Abstract
To evaluate the population variation, individual plasticity, and local adaptability of Solidago canadensis in response to shade treatment, we conducted a common pots experiment with a total of 150 ramets (5 genets, 15 populations, and 2 treatments) subjected to both control (natural light) and shady treatment (10% of natural light). Shade treatment significantly reduced growth and content of defense metabolites in S. canadensis. Compared to control, shading led to increased height, decreased basal diameter, increased leaf width, increased leaf length, increased chlorophyll content, stronger photosynthetic rate (Pn), stronger stomatal conductance (gs), and lower root to shoot ratio. Three-way analysis of variance revealed geographical origin to significantly affect the basal diameter of S. canadensis, while genotype significantly affected plant height, intercelluar CO2 concentration (Ci), transpiration rate (Tr), and proline content. Significant interactive effects between shade and geographic origin were prevalent for most traits. The phenotypic differentiation coefficient of the plasticity of all traits was below 0.4, indicating that most of all variations can be found among individuals within populations. Phenotypic selection analysis revealed that fitness was significantly positively related to plant height, basal diameter, Ci, total flavonoid content, as well as the plasticity of plant height, leaf length, leaf width, gs, Ci, total flavonoid content, and malondialdehyde content under the control condition. However, subjected to shade, fitness was only significantly positively related to plant height, basal diameter, and the plasticity of basal diameter. Rather than local adaption, these results suggest that individual plasticity played a more prominent role in the shade response of the invasive S. canadensis.
Introduction
A growing consensus agrees that the environment has the ability to induce changes in the behavior of individual plants at a morphological and, or physiological level and that such changes might be crucial for survival in heterogeneous and variable conditions [1]. Functionally adaptive plasticity in allocational, morphological, and physiological traits that are involved in resource acquisition can allow individual plants to maximize their reproductive fitness in diverse environments. Plasticity plays a central role in biological invasions via allowing individuals to colonize environmentally diverse habitats and establish healthy populations [2, 3]. Furthermore, an invasive species may also spread across diverse habitats via local adaptation, evolving ecotypes with distinctive traits, and patterns of individual plasticity [4–7]. Although the majority of all published studies showed that both phenotypic plasticity and local adaptation contributed to invasion success [5, 8], the relative importance of both strategies has been subject to debate [9] and depends on species. Matesanz et al. verified that phenotypic plasticity (rather than locally adapted ecotypes) allows the Asian annual plant Polygonum cespitosum to colonize a wide range of habitats in northeastern North America [10].
Heterogeneous light environment is a dominant stress factor for plants and light is an extremely heterogeneous environmental factor, particularly in disturbed sites [11, 12]. Plants respond to changing light conditions by adjusting a series of morphological and physiological traits, such as leaf size, specific leaf area, net photosynthetic, and patterns of biomass allocation [13–15]. When confronted with low light intensities, invasive plants partitioned more area and biomass to leaves to increase light capture efficiency and improve light utilization efficiency to maximize carbon gain. Different responses to light can affect the process of invading and spreading, and further alter the competitive relationship between invasive and native plants. Consequently, the dynamics of population and community of invasive and native plants can be drastically altered.
Solidago canadensis L. (Asteraceae) is a perennial herb native to North America and was first introduced to Shanghai, China as an ornamental in 1935 [16]. Currently, it is widely distributed along the southeast coastal and the Yangtze River basin, and is also a major invasive plant species in many other countries [17, 18]. S. canadensis can severely damage local biodiversity, and the natural ecosystem. Furthermore, the very effective sexual and asexual reproduction can cause enormous economic losses. Several studies found that shade decreased growth and photosynthetic ability [19]. However, to the best of our knowledge, population variation of the phenotypic plasticity in response to shade has not been explored in S. canadensis. We hypothesized that different geographical populations would show different adaptive responses to shade. Our goal was to reveal the traits preferred by plants that thrive under shade and whether individual plasticity or local adaption plays the more important role in invasive species. Understanding the shade response of S. canadensis will help to predict invasive trends, and moreover provide basic references for the management and prevention of the invasive S. canadensis.
Materials and Methods
Plant sampling and propagation
In October 2012, rhizomes of S. canadensis were collected from 15 populations with a range of latitudes from 26.0968°N to 34.654°N and longitudes from 111.532°E to 121.804°E (Table 1). Within every population, 12 randomly selected ramets were dug out. The distances between collected rhizomes were at least 10 m to reduce the probability of sampling the same genet more than once. Our field studies did not involve any endangered or protected species and none of the population were privately owned or under nature protection. No specific permissions were required for these locations. Shoot bases with attached rhizomes were transferred to the Taizhou University in Linhai City, Zhejiang Province, China and kept moist until replanting. Rhizomes were individually planted in pots with diameters of 30 cm and depths of 30 cm. The soil mixture (yellow clay soil: sand: peat soil = 6:3:1) had a final pH of 6.80 ± 0.10, an organic matter content of 27.66 ± 0.69 g kg−1, a total nitrogen content of 361.00 ± 19.05 mg kg−1, an available phosphorus content of 8.00 ± 0.66 mg kg−1, and an available potassium content of 12.00 ± 0.58 mg kg−1. All plant material used in this study were propagated twice in a greenhouse for two years and three months and the environmental carryover effects were minimized.
