Abstract
In early August 2014, the municipality of Toledo, OH (USA) issued a ‘do not drink’ advisory on their water supply directly affecting over 400,000 residential customers and hundreds of businesses (Wilson, 2014). This order was attributable to levels of microcystin, a potent liver toxin, which rose to 2.5 μg L-1 in finished drinking water. The Toledo crisis afforded an opportunity to bring together scientists from around the world to share ideas regarding factors that contribute to bloom formation and toxigenicity, bloom and toxin detection as well as prevention and remediation of bloom events. These discussions took place at an NSF- and NOAA-sponsored workshop at Bowling Green State University on April 13 and 14, 2015. In all, more than 100 attendees from six countries and 15 US states gathered together to share their perspectives. The purpose of this review is to present the consensus summary of these issues that emerged from discussions at the Workshop. As additional reports in this special issue provide detailed reviews on many major CHAB species, this paper focuses on the general themes common to all blooms, such as bloom detection, modeling, nutrient loading, and strategies to reduce nutrients.
Keywords: Cyanobacteria, CHAB, Lake Erie, microcystin, cyanotoxin, phosphorus, nitrogen, eutrophication
1. Introduction
In early August 2014, the municipality of Toledo, OH (USA) issued a ‘do not drink’ advisory on their water supply directly affecting over 400,000 residential customers and hundreds of businesses (Wilson, 2014). This order was attributable to levels of microcystin, a potent liver toxin, which rose to 2.5 μg L-1 in finished drinking water and exceeded the 1 μg L-1 WHO advisory guideline value. This toxic drinking water was caused by a large bloom of cyanobacteria (CHAB: cyanobacterial harmful algal bloom) in the western basin of Lake Erie that was constrained by prevailing winds to the region around the city of Toledo’s water intake. CHABs are not new to Lake Erie; in fact they have recurred annually over the past two decades (Steffen et al., 2014a) and may be occurring with increasing intensity (Michalak et al., 2013; Ho and Michalak, 2015). Whereas this event gained great notoriety as national news in the US, CHABS occur annually on a global scale, promoting chronic and acute health hazards while concurrently producing serious economic effects (Roegner et al., 2014 and references therein). Wherever they are found, the suspected causes of CHABs are typically nutrients arising from various sources including fertilizer, wastewater, the atmosphere, and internal cycling from sediments (Paerl et al., 2011). Recent studies have developed models that predict CHAB intensity in Lake Erie from farm-derived nutrient loads linked to rainfall (Stumpf et al., 2012; Michalak et al., 2013; Obenour et al., 2014). The Toledo crisis afforded an opportunity to bring together scientists from around the world to share ideas regarding factors that contribute to bloom formation and toxigenicity, bloom and toxin detection as well as prevention and remediation of bloom events. These discussions took place at an NSF- and NOAA-sponsored workshop at Bowling Green State University on April 13 and 14, 2015.
The objective of the Workshop was to identify the major knowledge gaps regarding the understanding of bloom formation, detection and mitigation along with discussion of current bloom remediation efforts around the world. The workshop featured NSF- and NOAA-funded researchers and NOAA staff scientists who examined the biology of CHAB species, organism and toxin detection, nutrient management, and bloom forecasting in Lake Erie. Providing the broader international perspectives on bloom mitigation were scientists who have studied CHABs in China, Europe, Australia and Canada. In all, more than 100 attendees from six countries and 15 US states gathered together to share their perspectives. The purpose of this review is to present the consensus summary of the issues that emerged from discussions at the Workshop. Whereas the Workshop and this review focus largely on the 2014 Lake Erie CHAB as a case study, the general conclusions provided here can inform future research aims and mitigation strategies for diverse CHAB events globally. As additional reports in this special issue provide detailed reviews on many major CHAB species, this paper focuses sequentially on the general themes common to all blooms, such as nutrient loading, bloom detection methods, modeling, mitigation strategies, and economic incentives for bloom prevention.
2. Nutrient sources and watershed influences – Lake Erie case study
2.1. Phosphorus loads
Phosphorus (P) is a limiting nutrient widely responsible for controlling algal biomass in freshwater systems (Schindler, 1977). The Maumee River, which drains into the southwestern corner of Lake Erie, is the primary source of the P that is driving CHABs in the lake (Ohio EPA, 2013; US EPA, 2015a). With an area of 17,115 km2, its watershed is the largest draining into the Great Lakes. In 1975 the National Center for Water Quality Research at Heidelberg University initiated detailed nutrient-loading studies on the Maumee River at the USGS stream gaging station near Waterville, OH. Land use upstream from this station, which accounted for 95.8% of the watershed area, consisted primarily of row crop agriculture (73.3%), with smaller portions occupied by forests (6.5%), pasture/hay/grassland (6.3%) and urban areas (10.6%) (USDA Data Gateway for Ohio, 2006). Combined municipal and industrial point source inputs of total P (TP) upstream from Waterville accounted for only about 6.6% of the average annual TP export (Baker et al. 2014a). The TP load from the Maumee River was composed of 25% dissolved reactive P (DRP) and 75% total particulate P (TPP). Since DRP was considered to be 100% bioavailable while TPP in the Maumee River was potentially 25% bioavailable (Baker et al., 2014a), DRP is projected to account for ~56% of the total bioavailable P load based on the estimates at Waterville, OH.
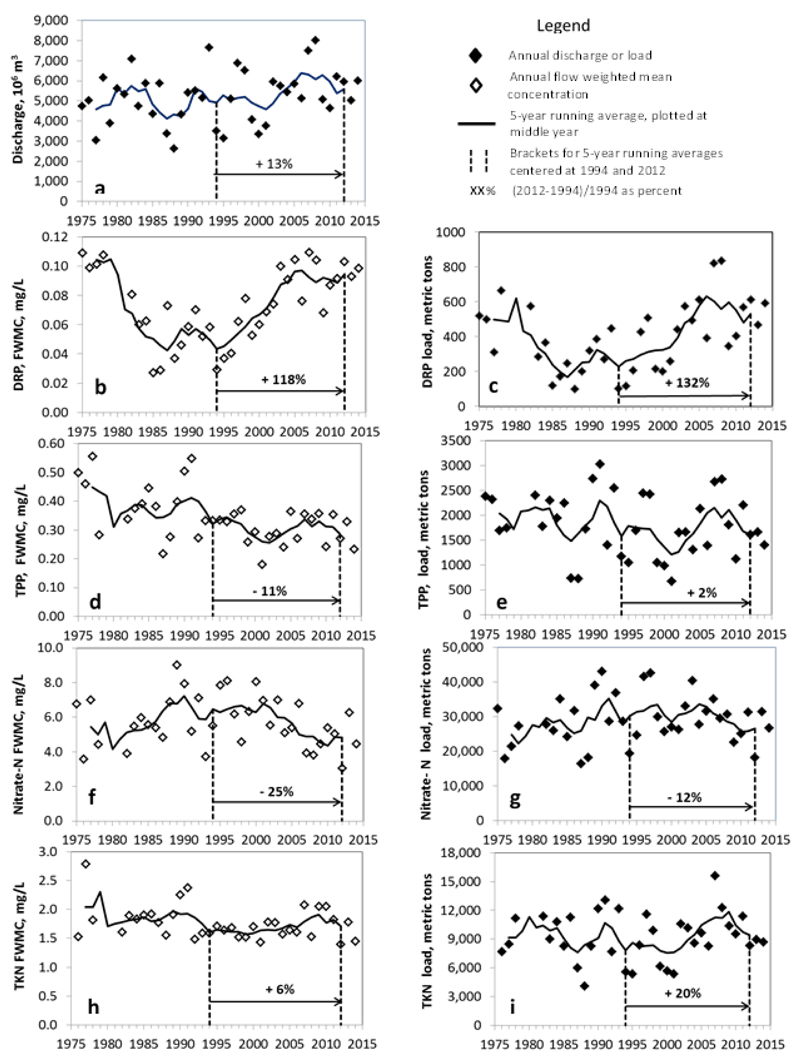
The annual discharge and annual loads and flow-weighted mean concentrations (FWMCs) for the period-of-record (water years 1975-1978 and 1982-2014) at the Waterville station are shown in Fig. 1. The Maumee River had large annual variability in discharge (Fig. 1A) due to variations in weather conditions, including the amounts, intensities and timing of rainfall or snow melt events. Although typically less variable than discharges, the annual FWMCs of nutrients also varied from year to year, due to the interactions of variable weather conditions with seasonal changes in land cover and land management activities (Figs. 1B, D, F, H). Annual nutrient loading, as the product of annual discharge and annual FWMC, was even more variable than discharge or FWMCs (Figs. 1C, E, G, and I). These annual variations in nutrient loading contributed to annual variations in CHAB size in the lake. Comparisons between annual loads of nutrients and annual variations in CHAB size support the development of the load-response curves that modelers have used to set revised Lake Erie target loads for both TP and DRP (US EPA, 2015; Ohio EPA, 2013; Stumpf et al., 2012).
Figure 1.
The relationships between annual discharge (A) and annual flow-weighted mean concentrations and loads of dissolved reactive phosphorus (DRP [B and C]), total particulate phosphorus (TPP [D and E]), Nitrate-N [F and G] and total Kjeldahl nitrogen (TKN [H and I]) for the Maumee River at Waterville, OH. The percent change between 5-year running average values for 1994 and 2012 are shown for each parameter.
Detection of trends in annual loading and in FWMCs of nutrients and sediments in rivers is complicated by short-term annual variability. A variety of techniques have been used to visualize and detect trends, including the use of locally weighted scatterplot smoothing (LOWESS) (Richards et al., 2008; Richards et al., 2009), linear regressions of flow adjusted, log transformed data (Richards and Baker, 2002), and running multi-year averages (Baker et al., 2014a; US EPA, 2015). The line graphs shown in Figure 1 represent running 5-year average values with each average calculated and plotted at the mid-point of its 5-year interval. Of particular interest are the changes in discharge, FWMCs and loads that occurred during the re-eutrophication of Lake Erie, beginning in the mid-1990s (Kane et al., 2014; Scavia et al., 2014). To estimate these changes, the 5-yr average value for 1994 was subtracted from that of 2012 and the difference expressed as a percentage of the 1994 value (Fig. 1). The FWMCs of DRP increased by 118% between the 5-year running averages of 1994 and 2012 (Fig. 1B). This increase in concentration, coupled with a 13% increase in discharge, resulted in a 132% increase in DRP loading. This suggests that the increase in DRP loading was primarily due to increases in FWMCs and, to a much lesser extent, to increases in discharge. In contrast with DRP, the FWMCs of TPP decreased by 11% (Fig. 1D). The TPP loads increased by 2% between these time periods due to the increasing discharge. These decreases in TPP FWMCs were attributed to farmer adoption of erosion control best management practices (BMPs) (Richards et al., 2009). Between 1994 and 2012, the TP loads (DRP + TPP) increased by 18%, due to the increase in DRP loading, while total bioavailable P loading increased by 48%, also in response to the increase in DRP loading. Suspended sediments, which bear the TPP, settled out of the water column rather quickly within the lower Maumee River, Maumee Bay and the lake (Baker et al., 2014b). As such, even the bioavailable portion of TPP becomes less available to support algal growth. In contrast, DRP remains in solution and is accessible to algae and bacteria. Therefore, increases in DRP loading may be even more important as a cause of re-eutrophication than its role in increasing total bioavailable P loading as measured at Waterville (Baker et al., 2014b).
Causes of increased flow-weighted mean concentrations of DRP
Of the multiple factors that may contribute to the increasing DRP loads (Daloglu et al., 2012 and Smith et al., 2015), those factors that are causing the increasing FWMCs appear to be particularly important (Joose and Baker, 2011). The processes by which DRP moves into the water that flows from croplands into streams have been described as either acute or chronic (Kleinman et al., 2011a). Acute losses occur when fertilizers or manure were broadcast onto the surface of fields, and runoff-inducing rainfall or snowmelt occurred shortly thereafter. The most commonly used forms of P fertilizer (mono- and di-ammonium phosphate [MAP and DAP]) are highly soluble and DRP concentrations increase to very high levels in the runoff water (Kleinman et al., 2011b). These losses decrease rapidly with successive runoff events. Chronic losses occurred after P from fertilizers and manures was equilibrated with the soil through either precipitation or adsorption. During runoff events, P moves from surficial soil particles into runoff water as DRP. These surficial soils form a zone of interaction between soil P and the runoff water (Sharpley, 1985). The resulting concentrations in the runoff water were much lower than from acute losses, but tended to be persistent (chronic) since most soils had the capacity to bind large amounts of P relative to that removed by either crops or runoff during a given year. The DRP concentrations in chronic losses are proportional to P soil test levels in the zone of interaction (Wang et al., 2010, Vadas et al., 2005a; Vadas et al., 2005b, Allen et al., 2006; Williams et al., 2015).
Changing farming practices in northwestern Ohio during the period of re-eutrophication can account for increased DRP losses by both acute and chronic pathways, although the relative importance of each is uncertain (OEPA, 2013). Fertilizer application by broadcasting has increased as farm size has increased, although watershed-specific changes in farm management practices are generally not available. Broadcasting is the least expensive and fastest way to apply fertilizer and it is compatible with modern variable-rate fertilizer applications and precision agriculture. As the number of rows on mechanical planters increased to accommodate planting on larger fields, fertilizer bins used for fertilizer banding were removed from planters. The resulting increased broadcasting of fertilizers increased the opportunities for acute losses of DRP.
