Abstract
Nonsurgical intratracheal instillation of 1 μg of purified, recombinant flagellin in several strains of mice stimulated a transient innate immune response in the lung characterized by the infiltration of neutrophils and the rapid production of tumor necrosis factor alpha, interleukin 6, granulocyte colony-stimulating factor, and the chemokines keratinocyte-derived chemokine, MIP1α, and MIP-2.
Flagellin, the major structural protein of bacterial flagella, signals via Toll-like receptor 5 (TLR5) (13, 26). Work from our laboratory (3, 4, 5, 19, 24) and those of others (6, 7, 10, 11, 13, 17, 18) has demonstrated that flagellin treatment stimulates the release of proinflammatory mediators such as tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), IL-6, IL-8, and nitric oxide (NO) in vitro and in vivo. Although previous studies have established that flagellin induces systemic inflammatory responses when administered intraperitoneally or intravenously, the effects of flagellin on mucosal immunity in the lung have not been explored. The impact of flagellin on innate and adaptive immunity in the lung is clearly important, given the role of flagellin as a virulence factor (2, 8, 27, 28) and as a potential adjuvant for vaccine therapy (1, 15, 16, 20, 21).
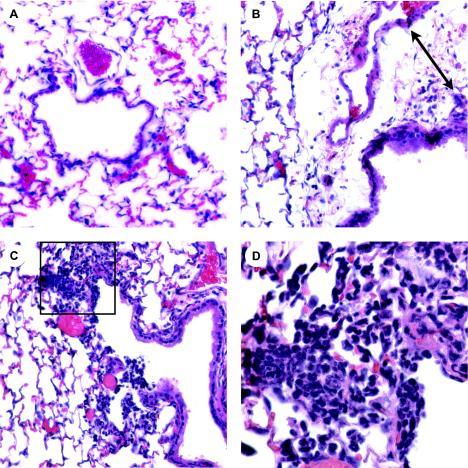
To determine the effects of flagellin on innate immunity in the lung, mice were anesthetized with Avertin (2,2,2-tribromoethanol; Sigma) and tert-amyl alcohol (Fisher) and their lungs were intratracheally (i.t.) instilled (9, 14) with 1 μg of soluble recombinant flagellin from Salmonella enterica serovar Enteritidis in a total volume of 50 μl of pyrogen-free phosphate-buffered saline (PBS) (19). Detoxi-Gel (Pierce) polymixin B columns were used to deplete endotoxin; residual levels in flagellin preparations were <1 pg/μg, as detected by the quantitative chromogenic Limulus amebocyte lysate assay (BioWhittaker). Mice were maintained in a specific-pathogen-free facility, and all research complied with federal and institutional guidelines set forth by the Wake Forest University Animal Care and Use Committee. Formalin-fixed, paraffin-embedded lung sections from groups of two mice were prepared at 1.5 h, 4 h, 12 h, 24 h, and 5 days and stained with hematoxylin and eosin for light microscopic examination (22). Figure 1A shows a representative control section with open alveolar spaces and few inflammatory cells. In contrast, sections from flagellin-treated mice at 12 h, the peak of the inflammatory response, revealed the presence of perivascular edema (Fig. 1B) and interstitial foci of leukocytes in the peribronchial space (Fig. 1C and D). Within 5 days postinstillation, these indicators of inflammation were not evident (data not shown).
FIG. 1.
Inflammatory effects of flagellin in the BALB/c lung. Female mice were anesthetized, and their lungs were i.t. instilled with 1 μg of soluble recombinant Salmonella flagellin in a total volume of 50 μl of pyrogen-free PBS. Preliminary studies using a colored dye established competency to instill at least 95% of the reagents into the lung. Lung sections were prepared and stained for histological analysis. Panels are representative sections from two mice per time point. (A) Control section from a mouse receiving only PBS. (B and C) Sections taken at 12 h after flagellin instillation (magnification, ×20). The arrow in panel B indicates perivascular edema. (D) ×60 magnification of the box in panel C, showing leukocyte infiltration of the peribronchial space.
