Abstract
We constructed an oral live vaccine based on the attenuated aroA mutant Salmonella enterica serovar Typhimurium strain SL3261 expressing outer membrane proteins F and I (OprF-OprI) from Pseudomonas aeruginosa and investigated it in a mouse model. Strains with in vivo inducible protein expression with the PpacC promoter showed good infection rates and immunogenicity but failed to engender detectable antibodies in the lung. However, a systemic booster vaccination following an oral primary immunization yielded high immunoglobulin A (IgA) and IgG antibody levels in both upper and lower airways superior to conventional systemic or mucosal booster vaccination alone. In addition, the proportion of IgG1 and IgG2a antibodies suggested that the systemic booster does not alter the more TH1-like type of response induced by the oral Salmonella primary vaccination. We conclude that an oral primary systemic booster vaccination strategy with an appropriate mucosal vector may be advantageous in diseases with the risk of P. aeruginosa airway infection, such as cystic fibrosis.
Chronic pulmonary infection with Pseudomonas aeruginosa is the leading cause of mortality in patients with cystic fibrosis (CF) (4, 32). Therapy of P. aeruginosa infection is complicated due to a biofilm formation on the mucosal surface, which effectively prevents eradication once chronic infection is established (17). Thus, prevention of chronic pulmonary P. aeruginosa airway infection is a primary goal for CF patients. Vaccines that offer an enhanced immunogenicity in the airway mucosa may, therefore, be particularly desirable.
Attenuated Salmonella species expressing heterologous antigens are promising candidates for the development of mucosal vaccines. This vaccination strategy is based on the ability of Salmonella bacteria to persist in the antigen-presenting cell (APC) during its migration to the lymphatic organs of the mucosal immune system. At this location, the desired antigen can be processed by the harboring APCs and presented to prime naïve T and B cells (34). Antibody-secreting cells (ASCs) primed in the mucosa-associated lymphatic tissue preferentially home at their induction site, leading to an enhanced mucosal immune response. Oral live vaccines based on recombinant Salmonella strains were successfully developed to induce a specific immune response against mucosal infections, such as human immunodeficiency virus, Helicobacter pylori, Clostridium difficile, and human papilloma virus in mice and humans (2, 10, 12, 25, 37).
After oral Salmonella administration, ASCs are primed in the gut-associated lymphoid tissue. Subsequently, the majority of ASCs home to the gut lamina propria, and, albeit less frequently, also to distant mucosal sites, such as the genitourinary tract or the respiratory mucosa (21). Thus, the antibody response is robust in the intestinal mucosa but less vigorous in the respiratory mucosa. Booster vaccinations with Salmonella do not appear to be sufficient to induce an enhanced immune response in the respiratory mucosa (1). In contrast, nasal administration of Salmonella was shown to have a strong immunogenicity in the airway mucosa (37).
Every given heterologous antigen interferes with the viability and immunogenicity of the vaccine strain. Among the many parameters relevant to the efficacy of a Salmonella vaccine are the immunogenicity of the foreign protein, the amount of the induced protein expression, and the induction of the protein expression (6). Thus, experiences from Salmonella constructs with other antigens cannot be easily transferred to the development of a new vaccine.
In this study, we developed a live attenuated Salmonella vaccine expressing a recombinant outer membrane fusion protein from P. aeruginosa (OprF-OprI). By modifications of the protein expression parameters, the newly constructed Salmonella vaccine showed a strong intestinal and systemic immunogenicity. We speculated that a systemic booster vaccination following a Salmonella primary vaccination enhances the immunogenicity in the respiratory mucosa. We show that a combined mucosal (oral) primary and systemic booster vaccination schedule is able to raise high mucosal antibody levels of both immunoglobulin A (IgA) and IgG isotypes in the respiratory mucosa, which is not achieved by mucosal or systemic vaccination alone. Moreover, the mucosal primary systemic booster vaccination schedule preserved the IgG subclass ratio typical for mucosal Salmonella vaccination, suggesting a more TH1-like type of response.
MATERIALS AND METHODS
Animals.
Male eight-week-old C57BL/6 mice were obtained from the animal facilities of Hannover Medical School and kept under pathogen-free conditions in accordance with German guidelines for animal care. All experiments were approved by the animal welfare committee of the local authorities.
Construction of plasmids and Salmonella live vaccine.
Attenuated Salmonella enterica serovar Typhimurium aroA SL3261 (18) was used as the vaccine carrier strain. Escherichia coli Electro10 (Stratagene) was used for cloning. The oprF-oprI fusion gene encoding Met-Ala-(His)6 OprF190-342-OprI21-83 from P. aeruginosa was cloned on pBR322-derived plasmids downstream of two constitutively active promoters (Pyz [14] in pDB3 and Ptrc in pDB4) or the in vivo inducible Salmonella PpagC promoter (7, 16) in plasmids pMW151, pMW209, and pMW210. These in vivo inducible constructs differed in the ribosomal binding site (33) upstream of oprF-oprI, resulting in differential in vivo OprF-OprI expression levels. The plasmids contained an ampicillin resistance gene for selection of plasmid-carrying bacteria. For use in humans, a maintenance system without antibiotic resistance, such as a balanced lethal complementation system, would be required.
For oral immunizations, plate-grown Salmonella strains were subcultured in a small overnight Luria-Bertani (LB) culture containing 90 μg of streptomycin per ml and 100 μg of ampicillin per ml, which was then used to inoculate a large liquid culture that was grown at 200 rpm and 37°C to the late logarithmic phase. Bacteria were harvested by centrifugation, washed in LB containing 3% NaHO3, and resuspended in the same medium at a density of 1010 CFU/ml. For immunization the mice were anesthetized by ether inhalation (Baker, Deventer, The Netherlands). A vaccination dose of 109 CFU of a Salmonella vaccine strain suspension in 100 μl was applied intragastrically with a round-tip stainless steel cannula.
