Abstract
In previous experiments on contextual memory, we proposed that the unreinforced re-exposure to the learning context (conditioned stimulus, CS) acts as a switch guiding the memory course toward reconsolidation or extinction, depending on reminder duration. This proposal implies that the system computes the total exposure time to the context, from CS onset to CS offset, and therefore, that the reminder presentation must be terminated for the switching mechanism to become operative. Here we investigated to what extent this requirement is necessary, and we explored the relation between diverse phases in the reconsolidation and extinction processes. We used the contextual memory model of the crab Chasmagnathus which involves an association between the learning context (CS) and a visual danger stimulus (unconditioned stimulus, US). Administration of cycloheximide was used to test the lability state of memory at different time points. The results show that two factors, no-reinforcement during the reminder (i.e., CS re-exposure) and CS offset are the necessary conditions for both processes to occur. Regardless of the reminder duration, memory retrieved by unreinforced CS re-exposure emerges intact and consolidated when tested before CS offset, suggesting that neither reconsolidation nor extinction is concomitant with CS re-exposure. Either process could only be triggered once the definitive mismatch between CS and US is confirmed by CS termination without the expected reinforcement.
According to the reconsolidation hypothesis, memory recalled by the presentation of a reminder enters a vulnerability phase (labilization) during which it is transiently sensitive to disruption, followed by a process of stabilization (reconsolidation) that returns memory to the former consolidated state (Nader et al. 2000a; Sara 2000a; Debiec et al. 2002; Pedreira et al. 2002). This hypothesis, initially supported by results obtained with rodents, was then confirmed with chick, fish, freshwater snail, and crab, all results showing that de novo protein synthesis was necessary (Nader et al. 2000a; Anokhin et al. 2002; Eisenberg et al. 2003; Pedreira and Maldonado 2003; Sangha et al. 2003). Therefore, learned behaviors of phylogenetically very diverse species proved to share the phenomenology as well as its molecular requirements, and such demonstration of universality provides a strong support for the hypothesis. However, other results with rodents conflicted with these findings, showing extinction instead of reconsolidation after reminder presentation (Berman and Dudai 2001; Vianna et al. 2001). The conflicting evidence of experimental outcomes nevertheless appears reconciled by our recent results with the crab model of contextual learning, concerning the relationship between reconsolidation and extinction (Pedreira and Maldonado 2003). It was demonstrated that crab re-exposure to the learning context (conditioned stimulus, CS) for a short time (5 to 40 min) induces labilization-reconsolidation, whereas re-exposure for a longer time (one or more hours) induces extinction, both depending on de novo protein synthesis. Based on these findings, we proposed that reminder (i.e., CS re-exposure) duration acts as a switch guiding the memory course toward reconsolidation (short reminder) or extinction (long reminder; Pedreira and Maldonado 2003). This proposal assumes that the system computes total exposure time to the context (the CS duration), from CS onset to CS offset, and therefore, that the reminder presentation must be terminated for the switching mechanism to become operative.
Here we investigated to what extent this requirement is necessary for triggering either reconsolidation or extinction. We also explored the relations between phases of the retrieval-labilization-reconsolidation sequence as well as those of the retrieval-extinction-consolidation sequence. The crab model of contextual learning is based on the crab's escape response elicited by the presentation of a visual danger stimulus (VDS; Maldonado 2002). The crab's escape response declines during repeated presentation of a VDS, and a strong freezing-to-VDS is built up which persists over time. This long-term memory implies an association between learning context (conditioned stimulus, CS) and the VDS (unconditioned stimulus, US), and is termed context-signal memory. Consolidation of this memory requires de novo protein synthesis (Pedreira et al. 1995, 1996), and is mediated by the cAMP signal pathway (Romano et al. 1996; Locatelli et al. 2002), by NFκB transcription factor (Freudenthal and Romano 2000; Merlo et al. 2002), and by NMDA-like glutamatergic receptors (Troncoso and Maldonado 2002).
RESULTS
Analysis of the Retrieval-Labilization-Reconsolidation Sequence
The first series of experiments (Figs. 1, 2) was focused on studying the retrieval-labilization-reconsolidation sequence. For revealing the lability state of memory at diverse time points, we took advantage of the fact that 15 μg of cycloheximide (CHX) per crab inhibits ∼90% of protein synthesis for 2 h (Pedreira et al. 1995) and blocks reconsolidation (Pedreira et al. 2002; Pedreira and Maldonado 2003).
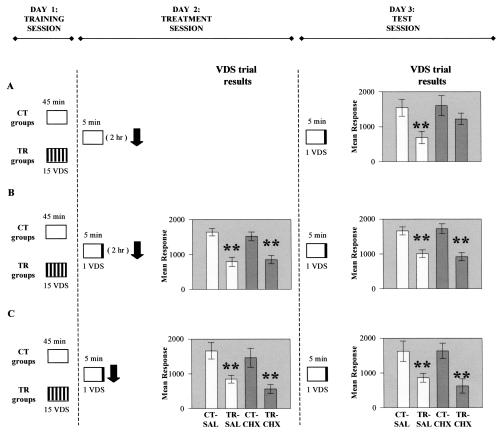
Figure 1.