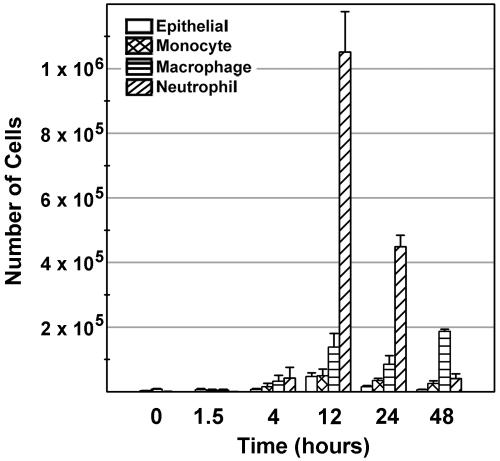
To determine the types of infiltrating cells and the kinetics of their recruitment, the lungs of female BALB/c mice in groups of four to six were i.t. instilled with 1 μg of flagellin and bronchoalveolar lavage fluid (BALF) was collected as previously described (23). Cell pellets from BALF of individual mice were resuspended in PBS containing 1 mg of bovine serum albumin per ml and dispersed onto slides using a Cytospin centrifuge. Slides were differentially stained, and cells were counted based on cell morphology. Figure 2 shows the numbers and types of cells in the BALF. Neutrophil accumulation in the lungs of flagellin-treated BALB/c mice peaked at 12 h postinstilllation, remained elevated for 24 h, and then decreased to relatively low levels. Forty-eight hours after flagellin treatment, macrophages were the predominant cell type recovered in the BALF. These results were consistent with our histological data as well as those of previously published studies demonstrating neutrophil infiltration of the lungs following intravenous flagellin administration (17, 18).
FIG. 2.
Flagellin-induced cellular infiltration in the lung. The lungs of female BALB/c mice in groups of four to six mice were i.t. instilled with 1 μg of flagellin, and BALF was collected at the indicated times postinstillation. Cells were dispersed onto slides and differentially stained for analysis by cell type. Cell numbers are shown as means with standard errors.
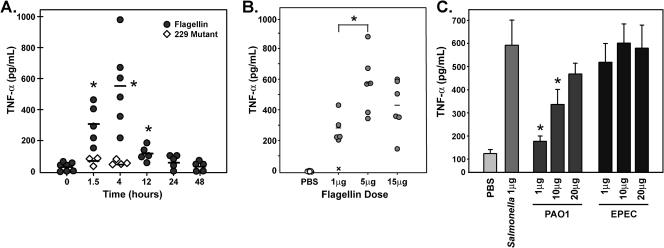
TNF-α is a pivotal mediator in the early response to pathogens and is important for neutrophil recruitment to the lung. In view of the stimulatory effect of flagellin on TNF-α production in vitro (3, 4), we investigated the effect of flagellin on TNF-α production in the lung. To examine the kinetics of the response, we determined the amount of TNF-α in the BALF by an enzyme-linked immunosorbent assay (ELISA) (OptEIA; BD Pharmingen) at various times postinstillation of 1 μg of flagellin in groups of four to seven BALB/c mice. As shown in Fig. 3A, flagellin induced TNF-α production in the lung as early as 1.5 h postinstillation, with peak levels occurring around 4 h. By 24 h, TNF-α levels were minimal. To confirm the specificity of the flagellin effect, an inactive mutant flagellin (19, 25) was also tested and was found to be negative. This truncated form of flagellin (designated 229) expresses amino acids 297 to 471 of the hypervariable region and thus is unable to signal through TLR5 (19, 25). Since 229 was prepared in the same manner as bioactive flagellin, it contained the same level of potentially stimulatory contaminants as the wild-type protein. Using different doses of flagellin (Fig. 3B), we found that the maximal TNF-α response was obtained with 5 μg. For comparative purposes, we assessed the TNF-α response with 1 μg of flagellin to those of two other TLR agonists, Salmonella lipopolysaccharide (10 μg; Sigma) and CpG oligodeoxynucleotide (10 μg of oligodeoxynucleotide 1826 with a phosphorothioate backbone; Integrated DNA Technologies). The CpG preparation contained <1 pg of endotoxin per μg. Instillation of 10 μg of lipopolysaccharide produced approximately 3.5 times the level of TNF-α as with 1 μg of flagellin, whereas the response to 10 μg of CpG was approximately 1.5-fold less than with flagellin.
FIG. 3.