SDS-PAGE, immunoblotting, and quantification of expression in vitro.
Samples of late log-phase LB cultures of SL3261, SL3261(pDB3), SL3261(pDB4), SL3261(pMW151), SL3261(pMW209), and SL3261(pMW210) or cultures in in vitro PpagC induction medium (M9 containing 10 μM Mg2+ [7]) were applied to standard sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-12% PAGE) gels, electroblotted onto nitrocellulose, and stained with monoclonal antibody to OprI (clone 2A1) (9), followed by a peroxidase-coupled antibody to mouse IgG and chemiluminescence detection. All antibodies were preadsorbed with SL3261 lysate to reduce nonspecific binding.
Production of the OprF-OrpI vaccine protein.
The recombinant vector pTrc-His-F-I, carrying the hybrid gene of Met-Ala-(His)6 OprF190-342-OprI21-83 from P. aeruginosa was expressed by E. coli XL-1 Blue and purified as described previously (11, 24). Subsequently, Met-Ala-(His)6 OprF190-342-OprI21-83 was adsorbed to aluminum hydroxide [Al(OH)3] (Superfos; Vedbaeck, Denmark). The final concentration of the vaccine was 0.1 mg of OprF-I/ml and 0.3 mg of Al(OH)3/ml with 0.05 mg of thimerosal/ml (Caesar & Lorenz, Hilden, Germany) as preservative.
Vaccination protocol.
The mucosal vaccination with Salmonella live bacteria was performed once at day 0. Infection was evaluated on day 7. Systemic immunization was performed with two applications of 15 μg of OprF-OrpI with a 27G cannula into the femoral muscle of the left hind leg on days 0 and 14. Groups with a combined mucosal primary-systemic booster vaccination schedule received Salmonella live vaccine on day 0 and a systemic (intramuscular) application of OprF-OprI on day 28. Immunogenicity was evaluated on day 28 in animals with a single oral vaccination and on day 42 in animals receiving booster vaccinations. The inoculation of all Salmonella strains was well tolerated without apparent signs of disease.
Determination of infection.
Mice were sacrificed in deep anesthesia with intraperitoneal application of xylazine (Bayer, Leverkusen, Germany) and ketamine (Albrecht, Aulendorf. Germany) by exsanguination of the inferior caval vein. The number of invasive bacteria was determined in the Peyer's patches (PP), the mesenteric lymph nodes (MLN), and the spleen as previously described (5, 6). The PP were cut off the intestinal wall and cleaned from remaining luminal contents. The recovered tissues were subsequently homogenized for 1 min (Ultra Turrax T8; IKA, Staufen, Germany) at 5,000 rpm in 1 ml (spleen and PP) or 2 ml (MLN) of phosphate-buffered saline (PBS). For lysis of the host cells containing Salmonella, 450 μl of 0.1% Triton X-100 (Sigma, Taufkirchen, Germany) was added to the suspension. To assess the number of viable Salmonella organisms, serial dilutions were plated on Önöz agar plates (Merck, Darmstadt, Germany) containing suitable antibiotics.
Determination of immunogenicity.
The induction of OprF-OrpI-specific antibodies was assessed in serum, the intestinal mucosa, bronchoalveolar lavage fluid (BALF), and nasal wash. Mice were sacrificed as described above. Blood obtained from the inferior caval vein was left for 2 h at room temperature and subsequently centrifuged at 10,000 × g for 10 min. The small intestine was taken out between the pylorus and the cecum, and the mesenteric tissue was removed. The remaining intestine was homogenized in PBS together with ethylenediamine tetraacetic acid (EDTA; Serva, Heidelberg, Germany) and phenylmethylsulfonyl fluoride (Sigma-Aldrich, Steinheim, Germany) as protease inhibitors. Subsequently, the suspension was centrifuged for 15 min at 10,000 × g. The supernatant was used for analysis. For lavage of the lung, the trachea was cannulated by a 22G Teflon cannula (Braun, Melsungen, Germany). The lung was subsequently flushed two times with 1 ml of PBS. Thereafter, the cannula was reverted to a cranial direction in the trachea to allow for a lavage of the nasopharyngeal cavity by 1 ml of PBS. The fluid was collected from the nostrils in head-down position. Again, EDTA and phenylmethylsulfonyl fluoride were added to the collected fluid. BALF and nasal wash were centrifuged at 60 × g for 10 min, and the supernatant was used for analysis. If not stated otherwise, all specimens were processed on ice or at 4°C and stored at −80°C until analysis.
ELISA.
OprF-OrpI-specific antibodies were determined by a biotin-avidin linked immunosorbent assay. Ninety-six-well microtiter plates (NUNC maxisorp; Greiner bio-one, Frickenhausen, Germany) were coated with OprF-OprI at a concentration of 10 mg/ml, blocked by a 0.2% bovine serum albumin solution (Sigma, Taufkirchen, Germany), and incubated with 50 μl of the various specimens overnight at 4°C. Serum was used in serial dilutions with 0.2% bovine serum albumin. The supernatant of the intestinal homogenate, BALF, and nasal wash were incubated without dilution. Further incubation steps were a goat anti-mouse detection antibody directed against various isotypes (Nordic Immunology, Tilburg, The Netherlands) at a dilution of 1: 5,000, followed by a biotin-linked donkey anti-goat IgG-specific secondary antibody (DPC Biermann, Bad Nauheim, Germany), diluted at 1:10,000. The latter was visualized with streptavidin and o-diadinisine (both Sigma). The reaction was stopped with 0.5 M sulfuric acid. The extinction was read at a λ of 492 nm. All assays were performed in duplicate. For assessment of serum reaction, the mean reciprocal value of the maximum dilution resulting in an extinction at least two times above background level was taken as the titer. In the analysis of all other specimens, the mean extinction was taken as an enzyme-linked immunosorbent assay (ELISA) unit.
