Abstract
Trichodesmium is a globally important marine diazotroph that accounts for approximately 60 − 80% of marine biological N2 fixation and as such plays a key role in marine N and C cycles. We undertook a comprehensive assessment of how the growth rate of Trichodesmium erythraeum IMS101 was directly affected by the combined interactions of temperature, pCO2 and light intensity. Our key findings were: low pCO2 affected the lower temperature tolerance limit (Tmin) but had no effect on the optimum temperature (Topt) at which growth was maximal or the maximum temperature tolerance limit (Tmax); low pCO2 had a greater effect on the thermal niche width than low-light; the effect of pCO2 on growth rate was more pronounced at suboptimal temperatures than at supraoptimal temperatures; temperature and light had a stronger effect on the photosynthetic efficiency (Fv/Fm) than did CO2; and at Topt, the maximum growth rate increased with increasing CO2, but the initial slope of the growth-irradiance curve was not affected by CO2. In the context of environmental change, our results suggest that the (i) nutrient replete growth rate of Trichodesmium IMS101 would have been severely limited by low pCO2 at the last glacial maximum (LGM), (ii) future increases in pCO2 will increase growth rates in areas where temperature ranges between Tmin to Topt, but will have negligible effect at temperatures between Topt and Tmax, (iii) areal increase of warm surface waters (> 18°C) has allowed the geographic range to increase significantly from the LGM to present and that the range will continue to expand to higher latitudes with continued warming, but (iv) continued global warming may exclude Trichodesmium spp. from some tropical regions by 2100 where temperature exceeds Topt.
Introduction
The ocean is a major sink for anthropogenic emissions [1], of which the capacity to store CO2 is strongly affected by biological processes [2]. As atmospheric CO2 increases, the dissolved inorganic carbon (DIC) concentrations in the oceans increases, the pH declines, and the inorganic speciation changes [3]. Such changes are expected to have diverse effects for marine ecosystems [4]. In addition to ocean acidification, climate change will concurrently lead to increases of sea surface temperature (SST) thereby enhancing water stratification and decreasing vertical mixing [5].
One of the most important phytoplankton groups in the open oceans are diazotrophic cyanobacteria, which convert N2 gas into ammonia (NH3) by nitrogenase activity prior to assimilation of this fixed N into organic matter. Their distributions range from the warm (~ 27°C) tropical waters around the equator (e.g. Trichodesmium and Crocosphaera spp.), to the colder (~ 5°C) waters of the Baltic Sea (e.g. Aphanizomenon sp. and Nodularia spp.) [6,7]. Diazotrophs found at cold temperatures possess specialised cell compartments (heterocysts) where N2 fixation occurs [8]. Conversely, all equatorial diazotrophic cyanobacteria are non-heterocystous. The non-heterocystous filamentous, diazotroph Trichodesmium spp. are estimated to contribute significantly to global productivity and to biogeochemical cycles [9], and are considered to be the dominant equatorial diazotrophs [10–12], representing up to 50% of new nitrogen in some regions [13,14], and contributing between 80 and 110 Tg of fixed N2 to the open ocean ecosystems per year [11], although there is evidence for significant contributions to N2 fixation by unicellular cyanobacteria [15].
Trichodesmium spp. often form extensive surface blooms where cells are exposed to high temperatures, high light intensities, fluctuating nutrient regimes and water column mixing [16,17], all of which can contribute to the high degree of spatial and temporal variability in trichome densities [13,18]. In the eastern Atlantic Ocean, Trichodesmium spp. are most abundant from 0 to 15°N, with complete absence south of 30°S [19]. Trichodesmium is commonly observed from the upper few meters of the water column where the surface light intensity is ~ 2500 μmol photons m-2 s-1, to ~ 100 meters depth where the 0.5 to 1% light level is reached (~ 12.5–25 μmol photons m-2 s-1) and temperatures are between 21 and 23°C [20,21].
Knowledge of how the filamentous diazotroph Trichodesmium responds to temperature, light and pCO2 is critically important to understand the potential implications of global warming and ocean acidification on future global biogeochemical cycles, food web dynamics, and overall productivity of the open oceans [22].
The effect of temperature on growth rate can be characterised by a thermal tolerance curve (μ-T curve); where an organism’s growth is constrained between a maximum (Tmax) and minimum (Tmin) temperature limit. The temperature range between Tmin and Tmax is defined as the thermal niche, and can vary subject to an organism’s physiological plasticity and evolutionary history [23]. Two features of a growth-temperature curve common to all ectotherms are unimodality and negative skewness [24,25]. Negative skewness describes the sudden sharp decline in fitness above Topt, and indicates that when acclimated to Topt, growth is more significantly reduced by warming than cooling, a trait of particular relevance given the predicted increase in SST over the coming decades.
The constraint that temperature imposes on the physiology of diazotrophs in general and Trichodesmium spp. in particular is still not fully understood, and yet is fundamentally important when trying to predict future productivity and global distributions. Recent research suggests that the minimum temperature limit (Tmin), optimum temperature (Topt), and maximum temperature limit (Tmax) for Trichodesmium erythraeum growth occurs between 20 − 22°C, 26 − 27°C and 32 − 35°C, respectively; with little variation found between strains or isolates (IMS101, KO4-20, RLI and 2175) [26–28].
Although some research has shown that the growth rate of Trichodesmium erythraeum IMS101 is unchanged or decreases with increased CO2 concentrations above current ambient levels, the majority of research indicates that an increase in CO2 whilst all other factors are kept constant, will result in an increase in growth (Table 1). As is the case for the majority of experiments investigating the effects of ocean acidification [29], most research involving Trichodesmium has used a single independent variable (e.g. CO2 whilst keeping temperature and light constant or temperature whilst keeping CO2 and light constant), of which there are typically 3 to 8 treatments and all cultured for short time periods and often with several undefined growth conditions (Table 1).
Table 1. The current literature regarding the effects of varying temperature (°C), CO2 (ppm), light intensity (μmol photons m-2 s-1) and L:D (hr:hr) period on Trichodesmium erythraeum IMS101 growth.
| Growth Conditions | Acclimation (Generations) | μ (d-1) | Effect of OA on Growth | Experimental Methods | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | Light | CO2 | L:D | Carbon Chemistry | Parameters Defined | Growth Index | ||||
| 20 | 100 | − | 12:12 | 15 | 0.04 | − | − | − | Chl a/C-specific | [26] |
| 21 | 0.10 | |||||||||
| 24 | 0.18 | |||||||||
| 26 | 0.25 | |||||||||
| 30 | 0.21 | |||||||||
| 31 | 0.15 | |||||||||
| 34 | 0.07 | |||||||||
| 24 | 140 | − | 14:10 | − | 0.38 | − | − | − | Chl a-specific | [30] |
| 26 | 0.65 | |||||||||
| 28 | 0.77 | |||||||||
| 31 | 0.64 | |||||||||
| 25 | 70 | − | 8:16 | 10–40 | 0.21 | − | − | − | Chl a-specific | [31] |
| 12:12 | 0.30 | |||||||||
| 16:8 | 0.45 | |||||||||
| 350 | 8:16 | 0.30 | ||||||||
| 12:12 | 0.29 | |||||||||
| 16:8 | 0.12 | |||||||||
| 25 | 150 | 180 | 14:10 | 35 | 0.26 | → | NaOH Additions | TA & TCO2 | Chl a-specific | [32] |
| 380 | 0.41 | |||||||||
| 550 | 0.44 | |||||||||
| 720 | 0.45 | |||||||||
| 800 | 0.46 | |||||||||
| 26 | 120 | 380 | 12:12 | > 800 | 0.26 | ↑ | BubblingGas Mixture | pH & TCO2 | Cell-specific | [33] |
| 750 | 0.37 | |||||||||
| 25 | 80–120 | 250 | 12:12 | 5 | 0.13 | ↑ | BubblingGas Mixture | Headspace pCO2 | Chl a/C-specific | [34] |
| 400 | 0.16 | |||||||||
| 900 | 0.26 | |||||||||
| 25 | 150 | 150 | 12:12 | 5 | 0.35 | →/↓ | BubblingGas Mixture | TA & pH | Chl a/C-specific | [35] |
| 370 | 0.29 | |||||||||
| 1000 | 0.32 | |||||||||
| 27 | 90 | 380a | 14:10 | − | 0.26 | ↓ | Bubbling Gas Mixture/NaOH Additions | − | Chl a/C-specific | [36] |
| 750a | 0.19 | |||||||||
| 380b | 0.46 | ↓ | ||||||||
| 750b | 0.37 | |||||||||
| 25 | 150 | 180 | 12:12 | 7 | 0.36 | ↓ | Bubbling Gas Mixture | pH & TCO2 | Chl a-specific | [37] |
| 380 | 0.34 | |||||||||
| 980 | 0.32 | |||||||||
| 1400 | 0.27 | |||||||||
| 180c | 11 | 0.34 | ↓ | |||||||
| 380c | 0.37 | |||||||||
| 980c | 0.35 | |||||||||
| 1400c | 0.29 | |||||||||
| 25 | 80–100 | 400 | 12:12 | 47 | 0.37 | ↑ | Bubbling Gas Mixture | − | Cell-specific | [38] |
| 900 | 0.58 | |||||||||
| 400d | 0.22 | ↑ | ||||||||
| 900d | 0.31 | |||||||||
| 25 | 50 | 150 | 12:12 | 10 | 0.15 | ↑ | Bubbling Gas Mixture | TA & pH | Chl a/C-specific | [39] |
| 900 | 0.24 | |||||||||
| 200 | 150 | 0.38 | ↑ | |||||||
| 900 | 0.42 | |||||||||
| 24 | 38 | Ambient | 12:12 | 7–10 | 0.12 | ↑ | Bubbling Gas Mixture | pH & TCO2 | Cell-specific | [40] |
| 100 | 0.25 | |||||||||
| 220 | 0.30 | |||||||||
| 38 | 750 | 0.12 | ||||||||
| 100 | 0.32 | |||||||||
| 220 | 0.38 | |||||||||
| 26 | 260e | − | 12:12 | − | 0.35 | − | − | − | Cell-specific | [41] |
| 670e | 0.48 | |||||||||
| 260f | 0.38 | |||||||||
| 670f | 0.49 | |||||||||
| 25 | 100 | 380 | 12:12 | 7–10 | 0.35 | ↑ | Bubbling Gas Mixture | pH & TCO2 | Cell-specific | [42] |
| 750 | 0.39 | |||||||||
| 29 | 380 | 0.36 | ↑ | |||||||
| 750 | 0.41 | |||||||||
| 25 | 80 | 400 | 12:12 | 10 | 0.18 | ↑ | Bubbling Gas Mixture | Headspace pCO2 | Chl a-specific | [43] |
| 900 | 0.32 | |||||||||
| 31 | 250 | 0.26 | ↑ | |||||||
| 400 | 0.27 | |||||||||
| 900 | 0.38 | |||||||||
A dash (−) represents no method for controlling the carbon chemistry or an undefined growth condition. Arrows represent an increase (↑), decrease (↓) or negligible (→) effect of ocean acidification (OA) on growth. ImageJ was used to obtain growth rates from figures reported in literature. Note that superscripts in the CO2 column indicate the following differences from the standard YBCII culture medium
a = 40 pM Fe´
b = 1250 pM Fe´
c = 100 μM NO3-
d = 0.5 μM P
e = 20 nM Ni and
f = 100 nM Ni.
