Abstract
Yersinia pseudotuberculosis mutants that overproduce the DNA adenine methylase (DamOP Yersinia) are attenuated, confer robust protective immune responses, and synthesize or secrete several Yersinia outer proteins (Yops) under conditions that are nonpermissive for synthesis and secretion in wild-type strains. To understand the molecular basis of immunity elicited by DamOP Yersinia, we investigated the effects of Dam overproduction on the synthesis and localization of a principal Yersinia immunogen, LcrV, a low-calcium-responsive virulence factor involved in Yop synthesis, localization, and suppression of host inflammatory activities. Dam overproduction relaxed the stringent temperature and calcium regulation of LcrV synthesis. Moreover, the LcrV-dependent synthesis and localization of the actin cytotoxin, YopE, were shown to be relaxed in DamOP cells, suggesting that the synthesis and localization of Yops can occur via both LcrV-dependent and -independent mechanisms. Last, the immunity conferred by DamOP Yersinia was strictly dependent on the presence of LcrV, which may result from its role (i) as an immunogen, (ii) as an immunomodulator of host anti-inflammatory activities, or (iii) in the altered synthesis and localization of Yops that could contribute to immunogen repertoire expansion.
Yersinia pestis is the causative agent of human plague (5, 7), whereas enteropathogenic Yersinia pseudotuberculosis and Yersinia enterocolitica are the causative agents of mesenteric lymphadenitis and gastroenteritis, respectively (6). The pathogenicity of Yersinia is dependent on the presence of a virulence plasmid, pCD in Y. pestis or pYV in enteropathogenic species, that encodes a type III secretion apparatus and antihost effector proteins, termed Yersinia outer proteins (Yops) (6, 9, 24). Upon host cell contact, the effectors are injected by the type III secretion apparatus into the host cytoplasm of target cells, where they inhibit phagocytosis and engage in anti-inflammatory activities (6, 8, 10, 15, 42, 49). The secretion of Yops is normally under strict regulatory control by the low-calcium response, whereby Yop secretion maximally occurs under conditions of low calcium (Ca2+) and high temperature (37°C) in vitro (12, 45).
Alteration of DNA adenine methylase (Dam) activity has been shown to attenuate the virulence of several pathogens and confer protective immune responses in vaccinated animals (13, 17, 18, 26). The molecular basis of virulence attenuation and protection conferred in Dam mutant strains appears to involve ectopic gene expression and the resultant elaboration of an expanded repertoire of antigens. In Y. pseudotuberculosis, Dam overproduction has been shown to attenuate virulence, confer protective immune responses, cause the secretion of several Yops under conditions that are nonpermissive for secretion in wild-type strains, and alter host immune responses to Yersinia antigens (22, 23). One of the low-calcium-responsive Yersinia antigens whose synthesis is affected by Dam overproduction is YopE, a 23-kDa actin cytotoxin involved in antiphagocytosis that is secreted under low-calcium conditions (1, 3, 48). Dam overproduction relaxed the high-temperature and low-calcium dependence of YopE synthesis and relaxed the high-temperature but not the low-calcium dependence of YopE secretion (22, 23). Such patterns of altered expression and secretion may contribute to the attenuated virulence and robust immunity observed in vaccinated animals.
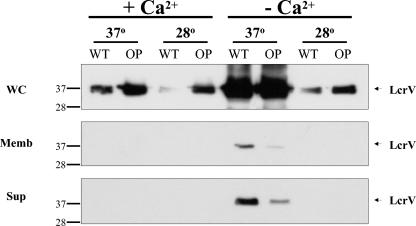
Dam overproduction in Yersinia relaxes the temperature and calcium dependence of LcrV synthesis.