Reconsolidation is triggered after completion of short unreinforced context re-exposure. Experimental protocols and results. Day 1 (Training session): (A,B,C) Trained (TR) groups received 15 trials of the visual danger stimulus (VDS), intertrial interval: 3 min; control (CT) groups remained in the context for the entire session (45 min). Day 2 (Treatment session): (A) No VDS during context re-exposure; saline (SAL) injection to one-half of the CT-TR groups and cycloheximide (CHX) to the other half, given 2 h after re-exposure. (B) As in A but VDS during re-exposure. (C) As in B but SAL or CHX injections immediately after re-exposure. Day 3 (Test session): (A,B,C) One test trial. Open boxes represent context exposure; a set of several black bars inside the box represents 15 VDS presentations; only one black bar indicates one VDS during the last minute of exposure; a black arrow for saline (SAL) or cycloheximide (CHX) injection; numbers on the boxes indicate duration of the context exposure; numbers in brackets indicate time interval in hours. VDS trial (results) on Day 2 and 3: graph ordinates: mean response to VDS presentation ± SEM in arbitrary units; white bars for CT and TR groups, both SAL-injected; gray bars for CT and TR groups, both CHX-injected. **, P < 0.01.
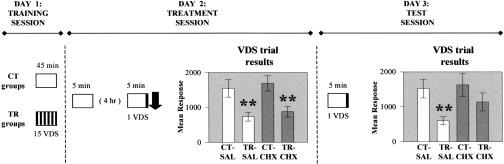
Figure 2.
Double context re-exposure. Experimental protocol and results. Day 1 (Training session) and Day 3 (Test session) as in Figure 1. Day 2 (Treatment session): Animals twice reexposed for 5 min: first time without VDS and second time, 4 h later, with VDS presentation. Once removed from container for the second time, animals were SAL- or CHX-injected. Symbols and VDS trial (results) on Days 2 and 3 as in Figure 1.
In Experiment A (Fig. 1A), trained (TR) and control (CT) groups were reexposed on Day 2 for 5 min to the learning context (CS) without reinforcement (VDS, i.e., the US), and injected 2 h later with saline (SAL) or cycloheximide (CHX). A significant control versus trained response difference (CT > TR) was found at test trial on Day 3 for the SAL group but not for the CHX group [ANOVA F(3,116) = 3.52, P < 0.01; P < 0.001 for SAL, P = 0.23 for CHX]. In addition, no significant difference between SAL and CHX control groups was disclosed, a result confirmed throughout this study for all comparisons of control groups in the same experiment. Thus, context signal memory, defined by the significant difference CT > TR, is abolished when CHX is injected 2 h after the end of unreinforced CS re-exposure. This finding is in line with previous ones showing memory impairment when the drug was given 1 h before and up to ∼5 h after 5 min re-exposure, that is, within the time window of susceptibility to CHX (Pedreira et al. 2002; Pedreira and Maldonado 2003). In Experiment B (Fig. 1B), the VDS was presented during the last minute of context re-exposure on Day 2. Both SAL and CHX groups showed retention (CT > TR) at both the VDS trial on Day 2 [ANOVA F(3,156) = 13.10, P < 10-6; P < 0.0001 for SAL and CHX] and the VDS trial on Day 3 [ANOVA F(3,156) = 12.26, P < 10-6; P < 0.0001 for SAL and CHX]. Hence, the CHX injection had no effect though given within its time window, suggesting that no reconsolidation, and thus no labilization, is produced by the 5-min CS re-exposure when it includes a VDS trial (US). However, our hypothesis could be challenged by arguing that CS re-exposure actually produced labilization from CS onset (crab placement in the container) but that US presentation before CS offset (crab removal) induced an accelerated reconsolidation process, so that CHX after 2 h arrived too late to disrupt memory. This alternative interpretation was tested in Experiment C (Fig. 1C), by injecting CHX immediately after CS offset, and, as in Experiment B, memory retention (CT > TR) was shown at the VDS trial on both Day 2 [ANOVA F(3,116) = 6.79, P < 0.001; P < 0.01 for SAL and CHX] and Day 3 [ANOVA F(3,116) = 5.62, P < 0.01; P < 0.01 for SAL and CHX]. Therefore, memory tested at the last minute of learning context re-exposure remains intact (i.e., memory retention was displayed) and consolidated (i.e., subsequent injection of CHX had no effect). This result rules out the hypothesis of a labilization triggered by CS onset and reconsolidated by VDS before CS offset. In short, memory labilization appears to be strictly dependent on the fulfillment of two conditions: first, the closure of the learning context re-exposure (CS offset) and second, the absence of VDS (US) during the entire CS presentation. In other words, labilization is not brought on by CS onset (retrieval) but by CS offset plus lack of reinforcement.