To investigate the integrated effects of key physical/chemical variables (temperature, pCO2 and light) that will be altered by climate change on the growth rate of Trichodesmium, we performed a systematic, multivariable experiment, where Trichodesmium IMS101 was cultured over long durations (> 9 months), at multiple treatments (n = 174) with controlled and defined growth conditions, thus ensuring that balanced growth and complete physiological acclimation was achieved (S1 Table). The aims were to assess the response of Trichodesmium IMS101 growth to temperature, CO2 and light intensity. We tested the hypotheses that (i) the thermal niche width of Trichodesmium IMS101 is reduced (i.e. Tmin increased and/or Tmax decreased) under suboptimal light or pCO2, (ii) the optimal temperature (Topt) for growth is unaffected by light or pCO2, (iii) the maximum photochemical efficiency of PSII (Fv/Fm) is affected by suboptimal and supraoptimal temperature, light and CO2, and (iv) when light is limiting, suboptimal pCO2 further reduces growth rate.
Materials and Methods
Trichodesmium erythraeum IMS101 was semi-continuously cultured to achieve fully acclimated balanced growth across a range of temperatures (19 − 32°C), light intensities (10–1400 μmol photons m-2 s-1) and targeted pCO2 concentrations (180, 380 and 720 ppm).
Experimental setup
Cultures were grown at low volumes (5 mL) in 12 mL glass test tubes. Test tubes were acid washed and autoclaved prior to culturing, and each dilution was made into a new tube to avoid the build-up of contaminants. Growth rates were quantified from changes in fluorescence (Fo) measured daily (between 09:00 to 10:30) on dark-adapted cultures (20 minutes) using a FRRfII Fastact Fluorometer (Chelsea Technologies Group Ltd, UK). The FRRfII parameters were optimised prior to the experiment to ensure a saturating fluorescence curve was achieved for both low (post-dilution) and high (pre-dilution) cell density cultures.
Cultures were kept at the lower section of the exponential growth phase (S1 Fig) and optically thin to avoid nutrient limitation, self-shading and minimise CO2 drift [32]. Tubes were gently inverted twice a day to minimise trichomes aggregating at the meniscus. Subject to the temperature and CO2, high light cultures were usually diluted every fourth to fifth day, while low light cultures every tenth to twelfth day. When Fo declined at an extreme growth condition (e.g. high temperature), three attempts were made to re-grow that treatment, using culture from the closest growth condition.
Culture medium and carbonate chemistry
A single batch (25 L) of filter-sterilised (0.25 μm pore) YBCII media [44] was made and stored in acid-washed, autoclaved Duran bottles (no headspace). The inorganic carbon chemistry of each bottle was determined via CO2SYS [45]; using a 15 mL sample for TCO2 analysis (Shimadzu TOC-V Analyser & ASI-V Autosampler), and a 10 mL sample for pH (Thermo Scientific Orion Ross Ultra pH Electrode EW-05718-75, UK). The pH probes were rinsed and calibrated with fresh (< 2 weeks) artificial seawater buffers (TRIS and AMP) prior to use [46].
Once a culture reached a pre-determined Fo value, it was diluted (0.5 mL culture to 4.5 mL media) with filter-sterilised (0.2 μm pore) YBCII media that had been adjusted to a target pH (and thus target CO2) to return the culture to a starting Fo value. To obtain a targeted CO2 concentration in the YBCII media used to dilute the semi-continuous cultures, the medium was bubbled with a CO2-air mixture to a targeted pH (precision of ± 0.002). Once the media reached the desired CO2 concentration (± 1%) it was immediately distributed into the test tubes, already containing the culture (S1 File). Test tubes were sealed via a PTFE lined screw cap and PTFE tape on the test tube threads, ensuring a gas-tight seal and preventing exchange of CO2 with the atmosphere. Prior to screwing the cap on, the gaseous headspace was flushed with a filtered (0.2 μm pore) standard gas mixture of a target CO2 concentration (BOC Industrial Gases, UK). All carbon chemistry calculations were made in CO2SYS [45], using the 1st and 2nd equilibrium constants (K1 and K2) for carbonic acid [47], the dissociation constant for KSO4 [48], the boric acid constant (KB) [49], and the total pH scale. The CO2SYS program calculates CO2 concentrations as μatm; however, as the CO2 concentrations reported here are to zero decimal places the equivalent units of parts per million are used (ppm).
Prior to every dilution, 3.5 mL of culture was collected in a 5 mL plastic cryogenic vial (Sigma-Aldrich V5257-250EA) and was used to measure the post-culturing pH. Assuming alkalinity remained constant throughout the entire growth phase [50], a post-culturing CO2 was calculated using the post-culturing pH and initial alkalinity.
Temperature gradient
To measure the effect of temperature on growth, a custom-made water-jacketed aluminium temperature block was used to house test tube cultures. The temperature ranged from 18 to 33°C, and the temperature steps along the gradient were at ~ 0.5°C increments. The temperature (± 0.1°C) of each tube was measured using a temperature probe (Sper Scientific 840038, Arizona USA), and varied < 0.2°C over a diel period for any given culture along the gradient. A clear Perspex sheet was secured beneath of the block to hold the tubes in place and allow illumination from below. Light was provided by four fan-cooled LED strips (The Optoelectronic Manufacturing Corporation Ltd. 3ft T5 Daylight, UK), and was adjusted using neutral density filters, with half of the test tubes illuminated at low light (40 μmol photons m-2 s-1 ± 0.4), and the remaining tubes at high light (400 μmol photons m-2 s-1 ± 4), all at a 12:12 L:D cycle. The light was measured for each test tube using a light meter (Li-Cor Li-250A, Nebraska USA), and was checked weekly throughout the experiment. For each of the low and high light treatments, and for each temperature treatment, Trichodesmium IMS101 was cultured at three targeted CO2 concentrations (180, 380 and 720 ppm), giving a total of 120 treatments.
Light gradient
To measure the light response of growth a second temperature block was setup and run concurrently. All test tube cultures were maintained at 26°C. Using neutral density filters, a light gradient was setup ranging from 10 to 1400 μmol photons m-2 s-1, at a 12:12 L:D cycle. The light steps along the gradient were distributed in order to resolve the light-limited and saturated sections of the growth curve. For each light treatment, Trichodesmium IMS101 was cultured at three targeted CO2 concentrations (180, 380 and 720 ppm), giving a total of 54 treatments.
The measured pCO2 in the cultures just prior to dilution into fresh medium were between 20% and 30% lower than the target pCO2 concentrations of 180, 380 and 720 ppm, and were similar across all temperatures and light intensities (Table 2).
