Abstract
Individuals living in regions where malaria is endemic develop an acquired immunity to malaria which enables them to remain asymptomatic while still carrying parasites. Field studies indicate that cumulative exposure to a variety of diverse Plasmodium parasites is required for the transition from symptomatic to asymptomatic malaria. This study used a simulation model of the within-host dynamics of P. falciparum to investigate the development of acquired clinical immunity under different transmission conditions and levels of parasite diversity. Antibodies developed to P. falciparum erythrocyte membrane protein 1 (PfEMP1), a clonally variant molecule, were assumed to be a key human immunological response to P. falciparum infection, along with responses to clonally conserved but polymorphic antigens. The time to the development of clinical immunity was found to be proportional to parasite diversity and inversely proportional to transmission intensity. The effect of early termination of symptomatic infections by chemotherapy was investigated and found not to inhibit the host's ability to develop acquired immunity. However, the time required to achieve this state was approximately double that compared to when no treatment was administered. This study demonstrates that an immune response primarily targeted against PfEMP1 has the ability to reduce clinical symptoms of infections irrespective of whether treatment is administered, supporting its role in the development of acquired clinical immunity. The results also illustrate a novel use for simulation models of P. falciparum infections, investigation of the influence of intervention strategies on the development of naturally acquired clinical immunity.
Plasmodium falciparum malaria is a common disease in many tropical countries. The majority of clinical P. falciparum cases are uncomplicated, but some individuals will develop complications, including severe anemia, coma, and death (24). These complications are more prevalent in populations such as the young, pregnant women, and nonimmune adults. Residents of regions where malaria is endemic acquire partial immunity to the disease that reduces the frequency of clinical attacks, although not necessarily reducing parasite prevalence (14). This antidisease immunity is not sterile and requires continual boosting. The age at which acquired immunity becomes evident varies depending on the level of exposure, with higher exposure resulting in earlier development of clinical immunity (26). Children living in areas of high endemicity experience less frequent episodes of malaria after the age of 5 years (26), while people in areas of hypoendemicity may never develop clinical immunity. Since clinical immunity to malaria is highly strain specific (30, 37), the speed with which immunity develops is inversely correlated to the size and diversity of the parasite population. It has also been shown that the rate of the development of clinical immunity correlates with the length of infection; asymptomatic status is reached sooner when the infections are longer (30).
Asexual malaria parasites exist in two distinct stages within the human host, a developmental stage in hepatocytes and the blood-stage parasites. Immune responses are developed to both stages (7, 31, 35), but long-term antidisease immunity has been linked to exposure to blood-stage parasites (36). During blood-stage infections, parasites insert a number of proteins into the membranes of infected erythrocytes (15). Although many of these proteins are immunogenic (8, 13, 16), the gradual development of acquired immunity and its reliance on repeated exposure suggest that proteins that undergo antigenic variation may be fundamental to the development of acquired immunity (9, 27). P. falciparum erythrocyte membrane protein 1 (PfEMP1) is the best characterized of these proteins.
PfEMP1 is a clonally variant protein encoded by ≈60 var genes per parasite (17). The development of specific antibody responses to PfEMP1 variants occurs after exposure to parasites expressing those variants (10, 20, 21), with sera from individuals from regions where malaria is hyperendemic able to recognize many variants (12, 28).
Some of the intervention factors potentially affecting the development of clinical immunity have been investigated. While the effect of insecticide-treated bed nets is relatively easy to investigate in field studies (1, 36), other factors such as repeated chemotherapy are not as easily assessed in field studies due to ethical considerations. In these circumstances, mathematical modeling is one of the few tools available to investigate the effect that various intervention measures may have on the development of acquired immunity.
This study explores the development of acquired clinical immunity with a computer simulation model of P. falciparum infections within individuals. The immune response incorporated is skewed towards the production of anti-PfEMP1 antibodies and demonstrates that clinical immunity develops with repeated exposure to a variety of parasites. The time required to develop this immunity was found to be proportional to parasite diversity and inversely proportional to transmission intensity, features commonly found in field studies. Simulation of repeated chemotherapy of symptomatic infections indicated that clinical immunity took almost twice as long to develop as in infections where no treatment was provided. This study demonstrates that an immune response primarily targeting PfEMP1 has the ability to reduce clinical symptoms of infection irrespective of whether treatment is administered, supporting its role in the development of acquired clinical immunity.
MATERIALS AND METHODS
Model.
