Abstract
Platelet binding by Streptococcus gordonii strain M99 is dependent on expression of the cell wall-anchored glycoprotein GspB. This large cell surface protein is exported from the M99 cytoplasm via a dedicated transport system that includes SecA2 and SecY2. GspB is highly similar to Hsa, a protein expressed by S. gordonii Challis that has been characterized as a sialic acid binding hemagglutinin. In this study, we compared the contribution of GspB and Hsa to the adherence of S. gordonii to selected glycoproteins. Our results indicate that GspB can mediate binding to a variety of sialylated glycoproteins. GspB facilitates binding to carbohydrates bearing sialic acid in either α(2-3) or α(2-6) linkages, with a slight preference for α(2-3) linkages. Furthermore, GspB readily mediates binding to sialic acid residues on immobilized glycocalicin, the extracellular portion of the platelet membrane glycoprotein (GP) Ibα (the ligand binding subunit of the platelet von Willebrand factor receptor complex GPIb-IX-V). Although Hsa is required for the binding of S. gordonii Challis to sialic acid, most of the Hsa expressed by Challis is retained in the cytoplasm. The deficiency in export is due, at least in part, to a nonsense mutation in secA2. Hsa export can be enhanced by complementation with secA2 from M99, which also results in significantly greater binding to sialylated glycoproteins, including glycocalicin. The combined results indicate that GspB and Hsa contribute similar binding capabilities to M99 and Challis, respectively, but there may be subtle differences in the preferred epitopes to which these adhesins bind.
The interaction of microbes with platelets is thought to play a central role in the pathogenesis of infective endocarditis. Platelet binding by Streptococcus gordonii strain M99 is predominantly mediated by the cell surface glycoprotein GspB (3). The 9.2-kb gspB gene encodes a protein of 3,072 amino acids, with a predicted molecular mass of 286 kDa. The amino-terminal end of this extremely large protein is predicted to form a 90-amino-acid signal peptide, which is followed by a short serine-rich region (srr1), a region rich in basic residues, an extensive region consisting of approximately 190 semiconserved repeats of the sequence SASESASTSASV (srr2), and a carboxy-terminal cell wall anchoring domain (Fig. 1).
FIG. 1.
Domain structure of the GspB and Hsa polypeptides. SP, putative signal peptide; srr1, first serine-rich region; BR, basic region; srr2, second serine-rich region; CWA, cell wall anchoring domain (an LPXTG motif, hydrophobic region, and charged tail, characterized as a signal for covalent linkage to the cell wall peptidoglycan [9, 20, 21]). The predicted molecular mass of each protein is indicated.
When expressed by M99, GspB is heavily glycosylated, primarily with glucose and glucosamine resides, and consists of ∼10% carbohydrate (2). It is not yet known whether the GspB-associated carbohydrate residues are directly involved in platelet binding, as nonglycosylated forms of GspB are highly unstable. The cellular components required for the glycosylation of GspB are encoded within a 14-kb region just downstream of gspB (Fig. 2). These include two proteins (Gly and Nss) that affect the carbohydrate composition of GspB and two proteins (Gtf and Orf4) that may be essential for glycosylation (27). These enzymes appear to function cytoplasmically, since glycosylation occurs independently of GspB export (2).
FIG. 2.
Comparison of the gspB-secY2/A2 locus of M99 with the hsa-secY2/A2 locus of Challis. Gly, Nss, and Gtf are likely to function in carbohydrate metabolism: Gly is predicted to be a cytoplasmic glycosyl transferase (family 8); Nss is similar to nucleotide sugar synthetases; Gtf is a likely glucosyl transferase; SecA2 and SecY2 are similar to the SecA ATPase and the SecY transmembrane translocase of various organisms, respectively (components of the general secretory pathway), and are required specifically for the export of GspB. Asp1, Asp2, and Asp3 are additional accessory secretory proteins. Orf4 is not similar to any protein of known function. The asterisk denotes a frameshift mutation at codon 361 of 682 in gly; the double asterisk indicates a nonsense mutation at codon 770 of 793 in secA2.
Another interesting feature of GspB is that the presentation of this unusual glycoprotein on the cell surface requires a dedicated transport system, components of which are also encoded in the region downstream of gspB (3). Proteins that facilitate GspB transport from the cytoplasm include SecA2 and SecY2 (paralogues of the canonical SecA and SecY, respectively), along with Asp1, Asp2, and Asp3 (27). The latter three proteins are not similar to any proteins of known function, and their precise role in GspB export has yet to be elucidated.
Homologues of GspB, Gtf, Orf4, and the accessory Sec system are present in a number of other streptococcal and staphylococcal species (27). In most cases, little is known about the glycosylation, export, and function of these GspB homologues. However, Fap1, a GspB homologue expressed by Streptococcus parasanguis, has been shown to be glycosylated and to mediate binding to saliva-coated hydroxyapatite (22, 28). In addition, the homologue of GspB in S. gordonii strain Challis (Hsa) has been characterized as a sialic acid binding hemagglutinin (24) and has also recently been shown to mediate the agglutination of platelets (26). Of note, Hsa is nearly identical to GspB at the N-terminal end and throughout the serine-rich repeat regions, but the intervening basic regions are less than 50% similar.
Our group has been interested in characterizing the receptor for GspB on human platelets. The close similarity of GspB to Hsa suggested that these proteins might have related binding properties. Results presented here indicate that GspB and Hsa do indeed confer similar binding capabilities on their respective hosts, including the ability to bind sialic acid residues on the platelet membrane glycoprotein (GP) Ibα. In addition, our findings indicate that Challis has a defect in Hsa export which can be partially corrected by SecA2 of M99. Furthermore, SecA2-enhanced surface expression of Hsa leads to a marked increase in sialic acid binding by Challis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and reagents.