Table 2. The mean (± S.E.) growth conditions of Trichodesmium erythraeum IMS101 cultures for the temperature and light response.
| Temperature response | Light response | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low CO2 | Mid CO2 | High CO2 | Low CO2 | Mid CO2 | High CO2 | |||||
| Variables | Units | LL | HL | LL | HL | LL | HL | |||
| pH | Total | 8.506 | 8.477 | 8.171 | 8.175 | 7.901 | 7.908 | 8.524 | 8.214 | 7.957 |
| H+ | nM | 3.1(±0.02) | 3.3(±0.032) | 6.7(±0.044) | 6.7(±0.041) | 12.6(±0.074) | 12.3(±0.056) | 3.0(±0.016) | 6.1(±0.033) | 11.0(±0.074) |
| AT | μM | 3275(±22) | 2993(±25) | 2723(±21) | 2796(±16) | 2549(±17) | 2626(±11) | 3004(±6) | 2963(±9) | 2936(±93) |
| TCO2 | μM | 2387(±23) | 2187(±18) | 2242(±19) | 2298(±14) | 2264(±15) | 2332(±10) | 2146(±5) | 2410(±7) | 2583(±14) |
| HCO3- | μM | 1765(±23) | 1639(±15) | 1922(±17) | 1962(±12) | 2067(±13) | 2127(±9) | 1562(±5) | 2031(±5) | 2330(±12) |
| CO32- | μM | 618(±5) | 544(±9) | 313(±4) | 326(±4) | 178(±3) | 186(±2) | 580(±2) | 370(±2) | 235(±2) |
| CO2 | μM | 3.89(±0.09) | 3.85(±0.08) | 9.14(±0.11) | 9.17(± 0.10) | 18.41(±0.18) | 18.64(±0.15) | 3.25(±0.03) | 8.62(±0.05) | 17.86(±0.06) |
| pCO2 | ppm | 139(±2) | 138(±2) | 325(±3) | 330(±2) | 649(±3) | 658(±2) | 118(±1) | 312(±2) | 647(±2) |
| Chl a | μg L-1 | 15.9(±1.1) | 16.2(±1.0) | 23.0(±1.7) | 19.6(±1.5) | 25.4(±1.8) | 20.6(±1.7) | 21.8(±1.3) | 28.8(±1.6) | 36.3(±2.1) |
| n | 46 | 63 | 74 | 78 | 83 | 98 | 107 | 124 | 102 | |
For the temperature response, the growth conditions per CO2 and light treatment is the average of all temperature treatments. For the light response, the growth conditions per CO2 treatment is the average of all light treatments. The total inorganic carbon (TCO2), bicarbonate (HCO3-), carbonate (CO32-) and pCO2 was calculated via CO2SYS using the pH and AT concentration. Temperature response growth conditions; low (180 ppm), mid (380 ppm) and high (720 ppm) CO2, 40 μmol photons m-2 s-1 (LL) and 400 μmol photons m-2 s-1 (HL), ranging between 18–31°C. Light response growth conditions; low (180 ppm), mid (380 ppm) and high (720 ppm) CO2, 26°C, ranging between 20–1400 μmol photons m-2 s-1)
Chlorophyll fluorescence
In addition to the minimum fluorescence (Fo), the FastPro software (Chelsea Technologies Group Ltd, UK) generated dark-adapted values of maximum fluorescence (Fm). The maximum photochemical efficiency of PSII in the dark-adapted state (Fv/Fm) was calculated using the following:
| (1) |
Data processing to obtain balanced growth rates
Acclimation took ~ 12 to 16 weeks with up to 20 dilutions required to allow cultures to achieve balanced growth at the extreme temperature and light limits. For each dilution, a growth rate was calculated from linear regression of ln(Fo) versus time. Balanced growth was assumed to have been achieved once growth rates from a minimum of three successive growth curves had stabilised (S2 File, S2 Fig). A script written in the open source statistical software R [51] was used to process and analyse the growth rate data for each treatment [52] (S4 File). This objective approach improved the efficiency of data processing, given the large number of treatments (n = 174) and the duration of the culturing (~ 9 months) and removed potential bias or subjectivity when determining a growth rate from numerous data points.
Growth rate versus light curve
The growth-light (μ-I) curves were modelled using the following function [53]:
| (2) |
where μmax’ is the hypothetical maximum growth rate (d-1); α is the initial light-limited slope of the growth-light curve (d-1 (μmol photons m-2 s-1)-1); β is the parameter that characterises photoinhibition at supraoptimal light intensities (d-1 (μmol m-2 s-1)-1); I is the light intensity (μmol photons m-2 s-1); and Ic is the compensation light at which the light-limited growth rate extrapolates to zero (μmol photons m-2 s-1).
The achieved maximum growth rate (μmax), light at which growth is maximal (Iopt), light-saturation parameter (Ik), and light inhibition parameter (Ip) were calculated from the fitted parameters as follows:
| (3) |
| (4) |
| (5) |
| (6) |
Growth versus temperature curve
The growth-temperature (μ-T) curves were modelled using a newly formulated modified sine function, which returns estimates for Tmin, Tmax and the maximum growth rate at Topt:
| (7) |
where μmax is the maximum growth rate (d-1); T is the temperature of the culture (°C); Tmin is the minimum temperature limit for growth (°C); Tmax is the maximum temperature limit for growth (°C); θ is a shape determining parameter, which alters the skewness; and Φ is a shape determining parameter, which alters the kurtosis.
The optimum temperature (Topt) is calculated from the fitted parameters as follows:
| (8) |
The growth-light and growth-temperature curve fits for each CO2 or light treatment were fitted using a weighted non-linear squares algorithm, where weights were the reciprocals of the standard errors associated with the median growth rates. Standard errors were propagated when parameters (e.g. Ik, Topt) were calculated from curve fit values (S3 Table). Further statistical analysis (Sigmaplot 11.0) was used to assess differences between CO2 and light treatments. When appropriate the data was log transformed to ensure normality, and a Two or Three-way ANOVA and post hoc Tukey test was applied on all of the growth rate data per light, CO2 treatment, as opposed to the single calculated curve fitted parameter values.
Results
Growth rates increased with increasing CO2 at all temperatures (Three-way ANOVA, Tukey post hoc test; P < 0.001). The growth responses were non-symmetrical around the optimum temperature for growth (Topt ranging between 24.7 to 26.9°C); specifically the effect of pCO2 on growth was more pronounced at suboptimal than supraoptimal temperatures (Fig 1A and 1B). Growth rates at temperatures below Topt were markedly lower at mid than high pCO2, whereas similar rates were observed at both mid and high pCO2 at temperatures above Topt.
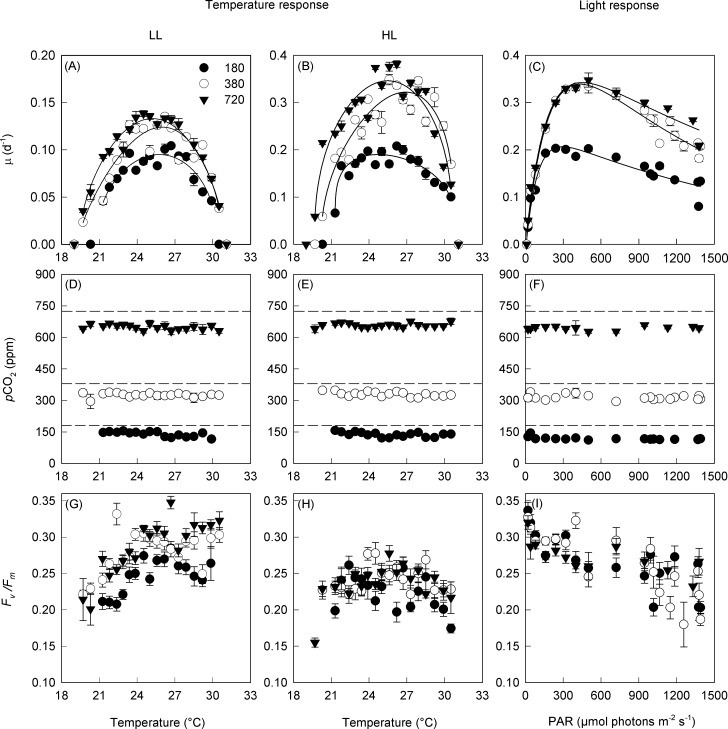
Fig 1. The median growth rate, and mean (± S.E.) pCO2 and maximum dark-adapted photochemical efficiency (Fv/Fm) of Trichodesmium erythraeum IMS101 when acclimated across a range of temperatures, light intensities, and at three target pCO2 concentrations (Low = 180 ppm, Mid = 380 ppm and High = 720 ppm) (174 treatments).
For the temperature response: LL = 40 μmol photons m-2 s-1; HL = 400 μmol photons m-2 s-1. Note the dashed line represents the initial pCO2 concentration for each treatment culture once diluted (T = 0), while the data points are the final CO2 concentration post culturing.
The growth-temperature curves exhibited marked reductions of growth rate (μ) accompanying small changes in temperature near the lower and upper tolerance limits, and smaller rates of change of growth with changes of temperature closer to the optimum (Fig 1A and 1B). With the exception of the high light-low pCO2 treatment, all other curves exhibited negative skewness (θ > 1) (Table 3).