Statistics.
Groups were compared with respect to serum titers by the Mann-Whitney test, because these discrete interval data were not normally distributed as assessed by the Shapiro-Wilk test. Differences of mean values of continuous quantitative data (ELISA units and IgG1/IgG2a ratios) were assessed with a two-sided Student's t test after estimation of equality of variance by the Levene's test or with a one-way analysis of variance for multiple analyses with the Bonferroni and Dunnett post hoc correction for groups with and without equality of variances, respectively. Differences were considered significant and highly significant with a P value < 0.05 and < 0.001, respectively. The calculations were performed with the SPSS program, version 12.0 (SPSS Inc., Chicago, Ill.).
RESULTS
Vaccine strains with constitutive OprF-OprI overexpression.
Protective immunity against airway infection with P. aeruginosa is believed to be based on mucosal antibody responses. Oral vaccination with recombinant attenuated Salmonella carrying a foreign antigen is an attractive option for inducing such mucosal immune responses. We therefore expressed the outer membrane fusion protein antigen OprF-OprI in a suitable Salmonella enterica serovar Typhimurium aroA strain. In order to achieve a high immunogenicity, we constructed in the first series two strains, SL3261(pDB3) and SL3261(pDB4), with a constitutive high level of OprF-OrpI expression (Fig. 1). Both strains carried plasmids with constitutively active promoters (Pyz in plasmid pDB3 and Ptrc in plasmid pDB4). However, these strains colonized the PP and MLN only poorly upon oral immunization (Fig. 2). Accordingly, despite the high levels of protein expression, the immunization with SL3261(pDB3) showed only occasionally a detectable antibody response in serum, while serum levels remained undetectable upon immunization with strain SL3261(pDB4). Moreover, there was no immune response in either intestinal or respiratory mucosa (Fig. 3).
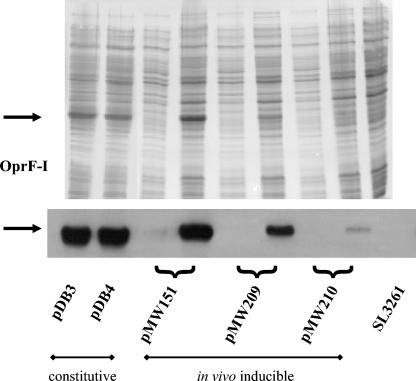
FIG. 1.
SDS-PAGE of late log-phase cultures of SL3261(pDB3), SL3261(pDB4), SL3261(pMW151), SL3261(pMW209), SL3261(pMW210), and the SL3261 parent strain (upper panel). Constructs with in vivo inducible protein expression were cultured in standard LB medium and under magnesium-free conditions (left and right lanes, respectively), demonstrating the induction of the desired OprF-OprI antigen as shown by immunoblot (lower panel). For clarity of the figure, vaccine strains are indicated with the name of their plasmid constructs only. Arrows indicate the apparent molecular weight of OprF-OprI.
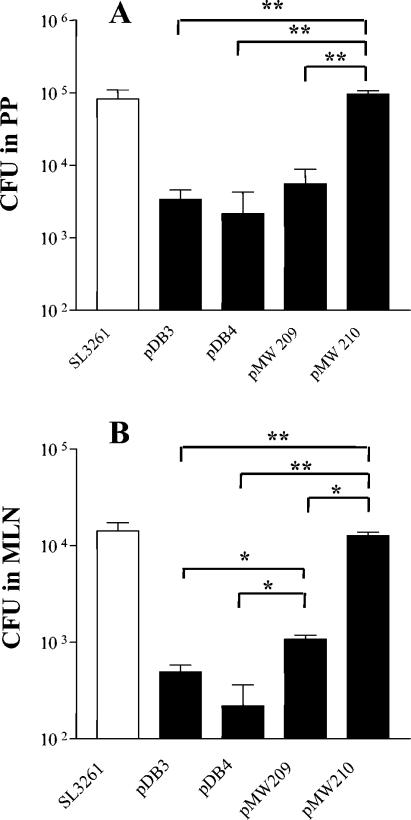
FIG. 2.
Infection by SL3261 and OprF-OprI vaccine constructs of PP and MLN on day 7 after gastric inoculation of 109 CFU. Bars represent mean CFU ± standard error of the means of 5 (pMW209 and pMW210) to 17 animals. In the spleen, Salmonella was found only occasionally at numbers <100 CFU. In vivo induction of OprF-OprI protein expression resulted in a significantly higher infection rate of SL3261(pMW209) in MLN. The infection level of strain SL3261(pMW210) was indifferent to parent strain SL3261 and higher than all other vaccine strains. Asterisks indicate significantly different mean values as obtained in two-sided Student's t tests. *, P < 0.05; **, P < 0.001. For clarity of the figure, vaccine strains are indicated with the name of their plasmid constructs only.