The foregoing interpretation leads to the following prediction. The CS re-exposure with US (which above showed memory intact and consolidated) would show memory intact but labile if it were preceded by a CS re-exposure without a US, that is, by a previous unreinforced CS re-exposure that triggers reconsolidation. This prediction was confirmed by the results of the experiment illustrated in Figure 2. All groups were twice exposed to CS for 5 min, separated by a 4-h interval: the first time without the VDS but the second with the VDS at the last minute. SAL or CHX was injected immediately after the CS offset of the second CS exposure, that is, within the window of susceptibility initiated by the CS offset of the first CS exposure. Memory emerged intact (CT > TR) for both the SAL and CHX groups at the VDS trial on Day 2 [ANOVA F(3,124) = 5.97, P < 0.001; P < 0.01 for SAL and CHX], but impaired (CT ≅ TR) only for the CHX groups, at the VDS trial on Day 3 [ANOVA F(3,124) = 3.49, P < 0.02; P < 0.01 for SAL, P = 0.16 for CHX]. Therefore, in keeping with the prediction, memory at the last minute of the second re-exposure remained intact but labile, as a consequence of the previous unreinforced re-exposure.
Analysis of the Retrieval-Extinction-Consolidation of Extinction Sequence
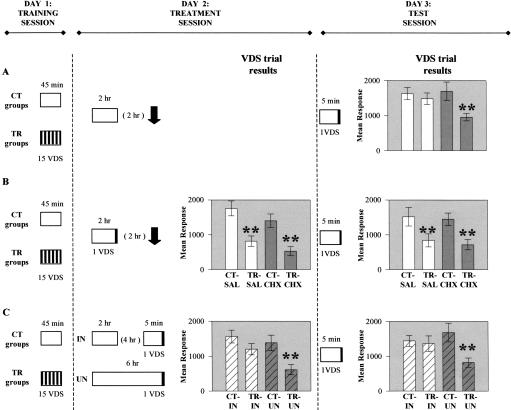
To explore the issues under study in connection with extinction, a second series of three experiments was carried out. The first two experiments (Fig. 3A,B) were similar to those of the first series but with a learning context re-exposure of 2 h instead of 5 min. Results of Experiment A at test trial on Day 3 (Fig. 3A) showed extinction (CT ≅ TR) for SAL groups but retention for CHX groups [ANOVA F(3,116) = 4.83, P < 0.01; P = 0.5 for SAL, P < 0.001 for CHX]. Thus, memory extinguishes when CS re-exposure lasts 2 h, but extinction is abolished by CHX injected 2 h after CS offset. This result is consistent with a previous one demonstrating that extinction induced by 1 h of context re-exposure is blocked by CHX given 1 h before or less than 5 h after context re-exposure (Pedreira and Maldonado 2003). In Experiment B (Fig. 3B), a single VDS trial was included during the last minute of CS re-exposure, and both SAL and CHX groups showed retention (CT > TR) at the VDS trial both on Day 2 [ANOVA F(3,128) = 10.1, P < 5×10-5; P < 0.001 for SAL and CHX] and on Day 3 [ANOVA F = 4.25, P < 0.01; P < 0.01 for SAL and CHX]. These results agree with those following 5-min learning context re-exposure (Fig. 1B): CHX injection has no effect though given within the time window of susceptibility, indicating that inclusion of a reinforcement during CS exposure leaves the old memory not only intact but also insensitive to CHX, that is, intact and consolidated. However, this lack of extinction could be accounted for in terms of the old memory reinstatement by the reinforcer, that is, recovery of behavior when the subject is exposed to US after extinction (Bouton 2002). To address this alternative explanation, Experiment C (Fig. 3C) was performed. A trained group and its respective control received on Day 2 a context re-exposure of 2 h without reinforcement, followed 4 h later by 5-min re-exposure with a VDS during the last minute (interrupted groups, IN). Instead, other TR-CT groups were context-reexposed on Day 2 for 6 h and received a VDS trial during the last minute of this long period (uninterrupted groups, UN). Extinction was shown in IN groups but not in UN groups at the VDS trial on both Day 2 [ANOVA F(3,156) = 5.09, P < 0.01; P = 0.16 for IN, P < 0.01 for UN] and Day 3 [ANOVA F(3,156) = 3.21, P < 0.02; P = 0.79 for IN, P < 0.01 for UN]. Thus, a VDS trial given 4 h after the CS offset that follows the first 2-h CS re-exposure (i.e., 6 h after the first CS onset) shows extinction, whereas the same trial given simultaneously but during the last minute of an uninterrupted 6-h re-exposure shows retention. Therefore, extinction, as reconsolidation, is only produced by the interaction of CS offset with lack of reinforcement; that is, extinction, as reconsolidation, is induced not by CS onset (retrieval) but by CS offset of an unreinforced reminder.