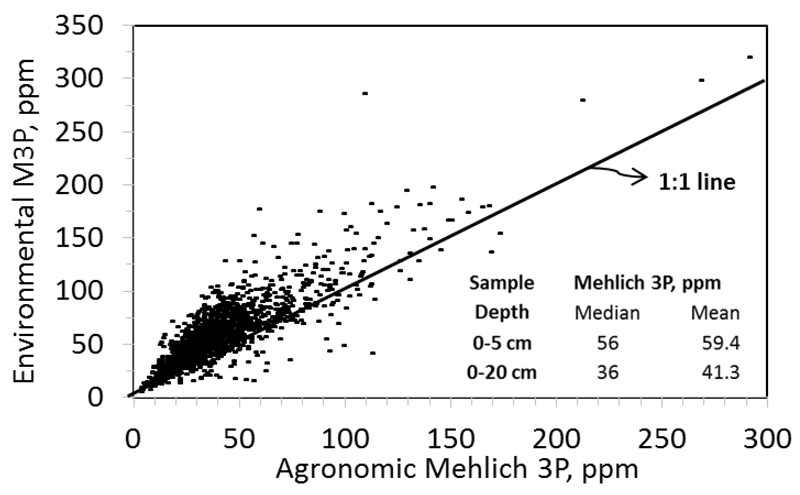
Broadcasting of P fertilizers also contribute to increased chronic losses of DRP. Soils have a large binding capacity for P and the P distribution in soils can become very uneven. Surface application of fertilizer, coupled with P release from the breakdown of crop residues at the surface, can result in P stratification wherein surficial soils have higher soil test levels than deeper soils (Sharpley, 2003; Soldat et al., 2007). By adopting practices to reduce erosion and associated losses of TPP, farmers have largely abandoned moldboard plowing that inverted soils and reduced this stratification. They have replaced it with no-till and mulch till operations that can increase the stratification (Kleinman et al., 2015). Consequently, reductions in erosion and TPP losses are often accompanied by increased DRP losses (Kleinman et al., 2015). Soil tests in the Sandusky River watershed, just to the east of the Maumee Watershed, showed P stratification with P levels in the upper 5 cm (zone of interaction) of the soil column 45% higher than in the 0-20 cm column normally used for agronomic soil testing to support fertilizer management (Fig. 2).
Figure 2.
A comparison between Mehlich 3 phosphorus soil test levels in the agronomic zone (0-20 cm. cores) and the environmental zone (zone of interactions with runoff water – 0-5 cm. cores) for 1610 fields in the Sandusky River Watershed.
No-till and mulch till production is also accompanied by the development of macropores that provide pathways for surface runoff to move directly to tile drainage systems that lead to streams. The benefits of tile drainage to crop production in this region have led to a constantly increasing density of tile drainage (Kleinman et al., 2015). Tile drainage now constitutes the major pathway by which DRP moves from cropland to streams (Macrae et al., 2007; King et al., 2014; Smith et al., 2014).
Surficial applications of manure also contribute to both acute and chronic losses of DRP. In the Lake Erie Basin, this may be a minor contributor relative to more traditional fertilizer applications where fertilizer applications account for approximately 88% of the P applied to cropland, compared with 12% from manure (Han et al., 2012). Fertilizer application of P exceeds manure P application by a factor of five in the Maumee Watershed. (NRCS, 2009).
2.2. Nitrogen loading and CHABs
Whereas the importance of P loads contributing to CHAB biomass in freshwater has been well established, recent studies of various systems worldwide have also implicated nitrogen (N) as important in supporting both biomass, species composition and toxigenicity (Orr and Jones, 1998; Wilhelm et al., 2003; Van de Waal et al., 2009; Davis et al., 2010; Paerl et al., 2011; Harke and Gobler, 2013; Paerl et al., 2014; Van de Wall et al., 2014; Müller and Mitrovic, 2015; Gobler et al., this issue). Since N in aquatic systems can rapidly cycle through multiple oxidation states, there is often high variability of N species in the water column and sediments. For example, embayments of Lake Erie and Grand Lake St. Marys (OH, USA) often have nitrate levels range from > 100 μM following rainfall events to below detection limits due to assimilation and active denitrification (Steffen et al., 2014; Davis et al., 2015). Additionally, given that microcystin is an abundant secondary metabolite (Nagata et al., 1997), the bioavailability of N likely plays a role in toxin production. Indeed, analysis of the Microcystis microcystin synthetase (mcy) gene cluster reveals that they are, at least in part, controlled by the N-responsive NtcA transcription factor (Ginn et al., 2010). Recently, western Lake Erie has been shown to exhibit N limitation of chlorophyll a production in late summer (Chaffin et al., 2013, Chaffin et al., 2014a, 2014b; Gobler et al., this issue), and that the biomass and toxigenicity of the Planktothrix bloom in Sandusky Bay, Lake Erie, is seasonally controlled by N inputs (Davis et al., 2015). Indeed, most of the highly aggressive and persistent CHAB blooms now occurring in Lake Erie and worldwide are dominated by non-N2 fixing genera (Microcystis and Planktothrix). This sends researchers and managers a strong message externally supplied combined N may contribute to more intense bloom events (Paerl et al., 2014).
The long term trends in N loadings and N:P ratios will be useful in understanding toxin production in future bloom events. Stow et al., (2015) reported a steady decline in TN:TP molar ratios in the Maumee River since the late 1990s, with lowest TN:TP occurring in summertime. Assimilation by biomass and denitrification may explain the documented late summer N limitation in western Lake Erie. Given these trends and the known influences of N on microcystin gene expression, management of N is warranted along with P (US EPA, 2015b, Steffen et al. 2014a; Gobler et al., this issue).
3. Bloom and Toxin Detection
Timely detection of CHABs and their associated toxins is essential from both management and research perspectives. Detection of both organisms and toxins is critical, due to variation in intrinsic genetic differences and external environmental factors that may influence gene expression and toxin synthesis. Real- or near real-time surveillance of toxic CHAB events, along with accurate identification and quantification of the organisms and toxins present, are required for efficient and effective mitigation efforts as well as advancing understanding of environmental factors driving population growth and toxicity (Ouellette and Wilhelm 2003). The end result is increased protection of human and animal health, and support of predictive models used to forecast CHAB development and trajectories. Advanced technologies for bloom and toxin detection are becoming commercially available for in situ and field-portable deployment as well as for use in the laboratory (Seltenrich, 2014a). These powerful tools are allowing the management and research communities to address critical deficiencies in their ability to rapidly respond to and investigate CHAB events, respectively. In this section, selected technologies and methods are described in the context of potential applications, along with the rationale for adopting a given approach to address a specific management and research need. Several of these are also summarized in Ho and Michalak (2015) and Srivastava et al. (2013).
The noxious (toxins) and nuisance (taste and odor compounds) properties of cyanobacteria are controlled by flexible genes/operons that vary on a strain-by-strain basis (Humbert et al., 2013; Kurmayer et al., 2015; Simm et al., 2015). No one genus, species, or strain can be reliably considered a producer of any of these compounds without further analysis. The two principal approaches to cyanotoxin risk assessment are direct detection by ELISA, HPLC-UV or LC-MS, or indirect detection by PCR/QPCR of specific genes involved in the biosynthesis of these compounds (Table 1). Both strategies have merits and should not be viewed as mutually-exclusive. For example, many cyanotoxin classes can occur as multiple structural isoforms that are still being discovered (e.g., 6 anatoxins, 3 cylindrospermopsins, 100+ microcystins, 7 nodularins, and 21+ saxitoxins have been reported to date) (Pearson et al., 2010; Cusick and Sayler, 2013), making direct detection of all individual variants by HPLC and LC/MS difficult and expensive. Recent efforts have focused on LC-MS/MS methods that can detect a class of compounds, eliminating the need for individual standards for all of the variants (Foss and Aubel, 2015). Alternatively, ELISA can provide effective detection of some metabolites, including those of cylindrospermopsins and microcystins, as the antibodies employed recognize conserved motifs present across most variants (e.g., James et al., 2010). In other cases, however, such as the paralytic shellfish poisoning toxins (PSTs), these immunoassays react primarily with the parent compound saxitoxin and frequently exhibit low cross-reactivity with other key variants such as neosaxitoxin or the gonyautoxins (e.g., Laycock et al., 2010). Their response to the PST derivatives specific to cyanobacteria (e.g. Lyngbya PSTs) is currently unknown. Where they are available, functional assays (i.e., receptor or enzyme inhibition assays) provide an estimate of the integrated toxic potency of a sample regardless of the congeners present and results can correspond closely to values generated by analytical methods (e.g., Powell and Doucette, 1999; Turner et al., 2000). By comparison, PCR assays specifically targeting genes can estimate the potential for a compound to occur and may be useful in the identification of samples that require further testing by direct analysis using the appropriate method(s) (Rinta-Kanto et al., 2005, 2009; Davis et al., 2009, 2010; Otten et al., 2015). This strategy could eliminate the need to test samples that are likely to be negative, allowing effective implementation of a more cost-effective tiered response approach. Molecular detection methods can amplify the initial target gene over one trillion-fold, making it a robust and sensitive option for early warning surveillance.
Table 1.
Overview of detection methods for assessing cyanobacterial abundance and toxicity. ‘Prep’ is time required for preparation of a sample(s) for analysis. ‘Analysis’ is time required for analysis and initial processing of data into a usable form. ‘na’ = not applicable.
| Detection method | Location | Target | Prep (h) | Analysis (h) | Samples/Run | Instrument cost (USD) |
|---|---|---|---|---|---|---|
| ELISA | Lab/Field | Toxin class | 1 | 2 | 40 | $3,000 - $25,000* |
| Receptor Assay | Lab | Toxin class | 1 | 4 | 10 - 20 | $3,000 - $50,000*@ |
| Enzyme Inhibition Assay | Lab | Toxin class | 1 | 2 | 40 | $3,000 - $25,000* |
| HPLC-UV | Lab | Toxin congener | 1 | 0.5 | 1 | $35,000 - $70,000 |
| LC-MS/MS | Lab | Toxin congener | 1 | 0.5 | 1 | $250,000 - $500,000 |
| PCR/QPCR | Lab/Field | Toxin gene | 1 | 2 | 40 | $5,000 - $40,000 |
| Light Microscope | Lab/Field | Cells | 0.25 | 1 | 1 | $200 - $20,000 |
| FlowCAM | Lab/Field | Cells | 0.5 | 8 | 1 | $65,000 - $95,000 |
| Imaging Flow Cytobot | Field& | Cells | na | na | Continuous | $135,000 - $160,000 |
| Autonomous Underwater Vehicle | Field | Phys-Chem-Pigments | na | na | Continuous | $50,000 - $100,000 |
| Environmental Sample Processor | Field& | Genes & Toxins | 0.5 - 2.0§ | 1 | 1 | $180,000 - $300,000# |
same instrument – 96 well plate reader spectrophotometer
for radioreceptor assays a standard or microplate scintillation counter is required
cost range reflects core instrument to fully deployable assembly
pier-based or in-water deployment
prep time depends on sample volume
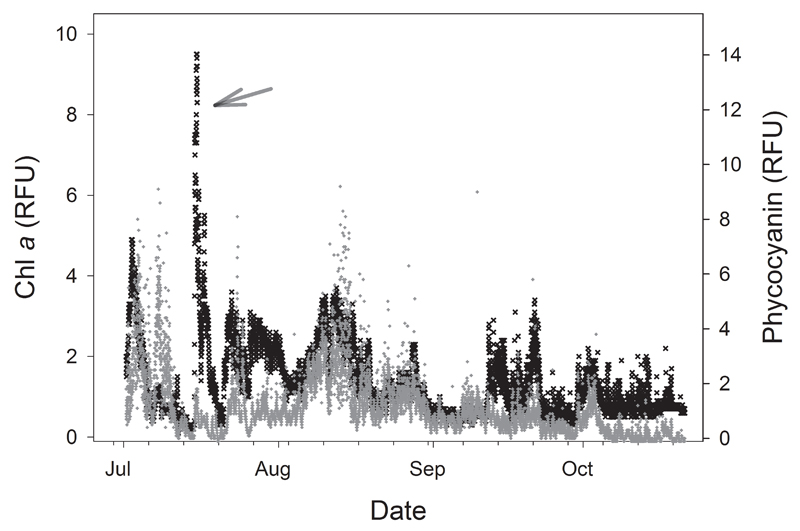
Some of the bloom and toxin detection methods identified above are now being deployed on in-water and field-portable platforms, providing greater flexibility in their application along with real- or near real-time data output. Lower cost, widely available sensors for in situ physicochemical analyses, including temperature, pH, dissolved oxygen, conductance, and photopigments, can provide data indicative of CHAB formation useful in guiding conventional sampling efforts. Buoy systems are routinely equipped with optical sensors for cyanobacterial pigments and their deployment in areas prone to blooms can provide an early alert for cyanobacterial blooms. A series of fifteen YSI EXO water quality sondes (YSI Inc., Yellow Springs, OH, USA) were deployed in the western basin of Lake Erie and linked by the Great Lakes Observing System (GLOS) network to provide real-time data to water treatment plant operators and the public at-large (habs.glos.us/map/). Outfitting such sensors with both in vivo chlorophyll a and phycocyanin sensors can permit the clear differentiation of eukaryotic and cyanobacterial algal blooms, as seen in an example from summer 2015 in Sandusky Bay (Fig. 3). Data generated throughout the summer allowed timely adjustment of treatment protocols as blooms migrated near Lake Erie municipal water intakes. Similar systems in the embayments of Lake Ontario allowed recreational users to track the water quality and potential CHABs in waters near their homes and marinas. Instruments such as ISCO water samplers (Teledyne Isco, Lincoln, NE, USA) can be programmed to collect and preserve water samples autonomously at set times and depths for analyses where real-time results are not essential. Multispectral sensors for algal pigments have been installed in large ships and smaller boats suited for nearshore waters (Pavlac et al., 2012), providing spatial resolution of blooms on the order of tens of meters. This is especially important in the nearshore zone where interference from land reflectance may prevent or compromise the use of satellite imagery. Modern optical sensors such as the Optical Phytoplankton Discriminator (OPD) allow for cyanobacteria blooms to be identified at the genus level (Sullivan and Boyer, unpubl. data). Although the OPD provides very useful information about the causative organisms, determination of toxicity or potential toxicity still requires chemical or molecular testing. Advances are also occurring on this front. For example, the Environmental Sample Processor, or ESP (McLane Research Laboratories, East Falmouth MA, USA), can be deployed subsurface on a mooring and can autonomously determine cell, gene and metabolite (e.g., toxin) concentrations via molecular (sandwich hybridization and/or QPCR; Greenfield et al., 2008; Robidart et al., 2014) and antibody-based (ELISA; Doucette et al., 2009) methods. The ESP has been deployed in marine systems as part of ocean observing networks, providing insights regarding environmental factors influencing bloom dynamics (Ryan et al., 2014) and toxin production. Efforts are now underway to develop ESP applications in freshwaters for CHAB and cyanotoxin detection in Lake Erie at the Toledo, OH water intake (Davis and Doucette, unpubl. data), and to adapt the ESP for deployment on autonomous underwater vehicles for improved spatial coverage (Seltenrich, 2014a). Real-time ESP data could ultimately be assimilated into predictive models for forecasting applications that currently rely primarily on satellite-based observations of surface waters (Stumpf et al., 2012). Although wider application of this technology is currently cost-prohibitive, targeted siting of ESPs along with selective deployment of autonomous underwater vehicles can alert managers to toxic bloom development and improve sampling efficiency for shore-based testing.