Table 3. The temperature dependent growth curve parameters (± S.E.) for Trichodesmium erythraeum IMS101 generated by fitting a five-parameter function to each of the two light (LL = 40 μmol photons m-2 s-1; HL = 400 μmol photons m-2 s-1) treatments at the three CO2 (Low = 180 ppm, Mid = 380 ppm and High = 720 ppm) treatments.
| Low CO2 | Mid CO2 | High CO2 | |||||
|---|---|---|---|---|---|---|---|
| Parameters | Units | LL | HL | LL | HL | LL | HL |
| μmax | d-1 | 0.095 (±0.004) | 0.190 (±0.006) | 0.124 (±0.006) | 0.322 (±0.012) | 0.133 (±0.003) | 0.346 (±0.012) |
| Tmin | ° C | 20.80 (±0.66) | 21.29 (±0.01) | 19.29 (±0.44) | 20.18 (±0.13) | 19.37 (±0.21) | 19.66 (±0.05) |
| Tmax | ° C | 30.27 (±0.51) | 30.85 (±0.50) | 30.83 (±0.34) | 30.76 (±0.37) | 30.95 (±0.29) | 30.66 (±0.19) |
| w | ° C | 9.47 (±0.83) | 9.56 (±0.50) | 11.54 (±0.56) | 10.58 (±0.39) | 11.58 (±0.36) | 11.00 (±0.20) |
| Φ | − | 0.38 (±0.21) | 0.27 (±0.11) | 0.54 (±0.19) | 0.30 (±0.13) | 0.55 (±0.12) | 0.36 (±0.09) |
| θ | − | 1.07 (±0.19) | 0.68 (±0.11) | 1.28 (±0.16) | 1.53 (±0.35) | 1.01 (±0.08) | 1.09 (±0.12) |
| Topt | ° C | 25.75 (±0.69) | 24.74 (±0.16) | 26.00 (±0.46) | 26.91 (±0.31) | 25.20 (±0.23) | 25.48 (±0.13) |
| r2 | 0.846 | 0.861 | 0.843 | 0.857 | 0.946 | 0.890 | |
Units; μmax (d-1), the maximum growth rate; Tmin (° C), the minimum temperature limit for growth; Tmax (° C), the maximum temperature limit for growth; w (° C), the thermal niche width for growth; Φ, the peakedness-shape determining factor (higher value = more peaked); θ, the skewness-shape determining factor (< 1 = positive skewness, > 1 = negative skewness). Both shape determining factors, Φ and θ, influence the shape of the curve without modifying μmax, Tmin or Tmax.
The minimum temperature limit for growth (Tmin) was affected by both CO2 and light (Table 3). Under low light, Tmin declined from 20.8 to 19.4°C between low and high CO2, whilst at high light the Tmin declined from 21.3 to 19.7°C between low and high CO2. The maximum temperature limit for growth (Tmax) did not significantly vary between most treatments, averaging about 30.7°C (Table 3). The temperature niche width (w = Tmax—Tmin) was ~ 1.5 to 2°C smaller at low than at mid and high CO2.
There were significant differences in the growth rates between the low and high light treatment (40 versus 400 μmol photons m-2 s-1) at all CO2 treatments (Three-way ANOVA, Tukey post hoc test; P < 0.001). The maximum growth rate (μmax) at the optimum temperature increased by about 30% (low light) and 70% (high light) as CO2 increased from low to mid, and by an additional 7% (both low light and high light) from mid to high CO2 (Table 3).
A more comprehensive assessment of the light-dependence of growth rate was made at the optimum temperature of 26°C (Fig 1C). The initial slope (α) showed little response to pCO2, but the maximum growth rate (μmax) increased by 65% from about 0.21 d-1 at low CO2 to 0.33 d-1 at the mid and high CO2 (Table 4). The light intensity at which the highest maximum growth rate occurred (Iopt) also increased with pCO2 from 290 μmol photons m-2 s-1 at low CO2 to 434 μmol photons m-2 s-1 at high CO2. The light-saturation parameter (Ik) was much lower at low CO2 in comparison to both mid and high CO2 treatments (Table 4).
Table 4. The light-dependent growth curve parameters (± S.E.) for Trichodesmium erythraeum IMS101 generated by fitting a three-parameter P-I function to each of the three CO2 (Low = 180 ppm, Mid = 380 ppm and High = 720 ppm) treatments at optimal temperature (26°C).
| Parameters | Units | Low CO2 | Mid CO2 | High CO2 |
|---|---|---|---|---|
| μmaxʹ | d-1 | 0.252(±0.019) | 0.507(±0.061) | 0.437(±0.042) |
| μmax | d-1 | 0.207(±0.066) | 0.330(±0.060) | 0.330(±0.064) |
| α | d-1 (μmol photons m-2 s-1)-1 | 2.7·10−3(±4.9·10−4) | 2.5·10−3(±2.4·10−4) | 2.6·10−3(±3.2·10−4) |
| β | d-1 (μmol photons m-2 s-1)-1 | 1.3·10−4(±3.5·10−4) | 3.6·10−4(±9.1·10−5) | 2.1·10−4(±6.5·10−5) |
| Ik | (μmol photons m-2 s-1) | 78(±29) | 131(±27) | 125(±29) |
| Ip | (μmol photons m-2 s-1) | 1597(±4290) | 921(±287) | 1591(±585) |
| Ic | (μmol photons m-2 s-1) | 6.0(±5.5) | 2.6(±4.9) | 0.6(±5.8) |
| Iopt | (μmol photons m-2 s-1) | 290(±255) | 419(±76) | 434(±73) |
| r2 | 0.964 | 0.989 | 0.989 |
Units; μmax' (d-1), the hypothetical maximum growth rate in the absence of photoinhibition; μmax (d-1), the achieved maximum growth rate; α (d-1 (μmol photons m2 s-1)), the initial slope of the growth-light curve; β (d-1 (μmol photons m2 s-1)), the photoinhibition slope of the growth-irradiance curve; Ik (μmol photons m2 s-1), the light-saturating parameter; Ip (μmol photons m2 s-1), the photoinhibition parameter.
The maximum photochemical efficiency of PSII (Fv/Fm) was greatest at Topt, and was significantly higher (Three-way ANOVA, Tukey post hoc test; P < 0.001) for low light than high light treatments (Fig 1G and 1H). In addition, Fv/Fm increased as CO2 increased from low to mid and high CO2 (Three-way ANOVA, Tukey post hoc test; P < 0.001) (Fig 1G,H). At low light and for all CO2 treatments, Fv/Fm decreased as temperature decreased from Topt to Tmin, while there was no difference in Fv/Fm (~ 0.3) as temperature increased above Topt to Tmax (Two-way ANOVA, Tukey post hoc test; P < 0.001). Conversely at high light, there was no significant difference of Fv/Fm at the Tmin, Topt or Tmax values between CO2 treatments (Fig 1H). The maximum Fv/Fm recorded (~ 0.35) was at Topt and the lowest light intensity (20 μmol photons m-2 s-1) (Fig 1I).
Discussion
Our key findings for Trichodemisum IMS101 are: (i) at Topt, CO2 affected μmax but not the initial slope (α) of the growth-light curve; (ii) low CO2 constrained the thermal niche width more than low light; (iii) there was greater divergence in the temperature dependence of growth rate due to differences in CO2 below Topt than above Topt; (iv) the maximum photosynthetic efficiency (Fv/Fm) was influenced more strongly by varying temperature and light than CO2; and (v) CO2 affected Tmin while there was no effect of CO2 on Tmax. Here we discuss the physiological mechanisms that may explain these results.
Light and CO2 dependencies of growth at Topt
The observed increase in μmax with increasing pCO2 is qualitatively consistent with previous results [32,34], although there are slight quantitative differences in the percentage increase in growth between mid (~ 380 ppm) and high (~ 720 ppm) CO2 treatments.
Previous studies have attributed the low growth rates at low CO2 to high energy demands required to establish, maintain and operate a carbon concentrating mechanism (CCM), which in turn limits the energy available for N2 fixation, reducing growth [34,42,43]. Our observations of the effect of CO2 at light-saturation and optimal temperature support these findings, while the lack of variability in the light-limited initial slopes is inconsistent with this hypothesis because we would expect the initial slope to be lower at low CO2 if operation of the CCM imposed significant energetic cost. The lack of variation in the initial slopes were perhaps due to low data resolution (too few low-light data points) and the curve fitting process (more heavily weighted to the high-light data points). Consistent with this suggestion is our observation from the thermal response curves that growth rate at low light (40 μmol photons m-2 s-1) increased with increasing pCO2 by about 20% from low to mid CO2 with a further 10% increase from mid to high CO2.
Operation of the CCM is required to maintain high internal CO2 concentrations within the carboxysome to inhibit photorespiration. Conversely, at high CO2 concentrations, when the CCM is fully saturated, Trichodesmium spp. can down-regulate CCM activity and up-regulate other cellular processes (e.g. N2 fixation), which indirectly increases growth [40,42]. Although this is an attractive hypothesis, direct measurements of the magnitude and cost of operating the CCM in Trichodesmium spp. have not been reported. However, the effect on growth rate may be relatively small since the photon requirement for operating the CCM accounts for only 5–15% of the photon requirement for growth (S3 File, S2 Table).
The values of the light-saturation parameters (Ik) were similar to previous observations [21], and exhibited a clear CO2 response, which was driven largely by the changes in μmax. The photoinhibition parameter (Ip) was higher at high CO2 than mid CO2, indicating an increased capability at alleviating the effects of photoinhibition at high CO2, most likely attributed to CCM down-regulation and the up-regulation of photoprotective-related mechanisms. Interestingly, the Ip was also higher at low CO2 than mid CO2, which could be attributed to a low CO2 stress response triggering an up-regulation of photoprotective mechanisms. However, due to the standard error of the modelled slope of photoinhibition (β), the propagated error of the low CO2 Ip value is large, making a difference between the low and mid CO2 treatment a possible artefact of the curve fit.