The bacterial strains and plasmids used in this study are listed in Table 1. S. gordonii strains were grown in Todd-Hewitt broth (Difco Laboratories) at 37°C in a 5% CO2 environment. For strains that carry pM99secA2 (PS795 and PS797), the growth medium included 15 μg of erythromycin ml−1. Biotinylated lectins were purchased from Vector Laboratories. Streptavidin-conjugated horseradish peroxidase, Dulbecco's phosphate-buffered saline (DPBS), trypsin, bovine submaxillary mucin (BSM), fetuin, asialofetuin, N-acetylneuraminic acid, lactose, and sialyllactose (64% α2-3 isomer, 18% α2-6 isomer, and 17% α2-6 sialyllactosamine) were from Sigma. Multivalent sialyllactose (trisaccharides chemically attached to human serum albumin via an acetylphenylenediamine spacer) was purchased from Accurate Chemical Corporation. Glycocalicin, the soluble extracellular region of GPIbα, was purified from pooled donor platelets as described previously (18). Sialidase A (a neuraminidase capable of cleaving sialic acid linked α2-3, α2-6, α2-8, and α2-9) was obtained from Prozyme.
TABLE 1.
Strains and plasmids used in the present study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| M99 | S. gordonii endocarditis isolate | 23 |
| PS321 | M99 Δ[nss-gtf]; Ermr | 3 |
| PS436 | M99 gspB::pVA891 (nonpolar mutation); Ermr | 3 |
| Challisa | S. gordonii strain CH1 | D. Clewell |
| PS779 | Challis Δhsa::pEVP3;Cmr | This study |
| PS787 | Challis hsa::pM99B194His6int; Cmr | This study |
| PS795 | Challis (pM99secA2); Ermr | This study |
| PS796 | Challis Δ[nss-gtf];Ermr | This study |
| PS797 | PS787 (pM99secA2); secretes HsaH6; Ermr Cmr | This study |
| PS798 | Challis secA2::pM993′A2; Ermr | This study |
| PS799 | PS787 secA2::pM993′A2; secretes HsaH6; Ermr Cmr | This study |
| Plasmids | ||
| pBluescript | Ampr | Stratagene |
| pVA891 | erm (gram positive), cat tet ori (E. coli) | 14 |
| pEVP3 | cat (gram positive and E. coli), ori (E. coli), lacZ | 6 |
| pMSP3535 | erm, ori (gram positive and E. coli), nisin-inducible promoter | 5 |
| pB194His6int | SpeI-NheI fragment of gspB fused with six histidine codons and cloned in pEVP3 | 2 |
| pSgΔnss-gtf | pVA891 and flanking chromosomal DNA rescued from PS321 | This study |
| pSgΔBR | 5′ and 3′-end fragments of gspB in pEVP3 | This study |
| pM993′A2 | Sau3AI-EcoRV fragment spanning the M99 secA2codon 557 through codon 22 of gtf cloned in pVA891 | This study |
| pM99secA2 | secA2 of M99 in pMSP3535 | This study |
This strain, thought to be identical to DL1 and V288, may also be an endocarditis isolate (M. Vickerman and S. Gill [T1GR], personal communication).
Strain construction.
Hsa-nonexpressing derivatives of Challis were generated by deletion of either the hsa structural gene (PS779) or the accessory sec locus (PS796). To make a deletion in hsa, a SalI-BglII fragment from the 3′ end of gspB was cloned adjacent to a BglII-SalI fragment from the 5′ end of gspB in pEVP3. The resulting plasmid, pSgΔBR, was linearized with BglII and then introduced to Challis by natural transformation. PS796 was constructed as follows. Plasmid pVA891 and flanking chromosomal DNA were first rescued from PS321 by circular ligation of BglII-digested chromosomal DNA. The rescued plasmid (pSgΔnss-gtf) was propagated in Escherichia coli strain DH5α, linearized with BglII, and then used to transform Challis.
Strain PS787, which produces a C-terminally truncated and His6-tagged variant of Hsa (HsaH6), was derived by transformation of Challis with pB194his6. This plasmid consists of pEVP3 carrying a fragment of the gspB srr2 with an in-frame fusion of six histidine codons (2).
For complementation of secA2 in cis, a Sau3AI-EcoRV fragment of the M99 chromosome spanning the 557th codon of secA2 through the 22nd codon of gtf was cloned in pVA891. The resulting plasmid (pM993′A2) was propagated in E. coli strain DH5α and then used to transform Challis or PS787. For complementation in trans, the ribosome binding site and coding region of secA2 were amplified from M99 chromosomal DNA by PCR, using the upstream BamHI-linked primer 5′-AAGGATCCAGAGGAGTCAAATGGTTAAAAA-3′ and the SphI-linked downstream primer 5′-AAAAGCATGCTATGGGAAGTACATTACGAC-3′. The resulting 2.5-kb PCR product was cloned in the nisin-inducible expression vector pMSP3535 to create pM99secA2 and then introduced to Challis or PS787 by natural transformation. Complementation, as evidenced by the increased export of Hsa or HsaH6, occurred constitutively and was not enhanced by the addition of nisin (data not shown).
Transformation of S. gordonii.
Intact or linearized plasmid DNA was introduced to S. gordonii strains as described previously (3), except for omission of the competence-stimulating peptide. Plasmid or chromosomal DNA was extracted from S. gordonii strains as described elsewhere (3). Integration of transforming DNA segments at the expected site was confirmed by Southern blot analysis. For strains transformed with pM99secA2, plasmid content was verified by ethidium bromide staining of DNA that had been digested with EcoRV.
Analysis of cell wall, secreted, and protoplast proteins.
