Abstract
It has been suggested that retrieval during a nonreinforced test induces reconsolidation instead of extinction of the mnemonic trace. Reconsolidation would preserve the original memory from the labilization induced by its nonreinforced recall through a hitherto uncharacterized mechanism requiring protein synthesis. Given the importance that such a process would have in terms of maintaining, as part of the animal behavioral repertoire, a learned response that has been devalued by experience, we analyzed its existence for the memory associated with a one-trial, step-down inhibitory avoidance task (IA), a memory whose consolidation and extinction require protein synthesis in the CA1 region of the dorsal hippocampus (CA1) and involve the participation of the basolateral amygdala (BLA) and entorhinal cortex (ENT). Rats were trained in IA, and 24 h later they were submitted either to a pure reactivation session (retrieval without stepping down), which was unable by itself to initiate extinction of the avoidance response, or to a second training session. Fifteen minutes before or 3 h after either the reactivation or the retraining sessions, animals were infused with the protein synthesis inhibitor anisomycin (ANI) into CA1, BLA, or ENT. Contrary to the prediction of the reconsolidation hypothesis, none of these treatments affected subsequent memory retention. Because reconsolidation is regarded to be a direct consequence of retrieval, one would expect that, when given before a retention test or a pure reactivation session, enhancers of memory expression should permanently improve retention and, therefore, facilitate retrieval both in that and in subsequent sessions. Using two well-known retrieval enhancers, noradrenaline and adrenocorticotropin1-24, we could not find any evidence suggestive of reconsolidation. Hence, our results indicate that there is no retrieval-induced, protein synthesis-dependent process that would cause reconsolidation of IA memory.
Memories are stored through a process called consolidation. This process was postulated at the very beginning of the twentieth century (Müller and Pilzecker 1900) and was experimentally supported by the demonstration over the years that several agents induce amnesia when given shortly after acquisition, but not later. This hypothesis became a theory only after other posttraining treatments were found to facilitate retention, allowing the eventual investigation of putative mechanisms for consolidation (McGaugh 1966). These mechanisms are now known to consist of a tightly knit assortment of interdependent molecular processes (Izquierdo and Medina 1997) that are conserved across species or even phyla (Tully 1998; Menzel 2001). A central tenet of the consolidation theory is that consolidated memories are more stable and resistant to changes than are newly acquired ones. This does not mean that consolidated memories are fixed and cannot be modified, which would obviously be a rather ill-adaptive way to store information. Aside from changes induced by misleading or false information (Loftus and Palmer 1974; Schacter and Dodson 2001), learning of other tasks, and neurohumoral influences (Izquierdo 1989), it has been known for nearly a century (Pavlov 1927) that well-established conditioned responses (CRs) can be extinguished through the repeated presentation of the conditioned stimulus (CS) in the absence of the unconditioned stimulus (US) to which it had been associated (i.e., by nonreinforced retrieval). Although expressed as a weakening of the tendency to retrieve the CR on presentation of the CS, extinction does not involve forgetting but, rather, a new, additional learning in which a CS-no US pairing overrules the original CS-US association (Rescorla 2001). In fact, as happens with the consolidation of most types of new memories (see Myers and Davis 2002a), extinction is blocked by protein synthesis inhibitors infused into the hippocampus (Vianna et al. 2001) or the basolateral amygdala (BLA; Myers and Davis 2002b; Bahar et al. 2003) at the time of the first of a series of test sessions. The inhibition of extinction by ANI administered into BLA or the hippocampus is very consistent across the literature (Myers and Davis 2002a,b). One very recent paper, however, suggests that, in some conditions, intrahippocampal ANI may have an opposite effect (Fischer et al. 2004). Clearly, extinction is a retrieval-induced phenomenon that devalues learned behaviors that are no longer adaptive without erasing stored information that could still be useful in the future.
Building on previous findings indicating that trained animals reexposed to proper reminder cues followed by hypothermia or electroconvulsive shock temporarily show weakened retrieval (Misanin et al. 1968), it has been suggested that presentation of a nonreinforced CS initiates a process of reconsolidation rather than extinction. This early paper was decisively refuted by Dawson and McGaugh (1969), who, using a better controlled experimental design, were simply unable to reproduce the results presented by Misanin and coworkers.
Reconsolidation would reaffirm behaviors that could or would be extinguished (Nader 2003). In view of our longstanding knowledge about extinction, this suggestion may, at first sight, appear counterintuitive (Myers and Davis 2002b). It mainly derives from results indicating that, in some aversive tasks, the protein synthesis inhibitor anisomycin (ANI) given at the time of the first memory retention test may worsen performance during a second or subsequent tests (Nader et al. 2000; Debiec et al. 2002). Using a mechanism that requires protein synthesis, reconsolidation would rebuild the trace labilized by retrieval.