Figure 3.
Summer 2015 surface water in situ chlorophyll a (black) and phycocyanin (gray) profiles expressed as relative fluorescence units (RFU) measured with an EXO2 sonde (YSI Inc.) deployed from a buoy at the city of Sandusky (OH) principal water intake in coastal waters of Lake Erie. Use of a multiparameter sonde resolved an algal bloom in mid-July (arrow) that was not dominated by cyanobacteria, thus guiding water treatment options by city utilities personnel.
Progress has been made in the development and application of inexpensive field kits for HABs and their toxins. Hand–held, field-portable fluorometers (Algal Torch®, bbe Moldaenke GmbH, Schwentinental, Germany or AquaFluor®, Turner Designs, Sunnyvale, CA, USA) provide scientists with a quick determination if a bloom consists of cyanobacteria and can provide some indication of the level of cyanobacteria associated with the bloom. These tools can be utilized by citizen scientists and water quality managers to evaluate local water quality. Other options for water quality managers include qualitative "dip-stick" assays for cyanotoxins (e.g., Abraxis, Warminster, PA, USA) and field-portable devices for QPCR (e.g., Ubiquitome, Ltd., Dunedin, New Zealand) that could be paired with rapid DNA extraction methods such as Whatman FTA Elute cards (GE Healthcare Life Sciences, Marlborough, MA, USA) for on-site organism and/or target gene detection, producing valuable results in near real-time. While these rapid tools may not yield a definitive answer as to whether a bloom is toxic or not, they can be used to focus sampling and testing efforts and alert managers to potential problems in the early stages of bloom development.
The frequency of discrete sampling needed to detect the occurrence of CHABs should be guided by adaptive management principles, in which water sampling and water quality protocols are adjusted to changes in season. During non-bloom months such as winter and early spring in north temperate ecosystems, it is advisable to conduct occasional (e.g., bi-weekly) visual inspections of the water body and discrete samples should be collected and tested at least monthly and anytime the water appears discolored. In the spring, as rising water temperatures reach or exceed ~12 °C and lakes begin to stratify, discrete sampling frequency should be increased. Larger bodies of water can leverage remotely sensed satellite or airborne spectroradiometer data to ascertain bloom conditions and geographic location (Section 4 below), whereas autonomous monitoring of smaller water bodies will need to rely on deployment of in situ sensors, such as the water quality sondes currently networked in Lake Erie. Devices that are able to detect changes in photopigment concentrations (chlorophyll a, phycocyanin) can yield information diagnostic of potential CHAB development. Remote sensing technologies are improving rapidly. Drones fitted with cameras or other imaging devices can already be employed to survey smaller water bodies and relatively inexpensive, low orbiting satellites (e.g., CubeSats) are continually being launched, which will improve access to remotely sensed data by a wider audience (Hand, 2015; Van der Merwe and Price, 2015). Overall, the best strategy to monitor and measure concentrations of CHAB cells and toxins will depend on the management or research application. Specific factors that will drive decision making may include the type of data needed (e.g., qualitative vs. semi-quantitative vs. quantitative), the spatio-temporal coverage required, and how quickly results are desired (e.g., real-time, hours, days) in order to inform effective management decisions or resolve bloom population and toxin dynamics. The focus for those developing future detection technologies continues to be on developing less expensive, higher throughput, and more user-friendly tools that, in some cases, have some degree of field-portability and thus allow for greater flexibility in their application.
4. Bloom prediction and modeling
4.1. Remote sensing tools
Remote sensing using satellites and increasingly aircraft and drones provides the capability for rapid synoptic detection of blooms. Remote sensing is particularly effective for CHABs because many of these blooms exhibit surface aggregation. Modeling efforts employ a mixture of statistical and process models to provide hindcasts to understand the mechanisms of HAB formation, nowcasts of near-term changes in conditions or forecasts of potential future CHAB conditions. As remote sensing is limited to observations of the surface of the water (with the signal arising from roughly the uppermost third of the euphotic zone), there are distinct advantages when discrete sampling, subsurface sensors, and remote sensing data are used in conjunction with or assimilated into models, enhancing our ability to quantify errors and thus capitalizing on the strengths and minimizing the weaknesses of each individual component.
There are currently several CHAB-related reporting products that are regularly available for Lake Erie, which to varying degrees combine results from remote sensing, in situ field sampling and model simulations for comparison of observational and simulated fields. These approaches provide products to track map bloom intensity, surface scum distribution, suspended sediment and colored dissolved organic matter distribution and/or simulations of changes in the distribution of particles and environmental properties with time. These systems include the NOAA Lake Erie Experimental HAB Bulletin and associated Experimental HAB Tracker, which compare field, remote sensing and fields from process model simulations (see www.glerl.noaa.gov/res/HABs_and_Hypoxia/ and links therein). The Michigan Tech Developmental Harmful and Nuisance Algal Map product also provides a comprehensive interactive remote sensing atlas including cloud free daily maps of true color imagery, lake surface temperature, HAB extent, chlorophyll a, colored dissolved organic matter, dissolved organic carbon and suspended sediment (www.greatlakesremotesensing.org).
Several major challenges exist when working with remote sensing data. Because pigments are detectable in the visible part of the spectrum, atmospheric biases and cloud cover pose problems which must be addressed. CHAB forming environments have water that is a complex mixture of living and non-living particles and dissolved substances, referred to as Case II water. Case II water can absorb and scatter visible light in ways that are more complex than open ocean environments (Case I water), where traditional band ratio remote sensing methods were developed. Therefore, partitioning the remote sensing response found in conjunction with CHABs and other water constituents is important because the optical complexity of Case II waters (Ortiz et al., 2013, Ali and Ortiz, 2014; Ali et al., 2014b) can produce erroneous chlorophyll a biomass estimates when traditional band ratio methods are applied (Witter et al. 2009).
Additional problems arise from differences in instrument design and the variety of algorithms in use, which can make comparison of results from different locations challenging. Differences in spectral resolution and band placement on different instruments can influence the quality of remote sensing products. These vary from sensors such as Landsat and MODIS that have 4-10 bands in the visible, to the multi-band option of MERIS, to hyperspectral sensors, such as HICO. MERIS and its intended replacement, the European Space Agency’s Sentinel-3 Ocean and Land Color Instrument (OLCI) or proposed hyperspectral instruments such as HyspIRI and PACE offer the greatest capability to detect and quantify cyanobacterial blooms. Overall, instruments with global missions have been limited by a maximum pixel resolution of 300 m, which precludes their use in many smaller lakes. Landsat (versions 1 through 8 going back over 30 years) and the Sentinel-2 Multispectral Imager (MSI; launched June 2015) offer higher spatial resolution as low as 10 m, but lack frequency of imaging with repeat times on the order of 8-14 days, introducing a challenge in establishing consistency across sensors and availability of data for timely CHAB monitoring and prediction. Whereas Landsat effectively conveyed the intensity of the record summer 2015 CHAB in Lake Erie, its efficacy was limited to a single image representing only a west-central basin swath imaged by the satellite (Fig. 4). Indeed, the low frequency of Landsat overpasses in the lower Laurentian Great Lakes in particular has been an important limitation given frequency of cloud cover over the lakes (sensu Vincent et al., 2004). The typical availability of acceptable, cloud-free, summer-time Landsat images around the Lake Erie Islands in the western basin is about 25% due to convective cloud formation associated with thermal differences that arise between the land and water (Ortiz et al. 2013).
Figure 4.
Image of CHAB in west-central Lake Erie captured on July 28, 2015 by the Operational Land Imager (OLI) on Landsat 8. The CHAB enveloped the Lake Erie islands and continued into Canadian waters east of Point Pelee. The persistent CHAB in Sandusky Bay is also visible. Landsat imagery courtesy of NASA Goddard Space Flight Center and U.S. Geological Survey.
The wide variety of algorithms employed by different researchers also often makes intercomparison of results difficult. Remote sensing uses optical characteristics to model biomass (generally as chlorophyll a), inherent optical properties (absorption and scattering due to different consituents) or bloom characterization, including the composition of different algal pigment classes. Model specification, parameter settings (without overfitting), and robust validation methods must be identified. Several classes of algorithms have been developed for MERIS, all with particular strengths and weaknesses. There is no systematic way of addressing the use, the confidence or validation of these methods for multiple applications. Sensors such as Landsat, which may provide over 30 years of data, have rarely been applied via a systematic approach and each new study often creates another “algorithm” or tuning. In some cases attempts have been made to consistently apply multiple algorithms to one sensor (Witter et al., 2009), or one set of algorithms to multiple sensors (Wynne et al., 2013), demonstrating both the potential and the difficulties of employing a single algorithm or suite of algorithms across multiple sensors.
Building off the successful deployment of HICO on the International Space Station, the future can expect increasing use of hyperspectral sensors with higher spatial resolution, first on airborne sensors, but ultimately available from satellite platforms (Seltenrich, 2014b; Kudela et al., 2015). The development of algorithms that fully tap these sensors, thereby furthering bio-optical understanding of how different CHAB constituents can be observed spectrally, is the next challenge in the field (Ortiz et al., 2013; Ali et al., 2014a,b). Hyperspectral data can be used to simulate other sensors, so assimilation of hyperspectral aircraft with MERIS or OLCI should become easier and provides an opportunity to standardize algorithms, uncertainty estimates and validation methods.
Whereas satellites provide both the historical and regional context for CHABs, manned and unmanned aircraft hold the prospect of introducing a key enhancement to satellite data. Aircraft can target small water bodies, provide greater flexibility in deployment and can provide data during cloudy conditions by flying under cloud cover. The effectiveness of an aerial hyperspectral sensing strategy was successfully demonstrated during the summers of 2014 and 2015 by scientists at the NASA Glenn Research Center (Cleveland, OH) and their field collaborators, who conducted weekly overflights of Lake Erie aboard aircraft equipped with a hyperspectral imager and miniature spectrometers. Flights were coordinated with ground sampling and provided high resolution mapping of the 2014 and 2015 Lake Erie CHAB event. Operations coordinated aerial assets from NASA Glenn and the ONR during 2014 and with NOAA GLERL in 2015. The flexibility offered by aircraft was also key to extending the spatial coverage of the NASA mission to include a >900 km CHAB event that developed in the Ohio River some 300 km south of Lake Erie and which spanned three US states in late August 2015. The combination of satellite, aircraft, sensor, and models will lead to a critical new era of remotely detecting CHABs.
4.2. Future model development
The objectives of modeling are to provide nowcasts and forecasts for management scenarios, and modeling to synthesize what is understood, thereby allowing scientists to capture knowledge gaps. Without validation of the model prediction with independent observations, these objectives cannot be met. Box’s maxim that “all models are wrong, but some are useful” (Box and Draper, 1987) must drive the grand strategy for modeling: require clear model objectives and outcomes, robust calibration data for inputs to the models, and independent, quality data for validation of the models.
Currently, a few projects with modeling emphasis have focused on developing alternative loading scenarios for guiding nutrient management. The NOAA-funded “Ecological Forecasting: Hypoxia Assessment in Lake Erie” (EcoFore) Project incorporated field samples, remote sensing and models of varying levels of complexity to study the root causes of hypoxia in the Central Basin of Lake Erie, which largely arises from CHAB events that develop in the western basin of Lake Erie. EcoFore developed the load response curves needed to guide revised hypoxia-based reduction targets as required by the 2012 Great Lakes Water Quality agreement. Based on these curves, Ecofore recommended phosphorous load reductions to Lake Erie of 46% to reduce HAB occurrence, decrease hypoxia and enhance fish habitat/stocks (Scavia et al. 2014). Similarly, the LimnoTech Western Lake Erie Ecosystem Model (WLEEM; LimnoTech 2010, 2013, 2014; Verhamme et al., 2015), a high-resolution process-based scenario-forecasting model, used field and remote sensing data from 2008 and 2011-2015 for calibration to establish target P loads for reducing cyanobacteria biomass. Based on WLEEM’s success in assessing HAB distribution in the western basin and its successful seasonal CHAB forecast for 2015, it is currently being used to assess internal sediment P recycling in the western basin. Other modeling approaches, such as ELCOM-CAEDYM, also provide a coupled hydrodynamic and bio-geochemical modeling strategy that can be used for cyanobacteria (Leon et al., 2011). All these types of models will increasingly be relied on for assimilation of observations to guide nowcast and forecast products.
The success of future CHAB models will depend on a close coupling of models and observations, and the ability to represent processes related to anthropogenic climate forcing. Both statistical and process models can be used to develop and test hypotheses regarding the key factors driving both seasonal and interannual CHAB dynamics. Models will increasingly become assimilative—integrating new data to adjust the forecasts. This is happening now with interannual nutrient loads driving forecasts of bloom severity (Stumpf et al., 2012; Obenour et al., 2014) and process models that are calibrated against field and remote sensing data (Verhamme et al. 2015). Effective collaborations between modelers and observationists can also identify the most important datasets, sampling locations and intervals, ultimately leading to more cost-effective and sustainable monitoring.
Important priorities in developing CHAB models are (1) establishing the end result the models must ultimately predict; (2) identifying the spatio-temporal constraints that impact the end result; (3) adopting best practices for the creation and validation of the model; (4) and identifying the observational methods that will support the development, input, and validation of the model.