Here we examined the effect of Dam overproduction on the synthesis, localization, and secretion of LcrV, a low-calcium-responsive Yersinia virulence protein involved in Yop expression (30), Yop translocation (38), and the suppression of host inflammatory activities (6, 33, 43). LcrV is also a principal Yersinia immunogen, as robust levels of protection are conferred when LcrV is delivered as a subunit vaccine (7, 25, 32); additionally, administration of antibodies directed against LcrV epitopes confers passive immunity (reviewed in reference 6). Dam+ and DamOP Yersinia (Table 1; Fig. 1) were grown under conditions permissive for LcrV synthesis (low calcium, high temperature) and conditions nonpermissive for LcrV synthesis (high calcium, low temperature; high calcium, high temperature; and low calcium, low temperature). Whole-cell, membrane, and supernatant fractions were analyzed by immunoblotting using anti-LcrV antibody. Dam overproduction relaxed the temperature or calcium dependence of LcrV synthesis under all three nonpermissive conditions tested (Fig. 1, whole-cell fraction). Thus, Dam overproduction disrupts both the temperature and calcium control of LcrV synthesis in a manner similar to what has been observed for YopE (23).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype | Source or reference(s) |
|---|---|---|
| Y. pseudotuberculosis strain | ||
| YPIIIpYV | Wild type | Stanley Falkow |
| MT2294 | dam::Kn + pTP166-Cm | 22 |
| MT2394 | ΔlcrV | This work |
| MT2395 | ΔlcrV dam::Kn + pTP166-Cm | This work |
| Plasmid | ||
| pTP166-Cm | E. coli dam under tac promoter control; chloramphenicol- resistant derivative of pTP166 | 22, 29 |
FIG. 1.
The high-temperature and low-calcium dependence of LcrV synthesis is relaxed in Dam-overproducing Y. pseudotuberculosis. Whole-cell (WC), membrane (Memb), and supernatant (Sup) fractions (12) were prepared from wild-type (WT) and Dam-overproducing (OP) Y. pseudotuberculosis grown under the indicated conditions according to methods described previously (12, 44, 45). For each growth condition, total protein extracts corresponding to 2.0 × 106 cells (∼20 μg of protein/well) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane (Pierce), and probed with mouse anti-LcrV monoclonal antibodies (1:4,000 dilution). Peroxidase-conjugated sheep anti-mouse immunoglobulin G (1:40,000 dilution; Amersham Biosciences), was used as the secondary antibody, and hybridization was detected by chemiluminescence using Supersignal West Femto Maximum Sensitivity Substrate (Pierce) followed by a 2-min exposure to film. Inspection of corresponding Coomassie-stained gels showed similar band intensities of nonregulated proteins under all conditions tested (data not shown). Western analysis of lcrV+ and lcrV− strains confirmed that the 37-kDa protein was LcrV (data not shown).
The LcrV dependence of YopE synthesis and localization is relaxed under DamOP conditions.
Since LcrV is directly involved in Yop translocation (38) and indirectly involved in the positive control of Yop expression (30), we examined the effects of the presence and absence of LcrV on YopE synthesis and localization under DamOP conditions. LcrV is encoded on the Yersinia pYV virulence plasmid, within the lcrGVH-yopBD operon (2, 37). To assess the contribution of LcrV to Yop synthesis and localization under DamOP conditions, a nonpolar deletion was constructed in lcrV according to standard methods (18). Briefly, a PCR-based strategy was implemented such that 879 bp (293 codons) within lcrV were removed (bp 28 to 906 out of a total of 981 bp); the native lcrV reading frame was confirmed to be intact by DNA sequencing. The deletion strain (MT2394) showed no LcrV expression as assessed by Western analysis utilizing anti-LcrV antibodies (data not shown). This LcrV null mutant was used to discern whether Dam overproduction enabled Yersinia to override the strict LcrV dependence of Yop production and translocation (38).
Although the lack of LcrV resulted in a considerable reduction in YopE synthesis and localization to extracytosolic fractions in DamOP Yersinia, significantly more was observed under permissive conditions compared to Dam+ Yersinia (Fig. 2A and B). Further, when grown under nonpermissive conditions, the absence of LcrV did not abrogate the DamOP-mediated ectopic synthesis and localization of YopE (Fig. 2B). Taken together, these data suggest that the ectopic Yop synthesis and localization observed in DamOP cells (22, 23) can occur via LcrV-dependent and -independent mechanisms.