Cell wall proteins were extracted from S. gordonii strains by mutanolysin treatment (15). For analysis of secreted products, proteins were precipitated from culture supernatants by using trichloroacetic acid as described elsewhere (1). For analysis of cytoplasmic components, protoplasts (generated by digestion of the cell wall with mutanolysin) were lysed by suspension in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, followed by boiling for 10 min. Proteins were separated by SDS-PAGE through 3-to-8% Tris-acetate gels (Invitrogen) under reducing conditions and then transferred to BioTrace NT nitrocellulose membranes using the XCell SureLock transfer apparatus (Invitrogen). For Western blot analysis, membranes were incubated for 1 h in a suspension of 1× blocking reagent (Roche) in DPBS. Anti-GspB polyclonal goat serum (3) was used at a dilution of 1:5,000, and a peroxidase-conjugated anti-goat antibody (Sigma) was used at a 1:25,000 dilution. In some experiments, an anti-His6 monoclonal antibody (Sigma) was used at a dilution of 1:2,000 and a peroxidase-conjugated anti-mouse antibody (Sigma) was used at a 1:10,000 dilution. Blots were developed with the SuperSignal West Pico chemiluminescent substrate (Pierce).
Lectin binding assay.
The binding of biotinylated lectins to immobilized bacteria was performed as described previously (2). Results are reported as the mean ± the standard deviation of two independent experiments, with n = 8 for each strain tested. Differences in binding were compared by the unpaired t test, using the Welch modification.
Binding to immobilized glycoproteins.
Glycoproteins were immobilized in microtiter wells by adding 50 μl of glycoprotein solution at the following concentrations in DPBS: BSM, fetuin, and asialofetuin, 1 mg ml−1; sialyllactose-conjugated albumin, 100 μg ml−1; glycocalicin, 25 μg ml−1. For some experiments, glycocalicin was first treated with sialidase A (0.5 U per mg of glycocalicin) for 3 h at 37°C. The microtiter plates were incubated for 48 h at 4°C, unbound glycoprotein was removed by aspiration, and the wells were rinsed with 100 μl of DPBS. Fifty microliters of a blocking solution (1× blocking reagent [Roche] in DPBS) was then added to each well. After 1 h at room temperature, the blocking solution was replaced with 50 μl of a suspension of bacteria that had been grown for 18 h in Todd-Hewitt broth, washed twice with DPBS, sonicated briefly to disrupt aggregated cells, and then diluted to approximately 2 × 106 ml−1 in DPBS. For studies in which the ability of sugars to block binding was examined, the washed and sonicated bacteria were diluted into DPBS containing the indicated saccharide at a final concentration of 50 mM and pH 7.5. Plates were incubated for 2 h at room temperature with gentle rocking, unbound bacteria were removed by aspiration, and wells were washed three times with 100 μl of DPBS. Bound organisms were released by treatment of the wells with 50 μl of a solution of trypsin (1 mg ml of DPBS−1) for 1 h. The number of bound organisms and the precise number of bacteria in the inocula were determined by plating serial dilutions of the bacterial suspensions on sheep blood agar. Results are reported as the means ± standard deviations of two or three independent experiments, with a combined total of n = 12 for each strain tested. Differences in binding were compared by the unpaired t test, using the Welch modification.
Challis genome sequence analysis.
Sequence data from the incomplete S. gordonii Challis CH1 sequencing project were obtained by file transfer protocol from The Institute for Genomic Research (TIGR). The partially assembled sequence data were analyzed using the Gene Construction Kit 2 software (Textco).Protein sequences were compared using the Genetics Computer Group programs available from the Computer Graphics Laboratory at the University of California, San Francisco.
Confirmation of gly and secA2 mutations in S. gordonii CH1.
Primers 5′-GTATCTCGAGTAAGTTTGCTAAGTGGATAA-3′ and 5′-GCAATCAAGGCCGTACTCTG-3′ were used to amplify gly from CH1 chromosomal DNA by PCR. The resulting 2.1-kb PCR product was submitted for sequence analysis with primer 5′-TCCATCTCCCCATCTCCAAAACGAACC-3′. Primers 5′-GGTACTGCAGCTTAGTGCGGGTGTTGAGTC-3′ and 5′-GAATATTATCCGCTAAAATCATATCC-3′ were used to amplify the secA2 region of the CH1 chromosome. The 2.6-kb PCR product was submitted for sequence analysis with primer 5′-ATTACGACCTCTCCTTTGGGG-3′.
RESULTS
GspB facilitates binding to sialylated glycoproteins.
Using a qualitative assay for adherence, Takahashi et al. noted that S. gordonii DL1 (Challis) could bind to sialic acid configured in α(2-3), but not α(2-6), linkages (25) and that binding to α(2-3)-linked sialic acid was dependent on the expression of Hsa (24). In this study, we used a quantitative assay to monitor the binding of S. gordonii to sialylated glycoproteins. Binding of S. gordonii M99 to fetuin and BSM was first examined (Fig. 3). Fetuin is a highly sialylated glycoprotein with well-characterized carbohydrate chains that include terminal sialic acid linked in both α(2-3) and α(2-6) configurations (8, 17). The sialic residues linked to BSM are O-acetylated, which can affect recognition by some sialic acid binding lectins (7). The level of M99 binding to fetuin (0.56% ± 0.11% of the inoculum bound) was significantly higher than binding to wells coated with the blocking reagent only (0.19% ± 0.04%; P < 0.0001). M99 binding to fetuin was threefold higher than that of the gspB mutant strain PS436 (P < 0.0001). Binding of M99 to BSM was also significantly greater than that of PS436 (P = 0.0003) but was lower in magnitude than binding to fetuin, suggesting that GspB does not preferentially bind O-acetylated sialic acid residues.
FIG. 3.
Binding of M99 and the gspB mutant strain PS436 to immobilized glycoproteins. Microtiter wells were coated with the indicated amounts of each protein. Binding is expressed as the mean ± standard deviation of the results of three independent experiments. Binding of these strains to plastic alone was approximately 0.2% of the applied inoculum. BSM, bovine submaxillary mucin; SL, sialyllactose; glycocalicin, the extracellular portion of the platelet membrane GPIbα.