Minimum temperature for growth (Tmin)
The minimum temperature at which Trichodesmium spp. can grow is likely set by the ability to maintain anoxic conditions that are required to prevent nitrogenase inhibition [8]. Unlike heterocystous diazotrophs, Trichodesmium spp. do not possess a glycolipid barrier that prevents or reduces the diffusion of dissolved gases into the cell [8]. To prevent O2 inhibiting nitrogenase, Trichodesmium spp. uncouple photosynthesis from N2 fixation both temporally and spatially [54,55], maintains a high mitochondrial respiration rate [8,56] and may also use O2 scavenging processes (Mehler reaction and hydrogenase activity) to maintain an anoxic state within the diazocytes [34,57,58]. Stal [8] calculated that respiration would not be able to maintain anoxia at T < 17°C, which is slightly below the Tmin of 19 to 21°C that we (Table 3) and others [26] have observed. The higher Tmin that we observed at low CO2 may reflect lower availability of substrate for respiration due to lower photosynthesis rate, or it may reflect the higher metabolic demand for operation of a carbon concentrating mechanism, which diverts energy that would otherwise be available to fuel N2 fixation.
Supra-optimal temperatures for growth (Topt to Tmax)
The shape of the growth curve from Topt to Tmax exhibited two distinct sections, where growth steadily decreases to ~ 29°C, before plummeting to zero at Tmax. This two-part decline in growth was most pronounced at low light-low CO2, which may in part be due to an enhanced CCM activity limiting the efficacy of certain heat stress processes.
The maximum temperature limit for growth (Tmax) was similar across all light and CO2 treatments. The primary targets of thermal damage in vascular plants include the oxygen evolving complex along with the associated cofactors in photosystem II (PSII), the activity of the carboxylating enzyme Rubisco and the ATP generating system [59]. The maintenance of high values of Fv/Fm at temperatures above Topt under light-limiting conditions indicates that PSII was not damaged by high temperature in these benign low light conditions irrespective of pCO2. In contrast, under light-saturated conditions, Fv/Fm peaked near Topt under all pCO2 treatments.
Reduced growth rates caused by temperatures exceeding Topt may perhaps be due to the inhibition of Rubisco activity or an increase in the ratio of oxygenation to carboxylation by Rubisco. In comparison to other photosynthetic enzymes, Rubisco has a low turnover rate requiring high concentrations to maintain a sufficient rate of photosynthesis. Rubisco activase has a lower maximum temperature tolerance than Rubisco itself. Therefore, if the temperature exceeds the temperature limit to which an organism is adapted, the activity of Ruisco activase is severely reduced and unable to offset the rate of Rubisco deactivation, reducing or inhibiting photosynthesis [60–62]. Previous studies have shown Rubisco activase activity to significantly decrease at ~ 30°C, with maximum inefficiency occurring at 45°C [61]. Although the Tmax values reported here were ~ 31°C, it is likely that the concentration, substrate affinities and the temperature adaptations of Trichodesmium’s Rubisco activase and Rubisco itself would differ from terrestrial plants and other cyanobacteria.
Rubisco in Trichodesmium spp. is characterised by a low affinity for CO2 relative to ambient CO2 concentrations [63–65]. As temperatures increase, the solubility of CO2 decreases more rapidly than that of O2. As such, increasing temperature favours the oxygenation of Rubisco (photorespiration), which reduces the rate of 3-Phosphoglycerate production and requires significant amounts of energy and reductant to process the NH3 and potentially enzyme-inhibiting (i.e. 2-P glycolate) by-products. The solubility of CO2 and O2 within the carboxysomes is principally governed by temperature and not by light. Despite this there was a low light, low CO2 integrated effect on Tmax. A possible explanation for this observation could be that at low CO2, the CCM is up-regulated and functioning at maximum efficiency to maintain a sufficient internal CO2 concentration within the carboxysomes. At low light, photosynthetic rates are reduced, limiting the amount of energy that can be invested into CCM-related proteins. Thus, the combination of low light with low CO2 may make cells more susceptible to photorespiration, particularly at elevated temperatures where the solubility of CO2 is reduced.
To recap, we suggest that the observed two-part decline in growth between the Topt and Tmax values are due to two processes, photorespiration and Rubisco inhibition. The former primarily mediated by the temperature-driven changes to the solubility of O2 and CO2, where the response is compounded by other co-limiting factors (i.e. low CO2 and light); and the latter solely mediated by the temperature-driven response on enzyme kinetics, and is determined by Trichodesmium’s thermal tolerance and is not influence by other abiotic factors.
Potential effects of future climate change on the biogeography of Trichodesmium
Although temperature, CO2 and light intensity place limits on the potential for Trichodesmium growth, whether this potential is achieved depends on the availability of limiting nutrients (e.g. P, Fe). Trichodesmium spp. are most abundant in regions of the sea that receive high inputs of Fe via deposition of dust transported from arid source regions [66]. This may reflect Trichodesmium’s high metalloenzyme inventory, which suggests that iron, instead of phosphorus, may be the key nutrient in constraining Trichodesmium growth and productivity in the present and future oceans [67]. Increases in the supply of Fe to the ocean via increased dust deposition as desertification increases in a warmer climate may also favour Trichodesmium spp. [68–70]. However, this advantage may be negated if increases in the dust load occur where elevation of temperature above 26°C (Topt) inhibits growth of Trichodesmium.
Climate models indicate that the water column in oligotrophic regions of the ocean will become more stratified as global sea surface temperatures rise due to global warming, decreasing vertical mixing and thereby limiting the supply of new nutrients from the deep ocean [5]. The documented increase of water column transparency (decline of phytoplankton chlorophyll) in low to mid latitude oligotrophic oceans over the past 120 years suggests that phytoplankton abundance has declined. This is presumed to be due to an increase in vertical stratification [71], although this century scale observation does not necessarily appear to explain local changes of chlorophyll and stratification on shorter time scales [72]. Increased water column stratification with lower inorganic N concentrations in the surface mixed layer should give photosynthetic diazotrophs such as Trichodesmium spp. a competitive advantage over other phytoplankton [73]. However, exposure to high light intensities in shallower near surface mixed layers may reduce growth by requiring more energy be used for prevention or repair of damage due to photoxidative stress [74]. Although vertical inorganic P fluxes will also decline as the water columns become more stable, Trichodesmium spp. have several adaptations that allow it to exploit P-limited environments. These include an extracellular hydrolysis of dissolved organic P (DOP) by alkaline phosphatase activity [75], which can provide an additional source of P for growth. Trichodesmium spp. can also upregulate synthesis of proteins associated with high-affinity transport and hydrolysis of phosphonate compounds [76]. To date, this pathway is absent from other sequenced marine cyanobacterial genomes, and thus represents a unique adaption for scavenging and hydrolysing phosphorus compounds from organic sources, and growing in otherwise P-limited regions. In addition, Trichodesmium spp. may be capable of mining phosphate by increasing their density by carbohydrate production facilitating sinking to below the nutricline, where they take up phosphate before using gas vesicles to increase buoyancy enabling a return to the surface of the euphotic zone [77,78]. Finally, the additional N and energy investments required for exoenzymatic breakdown of DOP appears to give N2 fixers a competitive advantage in oligotrophic regions [79].
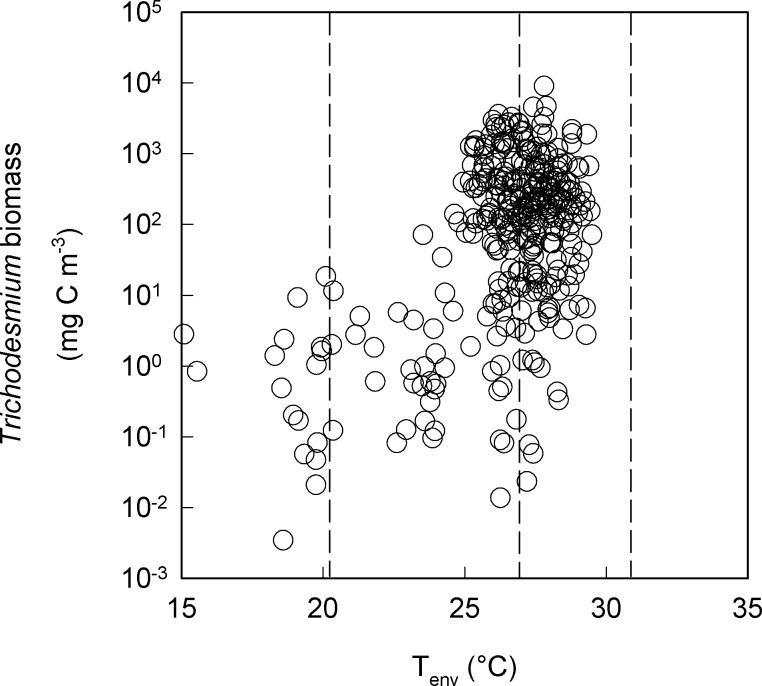
The thermal niche of Trichodesmium, which is confined to a relatively narrow temperature range from 19–30°C, is a key factor that sets the limits of its geographic distribution. It is clear that temperature plays a pivotal role in constraining Trichodesmium’s global distribution as i), peak abundance for Trichodesmium sp. occurs in regions that are supra-optimal temperature for growth of IMS101 (Fig 2) and other Trichodesmium isolates (e.g. GBRTRL 1101, KO4-20, RLI or 2175) that have been investigated [27,28] and ii), the growth response to temperature is negatively skewed. Growth rates decrease significantly with a 3 to 4°C increase above the optimal (26°C). The areal distribution of Trichodesmium spp. is predicted to increase as the 20°C isotherm slowly shifts pole-wards [80,81]. None-the-less, the negative effects of increased temperature and light at low latitudinal regions where temperature already exceeds Topt may lead to a contraction of the range at low latitudes. How future increases of SST will influence the distribution will depend on the capacity for Trichodesmium spp. to adapt by increasing its upper thermal tolerance limit.