The NSF Workshop identified two distinct end results, the modeling of bloom formation and the modeling of toxin forecasting. Including the requirements for modeling into the developing research priorities could lead to better integration between the observational and modeling strategies. For modeling bloom formation, the following actions are needed. First, the objectives of the model need to be defined, including the identification of the bloom biomass metrics, assessing how far in advance forecasts can be made, and the management and scientific needs of forecasts. Second, the models need to test the spatio-temporal occurrence of bloom-triggering events, the seasonality of bloom nutrient requirements, and the relationship between nutrients in the water column and sediments. Third, there is a need for creating substantive, rigorous and robust best practices for model development to provide guidance on a variety of factors, including the avoidance of model overfitting in accuracy assessment, scaling models between ecosystem levels, and integrating results from multiple models. Fourth, there is a need to better integrate simulated model fields with field sampling approaches to optimize sampling design (temporal and spatial acquisition) and methods (what data are best incorporated into models?). Simple sensors, such as pigment fluorescence or turbidity sensors, need to be assessed for their suitability for CHAB biomass estimation, especially given the general lack of inter-comparability between different metrics demonstrated previously (Ho and Michalak, 2015). Water sampling and sensors need to be integrated into model validation in a way that supports the modeling effort. Key sentinel sites or long-term monitoring sites (nutrients, biomass, etc.) need to be identified and maintained for consistency in model evaluation.
Despite the many challenges presented above regarding model development, useful tools are available for short-term forecasting and generating load reduction targets. With respect to Lake Erie, current efforts integrating remote sensing, regular sampling and particle tracking models have led to the development of HAB Tracker, which provides a 5-day forecast of surface bloom intensity and migration. Future refinement of the HAB Tracker will include validated distributions resulting from migration and wind mixing events that tend to drive Microcystis surface scums downward in the water column where municipal water intakes are located. Other groups have coupled different physical (hydrodynamic, wind-wave, sediment transport) and biological (nutrient ingestion, algal growth) models to link nutrient loads to cyanobacteria growth, allowing for the generation of load-reduction scenarios (Verhamme et al. 2015). Visible derivative spectroscopy of hyperspectral field and remote sensing data are also providing increasing specificity for estimation of multiple bloom components, with potential for model assimilation (Ortiz et al., 2013; Ali et al., 2014a,b; Ali and Ortiz, 2014). The challenge for these coupled-models will be to move beyond generating outputs of total biomass toward modeling of algal composition and toxicity.
The first priority for modeling the formation and release of toxins themselves is to develop models that will determine which toxins will appear and when. These models need to incorporate sufficient detail so that both long- and short-term spatio-temporal variability can be predicted. The models will need to incorporate factors such as grazing pressure and toxin gene expression with changing nutrient and environmental conditions, all of which can be expected to vary through lakes, seasons, years and CHAB species. Validation will push data collection into new areas. It will not be sufficient to simply estimate toxin concentrations from models, as the presence and expression of the genes or toxin-producing strains will be needed to address the uncertainties in the models. Biomolecular measurements from metatranscriptomics (e.g. Steffen et al., 2015) will need to be incorporated into the models, as it can provide not only resolution of what organisms are present, but also what each individual species is doing. Until there is a better understanding of why toxins are produced and what affects toxin production (e.g. grazing pressure, nutrient availability, oxidative stress [Rohrlack et al., 2001; Ginn et al., 2010; Zilliges et al., 2011]), mechanistic forecasting of toxin production will be unlikely.
Once models are developed, research should extend from predicting toxin biosynthesis within a single organism to focus on predicting spatial “patchiness” and seasonal and species variability in toxin production. Only then can long-term impacts on CHABS brought about by climate change or invasive species be widely understood.
5. Mitigation of blooms and CHAB management
5.1. Best management practices for reducing DRP loading
Best management practices (BMPs) for reducing DRP loading to Lake Erie from cropland can be divided based on their location of application. At the field scale, BMPs can be used to reduce the concentrations of DRP in runoff water or the amounts of runoff water leaving fields, while still others work by removing DRP from streams and rivers as they transport agricultural runoff to Lake Erie. Some examples of field level BMPs are nutrient management, water management, tillage management, and crop rotations that improve soil health. The optimal combination of these field-level approaches is unique to each field and farm operation.
Nutrient management options are included in the 4-R program of nutrient stewardship: Right product, Right rate, Right time, and Right place (Roberts, 2006; Bruulsema et al., 2009). Losses of DRP associated with direct dissolution of broadcast applications can be immediately reduced by fertilizer placement using injection or banding, and by avoiding broadcast applications at times when heavy rains are forecasted or when ground is frozen or snow covered ground. Where broadcasting is used, incorporation of the fertilizer by light vertical tillage to increase fertilizer-soil contact can minimize the time the broadcast application is vulnerable to direct dissolution. Although such incorporation might increase erosion and particulate P export, the trade-off in terms of reduction of bioavailable P loading would likely yield positive water quality benefits.
Application of the right amount of fertilizer requires information on soil test levels in both the agronomic zone (0-8 inches) and the surficial zone (0-2 inches; Fig. 2). It also requires information on the relationship between soil test levels and crop yields. Since current soil test levels reflect historical past management practices, their reduction can be both challenging and time consuming. Therefore, fertilizer application recommendations have been developed based on agronomic soil test levels and divided into build-up, maintenance, draw-down, and zero application (Lambert et al., 2007). Various guidelines for erosion and particulate P control have been developed to create awareness of the extent to which they could contribute to increased DRP runoff to Lake Erie and the effects of that runoff on CHABs (Lambert et al., 2007).
5.2. Cover crops and buffer zones
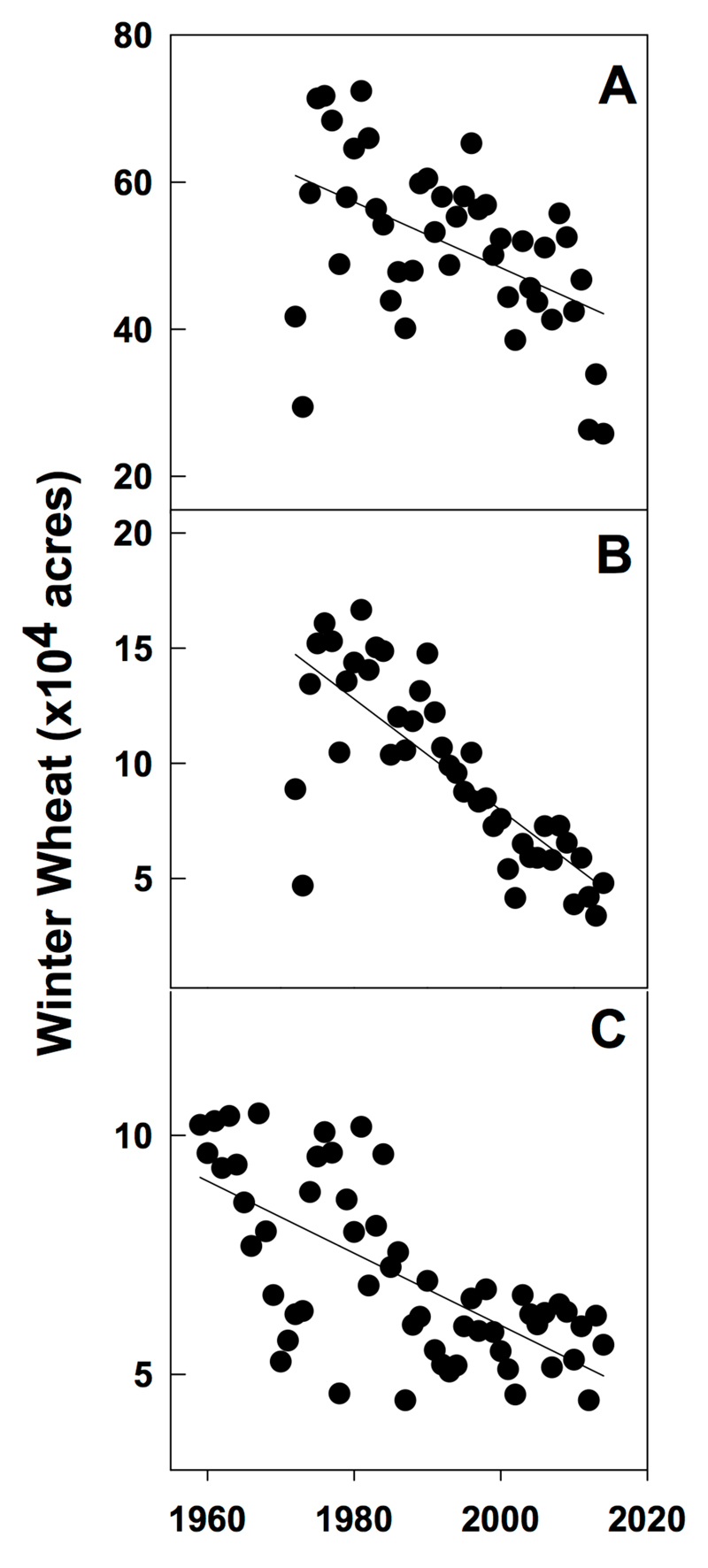
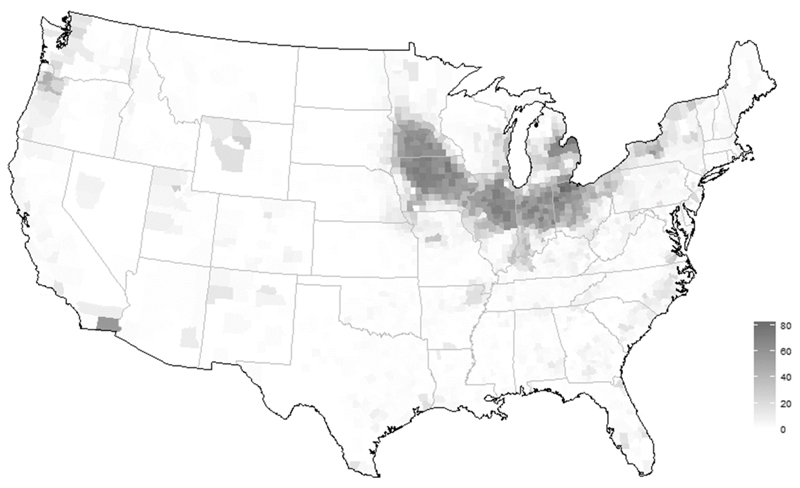
In most instances, the biggest sources of external N and P loading are diffuse non-point sources, dominated by agricultural and stormwater runoff. The most effective ways to reduce these sources are vegetative riparian buffers, construction of wetlands for treating and removing nutrients, and building of retention ponds for impervious runoff sources (Castelle et al., 1994). These land management strategies have been underutilized in the Great Lakes basin. As an example, concurrent with changes in Lake Erie during the last several decades there have been shifts in a variety of external forcing factors. Large shifts in farm management due to technological change and rising labor costs encouraged a shift from family farms of ~100 acres that dominated the landscape until the 1980s to the large farm operations that now exceed thousands of acres. At the same time, farmers have continued to use fewer crops in crop rotations (e.g., only corn and soybeans in Ohio) and they have changed the timing of fertilizer application in response to economic incentives, including fertilizer prices and labor costs (Huang et al., 1994; Sheriff, 2005). One observation amongst these changes has been the marked decrease in the use of winter cover crops (Fig. 5). Cover crops can provide a series of ecosystem services that historically include both a decrease both erosion and nutrient runoff (Schipanski et al., 2014). Whereas some questions remain concerning how crops in winter months may redistribute specific nutrients within the soil horizon (i.e., moving nutrients from the soil/roots to above ground plant components), their absence has likely lead to significant changes in the drainage landscape. This problem is in many ways exacerbated by the increasing density of field drainage tiles across the agricultural landscape (Fig. 6).
Figure 5.
Annual acreage of winter wheat planted in the Maumee River watershed for counties in the states of A) Ohio B) Indiana and C) Michigan. Data from the United States Department of Agriculture National Agriculture Statistical Service (www.nass.usda.gov, accessed on Feb 4, 2015).
Figure 6.
Tile drainage deployment in the USA. Key indicates percent of acreage drained by tile (data from 2012 US Census of Agriculture).
5.3. Bloom treatment strategies: benefits and limitations
Whereas efforts to prevent blooms must ultimately be directed toward the sources of external nutrient loading, whether they are agricultural, related to urban landscape management or coincident with wastewater treatment, numerous water management strategies have been developed and tested to prevent and mitigate cyanobacterial blooms in lakes and reservoirs. A detailed discussion on mitigation strategies is provided in a separate paper in this volume (Paerl et al., 2016), but a brief summary is provided in this section.
Development of re-established and constructed wetlands offers a geo-engineering strategy to mitigate nutrient loading into lakes to help prevent the occurrence of CHABs (Falconer, 2004). Adopting this strategy to address recurrent seasonal CHABs in Hungary’s Lake Balaton, the Kis-Balaton Water Protection System, a mitigation wetland constructed at the mouth of the River Zala, retains more than half of the non-point source nutrients that would otherwise enter the Lake (Tátrai et al., 2000). Combined with sewage diversion projects and geo-political changes resulting in major declines in Hungarian agriculture after 1989, the result has been marked improvement in trophic state for much of the Lake (Hatvani et al., 2014) and declines of formerly abundant CHABs (Istvánovics et al., 2007). Within the Lake Erie watershed, the Great Black Swamp dominated the landscape prior to the draining and reclamation of much of this land for agriculture in the 19th century. The few remnants of the original swamp provide ecosystem services ranging from their use as important stopovers on bird migration flyways to their role in water quality improvement. Indeed, their efficacy in reducing nutrient loads to Lake Erie is well-documented (Mitsch and Reeder, 1991, 1992 and Rea et al., 2015). More recent wetland restoration projects situated in this region (e.g. Egan et al., 2015) whose construction has been supported by the U.S. Environmental Protection Agency’s Great Lakes Restoration Initiative are expected to augment the ecosystem services of the remaining natural wetlands and should help to mitigate the external loading of both P and N into the lake.
Even when external nutrient loading is reduced to aquatic ecosystems, internal recycling of nutrients from the sediments can delay the desirable reduction of phytoplankton biomass (Søndergaard et al., 2003; Paerl et al., 2015). Multiple approaches can be used to reduce the release of P from sediment including dredging (Lürling and Faassen, 2012), hypolimnetic aeration (Beutel and Horne, 1999; Cooke et al., 2005; Singleton and Little, 2006) and chemical sequestration and capping using metal ions such as iron, lanthanum, calcium or aluminum to bind P (Spears et al., 2013; Mackay et al., 2014). In general, costs for aluminum and iron are much lower than other materials, including lanthanum-based products such as Phoslock® (Spears et al., 2013). The Fe-P bond has the disadvantage that P is released upon dissimilatory iron reduction under anaerobic conditions, whereas other ions have stronger bonding with P. The addition of P-binding metal ions is often combined with the addition of flocculants that attach to phytoplankton and aid their settling to the lake bottom. In lakes sufficiently isolated from surrounding water bodies that may otherwise supply the lake with nutrients, chemical sequestration and capping can be effective and keep the lake clear for many years (Akhurst et al., 2004; Lürling and Van Oosterhout, 2013). Reduction of internal N loads are best accomplished through selective enhancement of denitrification, by construction of artificial fringing wetlands and marshes adjacent to impacted water bodies, and (in reservoirs) optimizing hypolimnetic water discharge to vent the system of excessive hypolimnetic N and P.