TNF-α is induced by flagellin in the BALB/c lung. (A) Groups of four to seven BALB/c mice were i.t. instilled with 1 μg of purified flagellin or the inactive mutant flagellin 229 in a total volume of 50 μl of pyrogen-free PBS. At the indicated times postinstillation, BALF was collected for analysis of TNF-α production by ELISA. Bars represent means. An asterisk indicates statistical significance from time zero (P < 0.01) or 229 treatment (P < 0.05). (B) The lungs of BALB/c mice in groups of six were i.t. instilled with PBS alone or 1, 5, or 15 μg of flagellin. At 4 h, BALF was collected and analyzed for TNF-α production. Bars indicate means, and an asterisk indicates significance over the value obtained with 1 μg of flagellin. All doses were statistically greater than with PBS. (C) The lungs of BALB/c mice in groups of five were instilled with PBS alone, 1 μg of Salmonella flagellin, or 1, 10, or 20 μg of recombinant Pseudomonas strain PAO1 or EPEC flagellin. TNF-α in the BALF was determined at 4 h postinstillation by ELISA. Bars indicate standard errors, and an asterisk indicates significant difference from the value obtained with 1 μg of Salmonella flagellin. The F test for equality of variance and Student's one-sided t test were used to assign statistical significance at P values of <0.05 or <0.01.
In previous studies, we reported that flagellins from Salmonella strains exhibit comparable potencies in vitro. However, flagellins from Pseudomonas aeruginosa PAO1 and enteropathogenic Escherichia coli (EPEC) were approximately 1/10 as active (4, 5). To determine if these relationships held in vivo, we compared levels of TNF-α production after instillation of 1, 10, or 20 μg of purified, recombinant PAO1 or EPEC flagellin to 1 μg of Salmonella flagellin at 4 h. As shown in Fig. 3C, stimulation of TNF-α production by EPEC flagellin was comparable to that of Salmonella flagellin, whereas flagellin from PAO1 was approximately 1/10 to 1/20 as active as Salmonella flagellin. Although the basis for the reduced potency of Pseudomonas flagellin remains to be determined, we favor the hypothesis that this protein has a reduced affinity for TLR5.
The neutrophilic infiltration seen after flagellin instillation was consistent with the involvement of one or more chemoattractant factors. To determine the range of inflammatory cytokines induced by flagellin in the lungs of mice, we used a mouse cytokine array (RayBiotech, Inc.). Chemiluminescence was detected by using a Kodak Image Station 2000RT, and quantitation of spots was performed by using Kodak 1D software. The BALF from five BALB/c mice was pooled for analysis, and results were compared to those with the mutant flagellin (229) to determine an induction ratio. Increases of greater than fourfold were considered significant. The induced cytokines are shown in Table 1 with their respective induction ratios. As expected, there was a marked induction of TNF-α. In addition, there were increased levels of IL-6, granulocyte colony-stimulating factor (G-CSF), and the chemokines keratinocyte-derived chemokine (KC), MIP-1α, and MIP-2. IL-12p40 and IL-12p70 were also strongly induced in BALB/c mice.
TABLE 1.
Flagellin stimulates the production of a spectrum of cytokines in the mouse lunga
| Mouse strain | Fold induction of indicated cytokine (ratio of flagelline to 229)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| TNF-α | IL-6 | G-CSF | KC | MIP-2 | MIP-1α | IL-12p40 | IL-12p70 | Eotaxin | |
| BALB/c | 9 | 52 | 7 | 5 | 6 | 4 | 6 | 5 | 4 |
| C3H/HeN | 14 | 8 | 6 | 11 | 10 | 18 | ND | ND | ND |
| C3H/HeJ | 56 | 8 | 6 | 15 | 9 | 4 | ND | ND | ND |
| B6;129 | 1,428 | 33 | 32 | 493 | 12 | ND | ND | ND | |
| TNFR−/− | 35 | 40 | 37 | 54 | 14 | ND | ND | ND | ND |
BALF from groups of three to five mice were pooled for analysis by using Mouse Cytokine Array II from RayBiotech, Inc. Cytokines with a >4-fold increase in the ratio of flagellin induction to 229 induction are shown. ND, no difference.
In contrast to the requirement of TLR5 homomeric complexes in the induction of TNF-α production in response to flagellin, TLR5/TLR4 heteromeric complexes are required for the production of nitric oxide via an IFN-β- and STAT-1-dependent mechanism (24). To determine whether TLR5/TLR4 complexes are required for flagellin-induced cytokine production in the lung, we compared the response in C3H/HeJ mice, which have a nonfunctional mutant TLR4, to that of C3H/HeN mice (their wild-type counterpart). Both of these strains produced high levels of TNF-α in response to flagellin, a finding that supports the notion that only TLR5/TLR5 complexes are required for the induction of TNF-α. In addition, there were no significant differences in other cytokines induced by flagellin in C3H/HeJ mice versus those induced in C3H/HeN mice (Table 1), indicating that functional TLR4 is not required for the induction of IL-6, G-CSF, KC, MIP-2, or MIP-1α.