Figure 3.
Extinction is triggered after completion of long unreinforced context re-exposure. Experimental protocols and results. Day 1 (Training session) and Day 3 (Test session): (A,B,C) As in Figure 1. Day 2 (Treatment session): (A,B) As in Figure 1. (C) Half of the CT-TR groups follow the interrupted (IN) protocol: context re-exposed for 2 h, then removed, and 4 h later replaced in the context for 5 min with VDS at last minute; and the other half, the uninterrupted (UN) protocol: 6 h of re-exposure with VDS at last minute. Symbols as in Figure 1. VDS trial (results) on Days 2 and 3: Ordinates: mean response to VDS presentation ± SEM in arbitrary units; striped white bars represent CT or TR groups that followed IN protocol; striped gray bars represent CT or TR groups that followed UN protocol.
DISCUSSION
Two main conclusions stem from the present results: (1) no-reinforcement must be included during the entire CS presentation for either labilization-reconsolidation or extinction to become operative, and (2) both processes are dependent on CS offset. Therefore, at odds with the usual view, retrieval per se is unable to induce labilization of the old memory or to initiate extinction, that is, formation of a new memory that hinders the expression of the old one (extinction; Brooks and Bouton 1994).
Concerning extinction, the no-reinforcement requirement is demonstrated by the fact that retention of the old memory is disclosed at testing on Day 3 when a VDS trial is given at the last minute of a 2-h or 6-h re-exposure on Day 2 (SAL groups in Fig. 3B; UN groups in Fig. 3C). On the other hand, the conclusion that memory extinction depends on CS termination, namely, that the extinction process is not triggered by CS onset, is confirmed by several results from present and previous experiments. In the present study, extinction is shown by a VDS test trial during a 5-min re-exposure on Day 2, when such re-exposure is preceded by an unreinforced 2-h CS re-exposure, 4 h apart (IN groups, Fig. 3C), but not by a VDS test trial at the last minute of an uninterrupted 6-h re-exposure (UN groups, Fig. 3C). Incidentally, such results rule out an explanation of above findings as to the no-reinforcement requirement in terms of reinstatement effect (i.e., conditioned responding return after mere exposure to US; Bouton 2002). Moreover, a reiterated finding of our laboratory demonstrates that no extinction is disclosed despite a long CS exposure (12 or 24 h) if memory is tested before CS offset (Lozada et al. 1990; Tomsic et al. 1998), but extinction is shown if the animal is moved to another context for a while between CS re-exposure and test trial (Tomsic et al. 1998).
Concerning reconsolidation, the no-reinforcement requirement is mainly inferred from the finding that memory appears intact and consolidated at the testing on Day 3, despite 90% CHX-induced inhibition of protein synthesis on Day 2, only when a reinforcement is included (Fig. 1B,C). Cycloheximide was administered 2 h after, or even immediately after CS offset, which makes untenable an interpretation of the result in terms of US-induced accelerated reconsolidation. An alternative explanation for this failure of the CHX amnesic effect is that the VDS presentation (US) during re-exposure causes a new round of CS-US learning and formation of a new memory trace (Nadel and Land 2000). Therefore, the 15-μg CHX dose that proves sufficient to impair consolidation alone (Pedreira et al. 1995) or reconsolidation alone (Pedreira et al. 2002; Pedreira and Maldonado 2003) may now be insufficient to interfere with reconsolidation of the old memory plus consolidation of a new trace, both occurring at the same time. That is, addition of one VDS trial would require more protein synthesis and hence, the usually effective CHX dose would not be enough. Regardless of agreeing with the view that a higher requirement of de novo protein synthesis corresponds to a lower efficiency of the same inhibitor dose, the alternative interpretation seems to be at variance with previous results from our laboratory. Although 15 μg CHX blocks consolidation of 15 VDS trials, 10 μg is enough to block consolidation of 30 trials (Pedreira et al. 1995). Furthermore, 15 or 30 VDS trials induce, after training and for time periods similar to those for the protein synthesis requirement, a similar activation of the transcription factor NFκB, whereas no significant activation is detected after one to 10 trials (Freudenthal and Romano 2000).