To determine whether the configuration of the sialic acid linkage affected recognition by GspB, we examined the binding of M99 to two forms of sialyllactose, NeuAcα(2-3)Galβ(1-4)Glc and NeuAcα(2-6)Galβ(1-4)Glc, conjugated multivalently to human serum albumin (Fig. 3). M99 binding to both forms of sialyllactose was significantly greater than that of the gspB mutant strain PS436 [3.5-fold-higher binding to sialic acid linked α(2-3) and 3-fold-higher binding to sialic acid linked α(2-6); P < 0.0001]. When comparing the binding of M99 to the two types of linkages, M99 showed nearly a twofold-higher binding to sialic acid configured in α(2-3) versus α(2-6) linkages (a relative binding ratio of 1.7:1; P < 0.0001). In contrast, PS436 binding to the two forms was not significantly different (P = 0.24). The results indicate that binding between GspB and sialylated glycoproteins is not strictly due to nonspecific interactions (e.g., charge alone), since the two forms of sialyllactose differ only in the conformation of the NeuAc linkage to Gal. Rather, they indicate a conformational bias to GspB-mediated binding.
GspB mediates binding of S. gordonii to GPIbα.
The binding of M99 to platelet monolayers has been shown to depend in large part on the expression of GspB. The above results suggested that the platelet receptor for GspB was likely to be sialylated. Several platelet membrane glycoproteins are known to carry terminal sialic acid residues. Of these, glycoprotein Ib (GPIb) is the most abundant, estimated to be present at approximately 25,000 copies per platelet (4), and the most heavily glycosylated. The carbohydrate structures linked to GPIbα (one of two covalently linked polypeptide subunits that comprise GPIb) (Fig. 4) are well characterized. These include large, complex, tetraantennary N-linked carbohydrates within the amino-terminal portion of GPIbα (11) and short but numerous O-linkages throughout the membrane-proximal mucin-like core (12). The extracellular domain of GPIbα (glycocalicin) can be obtained by treatment of platelets with calpain (a calcium-dependent protease). Glycocalicin retains the known binding properties of GPIbα and has been characterized extensively (reviewed in reference 19).
FIG. 4.
Features of the platelet membrane glycoprotein GPIb. (A) Domains of the GPIb heterodimer (adapted from reference 13). GPIb consists of two covalently linked transmembrane proteins. The amino-terminal globular domain contains leucine-rich (leu-rich) repeats and binding sites for von Willebrand factor (vWF) and thrombin. The mucin-like core has approximately 60 O-linked oligosaccharide chains. (B) Diagram of the O-linked carbohydrates (12). (C) Diagram of the N-linked carbohydrates (11). NeuAc, N-acetyl neuraminic acid; Gal, galactose; GalNAc, N-acetyl galactosamine; GlcNAc, N-acetyl glucosamine.
We therefore examined whether GspB could mediate binding to purified glycocalicin. In the absence of GspB expression, binding of S. gordonii to glycocalicin was negligible (Fig. 3). GspB expression by M99 conferred a 20-fold increase in glycocalicin binding over that by the gspB mutant strain PS436. This indicates that GPIbα is likely to be a major receptor for M99 on platelets.
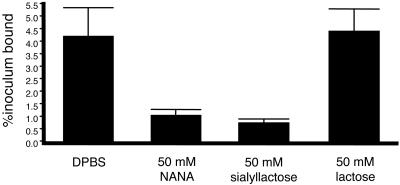
A common means to confirm binding to sialic acid residues on glycoproteins is to demonstrate a lack of binding to desialylated forms of the same glycoproteins. However, M99 and PS436 show significant levels of binding to asialofetuin and desialylated glycocalicin, indicating that M99 can bind these glycoproteins in a GspB-independent manner (data not shown). Therefore, it was not possible to verify the role of sialic acid in the adherence of S. gordonii M99 to glycocalicin by removal of sialic acid from this target glycoprotein. As an alternative approach, the abilities of various sugars to block the binding of M99 were examined (Fig. 5). Binding of M99 to glycocalicin in the presence of 50 mM N-acetyl neuraminic acid was reduced by a mean of 75%, compared with binding in the absence of the sugar. Sialyllactose was slightly more effective at inhibiting the binding of M99 to glycocalicin (mean reduction of 82%). Conversely, 50 mM lactose had no effect on the binding of M99 to glycocalicin. The ability of sialyllactose, but not lactose, to block binding to glycocalicin clearly demonstrated that M99 binds sialylated carbohydrates on GPIbα.
FIG. 5.
Inhibition of M99 binding to glycocalicin by carbohydrates. Wells were coated with 1.25 μg of glycocalicin. Binding is expressed as the mean ± standard deviation of the results of two independent experiments. NANA, N-acetyl neuraminic acid.
Hsa is not efficiently exported by S. gordonii Challis.
For comparison of the binding properties imparted by Hsa and GspB, two mutants of S. gordonii Challis that lack Hsa expression were generated. PS779 is a derivative of Challis in which most of hsa has been replaced by the integrative vector pEVP3. Strain PS796 carries a large deletion in the accessory sec locus. This latter type of mutation in M99 has been characterized extensively and has been shown to abolish expression of GspB but to have no effect on the expression of other surface proteins. Using a polyclonal anti-GspB serum to monitor expression of Hsa, a cell wall protein expressed by Challis (Fig. 6A, lane 1) was found to be absent from the two Hsa mutant strains (lanes 2 and 3). Unexpectedly, however, most of the Hsa expressed by Challis was retained within the protoplasts (Fig. 6B, lane 1). This suggested that Challis might have a defective accessory Sec system.
FIG. 6.
Expression of Hsa by S. gordonii Challis and derivative strains. Blots were probed with a polyclonal anti-GspB serum. (A) Proteins were extracted from the cell wall by using mutanolysin. Each lane was loaded with proteins extracted from bacteria in 730 μl of a broth culture. (B) Proteins associated with protoplasts. Lanes were loaded with protoplasts from 120 μl of the culture. Lane 1, S. gordonii Challis (parental strain); lane 2, PS779 (Δhsa); lane 3, PS796 (Δnss-gtf); lane 4, PS787 (srr2::his6).