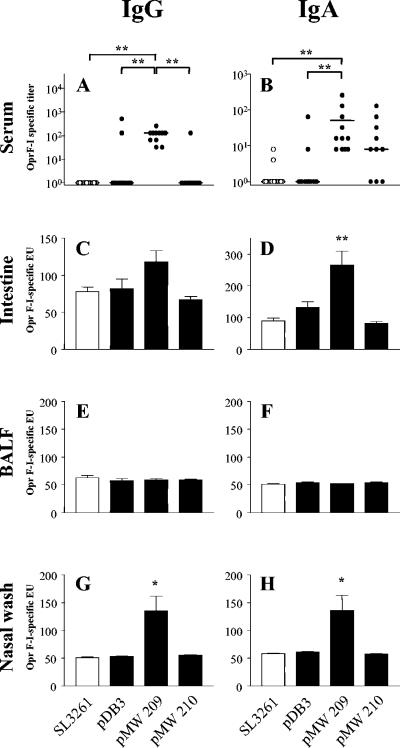
FIG. 3.
Formation of OprF-OrpI-specific antibodies of the IgG and IgA isotypes in serum, intestine, lung (BALF), and nose (nasal wash) 4 weeks after vaccination with 109 CFU of parent strain SL3261, of a strain with constitutive OprF-OrpI expression [SL3261(pDB3)], and of strains with in vivo inducible OprF-OrpI expression [SL3261(pMW209) and SL3261(pMW210)]. Dots represent individual titer values, bars indicate mean values of ELISA units (EU), error bars indicate the standard errors of the means. Groups comprised 11 to 17 animals from two independent sets of experiments. Asterisks indicate significantly different mean values with a P value of <0.05 (*) and a P value of <0.001 (**) as obtained by a Mann-Whitney test (for comparison of serum titers) or by a two-sided Student's t tests (for comparison of ELISA units). For clarity of the figure, vaccine strains are indicated with the name of their plasmid constructs only.
Vaccine strains with in vivo inducible OprF-OprI expression.
A number of previous studies have shown that the infection capability and immunogenicity of live Salmonella-based vaccines critically depend on the timing (6) and the level of antigen expression (33). We therefore modified our prototype vaccines by using an in vivo inducible expression cassette. In addition, we modulated the in vivo OprF-OprI expression levels by using a series of ribosomal binding site variants. Parallel testing of three different constructs (pMW151, pMW209, and pMW210) (Fig. 1) revealed that the strain with a high level of inducible protein expression [SL3261(pMW151)] (Fig. 1) still colonized poorly (data not shown). A moderate amount of protein expression upon in vivo induction [strain SL3261(pMW209)] (Fig. 1) resulted in a significantly improved infection rate in the MLN (Fig. 2). The very low level of inducible protein expression of the strain SL3261(pMW210) (Fig. 1) was related to a virtually unimpaired infection compared to the parent strain SL3261, which was in the order of 105 CFU in the PP and 104 CFU in the MLN (Fig. 2).
Immunogenicity turned out to be related to both infection rate and level of protein expression. The strain with the best infection rate, SL3261(pMW210), induced a moderate systemic immune response in the majority of, but not all, immunized mice, which was best with the IgA isotype (Fig. 3). However, there was no consistent mucosal immune response (Fig. 3). The strain with the highest level of in vivo inducible protein expression, SL3261(pMW151), failed to induce any detectable systemic or mucosal antibodies (data not shown). In contrast, the strain with moderate levels of both protein expression and infection, SL3261(pMW209), turned out to be the most effective vaccine strain in that it induced high levels of IgA and IgG antibodies in serum in all immunized mice. Moreover, SL3261(pMW209) was highly immunogenic in the intestinal mucosa, in particular for the IgA response. There were also moderate IgA and IgG responses in the nasal mucosa. However, this strategy failed to mount detectable levels of antibodies in the lung (Fig. 3).
Comparison of systemic, mucosal, and combined vaccination schedules.
In a clinical study a systemic booster vaccination with inactivated polio vaccine was shown to enhance mucosal immunogenicity following a primary vaccination with the oral polio vaccine (15). We speculated that a systemic booster with the present mucosal OprF-OprI vaccine can enhance the mucosal immunogenicity also in mice. In addition, we compared this combined schedule with both solely mucosal and conventional systemic immunization strategies.
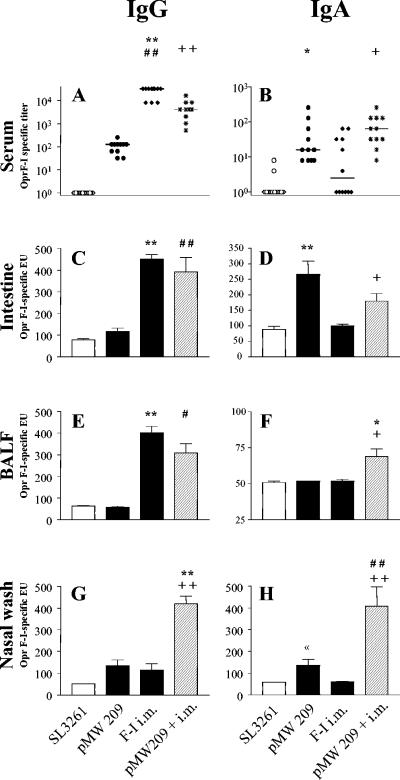
As expected, the systemic prime-booster schedule resulted in high levels of systemic antibodies of the IgG isotype and, though less pronounced, of the IgA isotype (Fig. 4). In addition, systemic vaccination induced a strong mucosal IgG response in the intestine and the bronchoalveolar lavage. There was also a moderate IgG response in the nasal mucosa comparable to the response with the mucosal vaccination with SL3261(pMW209). Systemic immunization alone could not induce any detectable mucosal IgA antibodies at any sites investigated. In contrast, the combined mucosal primary systemic booster vaccination strategy, which used the most efficient mucosal vaccine strain, SL3261(pMW209), substantially enhanced the immunogenicity in the respiratory mucosa of both the IgA and IgG isotypes. The combined strategy induced high levels of IgA antibodies in the upper and lower airways that were unachievable by any other approach used (Fig. 4). In addition, the combined schedule was also superior in the induction of IgG antibodies in the nasal mucosa, while the mucosal IgG response in the lung and the intestine was comparable to the response with conventional systemic vaccination (Fig. 4).