The conclusion that reconsolidation is triggered by CS offset stems from results shown in Figure 1A-C. These experiments show that memory at the last minute of the CS re-exposure on Day 2 is still intact and consolidated, thus ruling out the possibility that the labilization-reconsolidation process starts as early as CS onset (retrieval). Findings given in Figure 2 lend support to the same conclusion. In this experiment, a first 5-min re-exposure without reinforcement was followed, after a 4-h temporal interval, by a second 5-min re-exposure but with a VDS trial at the last minute. Results indicate that memory at the VDS trial of the second re-exposure emerges intact but labile, because the subsequent CHX injection blocks memory (test on Day 3). Thus, the CHX administration that proved to be without effect after reinforced re-exposure (Fig. 1B,C) here becomes efficient because the reinforced re-exposure is now preceded by the termination of an unreinforced re-exposure that triggers reconsolidation. However, another explanation of this finding could be posited. The experimental design shown in Figure 2 includes two CS onsets within the time window of susceptibility to the protein synthesis inhibitor, and it has been shown that a modification in the number of retrieval trials may influence the action of amnesic agents (e.g., Eisenberg et al. 2003). Therefore, the fact that CHX given after a reinforced CS re-exposure becomes efficient could be straightforwardly accounted for by the addition of a CS onset, regardless of the fact that the two re-exposures are not equivalent. Nonetheless, an increase in the number of retrieval trials is generally linked to a decrease in the sensitivity of the old trace to the consolidation blockers. In contrast, here the addition of an unreinforced context re-exposure to a reinforced one is followed by higher susceptibility to the protein synthesis inhibitor.
Grounded on the above conclusions, the following interpretative scheme is offered. Retrieved memory entails recovery of an organized knowledge (the context-signal memory) that implies predictions about reality (Dudai 2002), as it might be, in the present experiments, the prediction that VDS is coming. The interval between retrieval and CS offset is an expectation time during which the system computes the passage of time and the retrieved memory remains intact and consolidated. The completion of the CS re-exposure without reinforcement signals the irreversible mismatch between what was expected and what actually occurred. Should this confirmation of the mismatch come after a short expectancy time, then old memory is labilized, but should it occur after a long expectancy time, old memory is extinguished. Thus, the reconsolidation-or-extinction switch works at a key time point, that is, when the nonoccurrence of the expected reinforcement is definitely confirmed by the CS termination.
The offset of the unreinforced CS re-exposure signals a clear-cut differentiation between the first phase of the sequence, which includes retrieval and time computation, and the following phase, be it labilization-reconsolidation or extinction-consolidation. A caveat, however, is pertinent concerning the processes of retrieval and labilization. In our interpretative scheme, retrieval occurs in the first phase of the sequence triggered by CS onset, but nothing could be stated about how long retrieval is operating, as virtually nothing is known about the mechanisms that subserve it. The prevailing view is that retrieval would be an almost instantaneous episode, though it is likewise arguable that retrieval could last for minutes or more (Sara 2000a). On the other hand, labilization comes in our model after CS offset and is logically a necessary condition for reconsolidation, but as for retrieval, we have no information about duration, underlying mechanisms, or the kinetics linking labilization and reconsolidation. Regarding extinction, results from present experiments and from several previous reports from our laboratory (Lozada et al. 1990; Tomsic et al. 1998) suggest that the new memory (extinction) is built up not during the learning context re-exposure but rather after its termination. We demonstrate that this lack of extinction within the re-exposure interval could not be explained in terms of reinstatement by the reinforcer. In regard to the effect of cycloheximide, extinction is abolished when the drug is given 2 h after unreinforced CS offset (present results) or 1 h before (Pedreira and Maldonado 2003); that is, the old memory emerges intact. However, it is not possible to determine whether the drug effect is on extinction itself, that is, on acquisition of the new memory, or on consolidation of such acquisition, or on both.
At this juncture, it seems pertinent to ask for the probable biological meaning of labilization-reconsolidation after the offset of the unreinforced CS. We assume that a mismatch between what was expected and what actually occurred could result from a failed prediction. A wide range of memory flaws could account for such a failure, ranging from outdated to faulty or incomplete information. Therefore, it seems reasonable to suppose, in agreement with several authors (Nader et al. 2000b; Sara 2000b), that labilization-reconsolidation plays a repair role by enabling the system to integrate new information on the background of the past. This memory repair mechanism would not entail an obligatory phase of every retrieved memory but a mechanism of exception, triggered by the termination of an unreinforced CS and acting only after a short expectancy time. It would not work for cases where memory proved to be successful, as when reinforcement follows retrieval, or after a long expectancy time that installs a new memory but leaves the old memory intact though unexpressed (Brooks and Bouton 1994).
Our conclusions based on the present findings were drawn from experiments of contextual conditioning, but further studies would be required to test their validity in other models of CS-US associative memory, as cued conditioning. In this type of paradigm, the CS-US acquisition depends on the presentation of one or more trials, each including a punctuated CS paired with US, whereas the reminder consists of a trial similar to that of training but ending without the US. Eisenberg et al. (2003) reported, in experiments with the medaka fish, that a single reminder trial induces reconsolidation of the cued fear memory but 10 reminder trials result in massive extinction. These results could be interpreted as in keeping with those we previously reported (Pedreira and Maldonado 2003), as both studies showed that each process (reconsolidation and extinction) is selectively engaged by a different extension of the reminder. However, it is necessary to determine whether our interpretative model of a switching mechanism based on time computation of CS could be extended to paradigms of cued conditioning. It is possible to assume that in these paradigms, the course of memory toward labilization or extinction depends on the total expectancy time accumulated through successive unreinforced episodes, or on the number of such episodes, or on a more complicated algorithm that allows integration of the effect of successive unreinforced trials.
