be of relevance for specific aspects of infection or transmission. In the well-characterized dimorphic bacterium Bacillus anthracis (12), transition between forms with distinct functions (i.e., proliferation versus long-term survival) is accompanied by extensive changes in protein composition with de novo synthesis of many stage-specific proteins. Whether such dramatic protein expression changes also occur during the transition between rod-shaped and coccoid forms of Helicobacter cells remained largely unclear.
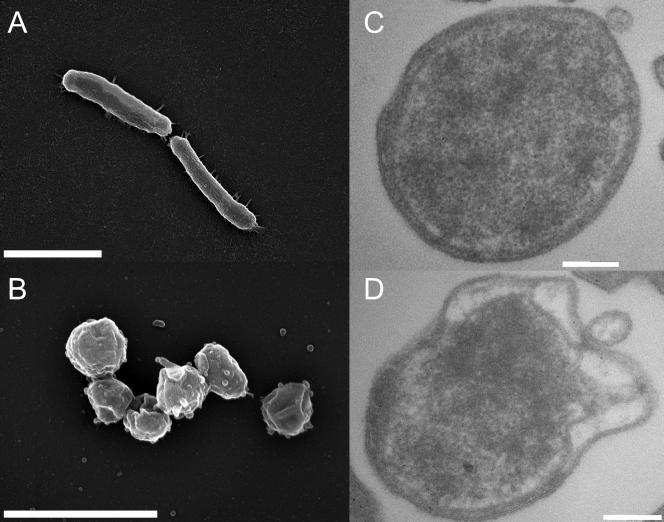
To address this issue on a global scale, we compared the protein compositions of rod-shaped Helicobacter pylori strain 26695 cells (25) from exponentially growing liquid cultures (Fig. 1A) and coccoid cells arising after 7 days of continuous liquid culture (Fig. 1B). Based on scanning electron microscopy data and the assumption of simple spherical or cylindrical shapes, coccoid Helicobacter had, on average, a 40% lower cell volume than rod-shaped Helicobacter in fresh cultures (0.23 versus 0.39 μm3). Transmission electron microscopy revealed that both A (Fig. 1C) and B forms (Fig. 1D) of coccoid Helicobacter (19, 20) were present in our long-term cultures. Two-dimensional gel electrophoresis (9, 10) of rod-shaped and coccoid cells revealed some 1,500 protein species that are reproducibly present under both culture conditions but also a few reproducibly stage-specific protein species (Fig. 2) (the pattern for fresh cultures is freely available at http://www.mpiib-berlin.mpg.de/2D-PAGE/) in agreement with previous low-resolution data (7). Peptide mass fingerprinting (9, 10) allowed the identification of all 16 detectable rod-shape-specific proteins and 11 of the 14 coccoid-specific proteins (Table 1).
FIG. 1.
Scanning (A, B) and transmission (C, D) electron microscopy of rod-shaped (A) and coccoid (B, C, D) Helicobacter cells. Aging cultures contained both apparently intact form A (C) and substantially damaged form B (D) coccoid cells. The scale bars represent 2 (A, B) or 200 nm (C, D).
FIG. 2.
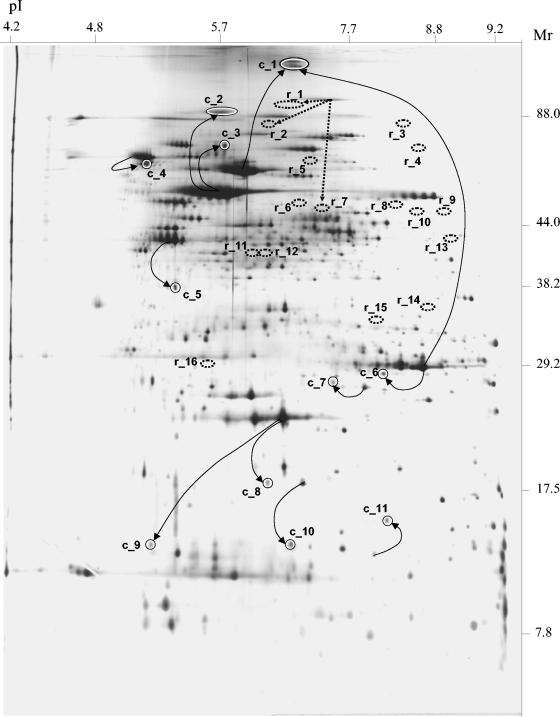
Protein composition of coccoid Helicobacter cells as determined by two-dimensional gel electrophoresis of bacteria harvested after 7 days of liquid culture. Proteins were detected by silver staining. The spot numbers correspond to those shown in Table 1. Spots r_1 to r_16 (rod shape specific) are absent in coccoid Helicobacter, while spots c_1 to c_11 are coccoid specific. The straight arrows indicate relationships between major and minor variants of individual proteins. The dotted arrows connect the major CagA variant with three minor variants that are only detected in rod-shaped Helicobacter.