To date, the plausibility of the reconsolidation hypothesis has been analyzed, using retrieval procedures that, at least in theory, should induce extinction of the original CR. In addition, several studies (Vianna et al. 2001; Lattal and Abel 2004) suggest that the retention impairment produced by different postretrieval treatments is typically transitory. For that reason, many consider the so-called reconsolidation process as representing just a temporary performance effect.
Given that reconsolidation would maintain an acquired CR devalued by experience (i.e., the opposite to extinction) as part of the animal behavioral repertoire, it is crucial to analyze its existence, using as the retrieval test either a pure reactivation session (one in which CS and CR are present but the possibility of extinction is neutrally prevented) or a retraining session (a session in which the animal is confronted again with a training procedure, such as the one from which the original memory stemmed and, hence, the one that should induce further consolidation). To do that, we used a one-trial, step-down inhibitory avoidance paradigm (IA), a form of fear conditioning much used for the study of consolidation, retrieval, and extinction.
RESULTS
Inhibition of Hippocampal Protein Synthesis at the Moment of, or 3 h After, Training Hinders IA Memory Consolidation
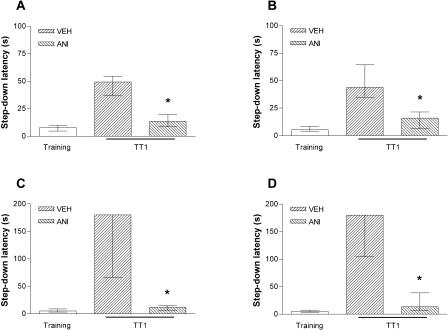
Confirming and extending previous results, we found that when given into the CA1 region of the dorsal hippocampus 15 min before or 3 h after training, ANI blocked consolidation of the IA memory, as evaluated in a retention test session carried out 24 h posttraining (Fig. 1). The amnesic effect of ANI was observed regardless of whether the animals had been trained using a 0.5-mA (Fig. 1A,B) or a 0.8-mA (Fig. 1C,D), 2-sec foot-shock.
Figure 1.
Consolidation of IA memory requires protein synthesis in the CA1 region of the dorsal hippocampus. Animals who had cannulas implanted and aimed toward the CA1 region of the dorsal hippocampus were trained in the IA task using a 0.5-mA (A,B) or 0.8-mA (C,D), 2-sec foot-shock. Fifteen minutes before (A,C) or 3 h after (B,D) the training session, the animals received 0.8 μL bilateral infusions of vehicle (VEH) or ANI (80 μg/side). Memory retention was evaluated in a test session carried out 24 h posttraining (TT1). Data represent median ± interquartile range of the step down latency time. *P < 0.005 versus VEH in Mann-Whitney test; n = 10 to 14 per group.
Inhibition of Protein Synthesis in CA1, the BLA, or the ENT 15 min Before or 3 h After a Pure Reactivation Session Does Not Affect Subsequent Memory Retention
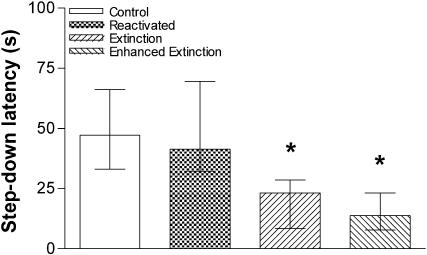
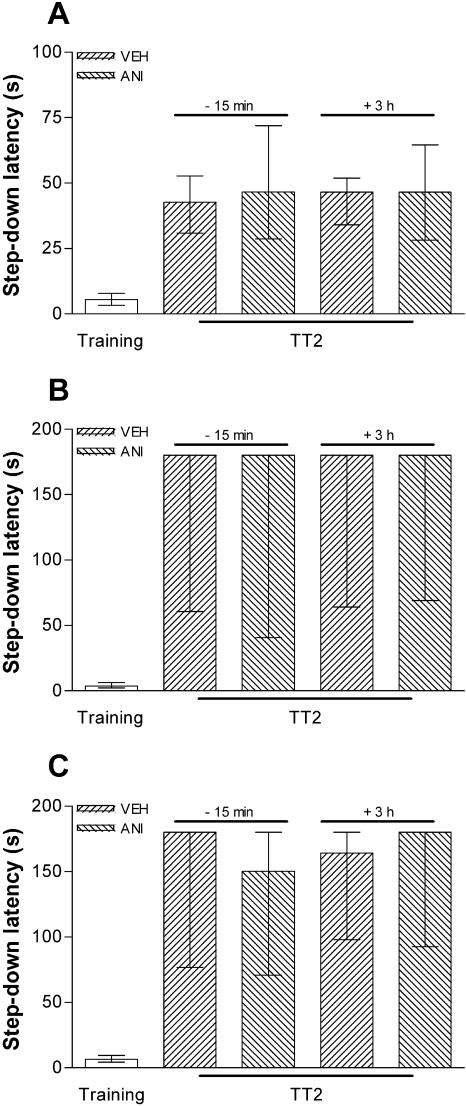
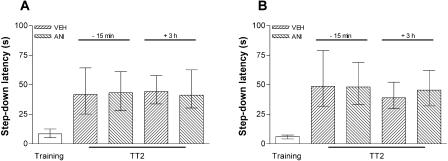
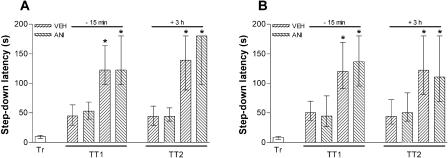
To study whether memory retrieval initiates a protein synthesis-dependent process, other than extinction in the hippocampus, that is able to influence further memory retention, animals were trained in IA using a 0.5- or 0.8-mA, 2-sec foot-shock and, 24 h later, were submitted to a pure reactivation session that, as can be seen in Figure 2, is unable to induce extinction of the IA response. Fifteen minutes before or 3 h after a 20-sec (Fig. 3A,B) or 40-sec (Fig. 3C) reactivation session, the animals received bilateral infusions