Fig 2. Trichodesmium is found most frequently and its biomass is highest where water temperature is supra-optimal for growth of IMS101.
The dashed lines represent the minimum (Tmin), optimal (Topt) and maximum (Tmax) temperature limits for Trichodesmium IMS101 growth for the mid CO2 (~ 380 ppm), high-light treatment. Data from the MARine Ecosystem Data archive (https://doi.pangaea.de/10.1594/PANGAEA.774851).
Increases in atmospheric CO2 are transmitted to the ocean, increasing the dissolved CO2, and causing ocean pH and carbonate concentration to decline and bicarbonate concentration to increase. Recent research suggests that ocean acidification has greater potential to increase phytoplankton growth rates in areas of the ocean where temperature is equal to or less than the Topt [82]. In regards to Trichodesmium IMS101, previous research indicates that increasing CO2 above 380–400 ppm can lead to modest increases in Trichodesmium growth rate [33,34,38–40,42,43], some studies have reported declines in growth at elevated CO2 [36,37] and others no change [32,35]. Our study suggests that ocean acidification will have a small to negligible effect for growth at the supraoptimal temperatures above 27°C. Differing growth responses to elevated CO2 between key phytoplankton types could cause sufficient shifts in competitive fitness to alter community structure [83]. Thus, the effect of ocean acidification, albeit indirectly, may still play a role in constraining Trichodesmium’s global distribution.
Supporting Information
All experimental cultures were semi-continuously cultured at balanced growth at the lower section of the exponential growth phase (dashed line). The highest Fo value a culture achieved prior to dilution was ~ 20% of the stationary phase Fo value.
(TIF)
The R code was applied to the full data set of each treatment (A). Criterion 1 identifies and discards data associated with crashed cultures (large circles) or lagged growth (B). Criterion 2 then identifies and discards individual data points (small circles) which, if incorporated, would significantly alter the gradient of a slope (C). Finally, criterion 3 identifies and discards slopes (dashed lines) associated with acclimation (D). The remaining slopes are associated with balanced growth, and were used to calculate a median growth rate (E).
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Temperature response growth conditions; low (~ 180 ppm), mid (~ 380 ppm) and high (~ 720 ppm) CO2, 40 μmol photons m-2 s-1 (LL) and 400 μmol photons m-2 s-1 (HL), ranging between 18–31°C. Light response growth conditions; low (~ 180 ppm), mid (~ 380 ppm) and high (~ 720 ppm) CO2, 26°C, ranging between 20–1400 μmol photons m-2 s-1). A circle (O) represents a growing culture; a cross (X) represents a condition where growth did not occur; a dash (-) represents a condition that was not used for culturing.
(DOCX)
Footnotes to S2 Table. a CO2 fixation to carbohydrate in the Calvin cycle according to the following stoichiometry. CO2 + 3 ATP + 2 NADPH → CH2O + H2O + 3ADP +3 Pi. The photon requirement (9 photons/CO2 fixed) is from Raven et al. [84]. b Carbon concentrating mechanism where the only energised step is the influx of HCO3- at one membrane between the medium and Rubisco. Lower value assumes no leakage, whereas the upper value assumes leakage rate equals to the rate of photosynthesis [84]. c The energetic cost of N2 fixation was calculated assuming complete recycling of H2 to recover ATP was calculated from the following stoichiometry: N2 + 6 H+ + 6 e- + 13 ATP → 2 NH3 + 13 ADP + 13 Pi.d The cost of ammonium assimilation into amino acids is 1 ATP/NH3 and 1 NADPH (2 reducing equivalents) assimilated via GOGAT. Protein synthesis would require an additional 4 ATP per peptide bond formed. e Based on a typical photosynthetic quotient of 1.2 O2 evolved per CO2 fixed for algae growing with ammonium as the inorganic N source. This accounts for the more reduced state of lipids and proteins relative to carbohydrates. f Total cost of synthesising 1 unit of C-biomass assumes a Redfield C:N ratio of 106C:16N and that protein accounts for all of the cell N. g Photon requirements were calculated based on 1/3 ATP generated per photon absorbed during linear photosynthetic electron transfer from H2O to O2, with the additional ATP requirement from provided either by LPET from H2O to H2O (water-water cycle) with 1/3 ATP generated per photon absorbed (higher estimate) or by cyclic photosynthetic electron transfer around photosystem I with 1 ATP generated per photon absorbed.
(DOCX)
Key; x is the calculated value, σx is the calculated error of uncertainty; a, b and c are known quantities; σa, σb and σc are errors of uncertainty for a, b and c, respectively; y is a constant with no measure of uncertainty.
(DOCX)
Acknowledgments
Tobias Boatman was supported by a UK Natural Environment Research Council PhD studentship (NE/J500379/1 DTB). The authors wish to thank Prof Graham Upton for the R scripts used for determining growth rate.
Data Availability
All growth rate data files and the inorganic carbon chemistry calculation datasheet are available from Figshare at the following link: https://figshare.com/s/f89d34207813af383790 (DOI: 10.6084/m9.figshare.4299230).
Funding Statement
Toby Boatman was supported by a UK Natural Environment Research Council(NERC) PhD studentship (NE/J500379/1 DTB) held at the University of Essex.
References
- 1.Sabine CL, Feely RA, Gruber N, Key RM, Lee K, et al. (2004) The oceanic sink for anthropogenic CO2. science 305: 367–371. 10.1126/science.1097403 [DOI] [PubMed] [Google Scholar]
- 2.Raven J, Falkowski P (1999) Oceanic sinks for atmospheric CO2. Plant, Cell and Environment 22: 741–755. [Google Scholar]
- 3.Caldeira K, Wickett ME (2003) Oceanography: anthropogenic carbon and ocean pH. Nature 425: 365–365. 10.1038/425365a [DOI] [PubMed] [Google Scholar]
- 4.Rost B, Zondervan I, Wolf-Gladrow D (2008) Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: current knowledge, contradictions and research directions. Marine Ecology Progress Series 227: 227–237. [Google Scholar]
- 5.Doney SC (2006) Oceanography: Plankton in a warmer world. Nature 444: 695–696. 10.1038/444695a [DOI] [PubMed] [Google Scholar]
- 6.Pandey KD, Shukla SP, Shukla PN, Giri DD, Singh JS, et al. (2004) Cyanobacteria in Antarctica: ecology, physiology and cold adaptation. Cellular and molecular biology (Noisy-le-Grand, France) 50: 575–584. [PubMed] [Google Scholar]
- 7.Zielke M, Ekker AS, Olsen RA, Spjelkavik S, Solheim B (2002) The influence of abiotic factors on biological nitrogen fixation in different types of vegetation in the High Arctic, Svalbard. Arctic, Antarctic, and Alpine Research 34: 293–299. [Google Scholar]
- 8.Stal LJ (2009) Is the distribution of nitrogen-fixing cyanobacteria in the oceans related to temperature? Environmental Microbiology 11: 1632–1645. 10.1111/j.1758-2229.2009.00016.x [DOI] [PubMed] [Google Scholar]
- 9.Capone DG (2008) Nitrogen in the marine environment: Academic Press. [Google Scholar]
- 10.Campbell L, Carpenter E, Montoya J, Kustka A, Capone D (2005) Picoplankton community structure within and outside a Trichodesmium bloom in the southwestern Pacific Ocean. Vie et milieu 55: 185–195. [Google Scholar]
- 11.Capone DG, Zehr JP, Paerl HW, Bergman B, Carpenter EJ (1997) Trichodesmium, a globally significant marine cyanobacterium. Science 276: 1221–1229. [Google Scholar]
- 12.Carpenter EJ, Capone DG (1992) Nitrogen fixation in Trichodesmium blooms. Marine Pelagic Cyanobacteria: Trichodesmium and other Diazotrophs 362: 211–217. [Google Scholar]
- 13.Capone DG, Burns JA, Montoya JP, Subramaniam A, Mahaffey C, et al. (2005) Nitrogen fixation by Trichodesmium spp.: An important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Global Biogeochemical Cycles 19: GB2024. [Google Scholar]
- 14.Karl D, Letelier R, Tupas L, Dore J, Christian J, et al. (1997) The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature 388: 533–538. [Google Scholar]