To examine the export defect in Challis in greater detail, we engineered a derivative of this strain that produces a truncated variant form of Hsa (HsaH6), in which the C-terminal half of the protein (including the cell wall anchoring domain) has been replaced by a portion of the GspB srr2, along with a C-terminal His6 tag. When expressed by M99 derivative strains, similarly truncated forms of GspB are no longer covalently linked to the cell wall but are freely secreted into the culture medium (2). The corresponding Challis derivative strain (PS787) did produce a truncated form of Hsa, as evidenced by faster migration of the protein during SDS-PAGE (Fig. 6A, lane 4). However, HsaH6 was not detected in the culture medium (see below). Instead, HsaH6 was mainly retained within the protoplasts (Fig. 6B, lane 4). These findings confirmed that Challis does not efficiently export Hsa.
The secY2/A2 locus of Challis.
Although an accessory sec locus of S. gordonii Challis has not yet been characterized or deposited in GenBank, inspection of the genome sequence data for Challis (recently available from TIGR) indicated that the region immediately downstream of hsa is nearly identical to the secY2/A2 locus of M99 (Fig. 2). The proteins encoded within this downstream region show an overall sequence similarity to the corresponding M99 proteins of approximately 95%, with two notable exceptions. First, the C-terminal half of Gly is not likely to be expressed, due to a frameshift mutation. Second, SecA2 is apparently truncated by 23 amino acids, due to a nonsense mutation. Each of these mutations was confirmed by sequence analysis of chromosomal DNA extracted from our laboratory strain of Challis (data not shown). In our studies of GspB expression by M99, we have found that disruption of gly has a minor effect on the composition of carbohydrate linked to GspB (i.e., fewer glucose residues per GspB monomer) (27), but it has no effect on export. Overall, these findings suggested that the failure of Challis to export Hsa might be due to the mutation in secA2.
Hsa export is enhanced by correction of the secA2 mutation in Challis.
Discovery of a nonsense mutation in secA2 led to the hypothesis that the defect in export of Hsa by Challis could be corrected by providing secA2 from M99. This was accomplished by two independent means. In one approach, the entire M99 secA2 gene was cloned in pMSP3535 (a multicopy plasmid with a nisin-inducible promoter), which was then used to transform Challis, thus providing the orthologous gene in trans. As a second approach, a fragment spanning the 3′ end of secA2 and 5′ end of gtf from M99 was cloned in pVA891, which was subsequently used for insertion-duplication mutagenesis of Challis. In this case, generation of a full-length secA2 coding region in Challis was dependent upon recombination upstream of the nonsense mutation (Fig. 7).
FIG. 7.
Repair of the secA2 mutation in Challis by replacement of the 3′ region of secA2 with the corresponding region from M99. The Challis secA2 sequence has a nonsense mutation (asterisk) at codon 770 of 793. Recombination upstream of the nonsense mutation results in a chimeric secA2 sequence and leaves an intact gtf downstream of the integrated vector.
When provided either in trans or in cis, the M99 secA2 sequences were able to partially repair the Hsa export defect. The Challis derivative strains PS795 (carrying pM99secA2 in trans) and PS798 (carrying pM993′A2 in cis) had an increased amount of cell wall-associated Hsa and a reduced amount of cytoplasmic Hsa (Fig. 8A, lanes 3 and 4). The cell wall-associated Hsa expressed by the complemented strains showed a more heterogeneous apparent molecular mass, migrating well ahead of the relatively minor amount of Hsa exported by Challis (lane 2) and slightly ahead of GspB expressed by M99 (lane 5).
FIG. 8.
Export of Hsa by Challis and derivative strains complemented with M99 secA2 sequences. (A) Cell wall proteins and protoplasts of secA2-complemented Challis (lanes 1 to 4). Lanes were loaded with cell wall proteins extracted from 730 μl of culture (upper panel) or with protoplasts from 120 μl of culture (lower panel). GspB expressed by M99 was used for comparison (lane 5; the predicted molecular mass of GspB is 286 kDa, and the apparent mass is approximately 450 kDa). Lane 5 was loaded with protoplasts from 120 μl of culture (lower panel) or with cell wall proteins extracted from 150 μl of the M99 culture (upper panel). Membrane was probed with a polyclonal anti-GspB serum. Lane 1, PS779 (Δhsa); lane 2, Challis; lane 3, PS795 (pM99secA2); lane 4, PS798 (pM993′A2); lane 5, M99. (B) Secreted proteins and protoplasts of secA2-complemented PS787.Lanes were loaded with proteins from 500 μl of the spent culture medium or with protoplasts from 120 μl of the culture. Lane 1, PS779 (Δhsa); lane 2, PS787 (srr2::his6); lane 3, PS797 (srr2::his6 pM99secA2); lane 4, PS799 (srr2::his6 pM993′A2). Upper panels: proteins were detected using the polyclonal anti-GspB serum. Lower panels: proteins were detected using an anti-His6 monoclonal antibody.
Likewise, PS787 derivatives carrying the entire M99 secA2 gene in trans (PS797) or having the integrated M99 secA2 3′ segment (PS799) secreted markedly increased quantities of HsaH6 into the culture medium and had reduced amounts of the protein retained within the protoplasts (Fig. 8B, lanes 3 and 4). The combined results indicated that Hsa is exported more efficiently in the secA2-complemented strains and demonstrated that the surface expression of Hsa is dependent on components of an accessory Sec system.
Lectins that recognize GlcNAc readily bind Hsa expressed by the secA2-complemented strains.
Lectins are proteins that bind specific carbohydrate moeities on glycoproteins. Lectin affinity has long been used to characterize the carbohydrate constituents of mammalian and plant glycoproteins. Our own studies have shown that GspB, either on the surface of M99 or purified, is recognized by the lectins WGA, succinylated WGA (sWGA), and GSL-II (2). These lectins have an affinity for glycoproteins containing N-acetylglucosamine (GlcNAc), a major constituent of the GspB-associated carbohydrate.