FIG. 2.
The LcrV dependence of YopE synthesis and localization is relaxed under Dam-overproducing conditions in Y. pseudotuberculosis. Whole-cell (WC), membrane (Memb), and supernatant (Sup) fractions (12) were prepared from dam wild-type (WT) and Dam-overproducing (OP) Y. pseudotuberculosis containing (A) or lacking (B) the lcrV gene. For each growth condition (12, 44, 45), total protein extracts corresponding to 2.0 × 106 cells (∼20 μg of protein/well) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane (Pierce), and probed with rabbit anti-YopE polyclonal antibodies (1:50,000 dilution). Peroxidase-conjugated donkey anti-rabbit immunoglobulin G was used as the secondary antibody (1:20,000 dilution; Amersham Biosciences), and hybridization was detected by chemiluminescence using Supersignal West Femto Maximum Sensitivity Substrate (Pierce) followed by a 30-s (A) or 2-min (B) exposure to film. Inspection of corresponding Coomassie-stained gels showed similar band intensities of nonregulated proteins under all conditions tested (data not shown). Western analysis of yopE+ and yopE− strains confirmed that the 23-kDa protein was YopE (23).
The protection conferred by DamOP Yersinia is dependent on the presence of the LcrV antigen.
LcrV is a principal Yersinia immunogen as potent levels of immunity to Yersinia infection are conferred when LcrV is delivered as a subunit vaccine (7, 32). Thus, we examined whether LcrV is required for the heightened immunity observed in animals vaccinated with DamOP Yersinia (22, 23) by comparing the protection conferred by LcrV− DamOP Yersinia to that conferred by LcrV+ DamOP Yersinia. Table 2 shows that the protection conferred by DamOP Yersinia is highly dependent on the presence of LcrV, as BALB/c mice orally immunized with LcrV− DamOP Y. pseudotuberculosis were not protected against a challenge with the virulent strain at more than 700 or 7,000 times the 50% lethal dose, whereas LcrV+ DamOP Yersinia elicited complete protection at these challenge doses. Additionally, the time of death following virulent challenge was similar in LcrV− DamOP vaccinated mice and control (nonvaccinated) mice, indicating that the immune protection conferred by DamOP Yersinia requires LcrV (data not shown). Such dependence on LcrV may be due to its role as a principal immunogen and/or its role in the synthesis and localization of Yops, which may also contribute to the immunity observed in DamOP Yersinia-vaccinated hosts.
TABLE 2.
Protective immunity conferred by Dam-overproducing Y. pseudotuberculosis is dependent on the presence of the LcrV antigena
| Vaccine strain | Relevant genotypeb | No. of survivors after challenge with indicated no. of organisms/total no. of animals
|
|
|---|---|---|---|
| 1.8 × 1010 | 1.8 × 1011 | ||
| None | NA | 0/10 | 0/10 |
| MT2294 | DamOP | 11/11 | 11/11 |
| MT2395 | DamOP ΔlcrV | 0/12 | 0/12 |
Six- to eight-week-old BALB/c mice were perorally immunized via gastrointubation with a dose of 3 × 109 LcrV− DamOP or 2 × 109 LcrV+ DamOP Y. pseudotuberculosis organisms (16). Mice were perorally challenged with virulent Y. pseudotuberculosis (YPIIIpYV) at the dose indicated 8 weeks postimmunization [the peroral 50% lethal dose of YPIIIpYV is 2.5 × 107 organisms, determined by Monack et al. (31)]. DamOP Y. pseudotuberculosis are cleared from vaccinated animals between day 5 and day 21 (22, 23) postimmunization, and thus DamOP Yersinia were not present at the time of challenge.