- 15.Martinez-Perez C, Mohr W, Löscher CR, Dekaezemacker J, Littmann S, et al. (2016) The small unicellular diazotrophic symbiont, UCYN-A, is a key player in the marine nitrogen cycle. Nature Microbiology 1: 16163 10.1038/nmicrobiol.2016.163 [DOI] [PubMed] [Google Scholar]
- 16.Carpenter EJ, Romans K (1991) Major role of the cyanobacterium Trichodesmium in nutrient cycling in the North Atlantic Ocean. Science 254: 1356–1358. 10.1126/science.254.5036.1356 [DOI] [PubMed] [Google Scholar]
- 17.Monteiro FM, Dutkiewicz S, Follows MJ (2011) Biogeographical controls on the marine nitrogen fixers. Global Biogeochemical Cycles 25: 1–8. [Google Scholar]
- 18.Carpenter EJ, Subramaniam A, Capone DG (2004) Biomass and primary productivity of the cyanobacterium Trichodesmium spp. in the tropical N Atlantic ocean. Deep Sea Research Part I: Oceanographic Research Papers 51: 173–203. [Google Scholar]
- 19.Tyrrell T, Marañón E, Poulton AJ, Bowie AR, Harbour DS, et al. (2003) Large-scale latitudinal distribution of Trichodesmium spp. in the Atlantic Ocean. Journal of Plankton Research 25: 405–416. [Google Scholar]
- 20.Karl D, Michaels A, Bergman B, Capone D, Carpenter E, et al. (2002) Dinitrogen fixation in the world's oceans. Biogeochemistry 57: 47–98. [Google Scholar]
- 21.Breitbarth E, Wohlers J, Klas J, LaRoche J, Peeken I (2008) Nitrogen fixation and growth rates of Trichodesmium IMS-101 as a function of light intensity. Marine Ecology Progress Series 359: 25–36. [Google Scholar]
- 22.Mulholland M (2007) The fate of nitrogen fixed by diazotrophs in the ocean. Biogeosciences 4: 37–51. [Google Scholar]
- 23.Thomas MK, Kremer CT, Litchman E (2016) Environment and evolutionary history determine the global biogeography of phytoplankton temperature traits. Global Ecology and Biogeography 25: 75–86. [Google Scholar]
- 24.Kingsolver JG (2009) The Well-Temperatured Biologist. The American Naturalist 174: 755–768. 10.1086/648310 [DOI] [PubMed] [Google Scholar]
- 25.Eppley RW (1972) Temperature and phytoplankton growth in the sea. Fishery Bulletin 70: 1063–1085. [Google Scholar]
- 26.Breitbarth E, Oschlies A, Laroche J (2007) Physiological constraints on the global distribution of Trichodesmium—effect of temperature on diazotrophy. Biogeosciences 4: 53–61. [Google Scholar]
- 27.Boyd PW, Rynearson TA, Armstrong EA, Fu F, Hayashi K, et al. (2013) Marine phytoplankton temperature versus growth responses from polar to tropical waters–outcome of a scientific community-wide study. PLoS ONE 8: e63091 10.1371/journal.pone.0063091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu F-X, Yu E, Garcia NS, Gale J, Luo Y, et al. (2014) Differing responses of marine N2 fixers to warming and consequences for future diazotroph community structure. Aquatic Microbial Ecology 72: 33–46. [Google Scholar]
- 29.Wernberg T, Smale DA, Thomsen MS (2012) A decade of climate change experiments on marine organisms: procedures, patterns and problems. Global Change Biology 18: 1491–1498. [Google Scholar]
- 30.Chappell PD, Webb EA (2010) A molecular assessment of the iron stress response in the two phylogenetic clades of Trichodesmium. Environmental Microbiology 12: 13–27. 10.1111/j.1462-2920.2009.02026.x [DOI] [PubMed] [Google Scholar]
- 31.Cai X, Gao K (2015) Levels of daily light doses under changed day-night cycles regulate temporal segregation of photosynthesis and N2 fixation in the Cyanobacterium Trichodesmium erythraeum IMS101. PLoS ONE 10: e0135401 10.1371/journal.pone.0135401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barcelos e Ramos J, Biswas H, Schulz KG, LaRoche J, Riebesell U (2007) Effect of rising atmospheric carbon dioxide on the marine nitrogen fixer Trichodesmium. Global Biogeochemical Cycles 21: GB2028. [Google Scholar]
- 33.Hutchins DA, Walworth NG, Webb EA, Saito MA, Moran D, et al. (2015) Irreversibly increased nitrogen fixation in Trichodesmium experimentally adapted to elevated carbon dioxide. Nature communications 6: 8155 10.1038/ncomms9155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levitan O, Rosenberg G, Setlik I, Setlikova E, Grigel J, et al. (2007) Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Global Change Biology 13: 531–538. [Google Scholar]
- 35.Kranz S, Sültemeyer D, Richter KU, Rost B (2009) Carbon acquisition in Trichodesmium: The effect of pCO2 and diurnal changes. Limnology and Oceanography 54: 548–559. [Google Scholar]
- 36.Shi D, Kranz SA, Kim JM, Morel FMM (2012) Ocean acidification slows nitrogen fixation and growth in the dominant diazotroph Trichodesmium under low-iron conditions. Proceedings of the National Academy of Sciences 109: 3094–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eichner M, Kranz SA, Rost B (2014) Combined effects of different CO2 levels and N sources on the diazotrophic cyanobacterium Trichodesmium. Physiologia Plantarum 152: 316–330. 10.1111/ppl.12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spungin D, Berman-Frank I, Levitan O (2014) Trichodesmium's strategies to alleviate phosphorus limitation in the future acidified oceans. Environmental Microbiology 16: 1935–1947. [DOI] [PubMed] [Google Scholar]
- 39.Kranz SA, Levitan O, Richter KU, Prášil O, Berman-Frank I, et al. (2010) Combined effects of CO2 and light on the N2-fixing cyanobacterium Trichodesmium IMS101: physiological responses. Plant Physiology 154: 334–345. 10.1104/pp.110.159145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia NS, Fu F-X, Breene CL, Bernhardt PW, Mulholland MR, et al. (2011) Interactive effects of Irradiance and CO2 on CO2 fixation and N2 fixation in the Diazotroph Trichodesmium erythraeum (Cyanobacteria). Journal of Phycology 47: 1292–1303. 10.1111/j.1529-8817.2011.01078.x [DOI] [PubMed] [Google Scholar]
- 41.Ho T-Y, Chu T-H, Hu C-L (2013) Interrelated influence of light and Ni on Trichodesmium growth. Frontiers in Microbiology 4: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutchins D, Fu FX, Zhang Y, Warner M, Feng Y, et al. (2007) CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios: implications for past, present, and future ocean biogeochemistry. Limnology and Oceanography 52: 1293–1304. [Google Scholar]
- 43.Levitan O, Brown CM, Sudhaus S, Campbell D, LaRoche J, et al. (2010) Regulation of nitrogen metabolism in the marine diazotroph Trichodesmium IMS101 under varying temperatures and atmospheric CO2 concentrations. Environmental Microbiology 12: 1899–1912. 10.1111/j.1462-2920.2010.02195.x [DOI] [PubMed] [Google Scholar]
- 44.Chen YB, Zehr JP, Mellon M (1996) Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium Sp. IMS 101 in defined media: evidence for a circadian rhythm. Journal of Phycology 32: 916–923. [Google Scholar]
- 45.Lewis E, Wallace D (1998) CO2SYS Program. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory Environmental Sciences Division, Oak Ridge, Tennessee. [Google Scholar]
- 46.Dickson AG (1993) pH buffers for sea water media based on the total hydrogen ion concentration scale. Deep Sea Research Part I: Oceanographic Research Papers 40: 107–118. [Google Scholar]
- 47.Millero FJ (2010) Carbonate constants for estuarine waters. Marine and Freshwater Research 61: 139–142. [Google Scholar]
- 48.Dickson AG (1990) Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep Sea Research Part A Oceanographic Research Papers 37: 755–766. [Google Scholar]
- 49.Lee K, Kim T-W, Byrne RH, Millero FJ, Feely RA, et al. (2010) The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans. Geochimica et Cosmochimica Acta 74: 1801–1811. [Google Scholar]
- 50.Kranz S, Wolf-Gladrow D, Nehrke G, Langer G, Rost B (2010) Calcium carbonate precipitation induced by the growth of the marine cyanobacteria Trichodesmium. Limnology and Oceanography 55: 2563–2569. [Google Scholar]
- 51.R-Development-Core-Team (2014) R: A language and environment for statistical computing. Vienna, Austria.