MATERIALS AND METHODS
Animals
Animals were adult male Chasmagnathus crabs 2.7-3.0 cm across the carapace, weighing ∼17.0 g, collected from water less than 1 m deep in the rías (narrow coastal inlets) of San Clemente del Tuyú, Argentina, and transported to the laboratory, where they were lodged in plastic tanks (35 × 48 × 27 cm) filled to 2 cm depth with diluted marine water, to a density of 20 crabs per tank. Water used in tanks and other containers during experiments was prepared using hw-Marinex (Winex-Germany), salinity 10‰-14‰, pH 7.4-7.6, and maintained within a range of 22°-24°C. The holding and experimental rooms were maintained on a 12-h light-dark cycle (light on 07:00-19:00 h). Animals were fed rabbit pellets (Nutrientes) every 3 d, and after feeding the water was changed. Experiments were carried out within 10 d after the animals' arrival, from January to August, and between 08:00 and 18:00 h. Each crab was used in only one experiment. Experimental procedures are in compliance with the policies on the use of Animals and Humans in Neuroscience Research.
The Experimental Device (the Actometer)
The actometer (Maldonado 2002) consisted of a bowl-shaped opaque container with a steep concave wall 12 cm high (23 cm top dia and 9 cm floor dia) covered to a depth of 0.5 cm with artificial seawater, where the crab was lodged before each experiment. During each trial of 9 sec, an opaque rectangular screen (a strip 25 × 7.5 cm), termed the visual danger stimulus (VDS), was moved horizontally over the animal, cyclically from left to right and vice versa. The VDS provoked an escape response by the crab and consequent container vibrations, which were converted into electrical signals through a piezoelectric transducer placed on the external wall of the actometer. These signals were amplified, integrated during each 9-sec trial, and translated into arbitrary numerical units ranging from 0 to 6000, before being processed by computer. The activity of every crab was recorded during each entire trial time. The experimental room had 40 actometers, separated from each other by partitions.
Experimental Procedure and Design
Each experiment lasted 3 d and included three phases: the training session, treatment session, and test session, each corresponding to 1 d. Control (CT) or trained (TR) groups of 30-40 crabs each were used in each experiment.
Day 1: Training Session
Control animals (CT) were kept in the container of the actometer during the entire training session (∼50 min) without being trained, that is, without being presented the visual danger stimulus (VDS). Trained animals (TR), after being in the container for 5 min without VDS, received 15 training trials, each consisting of a 9-sec VDS presentation, separated by intertrial intervals of 3 min. The actometer container used during the training session is referred to as the learning context. Immediately after the training session, both CT and TR crabs were moved from the learning context and housed individually in the resting containers, that is, plastic boxes covered to a depth of 0.5 cm with water and kept inside dimly lit drawers.
Day 2: Treatment Session
Crabs were exposed to the learning context for 5 min, 2 h, or 6 h, with or without VDS presentation during the last 1 min. An injection with saline (SAL) or cycloheximide solution (CHX) was given immediately after or 2 h after learning context re-exposure. After treatment, crabs were returned to the resting containers.
Day 3: Test Session
All crabs were placed again in the learning context for 5 min and were given one VDS trial (a test trial). Before animals were assigned to an experiment, they underwent a selection test: Each crab was turned onto its back, and only animals that immediately returned to their normal position were used. The rationale behind this selection is that crabs with a slow righting reaction show a low responsiveness to a large diversity of stimuli, and at a later time, they usually present unhealthy symptoms. No more than 5% of tested crabs were discarded in each experiment. If the mean response of the TR group at first training trial was ≤500, the experiment was discontinued and not attempted with other animals from the same capture effort. This drawback is often presented from September to November.
Crabs' baseline responsiveness to the passing screen (VDS) proves remarkably consistent up to 10 d after arrival, but on occasion animals coming from different capture efforts present differences in response level. Therefore, only crabs belonging to the same capture were used in each experiment.
Escape Response and Freezing
The amount of container vibrations during the 9 sec of VDS presentation (a trial) depends on the magnitude of the defensive responses a crab displays when presented with an impeding threat. Two types of defensive responses are distinguished: escape and freezing response (Pereyra et al. 1999, 2000). The escape response is a directional run of the animal in an attempt to move away from the passing screen (VDS), whereas the freezing response consists of a rigid motionless display in which the crab lies flattened on the substratum. During repeated VDS presentations (training), the escape response decreases in intensity and is replaced by the progressive building up of a strong and long-lasting freezing. No defensive responses but exploration or wandering are shown during context exposures without VDS presentation. Throughout this study, data were only recorded during trial periods, that is, during the 9-sec VDS. No spontaneous activity during context exposures was recorded.