of ANI (80 μg/side; 0.8 μL) or vehicle into CA1. Memory retention was evaluated in a test session carried out 24 h after reactivation. By itself, the pure reactivation session had no effect on memory retention, a fact that was unaltered by ANI given into CA1 15 min before or 3 h after the reactivation event, regardless of the intensity of the foot-shock used in the training session or the time spent over the platform during the pure reactivation session. To analyze whether ANI was able to block the putative reconsolidation process when given into other brain regions known to be involved in the consolidation of IA long-term memory (Bernabeu et al. 1995; Bonini et al. 2003), we trained animals in the IA task using a 0.5-mA, 2-sec foot-shock and, either 15 min before or 3 h after a 20-sec reactivation session carried out 24 h posttraining, infused them with ANI (80 μg/side; 0.8 μL) or vehicle into BLA or ENT. As can be seen in Figure 4, ANI did not affect memory retention as evaluated in a test session carried out 24 h after reactivation. Therefore, by itself, retrieval of the avoidance response does not initiate any protein synthesis-dependent process that is able to influence further memory retention in CA1, BLA, or ENT.
Figure 2.
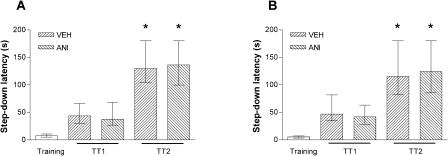
Memory reactivation without stepping down does not induce extinction of the IA response. Animals trained in the IA task (0.5 mA, 2-sec) were randomly assigned to one out of four experimental groups. The control group consisted of trained animals that were not submitted to any other behavioral procedure until they had been tested for IA retention. The reactivation group consisted of trained animals submitted to daily 20-sec pure reactivation sessions for 3 days. These animals were put on the training box platform and allowed to explore it freely. During the 20-sec period, rats avoided stepping down to the grid. The extinction group consisted of trained animals submitted to daily extinction sessions for 3 days. These animals were put on the training box platform and, at the very moment they eventually stepped down from it (no shock delivered), were removed to their home cages. The enhanced extinction group consisted of trained animals submitted to daily enhanced extinction sessions for 3 days. These animals were put on the training box platform and, after stepping down from it (no shock delivered), were allowed to freely explore the training box for 30 sec. Data represent median ± interquartile of the step-down latency time in a retention test session carried out 4 days after training. *P < 0.001 versus control group in Dunn's test after Kruskal-Wallis; n = 18 to 20 per group.
Figure 3.
Inhibition of protein synthesis in the CA1 region at the moment of or 3 h after a pure reactivation session does not affect an already consolidated mnemonic trace. Animals implanted with cannulas aimed to the CA1 region of the dorsal hippocampus were trained in the IA task using a 0.5-mA (A) or 0.8-mA (B,C), 2-sec foot-shock and 24 h later were submitted to a 20-sec (A,B) or 40-sec (C) pure reactivation session. Fifteen minutes before or 3 h after that session, the animals received 0.8 μL bilateral infusions of VEH or ANI (80 μg/side). Memory retention was evaluated in a test session carried out 24 h postreactivation (TT2); n = 12 to 15 per group.
Figure 4.