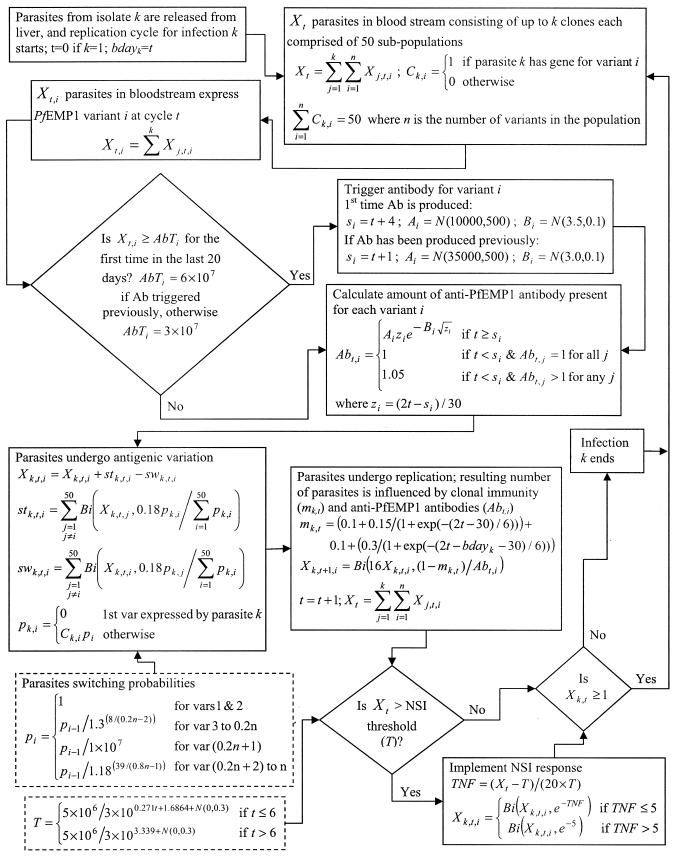
The simulation model reported previously (19) was used to mimic the within-host dynamics of P. falciparum infections. A 48-h time step is used in the model in line with the replication cycle of the parasite. Because this model was developed and calibrated with data from malaria-naïve hosts, several amendments were made to the assumptions of the model in order to incorporate repeated infections and the corresponding immune responses. These changes are summarized below, with details of the model given in Fig. 1.
FIG. 1.
Flowchart of the simulation model used to mimic the within-host dynamics of P. falciparum infections. Bi(a,b) represents the use of a binomial distribution with sample size a and probability b. When a was large, a normal distribution was used as an approximation of the binomial. N(a,b) represents the use of a normal distribution with mean a and standard deviation b. Ab, antibody; NSI, nonspecific immunity.
The model incorporated three types of immune responses; a nonspecific immune response that is responsible for the development of symptoms such as fever, a clonal immune response that is parasite specific and acts to reduce the net replication rate of the parasites, and an anti-PfEMP1 antibody immune response. The anti-PfEMP1 antibody response is produced after the number of parasites expressing a specific variant exceeded a given threshold and the corresponding time lag had elapsed. This time lag incorporated the time required for the stimulation and development of antibody. When a PfEMP1 variant had not previously elicited an immune response within the host, the threshold was assumed to be 12 parasites/μl, based on field data that antibody is produced prior to or about the time that clinical symptoms develop (27). A lag of 7 days was used between the triggering and the production of antibody. Following basic immunological principles that secondary antibody responses are triggered more rapidly with less antigen, these values were reduced to 6 parasites/μl and 2 days, respectively. The model assumed limited cross-reactivity between anti-PfEMP1 antibodies with antibodies to a given variant killing approximately 5% of parasites expressing other variants.
The function describing the clonal immune response consisted of two components. The first represented the response to conserved malaria antigens, and the second was a response to the antigens presented by a particular parasite genotype. Both components act to reduce the number of merozoites that survive one complete replication cycle, causing a reduction in the effective growth rate of the parasites. The first component develops during the initial infection and is maintained through subsequent infections, while the second component is assumed to be unique to each parasite genotype and is triggered by each new infection. Immune responses to parasite antigens other than PfEMP1 were grouped under this immune response.
To simulate infections by different parasites, it was assumed that there existed a pool of PfEMP1 variants (each encoded by a var gene) within the parasite population. Although it has been hypothesized that strong immune pressure is likely to result in independently transmitted strains (22), molecular studies of PfEMP1 support an alternative scenario of frequent gene recombination (38) occurring in randomly mating P. falciparum parasites (2). This scenario of frequent and random recombination of var genes is assumed in the model. The var genes within the population were assigned to one of two groups; 20% formed a fast-switching group, while the remaining 80% of the genes were classified as slow switching. The overall switch rate and the range of switching rates within the fast and slow groups were reported previously (19), but the probabilities of switching for each gene were adjusted to account for the large number of genes in the population compared to within one parasite. Fifty var genes were randomly selected from those in the population for each infecting parasite. This random allocation caused the characteristics of each parasite to differ, particularly with respect to the number of fast-switching variants in its genome.