- 52.Upton G (2014) A protocol for the determination of the growth rate of organisms subject to interrupted exponential growth. Journal of Computational Systems Biology 1: 103. [Google Scholar]
- 53.Platt T, Gallegos CL (1980) Modelling primary production Primary productivity in the sea: Springer; pp. 339–362. [Google Scholar]
- 54.Bergman B, Sandh G, Lin S, Larsson J, Carpenter EJ (2012) Trichodesmium–a widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiology Reviews 37: 286–302. 10.1111/j.1574-6976.2012.00352.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finzi-Hart JA, Pett-Ridge J, Weber PK, Popa R, Fallon SJ, et al. (2009) Fixation and fate of C and N in the cyanobacterium Trichodesmium using nanometer-scale secondary ion mass spectrometry. Proceedings of the National Academy of Sciences 106: 6345–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saino T, Hattori A (1982) Aerobic nitrogen fixation by the marine non-heterocystous cyanobacterium Trichodesmium (Oscillatoria) spp.: Its protective mechanism against oxygen. Marine Biology 70: 251–254. [Google Scholar]
- 57.Kana TM (1993) Rapid oxygen cycling in Trichodesmium thiebautii. Limnology and Oceanography 38: 18–24. [Google Scholar]
- 58.Carpenter J. E, Roenneberg T. (1995) The marine planktonic cyanobacteria Trichodesmium spp.: photosynthetic rate measurements in the SW Atlantic Ocean. Marine Ecology Progress Series 118: 267–273. [Google Scholar]
- 59.Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, et al. (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynthesis Research 98: 541–550. 10.1007/s11120-008-9331-0 [DOI] [PubMed] [Google Scholar]
- 60.Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proceedings of the National Academy of Sciences 97: 13430–13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salvucci ME, Crafts-Brandner SJ (2004) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiologia Plantarum 120: 179–186. 10.1111/j.0031-9317.2004.0173.x [DOI] [PubMed] [Google Scholar]
- 62.Salvucci ME, Crafts-Brandner SJ (2004) Mechanism for deactivation of Rubisco under moderate heat stress. Physiologia Plantarum 122: 513–519. [DOI] [PubMed] [Google Scholar]
- 63.Badger MR, Hanson D, Price GD (2002) Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Functional Plant Biology 29: 161–173. [DOI] [PubMed] [Google Scholar]
- 64.Badger MR, Price GD (2003) CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. Journal of Experimental Botany 54: 609–622. [DOI] [PubMed] [Google Scholar]
- 65.Price GD, Badger MR, Woodger FJ, Long BM (2008) Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. Journal of Experimental Botany 59: 1441–1461. [DOI] [PubMed] [Google Scholar]
- 66.Jickells T, An Z, Andersen KK, Baker A, Bergametti G, et al. (2005) Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 308: 67–71. 10.1126/science.1105959 [DOI] [PubMed] [Google Scholar]
- 67.Titman D, Kilham P (1976) Sinking in freshwater phytoplankton: some ecological implications of cell nutrient status and physical mixing processes. Limnology and Oceanography: 409–417. [Google Scholar]
- 68.Küpper H, Šetlík I, Seibert S, Prášil O, Šetlikova E, et al. (2008) Iron limitation in the marine cyanobacterium Trichodesmium reveals new insights into regulation of photosynthesis and nitrogen fixation. New Phytologist 179: 784–798. 10.1111/j.1469-8137.2008.02497.x [DOI] [PubMed] [Google Scholar]
- 69.Paerl HW, Prufert-Bebout LE, Guo C (1994) Iron-stimulated N2 fixation and growth in natural and cultured populations of the planktonic marine cyanobacteria Trichodesmium spp. Applied and Environmental Microbiology 60: 1044–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi T, Sun Y, Falkowski PG (2007) Effects of iron limitation on the expression of metabolic genes in the marine cyanobacterium Trichodesmium erythraeum IMS101. Environmental Microbiology 9: 2945–2956. 10.1111/j.1462-2920.2007.01406.x [DOI] [PubMed] [Google Scholar]
- 71.Boyce DG, Lewis MR, Worm B (2010) Global phytoplankton decline over the past century. Nature 466: 591–596. 10.1038/nature09268 [DOI] [PubMed] [Google Scholar]
- 72.Dave AC, Lozier MS (2013) Examining the global record of interannual variability in stratification and marine productivity in the low‐latitude and mid‐latitude ocean. Journal of Geophysical Research: Oceans 118: 3114–3127. [Google Scholar]
- 73.Sonntag S, Hense I (2011) Phytoplankton behavior affects ocean mixed layer dynamics through biological‐physical feedback mechanisms. Geophysical Research Letters 38. [Google Scholar]
- 74.Li G, Brown CM, Jeans JA, Donaher NA, McCarthy A, et al. (2015) The nitrogen costs of photosynthesis in a diatom under current and future pCO2. New Phytologist 205: 533–543. 10.1111/nph.13037 [DOI] [PubMed] [Google Scholar]
- 75.Stihl A, Sommer U, Post AF (2001) Alkaline phosphatase activities amoung populations of the colony-forming diazotrophic cyanobacterium Trichodesmium spp. (Cyanobacteria) in the Red Sea. Journal of Phycology 37: 310–317. [Google Scholar]
- 76.Dyhrman ST, Chappell PD, Haley ST, Moffett JW, Orchard ED, et al. (2006) Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 439: 68–71. 10.1038/nature04203 [DOI] [PubMed] [Google Scholar]
- 77.Romans KM, Carpenter EJ, Bergman B (1994) Buoyancy regulation in the colonial diazotrophic cyanobacterium Trichodesmium tenue: ultrastructure and storage of carbohydrate, polyphosphate, and nitrogen. Journal of Phycology 30: 935–942. [Google Scholar]
- 78.White AE, Spitz YH, Karl DM, Letelier RM (2006) Flexible elemental stoichiometry in Trichodesmium spp. and its ecological implications. Limnology and Oceanography 51: 1777–1790. [Google Scholar]
- 79.Landolfi A, Koeve W, Dietze H, Kähler P, Oschlies A (2015) A new perspective on environmental controls of marine nitrogen fixation. Geophysical Research Letters 42: 4482–4489. [Google Scholar]
- 80.Boyd PW, Doney SC (2002) Modelling regional responses by marine pelagic ecosystems to global climate change. Geophysical Research Letters 29: 53–51. [Google Scholar]
- 81.Boyd P, Ellwood M (2010) The biogeochemical cycle of iron in the ocean. Nature Geoscience 3: 675–682. [Google Scholar]
- 82.Thomas MK, Kremer CT, Klausmeier CA, Litchman E (2012) A global pattern of thermal adaptation in marine phytoplankton. Science 338: 1085–1088. 10.1126/science.1224836 [DOI] [PubMed] [Google Scholar]
- 83.Dutkiewicz S, Morris JJ, Follows MJ, Scott J, Levitan O, et al. (2015) Impact of ocean acidification on the structure of future phytoplankton communities. Nature Climate Change 5: 1002–1006. [Google Scholar]
- 84.Raven JA, Beardall J, Giordano M (2014) Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynthesis Research 121: 111–124. 10.1007/s11120-013-9962-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All experimental cultures were semi-continuously cultured at balanced growth at the lower section of the exponential growth phase (dashed line). The highest Fo value a culture achieved prior to dilution was ~ 20% of the stationary phase Fo value.
(TIF)
The R code was applied to the full data set of each treatment (A). Criterion 1 identifies and discards data associated with crashed cultures (large circles) or lagged growth (B). Criterion 2 then identifies and discards individual data points (small circles) which, if incorporated, would significantly alter the gradient of a slope (C). Finally, criterion 3 identifies and discards slopes (dashed lines) associated with acclimation (D). The remaining slopes are associated with balanced growth, and were used to calculate a median growth rate (E).
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Temperature response growth conditions; low (~ 180 ppm), mid (~ 380 ppm) and high (~ 720 ppm) CO2, 40 μmol photons m-2 s-1 (LL) and 400 μmol photons m-2 s-1 (HL), ranging between 18–31°C. Light response growth conditions; low (~ 180 ppm), mid (~ 380 ppm) and high (~ 720 ppm) CO2, 26°C, ranging between 20–1400 μmol photons m-2 s-1). A circle (O) represents a growing culture; a cross (X) represents a condition where growth did not occur; a dash (-) represents a condition that was not used for culturing.
(DOCX)
Footnotes to S2 Table. a CO2 fixation to carbohydrate in the Calvin cycle according to the following stoichiometry. CO2 + 3 ATP + 2 NADPH → CH2O + H2O + 3ADP +3 Pi. The photon requirement (9 photons/CO2 fixed) is from Raven et al. [84]. b Carbon concentrating mechanism where the only energised step is the influx of HCO3- at one membrane between the medium and Rubisco. Lower value assumes no leakage, whereas the upper value assumes leakage rate equals to the rate of photosynthesis [84]. c The energetic cost of N2 fixation was calculated assuming complete recycling of H2 to recover ATP was calculated from the following stoichiometry: N2 + 6 H+ + 6 e- + 13 ATP → 2 NH3 + 13 ADP + 13 Pi.d The cost of ammonium assimilation into amino acids is 1 ATP/NH3 and 1 NADPH (2 reducing equivalents) assimilated via GOGAT. Protein synthesis would require an additional 4 ATP per peptide bond formed. e Based on a typical photosynthetic quotient of 1.2 O2 evolved per CO2 fixed for algae growing with ammonium as the inorganic N source. This accounts for the more reduced state of lipids and proteins relative to carbohydrates. f Total cost of synthesising 1 unit of C-biomass assumes a Redfield C:N ratio of 106C:16N and that protein accounts for all of the cell N. g Photon requirements were calculated based on 1/3 ATP generated per photon absorbed during linear photosynthetic electron transfer from H2O to O2, with the additional ATP requirement from provided either by LPET from H2O to H2O (water-water cycle) with 1/3 ATP generated per photon absorbed (higher estimate) or by cyclic photosynthetic electron transfer around photosystem I with 1 ATP generated per photon absorbed.
(DOCX)
Key; x is the calculated value, σx is the calculated error of uncertainty; a, b and c are known quantities; σa, σb and σc are errors of uncertainty for a, b and c, respectively; y is a constant with no measure of uncertainty.
(DOCX)
Data Availability Statement
All growth rate data files and the inorganic carbon chemistry calculation datasheet are available from Figshare at the following link: https://figshare.com/s/f89d34207813af383790 (DOI: 10.6084/m9.figshare.4299230).