Induction of cytokine production by flagellin may be the result of direct stimulation of the cytokine-producing cells themselves or due to indirect stimulation by another cytokine, such as TNF-α. To examine the role of TNF-α in the induction of other cytokines in the lung by flagellin, the cytokine responses in TNFR1−/− mice (B6;129S-Tnfrsf1atm1Imx Tnfrsf1btm1Imx) and control B6;129SF2/J mice were compared. Similar subsets of cytokines were produced by both strains (Table 1), demonstrating that TNF-α signaling is not required for the induction of IL-6, KC, MIP-2, or G-CSF. TNF-α was reported to be important for IL-12 production in vitro and in vivo after infection with Listeria spp. (29). However, IL-12 was not detected in the BALF of TNFR−/− mice or the background B6;129 strain. This indicates a strain specificity for flagellin-induced IL-12 production in the lung.
Recently, a polymorphism that results in a premature stop codon was discovered in human TLR5 (12). This mutation predisposes individuals to Legionnaires' disease due to decreased proinflammatory cytokine production after exposure to Legionella pneumophila. Thus, flagellin signaling via TLR5 may play a crucial role in the host response to this organism and perhaps other flagellated bacteria. Our findings are consistent with the hypothesis that flagellin is an important signal for the induction of protective innate immune mechanisms that may also contribute to the development of a subsequent adaptive immune response (1, 15, 16, 20, 21). Finally, the results in this study provide a foundation for future studies of the potential use of flagellin as a mucosal adjuvant in the lung.
Acknowledgments
This study was supported by NIH grant R01-AI51319 (S.B.M.). A.N.H. was supported by a training grant from the NIH (T32-AI007401).
We thank Nancy Kock for assistance with the histological analyses.
Editor: F. C. Fang
REFERENCES
- 1.Ben-Yedidia, T., R. Tarrab-Hazdai, D. Schechtman, and R. Arnon. 1999. Intranasal administration of synthetic recombinant peptide-based vaccine protects mice from infection by Schistosoma mansoni. Infect. Immun. 67:4360-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carsiotis, M., D. L. Weinstein, H. Karch, I. A. Holder, and A. D. O'Brien. 1984. Flagella of Salmonella typhimurium are a virulence factor in infected C57BL/6J mice. Infect. Immun. 46:814-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciacci-Woolwine, F., I. C. Blomfield, S. H. Richardson, and S. B. Mizel. 1998. Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect. Immun. 66:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciacci-Woolwine, F., L. S. Kucera, S. H. Richardson, N. P. Iyer, and S. B. Mizel. 1997. Salmonellae activate tumor necrosis factor alpha production in a human promonocytic cell line via a released peptide. Infect. Immun. 65:4624-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciacci-Woolwine, F., P. F. McDermott, and S. B. Mizel. 1999. Induction of cytokine synthesis by flagella from gram-negative bacteria may be dependent on the activation or differentiation state of human monocytes. Infect. Immun. 67:5176-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaves-Pyles, T., K. Murthy, L. Liaudet, L. Virág, G. Ross, F. G. Soriano, C. Szabó, and A. L. Salzman. 2001. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: IκBα degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J. Immunol. 166:1248-1260. [DOI] [PubMed] [Google Scholar]
- 7.Eaves-Pyles, T. D., H. R. Wong, K. Odoms, and R. B. Pyles. 2001. Salmonella flagellin-dependent proinflammatory responses are localized to the conserved amino and carboxyl regions of the protein. J. Immunol. 167:7009-7016. [DOI] [PubMed] [Google Scholar]
- 8.Feldman, M., R. Bryan, S. Rajan, L. Scheffler, S. Brunnert, H. Tang, and A. Prince. 1998. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster, W. M., D. M. Walters, M. Longphre, K. Macri, and L. M. Miller. 2001. Methodology for the measurement of mucociliary function in the mouse by scintigraphy. J. Appl. Physiol. 90:1111-1118. [DOI] [PubMed] [Google Scholar]