A physical technique that may mitigate cyanobacteria is artificial mixing (for a recent review see Visser et al., 2015). Artificial mixing can lead to a shift in phytoplankton composition from cyanobacterial dominance to green algae and diatoms if the imposed mixing is strong enough to keep the cyanobacteria entrained in the turbulent flow (Huisman et al., 2004). The mixing should be deep enough to limit light availability and the mixing devices should be well distributed horizontally over the lake. Mixing techniques are often most effective in relatively small water bodies, where the force of mixing devices can be exerted over a large proportion of the surface area.
Another mitigation technique that works selectively on cyanobacteria in small bodies of water is the addition of low concentrations of hydrogen peroxide. In whole lake experiments, a single treatment with hydrogen peroxide (2 mg L-1) was effective at ridding the lake of cyanobacteria and cyanotoxin problems for an entire season (Matthijs et al., 2012). Other phytoplankton species (green algae, diatoms), zooplankton, macrophytes, birds and fish in the treated lake showed few, if any negative effects. Compared to other cyanocides that are currently in use (e.g. CuSO4, see Jancula and Marsalek, 2011), the advantage of hydrogen peroxide is that it rapidly decomposes leaving no permanent biocide traces as is seen with metal toxicants, such as copper salts which can accumulate in the sediments and food chain. Furthermore, cyanotoxins released by lysing cells degrade rapidly in the lake water following peroxide treatment (Matthijs et al., 2012). In the case of lakes too large to be treated as a whole such as Lake Erie, parts of the lake or secondary reservoirs could be treated with hydrogen peroxide to combat cyanobacterial blooms in areas near drinking water intakes. A ‘water harrow’ on a boat ensures homogeneous addition throughout the lake in very low concentrations (15,000 times dilution from a typical 3% over-the-counter drugstore formulation). The exact dose of hydrogen peroxide needed in the reservoir depends on the phytoplankton composition present and can be accurately tested directly before an application (Weenink et al., 2015). Despite the efficacy of these methods, none of these physical-chemical approaches should be viewed as a substitute for nutrient reductions that should accompany all these protocols.
6. Economic impacts and incentives for bloom prevention and mitigation
6.1. Reducing nutrient loads – what are the costs?
Given that efforts to significantly reduce nutrient loads are often costly, it is important to understand the relative costs of both reducing those loads as well as the costs of taking no action (i.e., the economic impacts of on-going CHABs). A recent estimate, based on the empirical model in Sohngen et al. (2015), suggests that the cost would be about $30 million per year to reduce P loading within the Lake Erie watershed by 40%. This model utilizes a tax on P inputs used by farmers, which is an economically efficient approach for pollution abatement. The 40% reduction recommended by policy makers could be achieved for about same costs with other approaches, as described below, but a reduction of this scale likely cannot be achieved for less.
What other economic instruments are available to mitigate nutrient loads and reduce the intensity of CHABs? The suite of BMPs employed in Midwestern cropping systems focuses mostly on nutrient management planning, cover crops, conservation rotations, buffer strips and riparian zones. These practices currently require a $20-$25 million per year investment from society through USDA conservation programs in the Maumee and Sandusky watersheds. Given the recurrent Lake Erie summertime CHABs, they are insufficient alone as mitigation strategies. Expanding the techniques already adopted in the Lake Erie region to achieve a 40% reduction from today's loads will cost more than is currently expended. If these efforts could be efficiently targeted to known nutrient loading hotspots, it is possible these expenditures could be reduced, but current policies for implementing the USDA conservation programs limit the most effective types of targeting (e.g. costs are not included in criteria for ranking implementation of proposed practices by farmers). The LUMINATE modeling system, which integrates agricultural decision-making in the Mississippi drainage with nutrient loading in the Gulf of Mexico (Kling et al., 2014), offers an assessment model that is relevant for the Lake Erie watershed. In this system, the model analyzes policy scenarios taking into consideration the costs of implementation and the resulting benefits to the receiving body of water.
Some emerging BMPs such as drainage water management and bioreactors show promise in mitigating CHABs although they are generally quite costly and have not been evaluated in practice. For example, using assumptions of the Ohio Phosphorus Task Force (2013), the cost of blind inlets was found to be ~$93 per kg P removed. Using the assumptions of the Huron County Soil and Water Conservation District, the cost is nearly $300 per kg P removed. These costs are about twice the costs of a P tax on a per kg basis. Hence, while drainage water management may be effective, it is costly and must be implemented broadly across the Lake Erie watershed to yield measurable results.
A regulated limit on P inputs to farms could reduce P emissions for the same overall cost as the P tax. Phosphorus limits would likely be more effective and less costly than a P tax because the regulatory system could be designed for both fertilizer and manure. In this case, regulators set a maximum input level, in pounds per acre, for an entire watershed. For instance, if the average input per acre in the Maumee River basin is currently 37 lbs per acre of P2O5, then the regulation can be adjusted downward to a maximum input of 32 lbs per acre, thus achieving a 14% reduction from current levels. This total nutrient input would include manure inputs and chemical inputs. Farmers would need to decide where best to apply their allotted amount of P (e.g. 32 lbs in this example multiplied by their total acres). The limit could be set on a watershed-by-watershed basis and could change over time, depending on environmental conditions downstream. Reductions in P would also yield parallel reductions in N as well, so this approach would provide additional value as a general strategy for nutrient management.
While many farmers test their soils for P as a regular protocol for agricultural productivity, a regulated limit on P inputs need not make this a requirement. A farmer's individual P application limit need not be tied to the soil test level on their farm. With a P application limit in place, individual farmers will have a strong incentive to conduct soil tests, but regulators do not need to require it.
A limit on P application can also be amended to denominate P limits for specific crops, or subsidize farmers to eliminate the costs of implementing P reductions. Creating a set of crop-by-crop limits, however, is more burdensome for regulators and it reduces flexibility for farmers to substitute crops that may help achieve P reduction.
Strategies to gain public support of initiatives targeting CHAB prevention and mitigation are also relevant to an economic analysis of this issue. Recently, Egan et al. (2015) reported greater success garnering public support by linking contingent valuation surveys distributed to residents of Ohio’s Lake Erie coast concerning proposed wetlands restoration funded by ongoing annual payments compared to a larger one-time payment.
7. Trends promoting future blooms
7.1. CHAB physiology and climate change
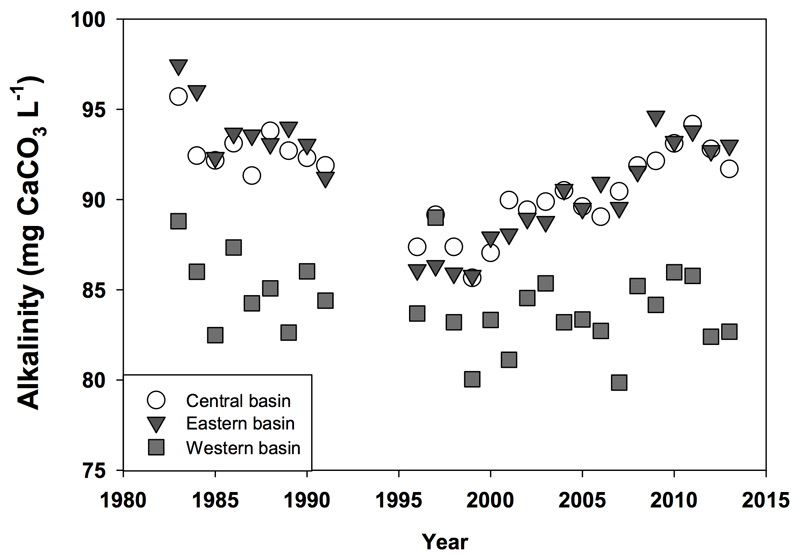
Whereas nutrient loadings from agricultural sources are the major drivers of CHAB events in Lake Erie and elsewhere, other trends in chemical and physical factors may favor the future dominance of cyanobacteria. Other changes in the Great Lakes basin may be less tightly linked to human activity and more closely related to changes in climate or biology. Regarding biological changes in the lake, in the late 1980s (and concurrent with the invasion and expansion of the Zebra mussel Dreissena polymorpha) there was a systematic decrease in alkalinity (in part due to dissolved inorganic carbonate species) in Lake Erie surface waters (Fig. 7). Concentrations continued to drop in all three basins until 1995, when a slow but steady recovery of alkalinity started in the central and eastern basins. The potential linkage here to cyanobacterial blooms is through the low concentration of dissolved carbon dioxide (CO2aq), as dense cyanobacterial blooms in low- and moderately alkaline waters can deplete the dissolved CO2 concentration to limiting levels (Verspagen et al. 2014). Highly buoyant CHAB taxa are able to directly intercept CO2 at the air-water interface, giving them a competitive advantage under C-limited conditions (Paerl and Ustach, 1982). Moreover, all cyanobacteria and many eukaryotic algae possess efficient carbon concentrating mechanisms (CCMs) that facilitate active uptake of inorganic carbon species against a concentration gradient of CO2 (Badger and Price, 2003; Giordano et al., 2005). Previous work has demonstrated that under low CO2 concentrations, CCMs in cyanobacteria are more efficient than other algae or higher plants (Badger and Price, 2003 and Badger et al., 2006) promoting cyanobacterial dominance under low CO2 conditions (Price et al., 2008). Recent insights in the genetic diversity of Ci uptake systems of cyanobacteria indicate that there is a large variation in the effectiveness of the cyanobacterial CCMs, even among different strains within the same genus (see Visser et al., 2016 this issue). Some cyanobacterial strains perform well at low CO2, whereas other strains are much better competitors under high CO2 conditions. As these previous findings are considered in the context of climate warming, increases in CO2 concentrations may have a more beneficial impact on species that have evolved to not have CCMs, have inferior CCMs, and/or rely primarily on CO2 transport (Fu et al., 2007). Furthermore, to date, only species-specific responses to rising CO2 levels have been evaluated for CHAB-forming cyanobacteria. Van de Waal et al. (2011) conducted competition experiments between toxic and non-toxic strains of cyanobacteria at high CO2 concentrations. This study suggested that non-toxic strains have an advantage under high CO2 conditions. Complicating this analysis was genetic analysis of several cyanobacterial strains by Sandrini et al. (2014) revealing that the two strains used in the competition experiments by Van de Waal et al. also differed in their Ci uptake systems. The strain with a high-affinity uptake system won the competition at low pCO2 levels, while the other strain was a Ci uptake generalist (both high and low-affinity uptake Ci systems) and won the competition at high pCO2 levels. Environmental factors affected by climate change will not occur individually but in parallel with each other. At present, little is known about the interactive effects between increased nutrients, higher temperatures (both predicted to positively influence CHAB formation and toxicity; see references below) and rising CO2 concentrations on the development of future CHABs (O’Neil et al., 2012).
Figure 7.
Mean summer alkalinity estimates for the three basins of Lake Erie since 1983. Data demonstrate a drop in CaCO3 equivalents since the early 1980’s that is coincident with the invasion of D. polymorpha into the system. While some level of recovery has been observed in the Central and Eastern basins, alkalinity in the western basin remains low. Data from the Great Lakes National Program Office (courtesy. R. Barbiero).
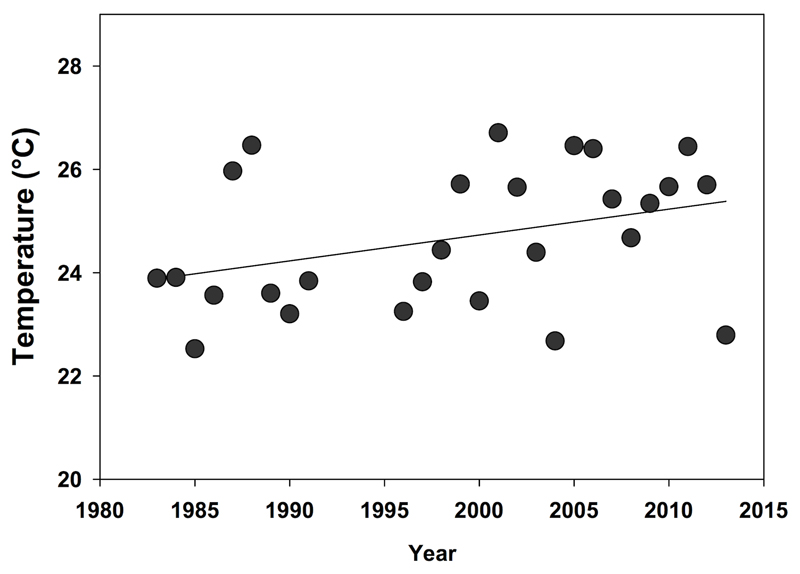
Climate change is predicted to result in increased precipitation and more intense storm events, leading to increased nutrient runoff (Michalak et al., 2013). At the same time, changes in surface water temperature are a potential environmental driver of cyanobacterial blooms. Blooms of toxic cyanobacteria are favored by increases in temperature (Paerl amd Huisman 2008; Paerl et al. 2010; Paerl et al. 2011). Temperature influences a variety of processes that shape microbial community structure, including absolute growth rates (Paerl and Huisman, 2008 and 2009), gas solubility (Hutchinson, 1957), water column nutrient chemistry (Hutchinson, 1957) and water column structure / mixing processes (Paerl et al., 2011). Several laboratory studies have found that cyanobacteria such as Microcystis perform better (i.e. have higher growth rates) at warmer temperatures (see Robarts and Zohary 1987). Furthermore, it was found that toxic strains of Microcystis grow faster than non-toxic strains at higher temperatures (Davis et al., 2009). Lake Erie has experienced a small but significant increase in surface waters temperatures (~ 0.05 °C per year over the last two decades) during recent decades (Fig. 8), consistent with global patterns of surface water temperature increases (O’Reilly et al., 2015; Sharma et al., 2015). Perhaps more important, the Great Lakes basin has experienced a period of unprecedented warmth, leading to not only higher seasonal averages, but record extreme temperature observations for both air and water during this period. While at this juncture the relationship between climate change and Microcystis blooms in Lake Erie remains an anecdotal observation (as there are not enough scientific data to support this), this is an area of inquiry for scientists. Indeed, changes in climate may influence CHAB distributions throughout the entire Great Lakes: Doblin et al. (2007) demonstrated that Microcystis cells could be transported to other Great Lakes in ships’ ballast water, and suggested that temperature may be one of the few limiting constraints in Great Lake embayments where blooms have not yet been observed.