We therefore used a lectin affinity assay to examine whether there were detectable differences in lectin binding to Challis and the secA2-complemented strains (Fig. 9). Binding of WGA and sWGA to the parental Challis strain was slightly higher than to strains lacking Hsa expression (PS779 and PS796), whereas binding of GSL-II was not significantly different among these strains (P > 0.05). The secA2-complemented strains (PS795 and PS798) showed greatly increased affinities for WGA, sWGA, and GSL-II (P < 0.0001). Increased surface expression of Hsa by these strains did not affect the affinity for concanavalin A (a lectin that recognizes glycoproteins containing glucose and mannose but that is also known to react with bacterial cell wall carbohydrates). The results suggested that Hsa is likely to be glycosylated with GlcNAc, as was found for GspB. Furthermore, the findings supported the results of Western blot analyses which indicated that there are quantitative differences in the amount of Hsa expressed on the surface of Challis versus the derivative strains.
FIG. 9.
Lectin binding to S. gordonii strain Challis and various derivative strains. Biotinylated forms of the indicated lectins were assessed for binding to bacteria that were immobilized in 96-well plates. WGA, wheat germ agglutinin (affinity for sialic acid and GlcNAc); sWGA, succinylated wheat germ agglutinin (affinity for GlcNAc); GSL-II, Griffonia simplicifolia lectin II (affinity for terminal GlcNAc); ConA, concanavalin A (affinity for glucose and mannose).
secA2-complemented Challis derivative strains show increased binding to sialylated glycoproteins.
The effect of increased Hsa export on binding to sialylated glycoproteins was next examined (Fig. 10). The parental Challis strain had levels of fetuin binding that were fourfold greater than those seen with the Hsa-nonexpressing strains PS779 and PS796 (P < 0.0001). Increased surface expression of Hsa by the secA2-complemented strains PS795 and PS798 resulted in a marked increase in fetuin binding. Both strains showed a 30-fold increase in fetuin binding compared with the Hsa− mutant strains and an 8-fold increase compared with Challis (P < 0.0001). Binding of each of the derivative strains to asialofetuin was not significantly different from that of Challis (P > 0.05). The increased binding of PS795 and PS798 to fetuin was not due to effects of secA2 complementation on events other than Hsa export and surface expression. Binding of strain PS797 was not significantly different from that of Challis (P = 0.8), and binding of strain PS799 was significantly lower than that of Challis (P < 0.0001). Of note, PS797 and PS799 carry the M99 secA2 sequences in trans and in cis, respectively, but have no cell wall-anchored Hsa. Thus, the increased binding of PS795 and PS798 to sialic acid appears to be solely due to increased Hsa surface expression.
FIG. 10.
Binding of Challis and derivative strains to immobilized fetuin and asialofetuin. Microtiter wells were coated with 50 μg of either protein. Binding is expressed as the mean ± standard deviation of the results of three independent experiments. For each of the strains, approximately 0.2% of the inoculum bound to wells that had been treated only with the blocking reagent.
The effect of the sialic acid linkage conformation on Hsa-mediated binding was also examined. For this and subsequent analyses, PS798 was used as the representative secA2-complemented strain, since the integrated M99 secA2 fragment is maintained in the absence of antibiotic selection (unpublished results). In this assay, Challis showed comparable levels of binding to sialyllactose configured in α(2-3) versus α(2-6) linkages (Fig. 11A). However, these levels of binding were not significantly different from that of the hsa mutant strain PS779 (P > 0.05 for all comparisons). Enhanced surface expression of Hsa led to a 10-fold increase in binding to α(2-3) sialyllactose and 2-fold-higher binding to α(2-6) sialyllactose (P < 0.0001). This corresponded to a fourfold preference for α(2-3) versus α(2-6) linkages, or a relative binding ratio of 4:1. Thus, Hsa imparts a greater bias towards the α(2-3) conformation than does GspB (relative binding ratio of 1.7:1).
FIG. 11.
Effect of Hsa expression on binding to sialyllactose and glycocalicin. Binding is expressed as the mean ± standard deviation of the results of two independent experiments. (A) Microtiter wells were coated with 5 μg of either NeuAcα(2-3)Galβ(1-4)-Glc or NeuAcα(2-6)Galβ(1-4)Glc multivalently conjugated to human serum albumin. (B) Microtiter wells were coated with 1.25 μg of either glycocalicin or desialylated glycocalicin.
Hsa mediates binding to GPIbα.
We also examined whether increased Hsa export affected binding to purified glycocalicin. As with GspB-dependent binding, Hsa expression by PS798 conferred a 20-fold increase in glycocalicin binding compared with the hsa mutant strain PS779 (Fig. 11B). When sialic acid was removed from the glycocalicin, binding of these strains was reduced to near background levels. To further examine the role of sialic acid in the binding of PS798 to glycocalicin, the ability of sugars to block binding was examined (Fig. 12). Unlike the effect of N-acetyl neuraminic acid on the binding of M99 to glycocalicin, the level of binding of PS798 to glycocalicin in the presence of the monosaccharide was just slightly lower than the level of binding seen in the absence of the sugar (mean reduction of 13%; P = 0.02). However, sialyllactose was highly effective at inhibiting the binding of PS798 to GPIb (a mean reduction of 96%). As was seen with M99, lactose had no effect on the binding of PS798 to glycocalicin (P = 0.10). These findings indicate that Hsa mediates binding to sialic acid residues on GPIbα and that the interaction of Hsa with GPIbα is subtly different from that of GspB.
FIG. 12.
Inhibition of PS798 binding to glycocalicin by carbohydrates. Wells were coated with 1.25 μg of glycocalicin. Binding is expressed as the mean ± standard deviation of the results of two independent experiments. NANA, N-acetyl neuraminic acid.