Bacterial strains are derivatives of Y. pseudotuberculosis YPIIIpYV. DamOP strains MT2395 and MT2294 contain E. coli dam on a chloramphenicol-resistant derivative of the high-copy-number recombinant plasmid pTP166 (22, 29) in dam mutant (dam::Kn) genetic backgrounds. Since dam is essential for viability in Y. pseudotuberculosis (22), the loss of the DamOP plasmids in dam mutant backgrounds is lethal for this pathogen. NA, not applicable.
The role of Dam in virulence and in the elicitation of protective immune responses may rely on its capability as a global regulator of gene expression (18, 26, 28, 36). Elucidation of the possible mechanisms by which Dam regulates gene expression comes from genetic analysis of the Escherichia coli pyelonephritis-associated pili (pap) operon (20, 26, 47), which encodes adherence factors (pili) that are essential for virulence in monkey and mouse models of pyelonephritis (35, 41). Dam target sites in the pap promoter are protected from methylation by the binding of regulatory proteins at or near these sites, forming specific DNA methylation patterns analogous to those exhibited in eukaryotes (4, 14, 19, 40, 46). These DNA methylation patterns regulate gene expression by modulating the binding of regulatory proteins to Dam target sites.
One possible outcome of Dam dysregulation is the production of an expanded repertoire of antigens that contribute to the potent state of immunity observed in vaccinated animals. Additionally, the low-grade persistence of dam mutant vaccines in appropriate lymphoid tissues (e.g., Peyer's patches) in Salmonella spp. (13, 18) and in Yersinia (22) may provide a stable source of antigens in sufficient quantity and duration for the transition to the development of potent adaptive immune responses (11, 26). This suggestion is supported by work with Salmonella wherein the loss of Dam function results in a number of changes in the bacterial physiology. dam− mutants appear to express in vitro a number of genes that are normally only produced in vivo during the initiation and progression of bacterial infection (17, 18, 27); additionally, both bacteria-associated and -secreted proteins are affected by the loss of Dam regulation (13, 17, 39).
Similarly, in Yersinia, Dam overproduction altered the expression of LcrV (Fig. 1) and the expression and/or secretion of YopE (Fig. 2A) as well as several other low-calcium-responsive Yersinia virulence proteins (22, 23). Additionally, since LcrV normally functions by suppressing inflammatory cytokines during infection, altered expression or localization of LcrV and/or Yops may contribute to the elicitation of protective responses by immunogen repertoire expansion and/or by altering pathogen-mediated modulation of host inflammatory activities (6, 21, 33, 34, 43).
Acknowledgments
We thank Bob Sinsheimer and Steve Julio for critically reading the manuscript and Robert Brubaker (Michigan State University) and Greg Plano (University of Miami, Miami, Florida) for the generous gifts of LcrV and YopE antibodies, respectively.
This work was supported by the University of California Biotech Program (grants no. 2001-15, 2002-14), the Santa Barbara Cottage Hospital Research Program, USDA grant no. 2000-02539, and the G. Harold & Leila Y. Mathers Foundation (grant to M.J.M).
Editor: A. D. O'Brien
REFERENCES
- 1.Andor, A., K. Trulzsch, M. Essler, A. Roggenkamp, A. Wiedemann, J. Heesemann, and M. Aepfelbacher. 2001. YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell. Microbiol. 3:301-310. [DOI] [PubMed] [Google Scholar]
- 2.Bergman, T., S. Hakansson, A. Forsberg, L. Norlander, A. Macellaro, A. Backman, I. Bolin, and H. Wolf-Watz. 1991. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J. Bacteriol. 173:1607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. [DOI] [PubMed] [Google Scholar]
- 4.Braaten, B. A., X. Nou, L. S. Kaltenbach, and D. A. Low. 1994. Methylation patterns in pap regulatory DNA control pyelonephritis- associated pili phase variation in E. coli. Cell 76:577-588. [DOI] [PubMed] [Google Scholar]
- 5.Brubaker, R. R. 1991. Factors promoting acute and chronic diseases caused by yersiniae. Clin. Microbiol. Rev. 4:309-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brubaker, R. R. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brubaker, R. R. 8 September 2000, posting date. Yersinia pestis and bubonic plague. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackelbrandt (ed.), The prokaryotes, an evolving electronic resource for the microbiological community. [Online.] Springer-Verlag, New York, N. Y. http://www.link.springer.de.