Inhibition of protein synthesis in the BLA or the ENT at the moment of or 3 h after a pure reactivation session does not affect an already consolidated mnemonic trace. Animals implanted with cannulas aimed to the BLA (A) or the entorhinal cortex (B) were trained in the IA task using a 0.5-mA, 2-sec foot-shock and, 24 h later, were submitted to a 20-sec pure reactivation session. Fifteen minutes before (-15 min) or 3 h after (+3 h) that session, the animals received 0.8 μL bilateral infusions of VEH or ANI (80 μg/side). Memory retention was evaluated in a test session carried out 24 h postreactivation (TT2); n = 10 to 12 per group.
Inhibition of Protein Synthesis in CA1, BLA, or ENT Does Not Block the Increase in Memory Retention Produced by a Retraining Session
The more powerful way to reactivate a consolidated memory is to confront the animal with a situation identical, in principle, to the one that originated the mnemonic trace under scrutiny. The procedure that more closely resembles such a situation consists, obviously, of submitting the animal to a second training session. The reconsolidation hypothesis predicts (see Duvarci et al. 2002) that the infusion of ANI, after a second training session, into an area of the brain where it is able to block memory consolidation (and reconsolidation) should render the animal totally amnesic. This would happen because during retraining, retrieval of the original trace would induce its own labilization, and hence ANI should block both reconsolidation of the memory associated with the first trial and consolidation of the second reinforced trial. Thus, in a different set of experiments, we trained animals in the IA task using a 0.5-mA, 2-sec foot-shock and then, 24 h later, submitted them to a retraining session. Fifteen minutes before or 3 h after the second training session, animals were infused with ANI (80 μg/side; 0.8 μL) or vehicle in CA1. Memory retention was evaluated in a test session carried out 24 h later. Retraining increased memory retention, but this enhancement was not blocked by ANI given either 15 min before (Fig. 5A) or 3 h after the second training session (Fig. 5B). The same results were obtained when the drug was administered into BLA or ENT 15 min before or 3 h after the second training session (Fig. 6). Therefore, although consolidation of the IA trace is blocked by ANI given 15 min before training or 3 h thereafter (Fig. 1), the improvement in retention caused by additional training is not.
Figure 5.
Inhibition of protein synthesis in the CA1 region does not block the enhancement in memory retention induced by retraining. Animals implanted with cannulas aimed to the CA1 region of the dorsal hippocampus were trained in the IA task using a 0.5-mA, 2-sec foot-shock and, 24 h later, were submitted to a retraining session (TT1). Fifteen minutes before (A) or 3 h after (B) that session, the animals received 0.8 μL bilateral infusions of VEH or ANI (80 μg/side). Memory retention was evaluated in a test session carried out 24 h after retraining (TT2). *P < 0.001 versus TT1 in Mann-Whitney test; n = 10 to 12 per group.
Figure 6.
Inhibition of protein synthesis in the BLA or the ENT does not block the enhancement in memory retention induced by retraining. Animals implanted with cannulas aimed to the BLA (A) or the ENT (B) were trained in the IA task using a 0.5-mA, 2-sec foot-shock and, 24 h later, were submitted to a retraining session (TT1). Fifteen minutes before (-15 min) or 3 h after (+3 h) the second training session, animals received 0.8 μL bilateral infusions of VEH or ANI (80 μg/side). Memory retention was evaluated in a test session carried out 24 h after retraining (TT2). *P < 0.001 versus TT1 in Mann-Whitney test; n = 10 to 12 per group.
Well-Known Retrieval Enhancers Given Before a Retention Test Session or a Pure Reactivation Session Do Not Affect Subsequent Memory Retention
If memory reactivation had any latent effect on subsequent retention resulting from an occult influence on retrieval, then retrieval enhancers given before a pure reactivation session should improve further performance of the task. Moreover, because the reconsolidation process has been postulated to be a direct consequence of retrieval, one could expect that treatments able to enhance retrieval during a reactivation session should also positively modulate the output of reconsolidation (i.e., they should permanently strengthen the mnemonic trace).
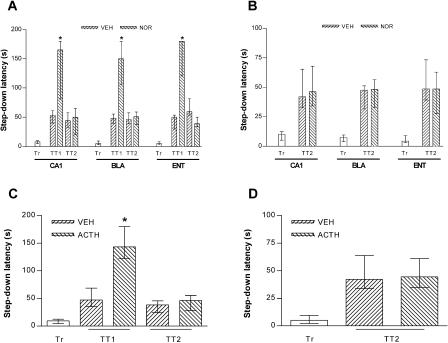
As can be seen in Figure 7, this was not the case for IA memory. The enhancement of memory retention induced by NA given into CA1, BLA, or ENT was limited to the test session before which it was given and did not extend to a subsequent retention test session carried out 1 day later (Fig. 7A). The same thing happened when NA was given into CA1, BLA, or ENT before a pure reactivation session, except that because of the design of this procedure, the retrieval enhancement produced by this treatment could not be seen during that session (Fig. 7B). Similarly, adrenocorticotropin1-24 (ACTH), given intraperitoneally 15 min before a retention test session, enhanced retention test performance only in that session (Fig. 7C), and when given 15 min before a pure reactivation session, it did not affect performance in a retention test session carried out 1 day later (Fig. 7D).