Infections were initiated in the model based on a specified infection rate. Prior to simulating the first infection, the model randomly assigned the days on which infection occurred based on the nominated infection rate. This was done by comparing random numbers to the infection rate and declaring that an infection started on the designated day if the random number was less than the infection rate. If this was not the case, the process was repeated for the next infection period (48 h later). The first infection always commenced on day 0, with subsequent infections starting on the allocated days. Up to five infections were permitted to commence on the same day, and there was no limit on the number of superinfections permitted within the host at one time. The model initially assumed a malaria-naïve host with no existing immunity.
Treatment was incorporated into the model by terminating all infections within the host when a fever occurred. It was assumed that every treatment was 100% effective and that no drug resistance was present.
The aim of this study was to investigate the onset of clinical immunity. For this purpose, clinical immunity was defined as existing when less than 5% of simulations had more than 1 day of fever (as defined in reference 19) in the first 30 days after an infection commenced. This definition allowed an immune person to develop an occasional fever.
Simulations.
Simulations were conducted for a variety of infection rates; the average intervals between infections were 14, 30, 60, 90, 150, and 200 days. The effect of parasite diversity was incorporated by changing the number of var genes present within the parasite population (500, 1,000, 1,500, and 2,000). Under the random-mating assumption, a population with 500 var genes (or PfEMP1 variants) would have originated from 10 unique parasite clones, while a pool of 2,000 variants would have originated from 40 unique parasite clones.
Each simulation mimicked up to 100 consecutive infections within the same host over a period of <20,000 days. The actual duration of the simulation varied depending on the specific infection rate, but all simulations were conducted for periods in excess of the time required for 95% of the last simulated infections to terminate. Simulations were conducted for two extreme situations: when no chemotherapy was administered during the simulation period, and when chemotherapy was applied whenever a fever was detected.
We conducted 500 simulations of the model for each of the 24 diversity-transmission combinations. From each simulation, a number of summary variables were extracted, including the number of days with parasite densities of >1,000 and >10,000/μl, length of the infection (days), number of days with a fever (as defined in reference 19), and maximum parasitemia. Data for all variables were obtained from model output for the 30 days following each infection and categorized by the infection number. An additional summary variable, the proportion of simulations in which an infection developed successfully (lasted >10 days) was calculated for each infection. A regression equation of the form y = y0 + a/{1 + exp[−(x − x0)/b]}, where x is the infection number, y is the proportion of infections that developed successfully, and a, b, x0, and y0 are fitted constants, was fitted to these data by using SigmaPlot (version 4.00; SPSS Inc.) to obtain an estimate of the asymptote (y0) for the proportion of infections that developed successfully.
RESULTS
Simulations with no chemotherapy.
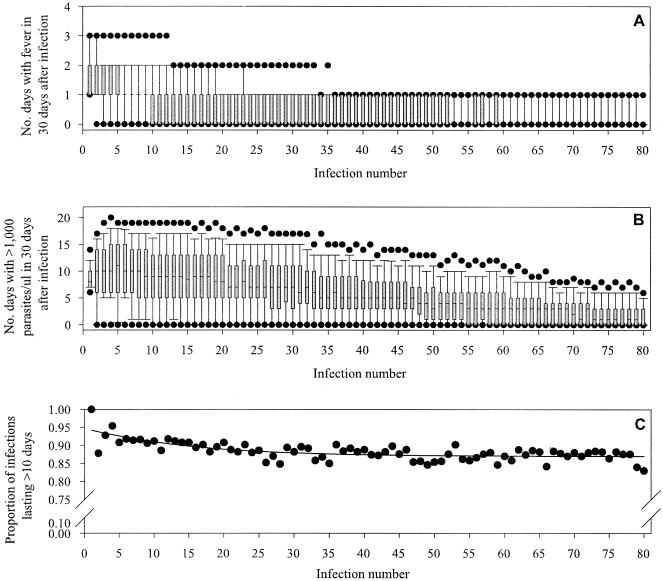
The number of occurrences of fever decreased with increasing infection number for all diversity-transmission combinations examined (Fig. 2). This pattern was also noted for the number of days with parasite densities of >1,000 and 10,000/μl (Fig. 2) and maximum parasitemia (data not shown).
FIG. 2.
Sample of output from 500 simulations with an infection approximately every 60 days and a population of 1,000 var genes. A) Number of fever occurrences, B) number of days with parasite density of >1,000/μl, and C) proportion of infections that were successful as blood-stage infections lasting more than 10 days. Shaded regions in A and B represent the interquartile range of the simulated data; dots represent the 5th and 95th percentiles. In C, the line represents the fitted regression model with an asymptote of 0.87.