Figure 8.
Mean summer temperature in Lake Erie’s western basin since 1983. Surface water temperatures for the western basin of Lake Erie have increased ~ 0.05 °C yr-1 (R2 = 0.129, p = 0.066).
8. Workshop synthesis
After two days of presentations and discussions in breakout groups, consensus was reached in the areas listed below. These points were shared with diverse stakeholders and the general public in a NOAA-sponsored open forum at the conclusion of the workshop:
1). Long term solutions for mitigating CHABs will involve integration of multiple approaches into models – from genes to ecosystems
To fully understand CHAB events and to predict where, when, and how intense and toxic blooms will be, integration of all areas of research from the cellular level to large spatial scales is necessary. There is a large and growing set of approaches that is providing these new data sources, ranging from monitoring cyanobacterial gene expression by transcriptomics to high resolution remote sensing. These emerging data sets must be integrated with more traditional data streams to create both mechanistic and quantitative models to better understand factors promoting the intensity and toxicity of CHABs.
2). Dual nutrient management is warranted in controlling CHAB events
Whereas management strategies in freshwaters to date have traditionally focused on controlling P inputs, emerging research from across the globe has provided a wealth of data demonstrating the role N loadings can play in controlling the growth and toxicity of CHABs. As such, N loads should be controlled to mitigate the intensity and toxicity of these events.
3). Treatment strategies exist for acute bloom control in smaller lakes, but they can be limited by scale and by regulatory barriers
For smaller lakes, multiple methods exist to mitigate bloom events. As an example, the Netherlands validated the performance of dilute hydrogen peroxide as an effective algaecide specific for cyanobacteria. Similar approaches may or may not be suitable in the Great Lakes basin due to individual state regulatory guidelines.
4). Land management decisions are central in preventing CHAB events
CHABs are strongly controlled by nutrient loading and within large lake ecosystems, the delivery of nutrients must be restricted to control these events. With respect to the Lake Erie watershed, the use of cover crops, buffer strips and construction of artificial wetlands divert nutrients away from lakes by supporting terrestrial biomass instead of algal biomass. In all cases, mitigating the largest nutrient sources will always be most important for lessening CHAB intensity.
5). Land use decisions can be incentivized
If the costs of mitigating CHAB events are understood, economic incentives can be established to encourage agricultural practices that reduce nutrient loadings from farms. Social scientists will play critical roles toward implementing practices and policies for CHAB prevention and mitigation.
As reviewed within this manuscript, these future research areas will provide information needed by policymakers and stakeholders to prevent of future CHAB events in the Great Lakes and elsewhere in the world. Given that Toledo Mayor D. Michael Collins has testified to the US Senate that the two-day Toledo crisis cost approximately $2.5 million in lost economic activity, even a brief event has considerable impact to a community (Congressional Record, December 4, 2014). In this paper, suggestions are presented regarding future research on CHAB events leading to prevention and control of blooms.
Of course, outcomes leading to a decline in bloom events require implementation of new practices, whether they include economic incentives, treatment strategies, management decisions or regulatory legislation. Whatever the outcome, scientific and social science research must inform public policy decisions. Indeed, the Toledo water crisis has proven that fundamental research can lead to sound legislation aimed at protecting the water supply. Important first steps included the passage of Ohio Senate Bill 1, cosponsored by State Sens. Randy Gardner and Rob Peterson, which regulate fertilizer application and disposal of dredged P-rich lake sediments. At the Federal level, H.R. 212, The Drinking Water Protection Act, was drafted by Rep. Bob Latta (R, OH), cosponsored by Sens. Rob Portman (R, OH), and Sherrod Brown (D, OH) and signed into law by President Obama. H.R. 212 charges the US EPA to develop a strategic plan to assess and manage risk associated with algal toxins. The fact that new laws emerged the year following the Toledo event demonstrates that government can respond in a timely manner, provided that lawmakers have ready access to the appropriate scientific information. To serve the public effectively, future legislation must continue to rely on effective communication between researchers and elected officials.
Acknowledgements
The CHAB Workshop was supported by the National Science Foundation under grant no. CBET-1515671 (GSB). Additional support was provided by the NOAA Great Lakes Environmental Research Laboratory, the University of Michigan Water Center and by the Office of the Vice President for Research & Economic Development at Bowling Green State University. Additional research support was provided by Ohio Department of Education grant R/HAB-2-BOR to GSB, RMM and JDO. GSB, RMM and SWW thank Mr. Jason Isakovic (Senior Legislative Assistant, office of U.S. Rep. Robert Latta) for helpful discussions on the legislative process. The authors also thank the many staff and students at BGSU who provided assistance prior and during the Workshop.
References
- Akhurst D, Jones GB, McConchie DM. The application of sediment capping agents on phosphorus speciation and mobility in a sub-tropical dunal lake. Marine and Freshwater Research. 2004;55:715–725. [Google Scholar]
- Ali KA, Ortiz JD. Multivariate approach for chlorophyll-a and suspended matter retrievals in Case II waters using hyperspectral data. Hydrological Sciences Journal. 2014;59:1–14. doi: 10.1080/02626667.2014.964242. [DOI] [Google Scholar]
- Ali KA, Witter DL, Ortiz JD. Multivariate approach to estimate color producing agents in Case 2 waters using first-derivative spectrophotometer data. Geocarto International. 2014a;29(2):102–127. doi: 10.1080/10106049.2012.743601. [DOI] [Google Scholar]
- Ali KA, Witter DL, Ortiz JD. Application of empirical and semi-analytical algorithms to MERIS data for estimating chlorophyll a in case waters of Lake Erie. Environmental Earth Sciences. 2014b;71(9):4209–4220. doi: 10.1007/s12665-013-2814-0. [DOI] [Google Scholar]
- Allen BL, Mallarino AP, Klatt JG, Baker JL, Camara M. Soil and surface runoff phosphorus relationships for five typical USA midwestern soils. Journal of Environmental Quality. 2006;35:599–610. doi: 10.2134/jeq2005.0135. [DOI] [PubMed] [Google Scholar]
- Badger MR, Price GD. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. Journal of Experimental Botany. 2003;54:609–622. doi: 10.1093/jxb/erg076. [DOI] [PubMed] [Google Scholar]
- Badger MR, Price GD, Long BM, Woodger FJ. The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. Journal of Experimental Botany. 2006;57:249–265. doi: 10.1093/jxb/eri286. [DOI] [PubMed] [Google Scholar]
- Baker DB, Confesor R, Ewing DE, Johnson LT, Kramer JW, Merryfield BJ. Phosphorus loading to Lake Erie from the Maumee, Sandusky and Cuyahoga rivers: the importance of bioavailability. Journal of Great Lakes Research. 2014a;40:502–517. [Google Scholar]
- Baker DB, Ewing DE, Johnson LT, Kramer JW, Merryfield BJ, Confesor R, Richards RO, Roerdink AA. Lagrangian analysis of the transport and processing of agricultural runoff in the lower Maumee River and Maumee Bay. Journal of Great Lakes Research. 2014b;40:479–495. [Google Scholar]
- Beutel MW, Horne AJ. A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality. Lake and Reservoir Management. 1999;15:285–297. [Google Scholar]
- Box GEP, Draper NR. Empirical Model Building and Response Surfaces. John Wiley & Sons; New York, NY: 1987. [Google Scholar]
- Bruulsema T, Lemunyon J, Herz B. Know your fertilizer rights. Crops and Soils. 2009;42:13–18. [Google Scholar]
- Castelle AJ, Johnson AW, Conolly C. Wetland and stream buffer size requirements—a review. Journal of Environmental Quality. 1994;23:878–882. doi: 10.2134/jeq1994.00472425002300050004x. [DOI] [PubMed] [Google Scholar]
- Chaffin JD, Bridgeman TB. Organic and inorganic nitrogen utilization by nitrogen-stressed cyanobacteria during bloom conditions. Journal of Applied Phycology. 2014;26:299–309. [Google Scholar]
- Chaffin JD, Bridgeman TB, Bade DL, Mobilian CN. Summer phytoplankton nutrient limitation in Maumee Bay of Lake Erie during high-flow and low flow years. Journal of Great Lakes Research. 2014;40:524–531. [Google Scholar]
- Congressional Record. Testimony of Mayor D. Michael Collins to the Senate Committee on Agriculture, Nutrition and Forestry. 2014 Dec 4; [Google Scholar]
- Cooke GD, Welch EB, Peterson SA, Nichols SA. In: Restoration and Management of Lakes and Reservoirs. 3rd edition. Cooke GD, editor. Taylor and Francis; Boca Raton, Florida: 2005. p. 591. [Google Scholar]
- Cusick KD, Sayler GS. An overview on the marine neurotoxin, saxitoxin: genetics, molecular targets, methods of detection and ecological functions. Marine Drugs. 2013;11:991–1018. doi: 10.3390/md11040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalogue I, Cho KH, Scavia D. Evaluating causes of trends in long-term dissolved reactive phosphorus loads to Lake Erie. Environmental Science and Technology. 2012;4:10660–10666. doi: 10.1021/es302315d. [DOI] [PubMed] [Google Scholar]
- Davis TW, Berry DL, Boyer GL, Gobler CJ. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae. 2009;8:715–725. [Google Scholar]
- Davis TW, Harke MJ, Marcoval MA, Goleski J, Orano-Dawson C, Berry DL, Gobler CJ. Effects of nitrogenous compounds and phosphorus on the growth of toxic and non-toxic strains of Microcystis during cyanobacterial blooms. Aquatic Microbial Ecology. 2010;61:149–162. [Google Scholar]
- Davis TW, Bullerjahn GS, Tuttle T, Rozmarynowycz M, McKay RM, Watson SB. Evaluation of increasing nitrogen and phosphorus concentrations on the toxicity and chlorophyll content during cyanobacterial blooms in Sandusky Bay, Lake Erie. Environmental Science and Technology. 2015;49:7197–7207. doi: 10.1021/acs.est.5b00799. [DOI] [PubMed] [Google Scholar]
- Doblin MA, Coyne KJ, Rinta-Kanto JM, Wilhelm SW, Dobbs FC. Dynamics and short-term survival of toxic cyanobacteria species in ballast water from NOBOB vessels transiting the Great Lakes-implications for HAB invasions. Harmful Algae. 2007;6:519–530. [Google Scholar]
- Doucette GJ, Mikulski CM, Jones KL, King KL, Greenfield DI, Marin R, Jensen S, Roman B, Elliott CT, Scholin CA. Remote, subsurface detection of the algal toxin domoic acid onboard the Environmental Sample Processor: assay development and field trials. Harmful Algae. 2009;8:880–888. [Google Scholar]
- Egan KJ, Corrigan JR, Dwyer DF. Three reasons to use annual payments in contingent valuation surveys: Convergent validity, discount rates, and mental accounting. Journal of Environmental Economics and Management. 2015;72:123–136. [Google Scholar]
- Foss AJ, Aubel MT. Using the MMPB technique to confirm microcystin concentrations in water measured by ELISA and HPLC (UV, MS, MS/MS) Toxicon. 2015;104:91–101. doi: 10.1016/j.toxicon.2015.07.332. [DOI] [PubMed] [Google Scholar]
- Fu FX, Warner ME, Zhang Y, Feng Y, Hutchins DA. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (Cyanobacteria) Journal of Phycology. 2007;43:485–496. [Google Scholar]
- Ginn HP, Pearson LA, Neilan BA. NtcA from Microcystis aeruginosa PCC 7806 is autoregulatory and binds to the microcystin promoter. Applied and Environmental Microbiology. 2010;76:4362–4368. doi: 10.1128/AEM.01862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M, Beardall J, Raven JA. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annual Review of Plant Biology. 2005;56:99–131. doi: 10.1146/annurev.arplant.56.032604.144052. [DOI] [PubMed] [Google Scholar]
- Greenfield DI, Marin R, Doucette GJ, Mikulski C, Jones K, Jensen S, Roman B, Alvarado N, Feldman J, Scholin C. Field applications of the second-generation Environmental Sample Processor (ESP) for remote detection of harmful algae: 2006-2007. Limnology and Oceanography Methods. 2008;6:667–679. [Google Scholar]
- Han H, Allen BL, Bosch NS. Historical pattern of phosphorus loading to Lake Erie watersheds. Journal of Great Lakes Research. 2012;38:289–298. [Google Scholar]
- Hand E. Startup Liftoff: How flocks of small, cheap satellites, hatched in Silicon Valley, will constantly monitor a changing Earth. Science. 2015;348:172–177. doi: 10.1126/science.348.6231.172. [DOI] [PubMed] [Google Scholar]
- Harke MJ, Gobler CJ. Global transcriptional responses of the toxic cyanobacterium, Microcystis aeruginosa, to nitrogen stress, phosphorus stress, and growth on organic matter. PLoS One. 2013;8(7):e69834. doi: 10.1371/journal.pone.0069834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatvani IG, Clement A, Kovács J, Kovács IS, Korponai J. Assessing water-quality data: The relationship between the water quality amelioration of Lake Balaton and the construction of its mitigation wetland. Journal of Great Lakes Research. 2014;40:115–125. [Google Scholar]