Figure 7.
Retrieval enhancers do not affect memory expression beyond the test session prior to which they were administered. Either animals implanted with cannulas aimed to the CA1 region of the dorsal hippocampus (CA1), the BLA, or the ENT or nonimplanted animals were trained in the IA task using a 0.5-mA, 2-sec foot-shock and, 24 h after training, were submitted to a retention test session (A,C; TT1) or to a 20-sec pure reactivation session (B,D). Fifteen minutes before those sessions, the animals received intra-CA1, intra-BLA, or intra-ENT infusions of noradrenaline (NOR; A,B) or intraperitoneal injections of ACTH (C,D). Memory was evaluated in a test session carried out 24 h later (TT2); that is, 48 h posttraining. *P < 0.001 versus VEH TT1 in Mann-Whitney test; n = 10 to 14 per group.
DISCUSSION
The major finding of this study is that neither retrieval nor enhanced retrieval facilitate further retention test performance, and they are not accompanied by protein synthesis-mediated events in CA1, BLA, or ENT that might be expected to induce reconsolidation as described by Nader et al. (2000) or Nader (2003).
Reconsolidation is a process that would be able to preserve a mnemonic trace from the labilization induced by its nonreinforced recall. The existence of such a process has been mainly speculated from experiments suggesting that it would be prevented by protein synthesis inhibitors given at the time of retrieval into areas of the brain involved in the consolidation of the memory under scrutiny (Judge and Quartermain 1982; Nader et al. 2000; see also Pedreira et al. 2002 and Pedreira and Maldonado 2003 for a description of this putative process in invertebrates). Here we observed that exposure to retrieval without extinction, facilitated or not, does not influence further retention test performance either in the presence or the absence of ANI given into the three major brain areas known to be involved in the consolidation of the memory for IA task: CA1, BLA, and ENT (Cammarota et al. 1998, 2000; Bonini et al. 2003; Rossato et al. 2004).
By extending previous results (Quevedo et al. 1999), we found that IA consolidation is blocked by the protein synthesis inhibitor ANI (80 μg/side; 0.8 μL) given into dorsal CA1 15 min before or 3 h after training. The amnesic effect of ANI was seen regardless of the intensity of the foot-shock used as a reinforcer and, hence, independent of the strength of the avoidance trace (Fig. 1). Notwithstanding that, and contravening the prediction of the reconsolidation hypothesis, when ANI was infused into CA1, BLA, or ENT 15 min before or 3 h after a pure reactivation session, although incapable by itself of altering memory retention (Fig. 2), this drug produced no effect on subsequent IA performance (Fig. 3). Interestingly, in this study we were unable to observe the temporary retention impairment produced by postretrieval ANI that we (Vianna et al. 2001) and others (Lattal and Abel 2004) have previously described. The reasons for this discrepancy are not clear, but they are probably related to the fact that, to produce memory expression, we employed a pure retrieval procedure instead of a regular test session that would lead to extinction.
Parenthetically, the findings presented in Figure 2 agree and supplement those of Krupa and Thompson (2003), who recently showed that inhibiting the expression of the CR prevents its extinction. Here we show that the mere presentation of the CS (the platform) without performance of the (wrong) CR (i.e., stepping down) does not induce extinction. It must be noted, however, that retrieval and extinction were measured here merely by assessing the presence or absence of the step-down response during a limited period of time. Probably, if other CRs (Hine and Paolino 1974) had been evaluated, quantitatively different results might have been observed. Most papers on retrieval and extinction usually measure just one of all the possible CRs.
There is no doubt that retrieval implies the reactivation of memories that otherwise would have remained dormant (Squire and Alvarez 1995; McGaugh 2000) and that a variety of factors may modify retrieval at the time of testing (see Loftus and Palmer 1974). However, there is doubt as to whether memory reactivation at the time of retrieval would necessarily mean reinstallment or even rekindling of the original memory, and there is no definitive proof that this happens.
Further training is the only undisputed procedure by which pre-existing memories may be enhanced, as happens in multitrial tasks or, as in the case of IA, by additional CS-US pairing. Notwithstanding that, and again contrary to what is predicted by the reconsolidation hypothesis (see Duvarci et al. 2002), ANI infused into the CA1 region of the dorsal hippocampus, BLA or ENT 15 min before or 3 h after a second training session was unable to block the enhancement of memory retention produced by further training (Fig. 4), indicating that real reconsolidation of the IA response by additional training does not require protein synthesis in these regions. These results could suggest, in principle, that learning the IA response twice involves different processes than learning it once or to a higher criterion. This matter is currently being investigated in our laboratory.