- 8.Cheng, L. W., and O. Schneewind. 2000. Type III machines of gram-negative bacteria: delivering the goods. Trends Microbiol. 8:214-220. [DOI] [PubMed] [Google Scholar]
- 9.Cornelis, G. R. 2000. Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. USA 97:8778-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelis, G. R. 2000. Type III secretion: a bacterial device for close combat with cells of their eukaryotic host. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:681-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants elicit protective immune responses to homologous and heterologous serovars in chickens. Infect. Immun. 69:7950-7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsberg, A., I. Bolin, L. Norlander, and H. Wolf-Watz. 1987. Molecular cloning and expression of calcium-regulated, plasmid-coded proteins of Y. pseudotuberculosis. Microb. Pathog. 2:123-137. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Del Portillo, F., M. G. Pucciarelli, and J. Casadesus. 1999. DNA adenine methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc. Natl. Acad. Sci. USA 96:11578-11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hale, W. B., M. W. van der Woude, and D. A. Low. 1994. Analysis of nonmethylated GATC sites in the Escherichia coli chromosome and identification of sites that are differentially methylated in response to environmental stimuli. J. Bacteriol. 176:3438-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haller, J. C., S. Carlson, K. J. Pederson, and D. E. Pierson. 2000. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 36:1436-1446. [DOI] [PubMed] [Google Scholar]
- 16.Heithoff, D. M., C. P. Conner, P. C. Hanna, S. M. Julio, U. Hentschel, and M. J. Mahan. 1997. Bacterial infection as assessed by in vivo gene expression. Proc. Natl. Acad. Sci. USA 94:934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heithoff, D. M., E. Y. Enioutina, R. A. Daynes, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect. Immun. 69:6725-6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heithoff, D. M., R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284:967-970. [DOI] [PubMed] [Google Scholar]
- 19.Hendrich, B., and A. Bird. 2000. Mammalian methyltransferases and methyl-CpG-binding domains: proteins involved in DNA methylation. Curr. Top. Microbiol. Immunol. 249:55-74. [DOI] [PubMed] [Google Scholar]
- 20.Hernday, A. D., B. A. Braaten, and D. A. Low. 2003. The mechanism by which DNA adenine methylase and PapI activate the pap epigenetic switch. Mol. Cell 12:947-957. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann, R., K. van Erp, K. Trulzsch, and J. Heesemann. 2004. Transcriptional responses of murine macrophages to infection with Yersinia enterocolitica. Cell. Microbiol. 6:377-390. [DOI] [PubMed] [Google Scholar]
- 22.Julio, S. M., D. M. Heithoff, D. Provenzano, K. E. Klose, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2001. DNA adenine methylase is essential for viability and plays a role in the pathogenesis of Yersinia pseudotuberculosis and Vibrio cholerae. Infect. Immun. 69:7610-7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julio, S. M., D. M. Heithoff, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2002. DNA adenine methylase overproduction in Yersinia pseudotuberculosis alters YopE expression and secretion and host immune responses to infection. Infect. Immun. 70:1006-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juris, S. J., F. Shao, and J. E. Dixon. 2002. Yersinia effectors target mammalian signalling pathways. Cell. Microbiol. 4:201-211. [DOI] [PubMed] [Google Scholar]
- 25.Leary, S. E., K. F. Griffin, E. E. Galyov, J. Hewer, E. D. Williamson, A. Holmstrom, Å. Forsberg, and R. W. Titball. 1999. Yersinia outer proteins (YOPS) E, K and N are antigenic but non-protective compared to V antigen, in a murine model of bubonic plague. Microb. Pathog. 26:159-169. [DOI] [PubMed] [Google Scholar]