FIG. 4.
Formation of OprF-OrpI-specific antibodies of IgG and IgA isotypes in serum, intestine, lung (BALF), and nose (nasal wash) after vaccination with 109 CFU of parent strain SL3261, with strain SL3261(pMW209) with in vivo inducible protein expression, with systemic vaccination twice (intramuscularly [i.m.]), and with a combined mucosal primary systemic booster vaccination [SL3261(pMW209)+ i.m.]. Dots represent individual titer values, bars indicate mean values of ELISA units (EU), and error bars indicate the standard errors of the means. Groups comprised 12 to 17 animals from two independent sets of experiments. Significantly different mean values are indicated as follows: *, comparison of SL3261(pMW209) versus i.m.; +, comparison of SL3261(pMW209)+i.m. versus i.m.; and #, comparison of SL3261(pMW209) versus SL3261(pMW209)+i.m. A single sign indicates a P value of <0.05, and a double sign indicates a P value of <0.001 as obtained by a Mann-Whitney test (for comparison of serum titers) or by a two-sided Student's t tests (for comparison of ELISA units). For clarity of the figure, vaccine strains are indicated with the name of their plasmid constructs only.
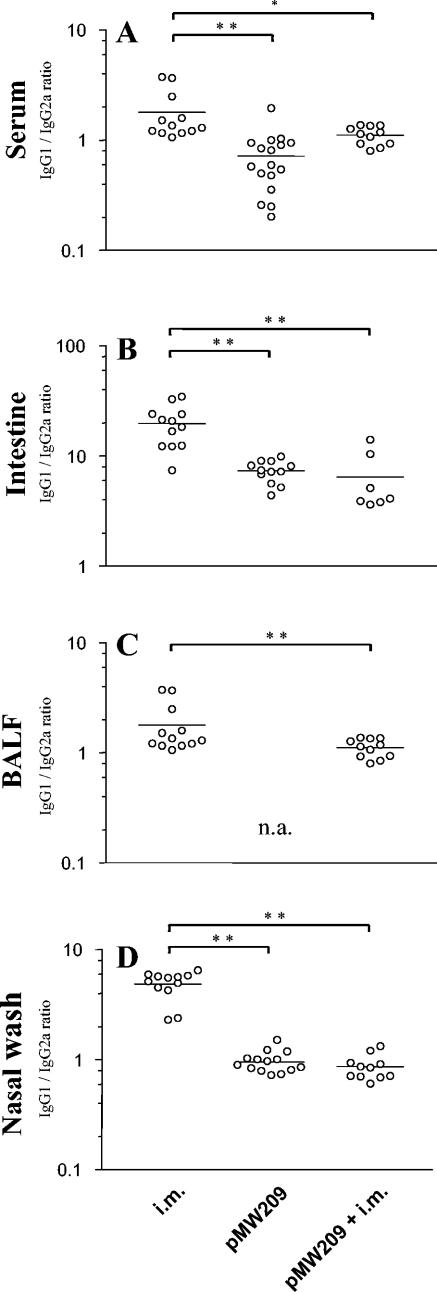
Ratio of IgG1 to IgG2a.
We further analyzed the pattern of immunogenicity achieved by the various immunization strategies as indicated by the IgG1/IgG2a ratio as a surrogate marker for the TH1-TH2 balance. Systemic vaccination resulted in a strong induction of IgG1 antibodies and a relatively weak IgG2a response, while the Salmonella live vaccine SL3261(pMW209) yielded a higher proportion of IgG2a antibodies. The different IgG1/IgG2a ratios were comparable in analyses of systemic and mucosal antibodies (Fig. 5). Interestingly, a systemic booster vaccination following the mucosal primary vaccination with SL3261(pMW209) did not change the mucosal pattern of the IgG1/IgG2a ratio (Fig. 5).
FIG. 5.
IgG1/IgG2a ratio of OprF-OrpI-specific antibodies in serum, intestine, lung (BALF) and nose (nasal wash). The ratio is considered a surrogate marker for the TH1-TH2 balance, with IgG1 in-dicating a more TH2-type response and IgG2a a more TH1-type response. Mice were vaccinated either twice systemically with an intramuscular application of OprF-I (i.m.), with a single oral application of attenuated recombinant Salmonella with in vivo inducible expression of OprF-OprI [SL3261(pMW209)], or with a combined mucosal primary systemic booster regimen (pMW209+i.m.). Dots represent individual IgG1/IgG2a ratios, and lines indicate the median values of each vaccination group. Groups were compared by one-way analysis of variance for multiple analyses with the Bonferroni and Dunnett post hoc correction for groups with and without equality of variances, respectively. Asterisks indicate a P value of <0.05 (*) and <0.001 (**). In BALF of mice vaccinated with SL3251(pMW209) alone, assessment of the IgG1/IgG2a ratio was not applicable (n.a.) due to the lack of relevant antibody levels. For clarity of the figure, vaccine strains are indicated with the name of their plasmid constructs only.