- Ho JC, Michalak AM. Challenges in tracking harmful algal blooms: a synthesis of evidence from Lake Erie. Journal of Great Lakes Research. 2015;41:317–325. [Google Scholar]
- Huang W-Y, Hansen L, Uri ND. The application timing of nitrogen fertilizer. Water, Air & Soil Pollution. 1994;73:189–211. [Google Scholar]
- Humbert JF, Barbe V, Latifi A, Gugger M, Calteau A, Coursin T, Lajus A, Castelli V, Oztas S, Samson G, Longin C, et al. A tribute to disorder in the genome of the bloom-forming freshwater cyanobacterium Microcystis aeruginosa. PLoS ONE. 2013;8:e70747. doi: 10.1371/journal.pone.0070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson GE. A Treatise on Limnology, Vol. I, Part: 1 Geography and Physics of Lakes. John Wiley & Sons; New York: 1957. [Google Scholar]
- Istvánovics V, Clement A, Somlyódy L, Specziár A, László G, Padisák J. Updating water quality targets for shallow Lake Balaton (Hungary), recovering from eutrophication. Hydrobiologia. 2007;581:305–318. [Google Scholar]
- James R, Gregg A, Dindal A, McKernan J. Environmental technology verification report. Abraxis microcystin test kits. Battelle/US EPA Advanced Monitoring Systems Center Technical Report. 73 pages. 2010 [Google Scholar]
- Jancula D, Marsalek B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere. 2011;85:1415–1422. doi: 10.1016/j.chemosphere.2011.08.036. [DOI] [PubMed] [Google Scholar]
- Johnson LT. National Center for Water Quality Research. Heidelberg University: 2015. Cumulative Tributary Loading Report Water Year 2014 (in preparation) www.heidelberg.edu/ncwqr. [Google Scholar]
- Joose PJ, Baker DB. Context for re-evaluating agricultural sources phosphorus loadings to the Great Lakes. Canadian Journal of Soil Science. 2011;91:317–327. [Google Scholar]
- Kane DD, Conroy JD, Richards RP, Baker DB, Culver DA. Re-eutrophication of Lake Erie: Correlations between tributary nutrient loads and phytoplankton biomass. Journal Great Lakes Research. 2014;40:496–501. [Google Scholar]
- King KW, Williams MR, Fausey NR. Contributions of systematic tile drainage to watershed-scale phosphorus transport. Journal of Environmental Quality. 2014;44:486–494. doi: 10.2134/jeq2014.04.0149. [DOI] [PubMed] [Google Scholar]
- Kleinman PJA, Sharpley AN, McDowell RW, Flaten DN, Buda AR, Tao L, Bergstrom L, Zhu Q. Managing agricultural phosphorus for water quality protection: principals for progress. Plant Soil. 2011a;349:169–182. [Google Scholar]
- Kleinman PJA, Sharpley AN, Buda AR, McDowell RW, Allen AL. Soil controls of phosphorus in runoff: management barriers and opportunities. Canadian Journal of Soil Science. 2011b;91:329–338. [Google Scholar]
- Kleinman PJA, Sharpley AN, Withers PJ, Bergström L, Johnson LT, Doody DG. Implementing agricultural phosphorus science and management to combat eutrophication. Ambio. 2015;44(Supplement 2):S297–S310. doi: 10.1007/s13280-015-0631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling CL, Panagopoulos Y, Rabotyagov SS, Valcu AM, Gassman PW, Campbell T, White MJ, et al. LUMINATE: linking agricultural land use, local water quality and Gulf of Mexico hypoxia. European Review of Agricultural Economics. 2014;41:431–459. [Google Scholar]
- Kosten S, Huszar VLM, Bécares E, Costa LS, van Donk E, Hansson LA, Jeppesen E, Kruk C, Lacerot G, Mazzeo N, De Meester L, et al. Warmer climates boost cyanobacterial dominance in shallow lakes. Global Change Biology. 2012;18:118–126. [Google Scholar]
- Kudela RM, Palacios SL, Austerberry DC, Accorsi EK, Guild LS, Torres-Perez J. Application of hyperspectral remote sensing to cyanobacterial blooms in inland waters. Remote Sensing of Environment. 2015;167:196–205. [Google Scholar]
- Kurmayer R, Blom JF, Deng L, Pernthaler J. Integrating phylogeny, geographic niche partitioning and secondary metabolite synthesis in bloom-forming Planktothrix. The ISME Journal. 2015;9:909–921. doi: 10.1038/ismej.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock MV, Donovan MA, Easy DJ. Sensitivity of lateral flow tests to mixtures of saxitoxins and applications to shellfish and phytoplankton monitoring. Toxicon. 2010;55:597–605. doi: 10.1016/j.toxicon.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Lambert DM, Lowenberg-DeBoer J, Malzer G. Managing phosphorus soil dynamics over space and time. Agricultural Economics. 2007;37:43–53. [Google Scholar]
- Leon LF, Smith REH, Hipsey MR, Bocaniov SA, Higgins SN, Hecky RE, Antenucci JP, Imberger J, Guildford SJ. Application of a 3D hydrodynamic-biological model for seasonal and spatial dynamics of water quality and phytoplankton in Lake Erie. Journal of Great Lakes Research. 2011;37:41–53. [Google Scholar]
- LimnoTech. Technical report prepared for the U.S. Army Corps of Engineers. Buffalo District: 2010. Development, Calibration, and Application of the Lower Maumee River – Maumee Bay Model; p. 113. (December 30, 2010) [Google Scholar]
- LimnoTech. Final Technical Report prepared for U.S. Army Corps of Engineers. Buffalo District under contract to Ecology & Environment, Inc: 2013. Development of an Integrated Modeling Approach for Quantifying the GLRI Deposition Metric: Pilot Application to Toledo Harbor; p. 125. [Google Scholar]
- LimnoTech and Ecology and Environment. Final Technical Report for Contract No. W912P4-10-D-0002. Prepared for U.S. Army Corps of Engineers. Buffalo District: 2014. Influence of Open-Lake Placement of Dredged Material on Western Lake Erie Basin Harmful Algal Blooms; p. 166. + Appendices. [Google Scholar]
- Lürling M, Faassen EJ. Controlling toxic cyanobacteria: Effects of dredging and phosphorus-binding clay on cyanobacteria and microcystins. Water Research. 2012;46:1447–1459. doi: 10.1016/j.watres.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Lürling M, Van Oosterhout F. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Research. 2013;47:6527–6537. doi: 10.1016/j.watres.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Mackay EB, Maberly SC, Pan G, Reitzel K, Bruere A, Corker N, Douglas G, et al. Geoengineering in lakes: welcome attraction or fatal distraction? Inland Waters. 2014;4:349–356. [Google Scholar]
- Macrae ML, English MC, Schiff SL, Stone M. Intra-annual variability in the contribution of tile drains to basin discharge and phosphorus export in a first-order agricultural catchment. Agricultural Water Management. 2007;92:171–182. [Google Scholar]
- Matthijs HC, Visser PM, Reeze B, Meeuse J, Slot PC, Wijn G, et al. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Research. 2012;46:1460–1472. doi: 10.1016/j.watres.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Michalak AM, Anderson EJ, Beletsky D, Boland S, Bosch NS, Bridgeman TB, et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proceedings of the National Academy of Sciences. 2013;110:6448–6452. doi: 10.1073/pnas.1216006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsch WJ, Reeder BC. Modelling nutrient retention of a freshwater coastal wetland: estimating the roles of primary productivity, sedimentation, resuspension and hydrology. Ecological Modelling. 1991;54:151–187. [Google Scholar]
- Mitsch WJ, Reeder BC. Nutrient and hydrologic budgets of a Great Lakes coastal freshwater wetland during a drought year. Wetlands Ecology and Management. 1992;1:211–222. [Google Scholar]
- Müller S, Mitrovic MM. Phytoplankton co-limitation by nitrogen and phosphorus in a shallow reservoir: progressing from the phosphorus limitation paradigm. Hydrobiologia. 2015;744:255–269. [Google Scholar]
- Nagata S, Tsutsumi T, Hasegawa A, Yoshida F, Ueno Y, Watanabe MF. Enzyme immunoassay for the direct determination of microcystins in environmental water. Journal of AOAC International. 1997;80:408–417. [Google Scholar]
- Natural Resources Conservation Service. Rapid Watershed Assessments. 2009 Available at www.oh.nrcs.usda.gov/programs/RWA/rapid_watershed_assessments.html. [Google Scholar]
- Obenour DR, Gronewold AD, Stow CA, Scavia D. Using a Bayesian hierarchical model to improve Lake Erie cyanobacteria bloom forecasts. Water Resources Research. 2014:50. doi: 10.1002/2014WR015616. [DOI] [Google Scholar]
- Ohio Environmental Protection Agency. Ohio Lake Erie Task Force 2. 2013 Final Report. Available at http://www.epa.state.oh.us. [Google Scholar]
- Ohio Phosphorus Task Force II Final Report. 2013 Available at http://lakeerie.ohio.gov/Portals/0/Reports/Task_Force_Report_October_2013.pdf. [Google Scholar]
- O’Neil JM, Davis TW, Burford MA, Gobler CJ. The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae. 2012;14:313–334. [Google Scholar]
- O’Reilly CM, Sharma S, Gray DK, Hampton SE, Read JS, Rowley RJ, et al. Rapid and highly variable warming of lake surface waters around the globe. Geophysical Research Letters. 2015;42 doi: 10.1002/2015GL066235. [DOI] [Google Scholar]
- Orr PT, Jones GJ. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnology and Oceanography. 1998;43:1604–1614. [Google Scholar]
- Ortiz JD, Witter DL, Ali KA, Fela N, Duff M, Mills L. Evaluating multiple color producing agents in Case II waters from Lake Erie. International Journal of Remote Sensing. 2013;34:8854–8880. [Google Scholar]
- Otten TG, Crosswell JR, Mackey S, Dreher T. Application of molecular tools for microbial source tracking and public health risk assessment of a Microcystis bloom traversing 300 km of the Klamath River. Harmful Algae. 2015;46:71–81. [Google Scholar]
- Ouellette AJA, Wilhelm SW. Toxic cyanobacteria: the evolving molecular toolbox. Frontiers in Ecology and the Environment. 2003;7:359–366. [Google Scholar]
- Paerl HW, Ustach J. Blue green algal scums: An explanation for their occurrence during freshwater blooms. Limnology and Oceanography. 1982;27:212–217. [Google Scholar]
- Paerl HW, Huisman J. Blooms like it hot. Science. 2008;320:57–58. doi: 10.1126/science.1155398. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Huisman J. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology Reports. 2009;1:27–37. doi: 10.1111/j.1758-2229.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Rossignol KL, Hall SN, Peierls BL, Wetz MS. Phytoplankton community indicators of short- and long-term ecological change in the anthropogenically and climatically impacted Neuse River Estuary, North Carolina, USA. Estuaries and Coasts. 2010;33:485–497. [Google Scholar]
- Paerl HW, Hall NS, Calandrino ES. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climactic-induced change. Science of the Total Environment. 2011;409:1739–1745. doi: 10.1016/j.scitotenv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Paerl HW. Mitigating harmful cyanobacterial blooms in a human-and climatically-impacted world. Life. 2014;4:988–1012. doi: 10.3390/life4040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl HW, Gardner WS, McCarthy MJ, Peierls BL, Wilhelm SW. New insights on old paradigms – shifting thoughts on freshwater eutrophication. Science. 2014;346:175. doi: 10.1126/science.346.6206.175-a. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Gardner WS, Havens KE, Joyner AR, McCarthy MJ, Newell SE, Qin B, Scott JT. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae. 2016 doi: 10.1016/j.hal.2015.09.009. (in press, this issue) [DOI] [PubMed] [Google Scholar]
- Pavlac MM, Smith TT, Thomas SP, Makarewicz JC, Lewis TW, Edwards WJ, Pennuto CM, Basiliko CP, Atkinson JF, Boyer GL. Assessment of phytoplankton distribution in the nearshore zone using continuous in situ fluorometry. Journal of Great Lakes Research. 2012;38(suppl 4):78–84. [Google Scholar]
- Pearson L, Mihali T, Moffitt M, Kellmann R, Neilan B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Marine Drugs. 2010;8:1650–80. doi: 10.3390/md8051650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CL, Doucette GJ. A receptor binding assay for paralytic shellfish poisoning toxins: recent advances and applications. Natural Toxins. 1999;7:393–400. doi: 10.1002/1522-7189(199911/12)7:6<393::aid-nt82>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Price GD, Badger MR, Woodger FJ, Long BM. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. Journal of Experimental Botany. 2008;59:1441–1461. doi: 10.1093/jxb/erm112. [DOI] [PubMed] [Google Scholar]
- Richards RP, Baker DB, Crumrine JP. Improved water quality in Ohio tributaries to Lake Erie: A consequence of conservation practices. Journal of Soil and Water Conservation. 2009;64:200–211. [Google Scholar]
- Richards RP, Baker DB. Trends in Water Quality in LEASEQ Rivers and Streams (Northwestern Ohio), 1975-1995. Journal of Environmental Quality. 2002;31:90–96. doi: 10.2134/jeq2002.9000. [DOI] [PubMed] [Google Scholar]
- Richards RP, Baker DB, Crumrine JP, Kramer JW, Ewing DE, Merryfield BJ. Thirty-year trends in suspended sediments in seven Lake Erie tributaries. Journal of Environmental Quality. 2008;37:1894–1908. doi: 10.2134/jeq2007.0590. [DOI] [PubMed] [Google Scholar]
- Rinta-Kanto JM, Ouellette AJA, Boyer GL, Twiss MR, Bridgeman TB, Wilhelm SW. Quantification of toxic Microcystis spp. during the 2003 and 2004 blooms in western Lake Erie using quantitative real-time PCR. Environmental Science and Technology. 2005;39:4198–4205. doi: 10.1021/es048249u. [DOI] [PubMed] [Google Scholar]
- Rinta-Kanto JM, Konopka EA, DeBruyn JM, Bourbonniere RA, Boyer GL, Wilhelm SW. Lake Erie Microcystis: relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae. 2009;8:665–673. [Google Scholar]