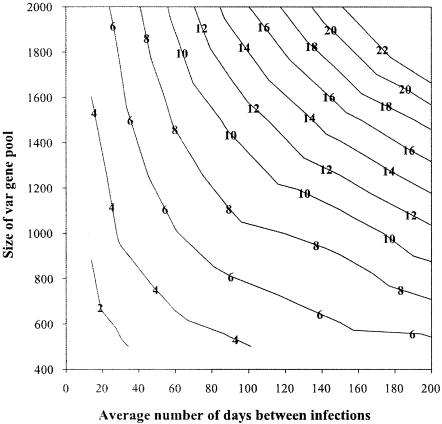
The number of infections required before immunity developed increased with parasite diversity and transmission rate. However, the average time taken to achieve clinical immunity (number of infections multiplied by transmission rate) decreased with increasing transmission (Fig. 3). At the onset of clinical immunity, between 34 and 70% of the simulations had no fever associated with infection. The highest values (67 to 70%) were associated with infections occurring every 14 days on average, even though superinfection was common at this transmission level. The lowest rates (34 to 39%) corresponded to less frequent infections occurring on average every 200 days.
FIG. 3.
Relationship between parasite diversity (size of the var gene pool) and transmission rate (expressed as the average number of days between infections) and the time (years) required to develop clinical immunity (contours) in the absence of chemotherapy.
Once immunity was established, all diversity-transmission combinations indicated that not every infection that was initiated developed into a successful blood-stage infection lasting more than 10 days. The highest proportion of successful infections (0.98) was associated with the combination of largest parasite diversity and lowest transmission rate. Conversely, the low-diversity and high-transmission setting provided the lowest success rate (<0.70). The proportion of infections that failed to develop almost perfectly reflected the ratio of the number of circulating anti-PfEMP1 variant antibodies within the host to the total number of var genes within the population. For the highest infection rate considered (one infection approximately every 14 days), a regression model was not fitted to the proportion of successful infections because the proportion of successful infections oscillated over time. These oscillations were greatest when combined with the lowest values of parasite diversity. This feature is primarily due to infections generating anti-PfEMP1 antibodies to a large proportion of the PfEMP1 variants within the population in a short time, causing the subsequent number of successful infections to decrease. Since most of these antibodies are developed over a relatively short period, they tend to wane at approximately the same time, which leaves the door open for new infections. These new infections again create a burst of antibody activity, and the cycle repeats itself.
Effect of chemotherapy on development of immunity.
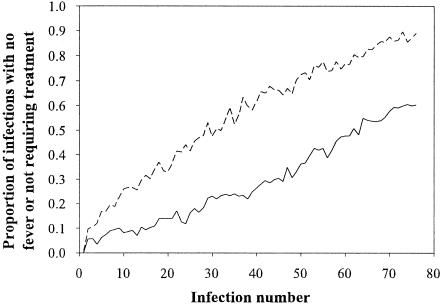
Simulations investigating the effect of repeated chemotherapy on the development of clinical immunity were only conducted at infection rates of one infection every 30 and 60 days. To compare the rate of development of clinical immunity between untreated and treated populations, the proportion of infections occurring with no fever in the untreated population was compared to the proportion of infections not requiring treatment in the treated population (since chemotherapy was administered whenever a fever presented) (Fig. 4, Table 1). In every case, the treated population showed a trend of decreasing treatment rate with increasing number of infections. However, the proportion of infections with fever requiring treatment (in the treated population) was always more than the number of fever occurrences in the untreated population. On average, the time required for 60% of simulations to not require treatment was double that for an equivalent immunological state to be achieved in the untreated population (Table 1).
FIG. 4.
Comparison of the number of infections without a fever (untreated population; dotted line) and the number not requiring treatment (treated population; solid line) for an infection rate of one infection every 60 days and a population of 1,000 var genes.
TABLE 1.
Number of infections required before 60% of simulations do not have a fever associated with infection or do not require chemotherapy for the untreated and treated populations, respectively
| No. of var genes in population | Avg interval between infections (days) | No. of infections (time in yrs) required for >60% of simulations to have no fever or require no treatment
|
Increase in time to reach 60% thresholda (-fold) | |
|---|---|---|---|---|
| Untreated | Treated | |||
| 500 | 30 | 21 (1.7) | 45 (3.7) | 2.2 |
| 60 | 19 (3.1) | 36 (5.9) | 1.9 | |
| 1,000 | 30 | 45 (3.7) | 79 (6.5) | 1.8 |
| 60 | 37 (6.1) | 73 (12.0) | 2.0 | |
Treated population compared to untreated population.
DISCUSSION
The development of acquired clinical immunity is an important feature of P. falciparum malaria because it reduces the frequency of symptomatic episodes and thus disease-associated morbidity and mortality. It is generally accepted that the gradual development of this immunity is directly related to the requirement that the host's immune system be repeatedly exposed to a wide variety of diverse parasite antigens. The development of immunity is therefore related to the transmission intensity and diversity of parasites. These two parameters often have a direct correlation, i.e., high transmission intensity is accompanied by a large parasite population repertoire with high diversity. The combined effect of these parameters on the speed of immunity development was the focus of this theoretical investigation. The outcome of the modeling indicated that increasing parasite diversity by increasing the number of unique PfEMP1 proteins within a population increases the time required to achieve immunity irrespective of transmission intensity. This is an intuitively obvious result if one assumes that disease occurs as a result of holes in the antigenic repertoire recognized by the host. Likewise, the model output indicated that increased transmission intensity decreased the time until acquired immunity developed. This result is supported by field observations of differences in the age when acquired immunity develops between areas of different transmission intensity (26), with older ages predicted under low-transmission-intensity conditions.