Drugs and Injection Procedure
Crustacean saline solution (Hoeger and Florey 1989) was used as vehicle. Fifty μL of saline or cycloheximide solution (15 μg per crab) was given through the right side of the dorsal cephalothoraxic-abdominal membrane, by means of a syringe fitted with a sleeve to control depth of penetration to 4 mm, thus ensuring that the injected solution was released in the pericardial sac. Cycloheximide was purchased from Sigma.
Data Analysis
Throughout this study, data analysis was aimed at testing a basic prediction stemming from our extensive work on the crab's context-signal memory (CSM). Namely, animals given 15 or more training trials with 3 min of intertrial interval (trained crabs, TR) show, at a test trial given up to 1 wk later, a level of response noticeably lesser than that of animals that received the same treatment but were untrained (control crabs, CT). Such a significant difference (P < 0.01) is invariably found, even when crabs were saline-injected pre- or posttraining, provided that the following requirements were fulfilled: Each group consisted of 30 or more individuals, the mean response of the TR group was ≥500, the groups were run simultaneously, and all animals came from the same capture effort. Therefore, a trained group is said to show context-signal memory retention when the basic assumption is confirmed. Rescorla (1988) convincingly argued in favor of this sort of analysis in which comparisons are confined to testing results, instead of using paired training-testing contrasts, stressing the need to clearly distinguish between time of input (training session) and time of assessment (test session).
Because all of the analysis is grounded on the CT > TR prediction, a test of a priori planned comparisons was used (Rosenthal and Rosnow 1985; Howell 1987). In each experiment, which includes two CT-TR pairs of groups, three contrasts were carried out: One comparison was performed between the two CT groups, and the other two between each CT and its respective TR group. No significant difference between CT groups was disclosed throughout this study. Each set of planned comparisons was performed following a significant main effect in one-way analysis of variance (ANOVA; α < 0.05). All response scores are represented as means ± the standard error of the means.
Acknowledgments
This research was supported by Fundación Antorchas, FONCYT (PICT-1-06602) and University of Buenos Aires (X-619). We thank A. Delorenzi, D. Tomsic, and A. Romano for reading the manuscript and for helpful criticism.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.76904.
References
- Anokhin, K.V., Tiunova, A.A., and Rose, S.P. 2002. Reminder effects-reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive avoidance task in young chicks. Eur. J. Neurosci. 15: 1759-1765. [DOI] [PubMed] [Google Scholar]
- Berman, D.E. and Dudai, Y. 2001. Memory extinction, learning anew, and learning the new: Dissociations in the molecular machinery of learning in cortex. Science 291: 2417-2419. [DOI] [PubMed] [Google Scholar]
- Bouton, M. 2002. Context, ambiguity, and unlearning: Source of relapse after behavioural extinction. Biol. Psychiatry 52: 976-986. [DOI] [PubMed] [Google Scholar]
- Brooks, D.C. and Bouton, M.E. 1994. A retrieval cue for extinction attenuates response recovery (renewal) caused by return to the conditioning context. J. Exp. Psychol. Anim. Behav. Process 20: 366-379. [DOI] [PubMed] [Google Scholar]
- Debiec, J., LeDoux, J.E., and Nader, K. 2002. Cellular and systems reconsolidation in the hippocampus. Neuron 36: 527-538. [DOI] [PubMed] [Google Scholar]
- Dudai, Y. 2002. Memory from A to Z. Keywords, concepts, and beyond. Oxford UP, Oxford, UK.
- Eisenberg, M., Kobilo, T., Berman, D.E., and Dudai, Y. 2003. Stability of retrieved memory: Inverse correlation with trace dominance. Science 301: 1102-1104. [DOI] [PubMed] [Google Scholar]
- Freudenthal, R. and Romano, A. 2000. Participation of Rel/NFκB transcription factors in long-term memory in the crab Chasmagnathus. Brain Res. 885: 274-281. [DOI] [PubMed] [Google Scholar]
- Hoeger, R. and Florey, E. 1989. Catecholamine degradation in the hemolymph of the Chinese crab, Eriocheir sinesis. Comp. Biochem. Physiol. 92C: 323-327. [Google Scholar]
- Howell, D.C. 1987. Statistical methods for psychology. Duxbury, Boston.