TABLE 1.
Identified H. pylori proteins with differential presence in rod-shaped and coccoid cells
| Spot no.a | Locus | Gene | Protein | Sequence coverage (%) | Theoretical mol wt | Theoretical pI |
|---|---|---|---|---|---|---|
| r_1 | HP0547 | cagA | CagA | 30 | 132,306 | 8.8 |
| r_2 | HP0547 | cagA | CagA | 29 | 132,306 | 8.8 |
| r_3 | HP0887 | vacA | Vacuolating cytotoxin | 18 | 139,227 | 9.0 |
| r_4 | HP0407 | bisC | Biotin sulfoxide reductase | 42 | 90,014 | 9.1 |
| r_5 | HP1527 | Hypothetical protein | 25 | 54,750 | 6.6 | |
| r_6 | HP0075 | ureC | Urease protein C | 39 | 49,055 | 6.4 |
| r_7 | HP0547 | cagA | CagA | 26 | 132,306 | 8.8 |
| r_8 | HP1019 | htrA | Serine protease | 47 | 47,954 | 9.0 |
| r_9 | HP0269 | Conserved hypothetical ATP-binding protein | 30 | 49,392 | 8.8 | |
| r_10 | HP0605 | Hypothetical protein | 33 | 54,553 | 9.0 | |
| r_11 | HP1179 | deoB | Phosphopentomutase | 38 | 46,151 | 5.9 |
| r_12 | HP0774 | tyrS | Tyrosyl-tRNA synthetase | 32 | 45,710 | 5.9 |
| r_13 | HP1126 | tolB | Colicin tolerance-like protein | 79 | 47,768 | 9.2 |
| r_14 | HP0854 | guaC | GMP reductase | 39 | 36,016 | 8.6 |
| r_15 | HP0353 | ansB | l-Asparaginase II | 46 | 35,516 | 8.6 |
| r_16 | HP0724 | fliH | Flagellar export protein | 15 | 29,303 | 5.4 |
| c_1 | HP0072/HP0073 | ureA/ureB | Urease alpha subunit/urease beta subunit | 52/37 | 26,523/61,645 | 8.5/5.6 |
| c_2 | HP0010 | groEL | Chaperone and heat shock protein 60 | 29 | 58,228 | 5.6 |
| c_3 | HP0010 | groEL | Chaperone and heat shock protein 60 | 28 | 58,228 | 5.6 |
| c_4 | HP0109 | dnaK | Chaperone and heat shock protein 70 | 36 | 67,011 | 5.0 |
| c_5 | HP1205 | tufB | Translation elongation factor EF-Tu | 43 | 43,620 | 5.2 |
| c_6 | HP0072 | ureA | Urease alpha subunit | 53 | 26,523 | 8.5 |
| c_7 | HP0561 | fabG | 3-Ketoacyl-acyl carrier protein reductase | 43 | 26,652 | 7.8 |
| c_8 | HP1563 | tsaA | Alkyl hydroperoxide reductase | 46 | 22,221 | 5.9 |
| c_9 | HP1563 | tsaA | Alkyl hydroperoxide reductase | 43 | 22,221 | 5.9 |
| c_10 | HP0390 | tagD | Adhesin-thiol peroxidase | 56 | 18,281 | 7.7 |
| c_11 | HP1246 | rps6 | Ribosomal protein S6 | 65 | 16,961 | 6.9 |
r_1 to r_16 designate rod-shape-specific spots; c_1 to c_11 designate coccoid-shape-specific spots.
Two rod-specific protein species, vacuolating toxin VacA (spot r_4) and the serine protease HtrA (spot r_8), had high staining intensities in fresh cultures. The important virulence factor VacA has previously been shown to be absent in coccoid Helicobacter (16) despite the presence of vacA mRNA (25). The outer membrane protein VacA is known to be prone to autoproteolysis resulting in at least two released fragments (2), which could explain its disappearance in coccoid cells. HtrA is secreted by Helicobacter (2), and this might contribute to its disappearance. In addition, destruction of this protease by autoproteolysis might also occur. All other protein species that were exclusively present in rod-shaped Helicobacter had a low abundance even at this stage. Among these weak spots there were three minor degradation products of the important virulence factor CagA (spots r_1, r_2, and r_7). However, the major full-length CagA species was preserved in coccoid Helicobacter in agreement with previous studies (3, 22). Other minor rod-shape-specific protein species included various enzymes and proteins of unknown function (Table 1).