DISCUSSION
The induction of a mucosal immune response in the airway mucosa remains a challenge for vaccine development. While conventional systemic immunization reliably raises strong systemic IgG and IgA responses, it induces IgA antibodies at mucosal sites poorly (15, 29). In the respiratory mucosa, IgA is the dominant isotype beyond the alveolar space. Mucosal IgA almost exclusively derives from ASCs located in the mucosal lamina propria, which have to be primed by a mucosal vaccination strategy. In addition, mucosal IgG antibodies originate, at least in part, from local ASCs (30, 31). The goal of a strong airway immunogenicity, therefore, seems to be best achieved with a mucosal vaccination strategy. Since mucosa-induced ASCs predominantly home to their induction site rather than to distant mucosal sites, nasal vaccination may be advantageous with respect to airway immunogenicity (21). However, nasal vaccines against various antigens showed variable success. Our own nasal vaccine based on the OprF-OrpI protein from P. aeruginosa showed a relatively weak systemic and mucosal immunogenicity in mice and humans (13, 22). Salmonella live vaccines applied in the nose may also raise safety issues with a potential infection of the central nervous system and the paranasal sinuses. We therefore pursued a strategy of enhancing airway immunogenicity based on an oral live vaccine with an attenuated Salmonella vector.
Salmonella are specialized to penetrate the gut epithelium and to survive intracellularly after phagocytosis in APCs, while being transported to the gut-associated lymphatic tissue (GALT), the PP and MLN. Salmonella vaccine constructs have been shown to express the desired antigens in the GALT for up to 3 weeks (5). These properties make them prototype mucosal vaccine vectors. However, heterologous antigen expression compromises both the ability to penetrate the gut epithelium and to persist in the lymphatic tissue. These limitations impair rational vaccine development, as the optimal balance between protein expression, viability, and immunogenicity unpredictably varies with every given heterologous protein.
In the present study, constitutive overexpression of OprF-OrpI severely compromised the infection rates of the Salmonella vaccine strains. Despite protein expression at high quantities, these strains mounted only an occasional immune response. We therefore established an in vivo inducible protein expression system in order to better allow the carrier to cross the difficult gut epithelium barrier without the burden of foreign protein expression (6, 16). By using the Salmonella PpagC promoter of the phoP/phoQ regulon that is activated by the intracellular milieu in eukaryotic cells, our vaccine strains had an improved ability to colonize and persist in the GALT. The most effective strain with in vivo inducible protein expression, SL3261(pMW209), could immunize mice at a 100% seroconversion rate and induced a pronounced mucosal response with IgA and IgG antibodies in the gut. Comparison with other strains with the same promoter but a different amount of protein expression demonstrated that the quantity of protein synthesis had a significant impact on immunogenicity in this system. Relatively minor alterations, therefore, can strongly influence the efficacy of an attenuated Salmonella live vaccine. Despite the good immunogenicity at the gut mucosa, oral SL3261(pMW209) vaccination mounted only a modest immune response in the upper airways and was unable to do so in the lung. This observation is consistent with findings with a different Salmonella vaccine (37).
In order to enhance the immunogenicity of the oral Salmonella vaccine in the airway mucosa, we introduced an oral primary systemic booster vaccination schedule. This schedule appeared promising because it has been reported that a systemic booster vaccination with inactivated polio vaccine yielded high levels of secretory IgA antibodies in saliva in humans after primary vaccination with an oral live polio vaccine but not after primary immunization with an inactivated polio vaccine (15). We speculated that a systemic booster enhances the mucosal antibody response in the airways also after an oral primary vaccination but not after a systemic primary vaccination, and we directly compared both schedules. Our data show that the combined mucosal primary systemic booster vaccination schedule can induce the desired high IgA and IgG antibody levels in both the upper and lower airways, which are not achieved by either oral or systemic vaccination alone.
We additionally assessed the relation of levels of IgG1 to IgG2a as a surrogate marker for the TH1 and TH2 balance (35). As expected, systemic vaccination resulted in a strong IgG1 response, suggesting a TH2-type response, while Salmonella vaccination appeared to induce a more TH1-type response with a higher proportion of IgG2a antibodies. Interestingly, the combined oral primary systemic booster vaccination schedule showed similar ratios to those of the mucosal vaccine alone.
The amplification of the mucosal antibody response by a systemic booster vaccination following an oral primary immunization may represent a more general principle of the interaction of mucosal and systemic immune systems. An enhanced mucosal antibody response and protection at both the induction site as well as at distant sites, together with an augmented systemic immunogenicity, have been reported in other studies of the nasal application of recombinant attenuated Salmonella or protein in mice (19, 23, 36). In our own clinical experience with a nasal gel vaccine with the recombinant OprF-OprI protein of P. aeruginosa, the serum antibody response also increased with use of a systemic booster schedule in humans (13). Our data suggest that the primary vaccination determines the type of response to the booster vaccination in terms of homing pattern, IgG-IgA distribution, and TH1-TH2 balance. It remains to be determined whether the enhanced airway immunogenicity following the systemic booster is due to a proliferation of memory ASCs located in the airway mucosa or to a higher number of circulating ASCs of intestinal or even systemic origin, some of which incidentally find their way to the airways.
How relevant are these findings for CF patients? The fact that CF patients virtually never suffer from a P. aeruginosa infection outside the respiratory mucosa suggests that local, but not systemic, immunity is insufficient to avert a P. aeruginosa infection in the presence of impaired innate pulmonary defense mechanisms (3). It is of note that P. aeruginosa infection of the lung is preceded by colonization of the upper airways (8). Thus, a vaccination that augments antibody responses in both the upper and lower airways may be particularly beneficial to prevent P. aeruginosa infection at an early stage in CF patients. In addition, the apparent, more TH1-like response may be favorable in CF. A TH1 phenotype was reported to relate to a better clinical outcome in a cross-sectional clinical study (28). Several animal studies demonstrated a better outcome of P. aeruginosa lung infection by interventions that initiate a shift towards a TH1-type response (20, 26, 27). It remains to be determined in experimental and clinical studies whether the enhanced airway immunogenicity of the present OprF-OprI live vaccine approach would translate into a better protection against P. aeruginosa.