- Locatelli, F., Maldonado, H., and Romano, A. 2002. Two critical periods for cAMP dependent protein kinase activity during long-term memory consolidation in the crab Chasmagnathus. Neurobiol. Learn. Mem. 77: 234-249. [DOI] [PubMed] [Google Scholar]
- Lozada, M., Romano, A., and Maldonado, H. 1990. Long-term habituation to a danger stimulus in the crab Chasmagnathus granulatus. Physiol. Behav. 47: 35-41. [DOI] [PubMed] [Google Scholar]
- Maldonado, H. 2002. Crustacean as model to investigate memory illustrated by extensive behavioral and physiological studies in Chasmagnathus. In The crustacean nervous system (ed. K. Wiese), pp. 314-327. Springer-Verlag Berlin, Heidelberg, Germany.
- Merlo, E., Freudenthal, R., and Romano, A. 2002. The IκB kinase inhibitor sulfasalazine impairs long-term memory in the crab Chasmagnathus. Neurosci. 112: 161-172. [DOI] [PubMed] [Google Scholar]
- Nadel, L. and Land, C. 2000. Memory traces revisited. Nat. Rev. Neurosci. 1: 209-212. [DOI] [PubMed] [Google Scholar]
- Nader, K., Schafe, G.E., and LeDoux, J.E. 2000a. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722-726. [DOI] [PubMed] [Google Scholar]
- ____. 2000b. The labile nature of consolidation theory. Nat. Neurosci. Rev. 1: 210-219. [DOI] [PubMed] [Google Scholar]
- Pedreira, M.E. and Maldonado, H. 2003. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38: 863-869. [DOI] [PubMed] [Google Scholar]
- Pedreira, M.E., Dimant, B., Tomsic, D., Quesada-Allué, L.A., and Maldonado, H. 1995. Cycloheximide inhibits context memory and long-term habituation in the crab Chasmagnathus. Pharmacol. Biochem. Behav. 52: 385-395. [DOI] [PubMed] [Google Scholar]
- Pedreira, M.E., Dimant, B., and Maldonado, H. 1996. Inhibitors of protein and RNA synthesis block context memory and long-term habituation in the crab Chasmagnathus. Pharmacol. Biochem. Behav. 54: 611-617. [DOI] [PubMed] [Google Scholar]
- Pedreira, M.E., Pérez-Cuesta, L.M., and Maldonado, H. 2002. Reactivation and reconsolidation of long-term memory in the crab Chasmagnathus: Protein synthesis requirement and mediation by NMDA-type glutamatergic receptors. J. Neurosci. 22: 8305-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyra, P., Saraco, M., and Maldonado, H. 1999. Decreased response or alternative defensive strategy in escape: Two novel types of long-term memory in the crab Chasmagnathus. J. Comp. Physiol. 184: 301-310. [Google Scholar]
- Pereyra, P., González Portino, E., and Maldonado, H. 2000. Conditioned defensive freezing in the crab is context-specific but not triggered by the context. Neurobiol. Learn. Mem. 74: 119-134. [DOI] [PubMed] [Google Scholar]
- Rescorla, R.A. 1988. Behavioral studies of pavlovian conditioning. Annu. Rev. Neurosci. 1: 320-352. [DOI] [PubMed] [Google Scholar]
- Romano, A., Locatelli, F., Delorenzi, A., Pedreira, M.E., and Maldonado, H. 1996. Effects of activation and inhibition of cAMP-dependent protein kinase on long-term habituation in the crab Chasmagnathus. Brain Res. 735: 131-140. [DOI] [PubMed] [Google Scholar]
- Rosenthal, R. and Rosnow, R.L. 1985. Contrast analysis focused comparisons in the analysis of variance. Cambridge UP, Cambridge, UK.
- Sangha, S., Scheibenstock, A., and Lukowiac, K. 2003. Reconsolidation for a long-term memory in Lymnea requires new protein and RNA synthesis and the soma of right pedal dorsal 1. J. Neurosci. 23: 8034-8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara, S.J. 2000a. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn. Mem. 7: 73-84. [DOI] [PubMed] [Google Scholar]
- ____. 2000b. Strengthening the shaky trace through retrieval. Nat. Neurosci. Rev. 1: 212-213. [DOI] [PubMed] [Google Scholar]
- Tomsic, D., Pedreira, M.E., Hermitte, G., Romano, A., and Maldonado, H. 1998. Context-US association as a determinant of long-term habituation in the crab Chasmagnathus. Anim. Learn. Behav. 26: 196-209. [Google Scholar]
- Troncoso, J. and Maldonado, H. 2002. Two related forms of memory in the crab Chasmagnathus are differentially affected by NMDA receptor antagonists. Pharmacol. Biochem. Behav. 72: 251-265. [DOI] [PubMed] [Google Scholar]
- Vianna, M.R.M., Szapiro, G., McGaugh, J.L., Medina, J.H., and Izquierdo, I. 2001. Retrieval of memory for fear-motivated training initiate extinction requiring protein synthesis in the rat hippocampus. Proc. Natl. Acad. Sci. 98: 12251-12254. [DOI] [PMC free article] [PubMed] [Google Scholar]