Coccoid-specific protein species were all moderately expressed and belong to diverse functional classes, including chaperones, enzymes, a ribosomal protein, and the important virulence factor urease. However, although the identified protein species were coccoid stage specific, they all were merely minor variants of previously identified proteins (9, 10) that are highly abundant in both rod-shaped and coccoid Helicobacter cells (Fig. 2), indicating that posttranslational modification, but not de novo synthesis during the transition to coccoid cells, resulted in coccoid-specific protein species. The majority of coccoid-specific protein species seem to have resulted from partial proteolysis to smaller fragments as indicated in Fig. 2. On the other hand, both ureases A and B (spot c_1) and Hsp60 (spots c_2 and c_3) formed high-molecular-weight complexes that were stable under the denaturing electrophoresis conditions, suggesting the formation of covalent bonds between different monomers in initially reversibly assembled tetradecamers of Hsp60 (1) or heterododecamers of ureases A and B (8), respectively. Finally, some protein species with shifted pI values indicate removal or introduction of charges, and the lower electrophoretic mobility of Rsp6 might suggest an altered three-dimensional conformation.
The apparent absence of stage-specific protein expression argues against major adaptive changes in coccoid Helicobacter, but the detected posttranslational modifications still might result in altered properties and functions that could have some relevance for Helicobacter infection biology. On the other hand, all detected modifications accounted for only tiny fractions of the respective proteins, whereas the overwhelming majority of the corresponding gene products was not differentially modified. In conclusion, our data argue against a specific adaptation of coccoid Helicobacter for particular tasks.
Acknowledgments
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (Bu 971/4-2 and SFB621-A9) to D.B. and T.F.M. and from the Bundesministerium für Bildung und Forschung (031U107C-031U207) to P.R.J. and T.F.M.
Editor: J. T. Barbieri
REFERENCES
- 1.Braig, K., Z. Otwinowski, R. Hegde, D. C. Boisvert, A. Joachimiak, A. L. Horwich, and P. B. Sigler. 1994. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature 371:578-586. [DOI] [PubMed] [Google Scholar]
- 2.Bumann, D., S. Aksu, M. Wendland, K. Janek, U. Zimny-Arndt, N. Sabarth, T. F. Meyer, and P. R. Jungblut. 2002. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun. 70:3396-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cellini, L., I. Robuffo, G. Spoto, E. Di Campli, M. Di Candia, and G. Donelli. 2004. Population dynamics in ageing Helicobacter pylori. New Microbiol. 27:29-35. [PubMed] [Google Scholar]
- 4.Chan, W. Y., P. K. Hui, K. M. Leung, J. Chow, F. Kwok, and C. S. Ng. 1994. Coccoid forms of Helicobacter pylori in the human stomach. Am. J. Clin. Pathol. 102:503-507. [DOI] [PubMed] [Google Scholar]
- 5.Costa, K., G. Bacher, G. Allmaier, M. G. Dominguez-Bello, L. Engstrand, P. Falk, M. A. de Pedro, and F. Garcia-del Portillo. 1999. The morphological transition of Helicobacter pylori cells from spiral to coccoid is preceded by a substantial modification of the cell wall. J. Bacteriol. 181:3710-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton, K. A., C. E. Catrenich, K. M. Makin, and S. Krakowka. 1995. Virulence of coccoid and bacillary forms of Helicobacter pylori in gnotobiotic piglets. J. Infect. Dis. 171:459-462. [DOI] [PubMed] [Google Scholar]
- 7.Figueroa, G., G. Faundez, M. Troncoso, P. Navarrete, and M. S. Toledo. 2002. Immunoglobulin G antibody response to infection with coccoid forms of Helicobacter pylori. Clin. Diagn. Lab. Immunol. 9:1067-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha, N. C., S. T. Oh, J. Y. Sung, K. A. Cha, M. H. Lee, and B. H. Oh. 2001. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat. Struct. Biol. 8:505-509. [DOI] [PubMed] [Google Scholar]
- 9.Jungblut, P. R., D. Bumann, G. Haas, U. Zimny-Arndt, P. Holland, S. Lamer, F. Siejak, A. Aebischer, and T. F. Meyer. 2002. Comparative proteome analysis of Helicobacter pylori. Mol. Microbiol. 36:710-725. [DOI] [PubMed] [Google Scholar]