In summary, the oral live vaccine with attenuated Salmonella, in vivo inducible expression of OprF-OprI, and a systemic booster is a promising vaccination strategy, as it induces high levels of OprF-OprI-specific antibodies in serum and in both upper and lower airways that are unattainable by conventional systemic or mucosal vaccination alone in a mouse model. Future studies will address the immunogenicity of this approach in humans and the protection in animal models.
Acknowledgments
This work was supported in part by the German Cystic Fibrosis Foundation (Mukoviszidose e.V.), the August and Erika Appenrodt Foundation, and Deutsche Forschungsgemeinschaft (grants Bu971/4-2 and SFB621-A9).
Editor: J. D. Clements
REFERENCES
- 1.Allen, J. S., G. Dougan, and R. A. Strugnell. 2000. Kinetics of the mucosal antibody secreting cell response and evidence of specific lymphocyte migration to the lung after oral immunisation with attenuated S. enterica var. typhimurium. FEMS Immunol. Med. Microbiol. 27:275-281. [DOI] [PubMed] [Google Scholar]
- 2.Baud, D., J. Benyacoub, V. Revaz, M. Kok, F. Ponci, M. Bobst, R. Curtiss III, P. De Grandi, and D. Nardelli-Haefliger. 2004. Immunogenicity against human papillomavirus type 16 virus-like particles is strongly enhanced by the PhoPc phenotype in Salmonella enterica serovar Typhimurium. Infect. Immun. 72:750-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher, R. C. 2004. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 23:146-158. [DOI] [PubMed] [Google Scholar]
- 4.Brennan, A. L., and D. M. Geddes. 2002. Cystic fibrosis. Curr. Opin. Infect. Dis. 15:175-182. [DOI] [PubMed] [Google Scholar]
- 5.Bumann, D. 2001. In vivo visualization of bacterial colonization, antigen expression, and specific T-cell induction following oral administration of live recombinant Salmonella enterica serovar Typhimurium. Infect. Immun. 69:4618-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bumann, D. 2001. Regulated antigen expression in live recombinant Salmonella enterica serovar Typhimurium strongly affects colonization capabilities and specific CD4+-T-cell responses. Infect. Immun. 69:7493-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bumann, D. 2002. Examination of Salmonella gene expression in an infected mammalian host using the green fluorescent protein and two-colour flow cytometry. Mol. Microbiol. 43:1269-1283. [DOI] [PubMed] [Google Scholar]
- 8.Burns, J. L., R. L. Gibson, S. McNamara, D. Yim, J. Emerson, M. Rosenfeld, P. Hiatt, K. McCoy, R. Castile, A. L. Smith, and B. W. Ramsey. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183:444-452. [DOI] [PubMed] [Google Scholar]
- 9.Eckhardt, A., M. M. Heiss, W. Ehret, W. Permanetter, M. Duchene, H. Domdey, and B. U. von Specht. 1991. Evaluation of protective mAbs against Pseudomonas aeruginosa outer membrane protein I by C1q binding assay. Zentbl. Bakteriol. 275:100-111. [DOI] [PubMed] [Google Scholar]
- 10.Fouts, T. R., A. L. DeVico, D. Y. Onyabe, M. T. Shata, K. C. Bagley, G. K. Lewis, and D. M. Hone. 2003. Progress toward the development of a bacterial vaccine vector that induces high-titer long-lived broadly neutralizing antibodies against HIV-1. FEMS Immunol. Med. Microbiol. 37:129-134. [DOI] [PubMed] [Google Scholar]
- 11.Gabelsberger, J., B. Knapp, S. Bauersachs, U. I. Enz, B. U. von Specht, and H. Domdey. 1997. A hybrid outer membrane protein antigen for vaccination against Pseudomonas aeruginosa. Behring Inst. Mitt. 123:302-314. [PubMed] [Google Scholar]
- 12.Gilbert, P. B., Y. L. Chiu, M. Allen, D. N. Lawrence, C. Chapdu, H. Israel, D. Holman, M. C. Keefer, M. Wolff, and S. E. Frey. 2003. Long-term safety analysis of preventive HIV-1 vaccines evaluated in AIDS vaccine evaluation group NIAID-sponsored phase I and II clinical trials. Vaccine 21:2933-2947. [DOI] [PubMed] [Google Scholar]
- 13.Göcke, K., U. Baumann, H. Hagemann, J. Gabelsberger, H. Hahn, J. Freihorst, and B. U. von Specht. 2003. Mucosal vaccination with a recombinant OprF-I vaccine of Pseudomonas aeruginosa in healthy volunteers: comparison of a systemic vs. a mucosal booster schedule. FEMS Immunol. Med. Microbiol. 37:167-171. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Duarte, O. G., B. Lucas, Z. X. Yan, K. Panthel, R. Haas, and T. F. Meyer. 1998. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine 16:460-471. [DOI] [PubMed] [Google Scholar]
- 15.Herremans, T. M., J. H. Reimerink, A. M. Buisman, T. G. Kimman, and M. P. Koopmans. 1999. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J. Immunol. 162:5011-5018. [PubMed] [Google Scholar]
- 16.Hohmann, E. L., C. A. Oletta, W. P. Loomis, and S. I. Miller. 1995. Macrophage-inducible expression of a model antigen in Salmonella typhimurium enhances immunogenicity. Proc. Natl. Acad. Sci. USA 92:2904-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoiby, N. 2002. New antimicrobials in the management of cystic fibrosis. J. Antimicrob. Chemother. 49:235-238. [DOI] [PubMed] [Google Scholar]