Although transmission intensity and parasite diversity both impact on the time required before immunity develops, parasite diversity was predicted to have a larger influence than transmission rate. For instance, a 20% increase in diversity (with transmission intensity remaining constant) was predicted to increase the time required to develop immunity by between ≈13 and 38%, depending on the initial level of diversity and transmission. In comparison, a decrease of 20% in the transmission rate (keeping diversity constant) increased the predicted time to immunity by ≈5 to 18%.
The parameters used in the model were calibrated based on infections of neurosyphilis patients with the P. falciparum El Limon and Santee Cooper parasite strains. The relationship between this parameter set and the characteristics of field isolates remains to be determined, but it has already been noted that differences exist in the pyrogenic thresholds (18), maximum parasitemia (19), and replication rates (34) of different parasites. Additionally, a febrile event has been defined here as a temperature of >40°C. This value is somewhat arbitrary because it is difficult to link the value of model parameters to actual body temperature. Hence, it should be viewed simply as an indicator of fever. As such, the model output represents a generalized system that can be used to look at the factors affecting the development of immunity; these factors would be expected to have the same effects under modified parasite-host parameters.
The model incorporates the detailed process of parasite antigenic variation in PfEMP1 and the corresponding host immune response. This is not meant to undervalue the contribution of other parasite antigens in the development of acquired clinical immunity. Although not specified individually, the host's immune responses to other parasite antigens are incorporated via the clonal immunity function. Emphasis has been placed on antigenic variation because this parasite feature appears to be one of the primary limiting factors in the speed of development of clinical immunity to a parasite population.
The PfEMP1 switching process and rates assumed in the model are similar to those reported previously to produce simulated infections with characteristics similar to those of observed clinical cases in malaria-naïve hosts (19). Recently it was proposed that a PfEMP1 variant may elicit a long-lived immune response unique to the variant and also short-lived transient responses to one or more shared epitopes (32). Our model currently contains a specific medium-lived antibody response to a particular variant and also a limited amount of cross-reactivity between this antibody and all other variants. The simplest assumption of cross-reactivity was used due to the lack of experimental data related to the degree of cross-reactivity between specific antibodies and PfEMP1 variants and the dynamics of such responses. Changes to the scope and/or magnitude of this cross-reactivity could affect the length of infection, which may in turn impact our predictions relating to the development of immunity. However, any such changes would be expected to be minimal.
Direct extrapolation of the model predictions to field situations requires further validation against field data. This is difficult due to the lack of data containing estimates of transmission intensity, parasite diversity, and age of onset of immunity for a particular field site. Additionally, the model assumes constant infection rates, while many regions exhibit seasonal transmission. There are, however, a limited set of field sites for which data can be extrapolated to validate the model output.
In western Kenya, the entomologic inoculation rate varied from ≈0.02 to 10.0 infected bites per person per night (equivalent to one infectious bite every 50 and 0.1 days, respectively), with at least 43 different merozoite surface protein 1 alleles detected (6). In very broad terms, this equates to ≈2,150 different PfEMP1 variants within the population, assuming that each parasite sampled had a unique repertoire of 50 var genes. In this setting, >10% of children 3 years of age experienced febrile outbreaks (6). The model output from simulations of a similar transmission environment (one infection every 14 days; 2,000 var genes in the population) predicted that the onset of immunity would occur at approximately 5 years of age. This value appears to be roughly in the correct time frame, since clinical immunity at the field site (by our definition) occurred after 3 years of age. Additionally, the actual infection rate is probably lower than the estimated entomologic inoculation rate because not all bites from sporozoite-infected mosquitoes result in infections (11, 33).
At the other end of the spectrum, Morong in the Phillipines has a much lower prevalence of P. falciparum. Although much of the region has little or no malaria, ≈5% of the population live in higher-transmission areas which have a point prevalence of ≈10% (5). Assuming a patency of 15 to 20 days before detection, this equates to an average probability of infection on the order of 0.005 to 0.006 per person per day, or one infection approximately every 160 to 200 days. Antibody seropositivity against P. falciparum antigen appears to plateau in this population at approximately 10 years of age, although this may not equate to clinical immunity because many adults still suffer symptomatic attacks (5). Parasite diversity within Morong appears to be relatively constrained, with only seven apical membrane antigen 1 sequence types and eight merozoite surface protein 1 sequence types detected in 113 and 103 parasite isolates, respectively (29). Assuming a total parasite reservoir of 10 to 20 different isolates, the var gene population would consist of approximately 500 to 1,000 different genes. According to the model output, with infections occurring approximately every 200 days and a var gene population of 1,000, acquired clinical immunity would be predicted by 11.5 years of age. This age generally reflects the plateau seen in antibody prevalence (5).