- 10.Krah, A., F. Schmidt, D. Becher, M. Schmid, D. Albrecht, A. Rack, K. Buttner, and P. R. Jungblut. 2003. Analysis of automatically generated peptide mass fingerprints of cellular proteins and antigens from Helicobacter pylori 26695 separated by two-dimensional electrophoresis. Mol. Cell. Proteomics 2:1271-1283. [DOI] [PubMed] [Google Scholar]
- 11.Kusters, J. G., M. M. Gerrits, J. A. Van Strijp, and C. M. Vandenbroucke-Grauls. 1997. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect. Immun. 65:3672-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, H., N. H. Bergman, B. Thomason, S. Shallom, A. Hazen, J. Crossno, D. A. Rasko, J. Ravel, T. D. Read, S. N. Peterson, J. Yates III, and P. C. Hanna. 2004. Formation and composition of the Bacillus anthracis endospore. J. Bacteriol. 186:164-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizoguchi, H., T. Fujioka, and M. Nasu. 1999. Evidence for viability of coccoid forms of Helicobacter pylori. J. Gastroenterol. 34(Suppl. 11):32-36. [PubMed] [Google Scholar]
- 14.Monstein, H. J., and J. Jonasson. 2001. Differential virulence-gene mRNA expression in coccoid forms of Helicobacter pylori. Biochem. Biophys. Res. Commun. 285:530-536. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson, H. O., J. Blom, W. Abu-Al-Soud, A. A. Ljungh, L. P. Andersen, and T. Wadstrom. 2002. Effect of cold starvation, acid stress, and nutrients on metabolic activity of Helicobacter pylori. Appl. Environ. Microbiol. 68:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson, I., M. Utt, H. O. Nilsson, A. Ljungh, and T. Wadstrom. 2000. Two-dimensional electrophoretic and immunoblot analysis of cell surface proteins of spiral-shaped and coccoid forms of Helicobacter pylori. Electrophoresis 21:2670-2677. [DOI] [PubMed] [Google Scholar]
- 17.Noach, L. A., T. M. Rolf, and G. N. Tytgat. 1994. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J. Clin. Pathol. 47:699-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panthel, K., W. Jechlinger, A. Matis, M. Rohde, M. Szostak, W. Lubitz, and R. Haas. 2003. Generation of Helicobacter pylori ghosts by PhiX protein E-mediated inactivation and their evaluation as vaccine candidates. Infect. Immun. 71:109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito, N., K. Konishi, F. Sato, M. Kato, H. Takeda, T. Sugiyama, and M. Asaka. 2003. Plural transformation-processes from spiral to coccoid Helicobacter pylori and its viability. J. Infect. 46:49-55. [DOI] [PubMed] [Google Scholar]
- 20.Sato, F., N. Saito, K. Konishi, E. Shoji, M. Kato, H. Takeda, T. Sugiyama, and M. Asaka. 2003. Ultrastructural observation of Helicobacter pylori in glucose-supplemented culture media. J. Med. Microbiol. 52:675-679. [DOI] [PubMed] [Google Scholar]
- 21.She, F. F., J. Y. Lin, J. Y. Liu, C. Huang, and D. H. Su. 2003. Virulence of water-induced coccoid Helicobacter pylori and its experimental infection in mice. World J. Gastroenterol. 9:516-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sisto, F., M. I. Brenciaglia, M. M. Scaltrito, and F. Dubini. 2000. Helicobacter pylori: ureA, cagA and vacA expression during conversion to the coccoid form. Int. J. Antimicrob. Agents 15:277-282. [DOI] [PubMed] [Google Scholar]
- 23.Sorberg, M., M. Nilsson, H. Hanberger, and L. E. Nilsson. 1996. Morphologic conversion of Helicobacter pylori from bacillary to coccoid form. Eur. J. Clin. Microbiol. Infect. Dis. 15:216-219. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi, H., M. Shirai, J. K. Akada, M. Tsuda, and T. Nakazawa. 1998. Nucleotide sequence and characterization of cdrA, a cell division-related gene of Helicobacter pylori. J. Bacteriol. 180:5263-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 26.Walker, M. M., and J. E. Crabtree. 1998. Helicobacter pylori infection and the pathogenesis of duodenal ulceration. Ann. N. Y. Acad. Sci. 859:96-111. [DOI] [PubMed] [Google Scholar]
- 27.Wang, X., E. Sturegard, R. Rupar, H. O. Nilsson, P. A. Aleljung, B. Carlen, R. Willen, and T. Wadstrom. 1997. Infection of BALB/c A mice by spiral and coccoid forms of Helicobacter pylori. J. Med. Microbiol. 46:657-663. [DOI] [PubMed] [Google Scholar]