- 18.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 19.Jespersgaard, C., P. Zhang, G. Hajishengallis, M. W. Russell, and S. M. Michalek. 2001. Effect of attenuated Salmonella enterica serovar Typhimurium expressing a Streptococcus mutans antigen on secondary responses to the cloned protein. Infect. Immun. 69:6604-6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen, H. K., H. P. Hougen, S. J. Cryz, Jr., J. Rygaard, and N. Hoiby. 1995. Vaccination promotes TH1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia in rats. Am. J. Respir. Crit. Care Med. 152:1337-1346. [DOI] [PubMed] [Google Scholar]
- 21.Kunkel, E. J., and E. C. Butcher. 2003. Plasma-cell homing. Nat. Rev. Immunol. 3:822-829. [DOI] [PubMed] [Google Scholar]
- 22.Larbig, M., E. Mansouri, J. Freihorst, B. Tümmler, G. Kohler, H. Domdey, B. Knapp, K. D. Hungerer, E. Hundt, J. Gabelsberger, and B. U. von Specht. 2001. Safety and immunogenicity of an intranasal Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers. Vaccine 19:2291-2297. [DOI] [PubMed] [Google Scholar]
- 23.Londono-Arcila, P., D. Freeman, H. Kleanthous, A. M. O'Dowd, S. Lewis, A. K. Turner, E. L. Rees, T. J. Tibbitts, J. Greenwood, T. P. Monath, and M. J. Darsley. 2002. Attenuated Salmonella enterica serovar Typhi expressing urease effectively immunizes mice against Helicobacter pylori challenge as part of a heterologous mucosal priming-parenteral boosting vaccination regimen. Infect. Immun. 70:5096-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansouri, E., J. Gabelsberger, B. Knapp, E. Hundt, U. Lenz, K. D. Hungerer, H. E. J. Gilleland, J. Staczek, H. Domdey, and B. U. von Specht. 1999. Safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers. Infect. Immun. 67:1461-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzger, W. G., E. Mansouri, M. Kronawitter, S. Diescher, M. Soerensen, R. Hurwitz, D. Bumann, T. Aebischer, B. U. von Specht, and T. F. Meyer. 2004. Impact of vector-priming on the immunogenicity of a live recombinant Salmonella enterica serovar typhi Ty21a vaccine expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine 22:2273-2277. [DOI] [PubMed] [Google Scholar]
- 26.Moser, C., P. O. Jensen, O. Kobayashi, H. P. Hougen, Z. Song, J. Rygaard, A. Kharazmi, and N. Hoiby. 2002. Improved outcome of chronic Pseudomonas aeruginosa lung infection is associated with induction of a Th1-dominated cytokine response. Clin. Exp. Immunol. 127:206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moser, C., H. K. Johansen, Z. Song, H. P. Hougen, J. Rygaard, and N. Hoiby. 1997. Chronic Pseudomonas aeruginosa lung infection is more severe in Th2 responding BALB/c mice compared to Th1 responding C3H/HeN mice. APMIS 105:838-842. [PubMed] [Google Scholar]
- 28.Moser, C., S. Kjaergaard, T. Pressler, A. Kharazmi, C. Koch, and N. Hoiby. 2000. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. APMIS 108:329-335. [DOI] [PubMed] [Google Scholar]
- 29.Muszkat, M., A. B. Yehuda, M. H. Schein, Y. Friedlander, P. Naveh, E. Greenbaum, M. Schlesinger, R. Levy, Z. Zakay-Rones, and G. Friedman. 2000. Local and systemic immune response in community-dwelling elderly after intranasal or intramuscular immunization with inactivated influenza vaccine. J. Med. Virol. 61:100-106. [PubMed] [Google Scholar]
- 30.Pilette, C., Y. Ouadriri, V. Godding, J. P. Vaerman, and Y. Sibille. 2001. Lung mucosal immunity: immunoglobulin-A revisited. Eur. Respir. J. 18:571-588. [DOI] [PubMed] [Google Scholar]
- 31.Quiding-Jabrink, M., I. Nordstrom, G. Granstrom, A. Kilander, M. Jertborn, E. C. Butcher, A. I. Lazarovits, J. Holmgren, and C. Czerkinsky. 1997. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J. Clin. Investig. 99:1281-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajan, S., and L. Saiman. 2002. Pulmonary infections in patients with cystic fibrosis. Semin. Respir. Infect. 17:47-56. [DOI] [PubMed] [Google Scholar]
- 33.Rollenhagen, C., M. Sorensen, K. Rizos, R. Hurvitz, and D. Bumann. 2004. Antigen selection based on expression levels during infection facilitates vaccine development for an intracellular pathogen. Proc. Natl. Acad. Sci. USA 101:8739-8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sirard, J. C., F. Niedergang, and J. P. Kraehenbuhl. 1999. Live attenuated Salmonella: a paradigm of mucosal vaccines. Immunol. Rev. 171:5-26. [DOI] [PubMed] [Google Scholar]
- 35.Stevens, T. L., A. Bossie, V. M. Sanders, R. Fernandez-Botran, R. L. Coffman, T. R. Mosmann, and E. S. Vitetta. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334:255-258. [DOI] [PubMed] [Google Scholar]
- 36.Walsh, E. E. 1994. Humoral, mucosal, and cellular immune response to topical immunization with a subunit respiratory syncytial virus vaccine. J. Infect. Dis. 170:345-350. [DOI] [PubMed] [Google Scholar]
- 37.Ward, S. J., G. Douce, D. Figueiredo, G. Dougan, and B. W. Wren. 1999. Immunogenicity of a Salmonella typhimurium aroA aroD vaccine expressing a nontoxic domain of Clostridium difficile toxin A. Infect. Immun. 67:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]