It must be noted that it is assumed in the model that the parasite population is constant. It is unlikely that the parasite population meets this assumption over long periods of time in all but the most isolated areas. If new parasites are introduced to a region, it is likely that individuals infected with this new parasite will become ill because they will have no recognition of the PfEMP1 variants expressed by the parasite. This predisposition to cause illness will persist until individuals start to be reinfected with the same parasite or until the antigenic repertoire of the new parasite is diluted through mating with other parasites. This may partially explain why many adults still experience clinical symptoms in regions of low transmission and why occasional epidemics occur in an otherwise immune population.
Studies of malaria incidence among transmigrants moving from regions of almost no malaria to where malaria is endemic suggest that protection can be established after a relatively brief period (4). This appears to contradict our results, but the criteria used to define immunity differ between the studies. Although Baird et al. (3) claim that clinical immunity occurred in individuals experiencing four infections in 2 years, they note that only 36 (62%) of the 58 subjects obtained clinical immunity (parasitemia without fever). Given that the study area was a newly developed village relatively isolated from other villages and all new residents received prophylaxis for 90 days after arrival (25), it is likely that parasite diversity is relatively limited. The model output for infections occurring every ≈150 days (that is, four times in 2 years) with 500 different var genes in the population indicated that after four infections, 21% of the simulated infections resulted in no febrile event, while 64% experienced only one febrile day. This represented a significant decrease in the risk of experiencing a fever after four infections, a finding similar to that of Baird et al. (3).
Use of a simulation model to mimic the development of clinical immunity within a host provided the opportunity to investigate the influence of early termination of infections through chemotherapy. Only the extreme situation of treating every infection that became symptomatic was considered. There was a consistent trend of delayed onset of immunity associated with repeated treatment, such that immunity took almost twice as long to develop compared to when infections were left untreated. This delay is due to the reduced exposure of the host to the range of parasite variant antigens. However, it is important to note that immunity did develop in the treated population. Hence, rapid treatment of symptomatic infections, which can potentially reduce the occurrence of severe complications and anemia, does not exclude the development of clinical immunity; it simply delays its onset. This trend of delayed immunity with frequent administration of drugs has been noted in Africa (23).
This study used a simulation model of the in-host dynamics of P. falciparum infections to explore the factors influencing the development of clinical immunity to P. falciparum malaria. Incorporating antibody responses to PfEMP1 along with general responses to other nonvariant antigens was sufficient to mimic the development of immunity and produce estimates of the age of immunity which appear to generally agree with that observed in the field. This supports the role of anti-PfEMP1 antibodies in the development of immunity. The simulation study also allowed the impact of regular chemotherapy to be assessed, concluding that clinical immunity will develop under such conditions but the age of onset is delayed.
Acknowledgments
This work was supported in part by NIH grant # AI47500-03. M.L.G. is funded by a University of Queensland Postdoctoral Research Fellowship.
The opinions expressed herein are those of the authors and do not necessarily reflect those of the Defense Health Services or any extant Defense policy.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Askjaer, N., C. Maxwell, W. Chambo, T. Staalsoe, M. Nielsen, L. Hviid, C. Curtis, and T. G. Theander. 2001. Insecticide-treated bed nets reduce plasma antibody levels and limit the repertoire of antibodies to Plasmodium falciparum variant surface antigens. Clin. Diagn. Lab. Immunol. 8:1289-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babiker, H. A., L. C. Ranford-Cartwright, D. Currie, J. D. Charlwood, P. Billingsley, T. Teuscher, and D. Walliker. 1994. Random mating in a natural population of the malaria parasite Plasmodium falciparum. Parasitology 109:413-421. [DOI] [PubMed] [Google Scholar]