- 10.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 11.Gewirtz, A. T., P. O. Simon, Jr., C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawn, T. R., A. Verbon, K. D. Lettinga, L. P. Zhao, S. S. Li, R. J. Laws, S. J. Skerrett, B. Beutler, L. Schroeder, A. Nachman, A. Ozinsky, K. D. Smith, and A. Aderem. 2003. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to Legionnaires' disease. J. Exp. Med. 198:1563-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 14.Ho, W., and A. Furst. 1973. Intratracheal instillation method for mouse lungs. Oncology 27:385-393. [DOI] [PubMed] [Google Scholar]
- 15.Jeon, S. H., T. Ben Yedidia, and R. Arnon. 2002. Intranasal immunization with synthetic recombinant vaccine containing multiple epitopes of influenza virus. Vaccine 20:2772-2780. [DOI] [PubMed] [Google Scholar]
- 16.Levi, R., and R. Arnon. 1996. Synthetic recombinant influenza vaccine induces efficient long-term immunity and cross-strain protection. Vaccine 14:85-92. [DOI] [PubMed] [Google Scholar]
- 17.Liaudet, L., K. G. K. Murthy, J. G. Mabley, P. Pacher, F. G. Soriano, A. L. Salzman, and C. Szabó. 2002. Comparison of inflammation, organ damage, and oxidant stress induced by Salmonella enterica serovar Muenchen flagellin and serovar Enteritidis lipopolysaccharide. Infect. Immun. 70:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liaudet, L., C. Szabó, O. V. Evgenov, K. G. Murthy, P. Pacher, L. Virag, J. G. Mabley, A. Marton, F. G. Soriano, M. Y. Kirov, L. J. Bjertnaes, and A. L. Salzman. 2003. Flagellin from gram-negative bacteria is a potent mediator of acute pulmonary inflammation in sepsis. Shock 19:131-137. [DOI] [PubMed] [Google Scholar]
- 19.McDermott, P. F., F. Ciacci-Woolwine, J. A. Snipes, and S. B. Mizel. 2000. High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect. Immun. 68:5525-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEwen, J., R. Levi, R. J. Horwitz, and R. Arnon. 1992. Synthetic recombinant vaccine expressing influenza haemagglutinin epitope in Salmonella flagellin leads to partial protection in mice. Vaccine 10:405-411. [DOI] [PubMed] [Google Scholar]
- 21.McSorley, S. J., B. D. Ehst, Y. Yu, and A. T. Gewirtz. 2002. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J. Immunol. 169:3914-3919. [DOI] [PubMed] [Google Scholar]
- 22.Medan, D., L. Wang, X. Yang, S. Dokka, V. Castranova, and Y. Rojanasakul. 2002. Induction of neutrophil apoptosis and secondary necrosis during endotoxin-induced pulmonary inflammation in mice. J. Cell. Physiol. 191:320-326. [DOI] [PubMed] [Google Scholar]
- 23.Miller, L. M., W. M. Foster, D. M. Dambach, D. Doebler, M. McKinnon, L. Killar, and M. Longphre. 2002. A murine model of cigarette smoke-induced pulmonary inflammation using intranasally administered smoke-conditioned medium. Exp. Lung Res. 28:435-455. [DOI] [PubMed] [Google Scholar]
- 24.Mizel, S. B., A. N. Honko, M. A. Moors, P. S. Smith, and A. P. West. 2003. Induction of macrophage nitric oxide production by gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J. Immunol. 170:6217-6223. [DOI] [PubMed] [Google Scholar]
- 25.Mizel, S. B., A. P. West, and R. R. Hantgan. 2003. Identification of a sequence in human toll-like receptor 5 required for the binding of gram-negative flagellin. J. Biol. Chem. 278:23624-23629. [DOI] [PubMed] [Google Scholar]
- 26.Sebastiani, G., G. Leveque, L. Lariviere, L. Laroche, E. Skamene, P. Gros, and D. Malo. 2000. Cloning and characterization of the murine Toll-like receptor 5 (TLR5) gene: sequence and mRNA expression studies in Salmonella-susceptible MOLF/Ei mice. Genomics 64:230-240. [DOI] [PubMed] [Google Scholar]
- 27.Wolfgang, M. C., J. Jyot, A. L. Goodman, R. Ramphal, and S. Lory. 2004. Pseudomonas aeruginosa regulates flagellin expression as part of a global response to airway fluid from cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 101:6664-6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng, H., A. Q. Carlson, Y. Guo, Y. Yu, L. S. Collier-Hyams, J. L. Madara, A. T. Gewirtz, and A. S. Neish. 2003. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J. Immunol. 171:3668-3674. [DOI] [PubMed] [Google Scholar]
- 29.Zhan, Y., and C. Cheers. 1998. Control of IL-12 and IFN-γ production in response to live or dead bacteria by TNF and other factors. J. Immunol. 161:1447-1453. [PubMed] [Google Scholar]