DISCUSSION
The combined results of the comparative analyses presented here indicate that there are similarities, as well as distinct differences, in the contributions of GspB and Hsa to adherence. As was reported for Hsa, expression of GspB confers an ability to bind sialic acid, with a preference for α(2-3) linkages. However, whereas Hsa imparts a 4-fold-higher binding to α(2-3)- versus α(2-6)-linked sialic acid, GspB imparts just a 1.7-fold-higher binding to α(2-3) versus α(2-6) linkages. Of particular relevance to the pathogenesis of infective endocarditis, GspB and Hsa both mediate binding to sialic acid residues on platelet membrane GPIbα. GPIb is therefore likely to be a major receptor for S. gordonii on platelets.
It is probable that the optimal receptors for Hsa and GspB differ slightly in the structure of the sialylated carbohydrate. That is, Hsa (as expressed by PS798) and GspB contribute comparably to glycocalicin binding (20-fold-higher binding than the respective mutant strains). However, Hsa mediates higher levels of binding to fetuin and albumin-conjugated sialyllactose than does GspB, as determined by either the percentage of the inoculum that binds or by the increase in binding compared with the corresponding mutant strain. This implies that the carbohydrate epitopes on glycocalicin to which GspB and Hsa bind may differ slightly. Nearly all of the polysaccharide chains linked to glycocalicin and fetuin terminate in sialic acid. In both glycocalicin and fetuin, the terminal branches of the N-linked structures consist of NeuAcα(2-3)Galβ(1-4)GlcNAc or NeuAcα(2-6)Galβ(1-4)GlcNAc (sia-lyllactosamine). However, the N-linked structures on glycocalicin are tetraantennary (Fig. 4C), whereas on fetuin they are triantennary. A second difference is that the O-linked oligosaccharides on glycocalicin (Fig. 4B) consist primarily of the branched hexasaccharide NeuNAcα(2-3)Galβ(1-3)[NeuAcα(2-3)Galβ(1-4)GlcNAcβ(1-6)]GalNAc along with minor amounts of the tetrasaccharide NeuNAcα(2-3)Galβ(1-3)[NeuAcα(2-6)]GalNAc and the trisaccharide NeuNAcα(2-3)Galβ(1-3)GalNAc. Conversely, fetuin primarily bears the trisaccharide and tetrasaccharide structures, with only minor amounts of the hexasaccharide. It is possible, therefore, that the O-linked hexasaccharide may be a preferred target for GspB, whereas the N-linked sialyllactosamine may be a preferred target for Hsa.
Results of other studies suggest that GPIb may be a common target on platelets for direct binding by viridans group streptococci. Of note, S. sanguis strain 133-79 has been shown to induce platelet aggregation via an interaction with GPIb (10). In that study, the interaction was localized to the amino-terminal portion of GPIbα (amino acids 1 to 225), suggesting that the epitopes on GPIb to which S. sanguis and S. gordonii adhere may be different. In a separate report, the O-linked trisaccharide NeuAcα(2-3)Galβ(1-3)GalNAc has been defined as a receptor for S. sanguis OMZ 9 on buccal epithelial cells (16). It would be interesting to determine whether S. sanguis OMZ 9 can also bind GPIbα and whether binding by these S. sanguis strains is mediated by a homologue of GspB and Hsa.
Confirmation of the differences in affinity of GspB and Hsa for sialylated glycoproteins will need to be assessed by using a purified protein-receptor system. While glycocalicin appears to be the best target receptor for such measurements, purification of full-length GspB has been problematic due to the low solubility of this heavily glycosylated protein. However, efforts to overexpress soluble forms of GspB, and to identify and characterize the binding domain of this adhesin, are in progress.
An unanticipated finding that has emerged from these studies is that wild-type Challis, commonly used as a representative S. gordonii strain, exports Hsa very inefficiently. This export defect is, at least in part, due to a nonsense mutation in secA2, a mutation that is not apparent in any of the other secY2/A2 loci of streptococci and staphylococci (3, 27). Correction of the defect by complementation with M99 secA2 sequences leads to a substantial increase in surface expression of Hsa, with a concomitant increase in sialic acid binding. The precise relationship between Hsa exported by Challis and Hsa exported by the complemented strains is difficult to determine because of the extremely small amounts of Hsa exported by Challis. However, our studies indicate that in the absence of a functional accessory Sec system, GspB and Hsa may not be properly processed and/or glycosylated (unpublished results). It is possible, therefore, that Hsa exported by the secA2-complemented strains PS795 and PS798 may have binding properties that differ from those of Hsa exported by Challis.
The relatively low levels of binding to fetuin by M99 and Challis may be significant. It is reasonable to speculate that indiscriminant high-affinity binding to certain sialylated compounds may be detrimental to survival in the bloodstream and that these two endocarditis-associated S. gordonii strains may have found different means to modulate affinity. The preferential binding of M99 to GPIb (compared with M99 binding to other sialylated glycoproteins) may enable the selective targeting of this organism to host tissues. Such specificity could allow the colonization of platelet-coated valve surfaces while avoiding adherence to cells or surfaces that might promote clearance. In comparison, Challis binds relatively poorly to both fetuin and GPIb, whereas PS798 adheres extremely well to both of these sialylated glycoproteins. Future studies using animal models of infection should help to determine whether these differences in binding affect virulence.
Acknowledgments
This work was supported by grants R01AI41513, R01AI057433, and P50-HL65967 from the National Institutes of Health and by the Department of Veterans Affairs.
We thank Prasenjit Guchhait for preparation of the glycocalicin.