Reconsolidation by retrieval testing rests mainly on the finding that ANI given at the time of testing into BLA (Nader et al. 2000) or CA1 (Debiec et al. 2002) or given systemically (Milekic and Alberini 2002) hinders performance in subsequent retention tests. The traditional consolidation hypothesis (Müller and Pilzecker 1900) was viewed as a real theory (McGaugh 1966) only after memory facilitation was described (McGaugh 2000). This validated the retrograde amnesic effects of electroconvulsive shock, hypoxia, head trauma, and other pre- or posttraining treatments previously studied. The inhibitory effect of an exogenous treatment is not enough to sustain the reconsolidation hypothesis, as it was not enough to sustain the original consolidation hypothesis. Behavioral inhibition can be caused by a performance effect or by an artifact. We suggest that further research attempting to find positive evidence for reconsolidation needs to be carried out (i.e., an experiment or experiments showing that retrieval leads to a subsequent sustained increase in retention test performance). In this respect, the experiments shown here constitute negative findings.
It has been suggested that perhaps a tendency to extinction and a tendency to reconsolidation may somehow coexist, and that one may predominate over the other, depending on the circumstances of testing or of the task (Nader 2003). Our experiments were unable to detect any such tendencies, in spite of the fact that they were designed to maximize the probability of occurrence of reconsolidation over that of extinction, although it is still possible that constraints related to the essence of the task (confinement, as in so-called contextual fear vs. the lack of it, as in IA) or other factors may lead retrieval to induce reconsolidation rather than the more habitual extinction process. Taken together with previous reports from our and other laboratories, these findings support the idea that extinction is the predominant outcome of nonreinforced retrieval, as indicated by Pavlov (1927) and Rescorla (2001), among many others. This is of importance because extinction is the basis for the treatment of mental disorders associated with learned fear. The treatment has been very successful with regard to phobias since the 1920s and, under the umbrella terms of “exposure” or “flooding” therapies, has been in use in the last several years for treatment of panic, posttraumatic stress disorder, and some forms of depression linked to acquired fear, with considerable success (Beckett 2002; Rothbaum and Schwartz 2002). These disorders would fare badly if re-presentation of a CS without the ensuing US induced reconsolidation of learned fear instead of extinction.
MATERIALS AND METHODS
Surgery and Drug Infusion
Three-month-old male Wistar rats (250-280 g) raised in our own facilities were used. They had free access to food and water, were housed 3-5 to a cage, and kept at 22°C under a 12-h light/dark cycle (lights on at 7:00 a.m.). To implant them with indwelling cannulas, rats were deeply anesthetized with thiopental (30-50 mg/Kg, intraperitoneally) and 27-gauge cannulas stereotaxically aimed to the pyramidal cell layer of the dorsal CA1 region, the BLA complex, or the ENT, using coordinates taken from the atlas of Paxinos and Watson (1986). Animals were allowed to recover from surgery for 4 d before submitting them to any other procedure. At the time of drug delivery, a 30-gauge infusion cannula was tightly fitted into the guides. Infusions (0.8 μL/side) were carried out over 30 sec, first on one side and then on the other; the infusion cannulas were left in place for 15 additional seconds to minimize backflow. Cannulas placement was verified postmortem: 2-4 h after the last behavioral test, 0.8 μL of a 4% methylene-blue solution was infused as described above, and the extension of the dye 30 min thereafter was taken as indicative of the presumable diffusion of the vehicle or drug previously given to each animal. Only data from animals with correct cannulas implants were included in statistical analyses. Intraperitoneal injections were carried out as described previously (Cammarota et al. 2003).
Drugs
ANI and noradrenaline (NA) were purchased from Sigma, and ACTH was from ICN Pharmaceuticals. The dose of ANI that was chosen inhibits protein synthesis in the hippocampus by more than 85% and blocks consolidation (Quevedo et al. 1999), extinction (Vianna et al. 2001), and reinstallment of the IA response (Cammarota et al. 2003). The doses of NA and ACTH used here have been previously found to facilitate retrieval of this task (Cammarota et al. 2003).
Behavioral Procedures
Rats were trained in a one-trial, step-down inhibitory avoidance task, as described elsewhere (Cammarota et al. 1998; Bevilaqua et al. 2003). The training apparatus was a 50 × 25 × 25-cm Plexiglas box with a platform that was 2.5-cm high, 8-cm wide, and 25-cm long and was on the left end of a series of bronze bars that constituted the floor of the box. The animals were gently placed on the platform facing the left rear corner of the training box (CS). When they stepped down and placed their four paws on the grid, they received a 2-secec, 0.5- or 0.8-mA scrambled shock to the foot (US). Depending on the experiment, 24 h after training, the rats were submitted to one out of five different behavioral procedures, as follows.