- Robarts RD, Zohary T. Temperature effects on photosynthetic capacity, inspiration and growth rates of bloom forming cyanobacteria. New Zealand Journal of Marine and Freshwater Research. 1987;21:391–399. [Google Scholar]
- Roberts TL. Fertilizer Best Management Practices. First edition. International Fertilizer Industry Association; Paris, France: 2007. Right product, right rate, right time and right place… the foundation of best management practices for fertilizer; pp. 29–32. [Google Scholar]
- Robidart JC, Church MJ, Ryan JP, Ascani F, Wilson ST, Bombar D, Marin R, Richards KJ, Karl DM, Scholin CA, Zehr JP. Ecogenomic sensor reveals controls on N2-fixing microorganisms in the North Pacific Ocean. The ISME Journal. 2014;8:1175–1185. doi: 10.1038/ismej.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegner AF, Brena B, Gonzalez-Sapienza G, Puschner B. Microcystins in potable surface waters: toxic effects and removal strategies. Journal of Applied Toxicology. 2014;34:441–457. doi: 10.1002/jat.2920. [DOI] [PubMed] [Google Scholar]
- Rohrlack T, Dittmann E, Börner T, Christoffersen K. Effects of cell-bound microcystins on survival and feeding of Daphnia spp. Applied and Environmental Microbiology. 2001;67:3523–3529. doi: 10.1128/AEM.67.8.3523-3529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JP, McManus MA, Kudela RM, Lara Artigas M, Bellingham JG, Chavez FP, Doucette G, Foley D, Godin M, Harvey JBJ, Marin R, et al. Boundary influences on HAB phytoplankton ecology in a stratification-enhanced upwelling shadow. Deep-Sea Research II. 2014;101:63–79. [Google Scholar]
- Scavia D, Allan JD, Arend KK, Bartell S, Beletsky D, Bosch NS, Brandt SB, Briland RD, Daloğlu I, DePinto JV, Dolan DM, et al. Assessing and addressing the re-eutrophication of Lake Erie: Central Basin Hypoxia. J Great Lakes Research. 2014;40:226–246. [Google Scholar]
- Schindler DW. Evolution of phosphorus limitation in lakes. Science. 1977;195:260–262. doi: 10.1126/science.195.4275.260. [DOI] [PubMed] [Google Scholar]
- Schipanski ME, Barbercheck M, Douglas MR, Finney DM, Haider K, Kaye JP, Kemanian AR, et al. A framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agricultural Systems. 2014;125:12–22. [Google Scholar]
- Seltenrich N. Keeping tabs on HABs: New tools for detecting, monitoring, and preventing harmful algal blooms. Environmental Health Perspectives. 2014a;122:A206–A213. doi: 10.1289/ehp.122-A206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltenrich N. Remote-sensing applications for environmental health research. Environmental Health Perspectives. 2014;122:A268–A275. doi: 10.1289/ehp.122-A268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Gray DK, Read JS, O’Reilly CM, Schneider P, Qudrat A, et al. A global database of lake surface temperatures collected by in situ and satellite methods from 1985–2009. Scientific Data. 2015;2 doi: 10.1038/sdata.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpley AN. Depth of surface soil-runoff interaction as affected by rainfall, soil slope and management. Journal of the Soil Science Society of America. 1985;49:1010–1015. [Google Scholar]
- Sharpley AN. Soil mixing to decrease surface stratification of phosphorus in manured soils. Journal of Environmental Quality. 2003;32:1375–1384. doi: 10.2134/jeq2003.1375. [DOI] [PubMed] [Google Scholar]
- Sheriff G. Efficient waste? Why farmers over-apply nutrients and the implications for policy design. Applied Economic Perspectives and Policy. 2005;27:542–557. [Google Scholar]
- Simm S, Keller M, Selymesi M, Schleiff E. The composition of the global and feature specific cyanobacterial core-genomes. Frontiers in Microbiology. 2015;6:219. doi: 10.3389/fmicb.2015.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Little JC. Designing hypolimnetic aeration and oxygenation systems—a review. Environonmental Science and Technology. 2006;40:7512–7520. doi: 10.1021/es060069s. [DOI] [PubMed] [Google Scholar]
- Smith DR, King KW, Johnson L, Francesconi W, Richards P, Baker D, Sharpley AN. Surface runoff and tile drainage transport of phosphorus in the midwestern United States. Journal of Environmental Quality. 2014;44:495–502. doi: 10.2134/jeq2014.04.0176. [DOI] [PubMed] [Google Scholar]
- Smith DR, King KW, Williams MR. What is causing harmful algal blooms in Lake Erie? Journal of Soil and Water Conservation. 2015;70:27A–29A. [Google Scholar]
- Sohngen B, King KW, Howard G, Newton J, Forster DL. Nutrient prices and concentrations in Midwestern agricultural watersheds. Ecological Economics. 2015;112:141–149. [Google Scholar]
- Soldat DJ, Petrovac AM. Soil phosphorus levels and stratification as affected by fertilizer and compost applications. Applied Turfgrass Science. 2007 doi: 10.1094/ATS-2007-0815-01-RS. [DOI] [Google Scholar]
- Søndergaard M, Jensen JP, Jeppesen E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia. 2003;506:135–145. [Google Scholar]
- Spears BM, Dudley B, Reitzel K, Rydin E. Geo-engineering in lakes - a call for consensus. Environmental Science & Technology. 2013;47:3953–3954. doi: 10.1021/es401363w. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Singh S, Ahn C-Y, Oh H-M, Asthana RK. Monitoring approaches for a toxic cyanobacterial bloom. Environ Sci Technol. 2013;47:8999–9013. doi: 10.1021/es401245k. [DOI] [PubMed] [Google Scholar]
- Steffen MM, Belisle BS, Watson SB, Boyer GL, Wilhelm SW. Review: Status, causes and consequences of cyanobacterial blooms in Lake Erie. Journal of Great Lake Research. 2014a;40:215–225. [Google Scholar]
- Steffen MM, Zhu Z, McKay RML, Wilhelm SW, Bullerjahn GS. Taxonomic assessment of a toxic cyanobacteria shift during the 2010 bloom in hypereutrophic Grand Lake St. Marys (Ohio, USA) Harmful Algae. 2014b;33:12–18. [Google Scholar]
- Steffen MM, Belisle BS, Watson SB, Bourbonniere RA, Boyer GL, Wilhelm SW. Metatranscriptomic evidence for co-occurring top-down and bottom-up controls on toxic cyanobacterial communities. Applied and Environmental Microbiology. 2015;81:3268–3275. doi: 10.1128/AEM.04101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow CA, Cha Y, Johnson LT, Confesor R, Richards RP. Long-term and seasonal trend decomposition of Maumee River nutrient inputs into western Lake Erie. Environmental Science and Technology. 2015;49:3392–3400. doi: 10.1021/es5062648. [DOI] [PubMed] [Google Scholar]
- Stumpf RP, Wynne TT, Baker DB, Fahnenstiel GL. Interannual variability of cyanobacterial blooms in Lake Erie. PLoS ONE. 2012;7:e42444. doi: 10.1371/journal.pone.0042444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tátrai I, Mátyás K, Korponai J, Paulovits G, Pomogyi P. The role of the Kis-Balaton Water Protection System in the control of water quality of Lake Balaton. Ecological Engineering. 2000;16:73–78. [Google Scholar]
- Tiessen KHD, Elliot JA, Yarotski J, Lobb DA, Flaten DN, Glozier NE. Conventional and conservation tillage: influence on seasonal runoff, sediment, and nutrient losses in the Canadian prairies. Journal of Environmental Quality. 2010;39:964–980. doi: 10.2134/jeq2009.0219. [DOI] [PubMed] [Google Scholar]
- Turner JT, Doucette GJ, Powell CL, Kulis DM, Keafer BA, Anderson DM. Accumulation of red tide toxins in larger size fractions of zooplankton assemblages from Massachusetts Bay, USA. Marine Ecology Progress Series. 2000;203:95–107. [Google Scholar]
- United States Department of Agriculture, National Agricultural Statistics Service. USDA Data Gateway for Ohio. 2006 Available at: www.nass.usda.gov/Research_and_Science/Cropland/metadata/metadata_oh06.htm.
- United States Environmental Protection Agency. Recommended phosphorus loading targets for Lake Erie. Annex 4 Objectives and Targets Task Team Final Report to the Nutrients Annex Subcommittee, Great Lake Water Quality Agreement of 2012. 2015a Available as PDF at www2.epa.gov. [Google Scholar]
- United States Environmental Protection Agency. Preventing eutrophication: scientific support for dual nutrient criteria. EPA Office of Water; Washington DC: 2015b. Document 820-S-15-001. [Google Scholar]
- Vadas PA, Kleinmann PJA, Sharpley AN, Turner BL. Relating soil phosphorus to dissolved phosphorus in runoff: a single extraction coefficient for water quality modeling. Journal of Environmental Quality. 2005a;34:572–580. doi: 10.2134/jeq2005.0572. [DOI] [PubMed] [Google Scholar]
- Vadas PA, Mallarino AP, McFarland A. The importance of sampling depth when testing soils for their potential to supply phosphorus to surface runoff. SERA17; 2005b. Position Paper http://sera17.org/publications/ [Google Scholar]
- Vadas PA, Good LW, Moore PA, Jr, Widman A. Estimating phosphorus loss in runoff from manure and fertilizer for a phosphorus loss quantification tool. Journal of Environmental Quality. 2009;38:1645–1653. doi: 10.2134/jeq2008.0337. [DOI] [PubMed] [Google Scholar]
- Van de Waal DB, Verspagen JMH, Lurling M, van Donk E, Visser PM, et al. The ecological stoichiometry of toxins produced by harmful cyanobacteria: an experimental test of the carbon-nutrient balance hypothesis. Ecology Letters. 2009;12:1326–1335. doi: 10.1111/j.1461-0248.2009.01383.x. [DOI] [PubMed] [Google Scholar]
- Van de Waal DB, Verspagen JM, Finke JF, Vournazou V, Immers AK, Kardinaal WEA, et al. Reversal in competitive dominance of a toxic versus non-toxic cyanobacterium in response to rising CO2. The ISME Journal. 2011;5:1438–1450. doi: 10.1038/ismej.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Waal DB, Smith VH, Declerck SAJ, Stam ECM, Elser JJ. Stoichiometric regulation of phytoplankton toxins. Ecology Letters. 2014;17:736–742. doi: 10.1111/ele.12280. [DOI] [PubMed] [Google Scholar]
- Van der Merwe D, Price KP. Harmful algal bloom characterization at ultra-high spatial and temporal resolution using small unmanned aircraft systems. Toxins. 2015;7:1065–1078. doi: 10.3390/toxins7041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhamme E, DePinto JV, Redder T, Schlea D. Western Lake Erie Ecosystem Model - connecting phosphorus loads to HABs biomass. International Association for Great Lakes Research Annual Conference; Burlington, VT: 2015. http://www.limno.com/pdfs/2015_IAGLR_Verhamme.pdf. [Google Scholar]
- Verspagen JMH, Van de Waal DB, Finke JF, Visser PM, Van Donk E, Huisman J. Rising CO2 levels will intensify phytoplankton blooms in eutrophic and hypertrophic lakes. PloS One. 2014b;9(8):e104325. doi: 10.1371/journal.pone.0104325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent RK, Qin X, McKay RML, Miner J, Czajkowski K, Savino J, Bridgeman T. Phycocyanin detection from LANDSAT TM data for mapping cyanobacterial blooms in Lake Erie. Remote Sensing of the Environment. 2004;89:381–392. [Google Scholar]
- Visser PM, Ibelings BW, Bormans M, Huisman J. Artificial mixing to control cyanobacterial blooms: a review. In: Visser PM, Ibelings BW, Fastner J, Bormans M, editors. Aquatic Ecology Special Issue ‘Cyanobacterial blooms. Ecology, prevention, mitigation and control.’. 2015. [DOI] [Google Scholar]
- Visser PM, Verspagen JMH, Sandrini G, Stal LG, Matthijs HCP, Davis TW, Paerl HW, Huisman J. Effects of rising CO2 and global warming on harmful cyanobacteria. Harmful Algae. doi: 10.1016/j.hal.2015.12.006. this issue. [DOI] [PubMed] [Google Scholar]
- Vitosh ML, Johnson JL, Mengel DB. Extension Bulletin E-2567. Michigan State University, The Ohio State University, Purdue University: 1995. Tri-State Fertilizer Recommendations for Corn, Soybeans, Wheat and Alfalfa. [Google Scholar]
- Wang YT, Zhang TQ, Hu QC, Tan CS, O’Halloran IP, Drury CF, Reid DK, Ma BL, Ball-Coelho B, Lauson JD, Reynolds WD, et al. Estimating dissolved reactive phosphorus concentration in surface runoff water from major Ontario soils. Journal of Environmental Quality. 2010;39:1771–1781. doi: 10.2134/jeq2009.0504. [DOI] [PubMed] [Google Scholar]
- Weenink EFJ, Luimstra VM, Schuurmans JM, Van Herk MJ, Visser PM, Matthijs HCP. Combatting cyanobacteria with hydrogen peroxide: a laboratory study on the consequences for phytoplankton community and diversity. Frontiers in Microbiology. 2015;6:714. doi: 10.3389/fmicb.2015.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm SW, DeBruyn JM, Gillor O, Twiss MR, Livingston K, Bourbonniere RA, Pickell LD, Trick CG, Dean AL, McKay R Michael L. Effect of phosphorus amendments on present day plankton communities in pelagic Lake Erie. Aquatic Microbial Ecology. 2003;32:275–285. [Google Scholar]
- Williams MR, King KW, Dayton E, LaBarge GA. Sensitivity analysis of the Ohio phosphorus risk index. Transactions of the American Society of Agricultural and Biological Engineers. 2015;58:93–102. [Google Scholar]
- Wilson EK. Danger from microcystins in Toledo water unclear. Chemical & Engineering News. 2014;92:9. [Google Scholar]
- Witter D, Ortiz JD, Palm S, Heath R, Budd J. Assessing the application of SeaWiFS ocean color algorithms to Lake Erie. Journal of Great Lakes Research. 2009;35:361–370. [Google Scholar]
- Wynne TT, Stumpf RP, Briggs TO. Comparing MODIS and MERIS spectral shapes for cyanobacterial bloom detection. International Journal of Remote Sensing. 2013;34:6668–6678. [Google Scholar]
- Zilliges Y, Kehr J-C, Meissner S, Ishida K, Mikkat S, Hagemann M, Kaplan A, Börner T, Dittmann E. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE. 2011;6:e17615. doi: 10.1371/journal.pone.0017615. [DOI] [PMC free article] [PubMed] [Google Scholar]