- 26.Low, D. A., N. J. Weyand, and M. J. Mahan. 2001. The roles of DNA adenine methylation in regulating bacterial gene expression and virulence. Infect. Immun. 69:7197-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahan, M. J., D. M. Heithoff, R. L. Sinsheimer, and D. A. Low. 2000. Assessment of bacterial pathogenesis by analysis of gene expression in the host. Annu. Rev. Genet. 34:139-164. [DOI] [PubMed] [Google Scholar]
- 28.Marinus, M. G. 1996. Methylation of DNA, p. 782-791. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Rezinkoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 29.Marinus, M. G., A. Poteete, and J. A. Arraj. 1984. Correlation of DNA adenine methylase activity with spontaneous mutability in Escherichia coli K-12. Gene 28:123-125. [DOI] [PubMed] [Google Scholar]
- 30.Matson, J. S., and M. L. Nilles. 2001. LcrG-LcrV interaction is required for control of Yops secretion in Yersinia pestis. J. Bacteriol. 183:5082-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motin, V. L., R. Nakajima, G. B. Smirnov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 62:4192-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Hanley, P., D. Low, I. Romero, D. Lark, K. Vosti, S. Falkow, and G. Schoolnik. 1985. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N. Engl. J. Med. 313:414-420. [DOI] [PubMed] [Google Scholar]
- 36.Oshima, T., C. Wada, Y. Kawagoe, T. Ara, M. Maeda, Y. Masuda, S. Hiraga, and H. Mori. 2002. Genome-wide analysis of deoxyadenosine methyltransferase-mediated control of gene expression in Escherichia coli. Mol. Microbiol. 45:673-675. [DOI] [PubMed] [Google Scholar]
- 37.Perry, R. D., P. A. Harmon, W. S. Bowmer, and S. C. Straley. 1986. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect. Immun. 54:428-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettersson, J., A. Holmstrom, J. Hill, S. Leary, E. Frithz-Lindsten, A. von Euler-Matell, E. Carlsson, R. Titball, A. Forsberg, and H. Wolf-Watz. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961-976. [DOI] [PubMed] [Google Scholar]
- 39.Pucciarelli, M. G., A. I. Prieto, J. Casadesus, and F. Garcia-del Portillo. 2002. Envelope instability in DNA adenine methylase mutants of Salmonella enterica. Microbiology 148:1171-1182. [DOI] [PubMed] [Google Scholar]
- 40.Ringquist, S., and C. L. Smith. 1992. The Escherichia coli chromosome contains specific, unmethylated dam and dcm sites. Proc. Natl. Acad. Sci. USA 89:4539-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts, J. A., G. M. Suarez, B. Kaack, G. Kallenius, and S. B. Svenson. 1985. Experimental pyelonephritis in the monkey. VII. Ascending pyelonephritis in the absence of vesicoureteral reflux. J. Urol. 133:1068-1075. [DOI] [PubMed] [Google Scholar]
- 42.Rosqvist, R., K. E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 168:1315-1321. [DOI] [PubMed] [Google Scholar]
- 44.Straley, S. C., and R. D. Perry. 1995. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 3:310-317. [DOI] [PubMed] [Google Scholar]
- 45.Straley, S. C., G. V. Plano, E. Skrzypek, P. L. Haddix, and K. A. Fields. 1993. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol. Microbiol. 8:1005-1010. [DOI] [PubMed] [Google Scholar]
- 46.Tavazoie, S., and G. M. Church. 1998. Quantitative whole-genome analysis of DNA-protein interactions by in vivo methylase protection in E. coli. Nat. Biotechnol. 16:566-571. [DOI] [PubMed] [Google Scholar]
- 47.van der Woude, M., B. Braaten, and D. Low. 1996. Epigenetic phase variation of the pap operon in Escherichia coli. Trends Microbiol. 4:5-9. [DOI] [PubMed] [Google Scholar]
- 48.Von Pawel-Rammingen, U., M. V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737-748. [DOI] [PubMed] [Google Scholar]
- 49.Yao, T., J. Mecsas, J. I. Healy, S. Falkow, and Y. Chien. 1999. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, YopH. J. Exp. Med. 190:1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]