- 3.Baird, J. K., M. J. Barcus, R. F. Elyazar, M. J. Bangs, J. D. Maguire, D. J. Fryauff, T. L. Richie, Sekartuti, and W. Kalalo. 2003. Onset of clinical immunity to Plasmodium falciparum among Javanese migrants to Indonesian Papua. Ann. Trop. Med. Parasitol. 97:557-564. [DOI] [PubMed] [Google Scholar]
- 4.Baird, J. K., T. R. Jones, E. W. Danudirgo, B. A. Annis, M. J. Bangs, H. Basri, Purnomo, and S. Masbar. 1991. Age-dependent acquired protection against Plasmodium falciparum in people having two years exposure to hyperendemic malaria. Am. J. Trop. Med. Hyg. 45:65-76. [DOI] [PubMed] [Google Scholar]
- 5.Belizario, V. Y., A. Saul, M. D. Bustos, M. A. Lansang, C. J. Pasay, M. Gatton, and N. P. Salazar. 1997. Field epidemiological studies on malaria in a low endemic area in the Philippines. Acta Trop. 63:241-256. [DOI] [PubMed] [Google Scholar]
- 6.Branch, O. H., S. Takala, S. Kariuki, B. L. Nahlen, M. Kolczak, W. Hawley, and A. A. Lal. 2001. Plasmodium falciparum genotypes, low complexity of infection, and resistance to subsequent malaria in participants in the Asembo Bay Cohort Project. Infect. Immun. 69:7783-7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branch, O. H., V. Udhayakumar, A. W. Hightower, A. J. Oloo, W. A. Hawley, B. L. Nahlen, P. B. Bloland, D. C. Kaslow, and A. A. Lal. 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am. J. Trop. Med. Hyg. 58:211-219. [DOI] [PubMed] [Google Scholar]
- 8.Bull, P. C., B. S. Lowe, M. Kortok, and K. Marsh. 1999. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect. Immun. 67:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bull, P. C., and K. Marsh. 2002. The role of antibodies to Plasmodium falciparum-infected-erythrocyte surface antigens in naturally acquired immunity to malaria. Trends Microbiol. 10:55-58. [DOI] [PubMed] [Google Scholar]
- 11.Charlwood, J. D., T. Smith, E. Lyimo, A. Y. Kitua, H. Masanja, M. Booth, P. L. Alonso, and M. Tanner. 1998. Incidence of Plasmodium falciparum infection in infants in relation to exposure to sporozoite-infected anophelines. Am. J. Trop. Med. Hyg. 59:243-251. [DOI] [PubMed] [Google Scholar]
- 12.Chattopadhyay, R., A. Sharma, V. K. Srivastava, S. S. Pati, S. K. Sharma, B. S. Das, and C. E. Chitnis. 2003. Plasmodium falciparum infection elicits both variant-specific and cross-reactive antibodies against variant surface antigens. Infect. Immun. 71:597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conway, D. J., D. R. Cavanagh, K. Tanabe, C. Roper, Z. S. Mikes, N. Sakihama, K. A. Bojang, A. M. Oduola, P. G. Kremsner, D. E. Arnot, B. M. Greenwood, and J. S. McBride. 2000. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat. Med. 6:689-692. [DOI] [PubMed] [Google Scholar]
- 14.Cox, M. J., D. E. Kum, L. Tavul, A. Narara, A. Raiko, M. Baisor, M. P. Alpers, G. F. Medley, and K. P. Day. 1994. Dynamics of malaria parasitaemia associated with febrile illness in children from a rural area of Madang, Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 88:191-197. [DOI] [PubMed] [Google Scholar]
- 15.Craig, A., and A. Scherf. 2001. Molecules on the surface of the Plasmodium falciparum infected erythrocyte and their role in malaria pathogenesis and immune evasion. Mol. Biochem. Parasitol. 115:129-143. [DOI] [PubMed] [Google Scholar]
- 16.Dodoo, D., T. G. Theander, J. A. Kurtzhals, K. Koram, E. Riley, B. D. Akanmori, F. K. Nkrumah, and L. Hviid. 1999. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect. Immun. 67:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. A. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatton, M. L., and Q. Cheng. 2002. Evaluation of the pyrogenic threshold for Plasmodium falciparum malaria in naive individuals. Am. J. Trop. Med. Hyg. 66:467-473. [DOI] [PubMed] [Google Scholar]
- 19.Gatton, M. L., and Q. Cheng. 2004. Investigating antigenic variation and other parasite-host interactions in Plasmodium falciparum infection in naïve hosts. Parasitology 128:367-376. [DOI] [PubMed] [Google Scholar]
- 20.Giha, H. A., T. Staalsoe, D. Dodoo, I. M. Elhassan, C. Roper, G. M. Satti, D. E. Arnot, T. G. Theander, and L. Hviid. 1999. Nine-year longitudinal study of antibodies to variant antigens on the surface of Plasmodium falciparum-infected erythrocytes. Infect. Immun. 67:4092-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giha, H. A., T. Staalsoe, D. Dodoo, C. Roper, G. M. Satti, D. E. Arnot, L. Hviid, and T. G. Theander. 2000. Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol. Lett. 71:117-126. [DOI] [PubMed] [Google Scholar]