Editor: J. N. Weiser
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 2.Bensing, B. A., B. W. Gibson, and P. M. Sullam. 2004. The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export. J. Bacteriol. 186:638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44:1081-1094. [DOI] [PubMed] [Google Scholar]
- 4.Berndt, M. C., C. Gregory, A. Kabral, H. Zola, D. Fournier, and P. A. Castaldi. 1985. Purification and preliminary characterization of the glycoprotein Ib complex in the human platelet membrane. Eur. J. Biochem. 151:637-649. [DOI] [PubMed] [Google Scholar]
- 5.Bryan, E. M., T. Bae, M. Kleerebezem, and G. M. Dunny. 2000. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid 44:183-190. [DOI] [PubMed] [Google Scholar]
- 6.Claverys, J. P., A. Dintilhac, E. V. Pestova, B. Martin, and D. A. Morrison. 1995. Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene 164:123-128. [DOI] [PubMed] [Google Scholar]
- 7.Denis, M., P. D. Palatty, N. R. Bai, and S. J. Suriya. 2003. Purification and characterization of a sialic acid specific lectin from the hemolymph of the freshwater crab Paratelphusa jacquemontii. Eur. J. Biochem. 270:4348-4355. [DOI] [PubMed] [Google Scholar]
- 8.Edge, A. S., and R. G. Spiro. 1987. Presence of an O-glycosidically linked hexasaccharide in fetuin. J. Biol. Chem. 262:16135-16141. [PubMed] [Google Scholar]
- 9.Fischetti, V. A., V. Pancholi, and O. Schneewind. 1990. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol. Microbiol. 4:1603-1605. [DOI] [PubMed] [Google Scholar]
- 10.Kerrigan, S. W., I. Douglas, A. Wray, J. Heath, M. F. Byrne, D. Fitzgerald, and D. Cox. 2002. A role for glycoprotein Ib in Streptococcus sanguis-induced platelet aggregation. Blood 100:509-516. [DOI] [PubMed] [Google Scholar]
- 11.Korrel, S. A., K. J. Clemetson, H. van Halbeek, J. P. Kamerling, J. J. Sixma, and J. F. Vliegenthart. 1988. Identification of a tetrasialylated monofucosylated tetraantennary N-linked carbohydrate chain in human platelet glycocalicin. FEBS Lett. 228:321-326. [DOI] [PubMed] [Google Scholar]
- 12.Korrel, S. A., K. J. Clemetson, H. Van Halbeek, J. P. Kamerling, J. J. Sixma, and J. F. Vliegenthart. 1984. Structural studies on the O-linked carbohydrate chains of human platelet glycocalicin. Eur. J. Biochem. 140:571-576. [DOI] [PubMed] [Google Scholar]
- 13.Lopez, J. A. 1994. The platelet glycoprotein Ib-IX complex. Blood Coagul. Fibrinolysis 5:97-119. [PubMed] [Google Scholar]
- 14.Macrina, F. L., R. P. Evans, J. A. Tobian, D. L. Hartley, D. B. Clewell, and K. R. Jones. 1983. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene 25:145-150. [DOI] [PubMed] [Google Scholar]
- 15.McNab, R., and H. F. Jenkinson. 1998. Lipoproteins and other cell-surface associated proteins in streptococci. Methods Cell Sci. 20:209-216. [Google Scholar]
- 16.Neeser, J. R., R. C. Grafstrom, A. Woltz, D. Brassart, V. Fryder, and B. Guggenheim. 1995. A 23 kDa membrane glycoprotein bearing NeuNAc alpha 2-3Gal beta 1-3GalNAc O-linked carbohydrate chains acts as a receptor for Streptococcus sanguis OMZ 9 on human buccal epithelial cells. Glycobiology 5:97-104. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson, B., N. E. Norden, and S. Svensson. 1979. Structural studies on the carbohydrate portion of fetuin. J. Biol. Chem. 254:4545-4553. [PubMed] [Google Scholar]
- 18.Romo, G. M., J. F. Dong, A. J. Schade, E. E. Gardiner, G. S. Kansas, C. Q. Li, L. V. McIntire, M. C. Berndt, and J. A. Lopez. 1999. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J. Exp. Med. 190:803-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggeri, Z. M. 1991. The platelet glycoprotein Ib-IX complex. Prog. Hemost. Thromb. 10:35-68. [PubMed] [Google Scholar]
- 20.Schneewind, O., D. Mihaylova-Petkov, and P. Model. 1993. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J. 12:4803-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneewind, O., P. Model, and V. A. Fischetti. 1992. Sorting of protein A to the staphylococcal cell wall. Cell 70:267-281. [DOI] [PubMed] [Google Scholar]
- 22.Stephenson, A. E., H. Wu, J. Novak, M. Tomana, K. Mintz, and P. Fives-Taylor. 2002. The Fap1 fimbrial adhesin is a glycoprotein: antibodies specific for the glycan moiety block the adhesion of Streptococcus parasanguis in an in vitro tooth model. Mol. Microbiol. 43:147-157. [DOI] [PubMed] [Google Scholar]
- 23.Sullam, P. M., F. H. Valone, and J. Mills. 1987. Mechanisms of platelet aggregation by viridans group streptococci. Infect. Immun. 55:1743-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi, Y., K. Konishi, J. O. Cisar, and M. Yoshikawa. 2002. Identification and characterization of hsa, the gene encoding the sialic acid-binding adhesin of Streptococcus gordonii DL1. Infect. Immun. 70:1209-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi, Y., A. L. Sandberg, S. Ruhl, J. Muller, and J. O. Cisar. 1997. A specific cell surface antigen of Streptococcus gordonii is associated with bacterial hemagglutination and adhesion to α2-3-linked sialic acid-containing receptors. Infect. Immun. 65:5042-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi, Y., A. Yajima, J. O. Cisar, and K. Konishi. 2004. Functional analysis of the Streptococcus gordonii DL1 sialic acid-binding adhesin and its essential role in bacterial binding to platelets. Infect. Immun. 72:3876-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takamatsu, D., B. A. Bensing, and P. M. Sullam. 2004. Genes in the accessory sec locus of Streptococcus gordonii have three functionally distinct effects on the expression of the platelet-binding protein GspB. Mol. Microbiol. 52:189-203. [DOI] [PubMed] [Google Scholar]
- 28.Wu, H., and P. M. Fives-Taylor. 1999. Identification of dipeptide repeats and a cell wall sorting signal in the fimbriae-associated adhesin, Fap1, of Streptococcus parasanguis. Mol. Microbiol. 34:1070-1081. [DOI] [PubMed] [Google Scholar]