Pure Reactivation Session
The animals were put on the training box platform for 20 or 40 s, after which they were retired from the apparatus. During this time, they explored the platform, avoiding stepping down from it.
Retention Test Session (Extinction Session)
The animals were put on the training box platform until they eventually stepped down from it. No foot-shock was given. After stepping down, animals were immediately retired from the training box.
Enhanced Extinction Session
The animals were put on the training box platform until they eventually stepped down from it. No foot-shock was given. After stepping down, animals were allowed to freely explore the training box during 30-sec.
Retraining Session
The animals were placed on the training box platform, and at the very moment they stepped down, placing their four paws on the grid, they received a 0.5-mA, 2-sec, scrambled foot-shock.
Acknowledgments
This work was supported by grants in Brazil from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento do Pessoal de Nível Superior (CAPES), and the Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS); and in Argentina from the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET) and the Agencia Nacional de Promoción Científica y Tecnológica.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.76804.
References
- Bahar, A., Samuel, A., Hazvi, S., and Dudai, Y. 2003. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. Eur. J. Neurosci. 17: 1527-1530. [DOI] [PubMed] [Google Scholar]
- Beckett, W.S. 2002. Post-traumatic stress disorder. N. Engl. J. Med. 346: 1495-1498. [PubMed] [Google Scholar]
- Bernabeu, R., Izquierdo, I., Cammarota, M., Jerusalinsky, D., and Medina, J.H. 1995. Learning-specific, time-dependent increase in [3H]phorbol dibutyrate binding to protein kinase C in selected regions of the rat brain. Brain Res. 685: 163-168. [DOI] [PubMed] [Google Scholar]
- Bevilaqua, L.R., Kerr, D.S., Medina, J.H., Izquierdo, I., and Cammarota, M. 2003. Inhibition of hippocampal Jun N-terminal kinase enhances short-term memory but blocks long-term memory formation and retrieval of an inhibitory avoidance task. Eur. J. Neurosci. 17: 897-902. [DOI] [PubMed] [Google Scholar]
- Bonini, J.S., Rodrigues, L., Kerr, D.S., Bevilaqua, L.R., Cammarota, M., and Izquierdo, I. 2003. AMPA/kainate and group-I metabotropic receptor antagonists infused into different brain areas impair memory formation of inhibitory avoidance in rats. Behav. Pharmacol. 14: 161-166. [DOI] [PubMed] [Google Scholar]
- Cammarota, M., Bernabeu, R., Levi De Stein, M., Izquierdo, I., and Medina, J.H. 1998. Learning-specific, time-dependent increases in hippocampal Ca2+/calmodulin-dependent protein kinase II activity and AMPA GluR1 subunit immunoreactivity. Eur. J. Neurosci. 10: 2669-2676. [DOI] [PubMed] [Google Scholar]
- Cammarota, M., Bevilaqua, L.R., Ardenghi, P., Paratcha, G., Levi de Stein, M., Izquierdo, I., and Medina, J.H. 2000. Learning-associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning: Abolition by NMDA receptor blockade. Mol. Brain Res. 76: 36-46. [DOI] [PubMed] [Google Scholar]
- Cammarota, M., Bevilaqua, L.R., Kerr, D., Medina, J.H., and Izquierdo, I. 2003. Inhibition of mRNA and protein synthesis in the CA1 region of the dorsal hippocampus blocks reinstallment of an extinguished conditioned fear response. J. Neurosci. 23: 737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, R.G. and McGaugh, J.L. 1969. Electroconvulsive shock effects on a reactivated memory trace: Further examination. Science 166: 525-527. [DOI] [PubMed] [Google Scholar]
- Debiec, J., LeDoux, J.E., and Nader, K. 2002. Cellular and systems reconsolidation in the hippocampus. Neuron 36: 527-538. [DOI] [PubMed] [Google Scholar]
- Duvarci, S., LeDoux, J.E., and Nader, K. 2002. Reactivation of a consolidated auditory fear memory during a reinforced trial returns the memory to a labile state. Program 85.19. 2002 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, 2002.