- 22.Gupta, S., M. C. J. Maiden, I. M Feavers, S. Nee, R. M. May, and R. M. Anderson. 1996. The maintenance of strain structures in populations of recombining infectious agents. Nat. Med. 2:437-442. [DOI] [PubMed] [Google Scholar]
- 23.Issifou, S., C. Rogier, M. Adjagba-Olakpo, N. Chabi-Worou, and F. Ntoumi. 2003. Complexity and genetic diversity of Plasmodium falciparum infections in young children living in urban areas of central and West Africa. Parasitol. Res. 90:423-428. [DOI] [PubMed] [Google Scholar]
- 24.Kitchen, S. F. 1949. Symptomology: general considerations, p. 966-994. In M. F. Boyd (ed.), Malariology: a comprehensive survey of all aspects of this group of diseases from a global standpoint, vol. 2. W. B. Saunders Company, Philadelphia, Pa. [Google Scholar]
- 25.Krisin, H. Basri, D. J. Fryauff, M. J. Barcus, M. J. Bangs, E. Ayomi, H. Marwoto, R. F. Elyazar, T. L. Richie, and J. K. Baird. 2003. Malaria in a cohort of Javanese migrants to Indonesian Papua. Ann. Trop. Med. Parasitol. 97:543-556. [DOI] [PubMed] [Google Scholar]
- 26.Miller, L. H., M. F. Good, and G. Milon. 1994. Malaria pathogenesis. Science 264:1878-1883. [DOI] [PubMed] [Google Scholar]
- 27.Ofori, M. F., D. Dodoo, T. Staalsoe, J. A. Kurtzhals, K. Koram, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect. Immun. 70:2982-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oguariri, R. M., S. Borrmann, M. Q. Klinkert, P. G. Kremsner, and J. F. J. Kun. 2001. High prevalence of human antibodies to recombinant duffy binding-like α domains of the Plasmodium falciparum-infected erythrocyte membrane protein 1 in semi-immune adults compared to that in nonimmune children. Infect. Immun. 69:7603-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasay, C. J. 1997. The genetic diversity of Plasmodium falciparum and Plasmodium vivax in an area of low malaria endemicity in the Philippines. Ph.D. thesis. University of Queensland, Brisbane, Australia.
- 30.Powell, R. D., J. V. McNamara, and K. H. Rieckmann. 1972. Clinical aspects of acquisition of immunity to falciparum malaria. Proceedings of the Helminthological Society of Washington 39 special issue:51-66.
- 31.Rasti, N., M. Wahlgren, and Q. Chen. 2004. Molecular aspects of malaria pathogenesis. FEMS Immunol. Med. Microbiol. 41:9-26. [DOI] [PubMed] [Google Scholar]
- 32.Recker, M., S. Nee, P. C. Bull, S. Kinyanjui, K. Marsh, C. Newbold, and S. Gupta. 2004. Transient cross-reactive immune responses can orchestrate antigenic variation in malaria. Nature 429:555-558. [DOI] [PubMed] [Google Scholar]
- 33.Rickman, L. S., T. R. Jones, G. W. Long, S. Paparello, I. Schneider, C. F. Paul, R. L. Beaudoin, and S. L. Hoffman. 1990. Plasmodium falciparum-infected Anopheles stephensi inconsistently transmit malaria to humans. Am. J. Trop. Med. Hyg. 43:441-445. [DOI] [PubMed] [Google Scholar]
- 34.Simpson, J. A., L. Aarons, W. E. Collins, G. M. Jeffery, and N. J. White. 2002. Population dynamics of untreated Plasmodium falciparum malaria within the adult human host during the expansion phase of the infection. Parasitology 124:247-263. [DOI] [PubMed] [Google Scholar]
- 35.Singer, L. M., L. B. Mirel, F. O. Ter-Kuile, O. H. Branch, J. M. Vulule, M. S. Kolczak, W. A. Hawley, S. K. Kariuki, D. C. Kaslow, and A. A. Lal. 2003. The effects of varying exposure to malaria transmission on development of antimalarial antibody responses in preschool children. XVI. Asembo Bay Cohort Project. J. Infect. Dis. 187:1756-1764. [DOI] [PubMed] [Google Scholar]
- 36.Smith, T., J. D. Charlwood, A. Y. Kitua, H. Masanja, S. Mwankusye, P. L. Alonso, and M. Tanner. 1998. Relationships of malaria morbidity with exposure to Plasmodium falciparum in young children in a highly endemic area. Am. J. Trop. Med. Hyg. 59:252-257. [DOI] [PubMed] [Google Scholar]
- 37.Taliaferro, W. H. 1949. Immunity to the malaria infections, p. 935-965. In M. F. Boyd (ed.), Malariology: a comprehensive survey of all aspects of this group of disease from a global standpoint, vol. 2. W. B. Saunders Company, Philadelphia, Pa. [Google Scholar]
- 38.Ward, C. P., G. T. Clottey, M. Dorris, D. D. Ji, and D. E. Arnot. 1999. Analysis of Plasmodium falciparum PfEMP1/var genes suggests that recombination rearranges constrained sequences. Mol. Biochem. Parasitol. 102:167-177. [DOI] [PubMed] [Google Scholar]