- Fischer, A., Sananbenesi, F., Schrick, C., Spiess, J., and Radulovic, J. 2004. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J. Neurosci. 24: 1962-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine, B. and Paolino, R.M. 1974. Electroshock amnesia to aversive heart-rate conditioning in rats. Behav. Biol. 10: 247-252. [DOI] [PubMed] [Google Scholar]
- Izquierdo, I. 1989. Different forms of post-training memory processing. Behav. Neural Biol. 51: 171-202. [DOI] [PubMed] [Google Scholar]
- Izquierdo, I. and Medina, J.H. 1997. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol. Learn. Mem. 68: 285-316. [DOI] [PubMed] [Google Scholar]
- Judge, M.E. and Quartermain, D. 1982. Characteristics of retrograde amnesia following reactivation of memory in mice. Physiol. Behav. 28: 585-590. [DOI] [PubMed] [Google Scholar]
- Krupa, D.J. and Thompson, R.F. 2003. Inhibiting the expression of a classically conditioned behavior prevents its extinction. J. Neurosci. 23: 10577-10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal, K.M. and Abel, T. 2004. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc. Natl. Acad. Sci. 101: 4667-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus, E.F. and Palmer, J.C. 1974. Reconstruction of automobile destruction: An example of interaction between language and memory. J. Verbal Learn. & Verbal Behav. 13: 585-589. [Google Scholar]
- McGaugh, J.L. 1966. Time-dependent processes in memory storage. Science 153: 1351-1358. [DOI] [PubMed] [Google Scholar]
- ____. 2000. Memory: A century of consolidation. Science 287: 248-251. [DOI] [PubMed] [Google Scholar]
- Menzel, R. 2001. Searching for the memory trace in a mini-brain, the honeybee. Learn. Mem. 8: 53-62. [DOI] [PubMed] [Google Scholar]
- Milekic, M.H. and Alberini, C.M. 2002. Temporally graded requirement for protein synthesis following memory reactivation. Neuron 36: 521-525. [DOI] [PubMed] [Google Scholar]
- Misanin, J.R., Miller, R.R., and Lewis, D.J. 1968. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160: 554-555. [DOI] [PubMed] [Google Scholar]
- Müller, G.E. and Pilzecker, A. 1900. Zeitschrift Psychol. 1: 1-288. [Google Scholar]
- Myers, K.M. and Davis, M. 2002a. Behavioral and neural analysis of extinction. Neuron 36: 567-584. [DOI] [PubMed] [Google Scholar]
- ____. 2002b. Systems-level reconsolidation: Reengagement of the hippocampus with memory reactivation. Neuron 36: 340-343. [DOI] [PubMed] [Google Scholar]
- Nader, K. 2003. Memory traces unbound. Trends Neurosci. 26: 65-72. [DOI] [PubMed] [Google Scholar]
- Nader, K., Schafe, G.E., and Le Doux, J.E. 2000. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722-726. [DOI] [PubMed] [Google Scholar]
- Pavlov, I.P. 1927. Conditioned reflexes. Oxford University Press, Oxford, UK.
- Paxinos, G. and Watson, C. 1986. The rat brain in stereotaxic coordinates. Academic Press, San Diego, CA.
- Pedreira, M.E. and Maldonado, H. 2003. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38: 863-869. [DOI] [PubMed] [Google Scholar]
- Pedreira, M.E., Perez-Cuesta, L.M., and Maldonado, H. 2002. Reactivation and reconsolidation of long-term memory in the crab Chasmagnathus: Protein synthesis requirement and mediation by NMDA-type glutamatergic receptors. J. Neurosci. 22: 8305-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo, J., Vianna, M.R., Roesler, R., de-Paris, F., Izquierdo, I., and Rose, S.P. 1999. Two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: Protection from amnesia by pretraining but not pre-exposure to the task apparatus. Learn Mem. 6: 600-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla, R.A. 2001. Retraining of extinguished Pavlovian stimuli. J. Exp. Psychol. Anim. Behav. Process 27: 115-124. [PubMed] [Google Scholar]
- Rossato, J.I., Bonini, J.S., Coitinho, A., Vianna, M.R., Medina, J.H., Cammarota, M., and Izquierdo, I. 2004. Retrograde amnesia induced by drugs acting on different molecular systems Behav. Neurosci. 118: 563-569. [DOI] [PubMed] [Google Scholar]
- Rothbaum, B.O. and Schwartz, A.C. 2002. Exposure therapy for posttraumatic stress disorder. Am. J. Psychother. 56: 59-75. [DOI] [PubMed] [Google Scholar]
- Schacter, D.L. and Dodson, C.S. 2001. Misattribution, false recognition and the sins of memory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356: 1385-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire, L.R. and Alvarez, P. 1995. Retrograde amnesia and memory consolidation: A neurobiological perspective. Curr. Opin. Neurobiol. 5: 169-177. [DOI] [PubMed] [Google Scholar]
- Tully, T. 1998. Toward a molecular biology of memory: The light's coming on! Nat. Neurosci. 1: 543-545. [DOI] [PubMed] [Google Scholar]
- Vianna, M.R., Szapiro, G., McGaugh, J.L., Medina, J.H., and Izquierdo, I. 2001. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc. Natl. Acad. Sci. 98: 12251-12254. [DOI] [PMC free article] [PubMed] [Google Scholar]