Table 1. Locations and habitats of 15 S. canadensis populations.
| No. | Population abbreviation | Location | Longitude | Latitude | Altitude (m) | Habitats |
|---|---|---|---|---|---|---|
| 1 | FZ | Fuzhou City, Fujian Province | N119.359° | E26.098° | 19 | Abandoned farmland |
| 2 | WZ | Wenzhou City, Zhejiang Province | N120.607° | E28.126° | 4 | Abandoned farmland |
| 3 | TZ | Taizhou City, Zhejiang Province | N121.397° | E28.656° | 6 | Abandoned farmland |
| 4 | JDZ | Jingdezheng City, Jiangxi Province | N117.166° | E29.318° | 40 | Green belts |
| 5 | JJ | Jiujiang City, Jiangxi Province | N116.283° | E29.985° | 18 | Abandoned vegetable garden |
| 6 | HZ | Xiaoshan District, Hangzhou City, Zhejiang Province | N120.297° | E30.161° | 9 | Abandoned farmland |
| 7 | WC | Wuchang District, Wuhan City, Hubei Province | N114.421° | E30.541° | 26 | Abandoned vegetable garden |
| 8 | YC | Yichang City, Hubei Province | N111.532° | E30.843° | 333 | Abandoned building land |
| 9 | WH | Hankou District, Wuhan City, Hubei Province | N114.350° | E30.878° | 25 | Abandoned farmland |
| 10 | HQ | Minhang District, Shanghai City | N121.433° | E31.307° | 5 | Green belts |
| 11 | WHu | Wuhu City, Anhui Province | N118.387° | E31.342° | 16 | Garbage dump |
| 12 | PD | Pudong District, Shanghai City | N121.804° | E31.354° | 3 | Abandoned farmland |
| 13 | NJ | Nanjing City, Jiangsu Province | N119.094° | E31.794° | 22 | Abandoned farmland |
| 14 | LYG | Lianyungang City, Jiangsu Province | N19.235° | E34.654° | 3 | Abandoned farmland |
| 15 | NT | Nantong City, Jiangsu Province | N120.843° | E32.070° | 5 | Abandoned farmland |
Experiment design
In June 2014, newly emerged S. canadensis ramets with similar height (approximately 15 cm) were cut off from their respective stock and individually planted in pots with diameters of 16 cm and depths of 14 cm filled with soil mixture mentioned above. A randomly chosen subset of pots was moved inside a shed covered with white nylon anti-fly net.
After 14 days, we recorded plant height (Ht1) and the number of leaves (Nt1) of S. canadensis and initiated the shade experiment. Two ramets from each of five genets of the 15 S. canadensis populations (in total 150 ramets, one replicate per genotype and per treatment) were allocated to one of two treatments: a control and a shade treatment. In the control treatment, plants were cultured under natural light. In the shade treatment, light availability was reduced by 90% via shading the plants with double-black and semi-transparent nylon nets clamped to a mental frame at 150 cm height [20]. Light intensities were measured via a handheld luxmeter. The positions of the pots were randomly changed every week to reduce position bias effects. No fertilizer was used and an adequate amount of water was provided.
Measurements
Sixty days after transplanting, plant height (Ht2), leaf length, and leaf width were measured via ruler with an accuracy of 0.1 cm and the leaf length/width ratio was calculated. The number of leaves (Nt2) was also recorded. The basal diameter was measured via vernier caliper with an accuracy of 0.02 cm. The rate of increase of the number of leaves was calculated as (Nt2-Nt1)/(t2-t1), and the increase rate in plant height was calculated as (Ht2-Ht1)/(t2-t1).
In situ photosynthesis measurements were made on the third fully expanded leaf, counted from the tip of the shoot and using a portable photosynthesis-measurement system (LI-6400 XT, Li-COR Inc., Lincoln, NE, USA). Measurements were obtained between 9:00 AM and 11:00 AM under a photosynthetically active radiation of 1,400 μmol m-2 s-1 (i.e. at light saturation) at a leaf temperature of 25°C, a CO2 concentration of 400 ppm, and relative humidity of 70%. Leaf area of effective photosynthesis was traced via leaf area analyzer (Win FOLIA, Regent Instruments Inc). Net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) were measured.
Three tender leaves per plant were collected and transferred to the lab immediately upon collection. Proline content was measured via acidic ninhydrin colorimetry [21]. The malondialdehyde and soluble sugar contents were measured via the thiobarbituric acid (TBA) method [22]. The chlorophyll content was measured spectrophotometrically following procedure published by Wintermans and De Mots [23]. All required weight was measured via balance with an accuracy of 0.1 mg.
Three matured leaves per plant at a similar position were collected and transferred to the lab immediately upon collection. The leaves were dried at 70°C and ground into powder. Experimental weight of each leaf was weighted via balance with an accuracy of 0.1 mg. Flavonoid content was measured via the rutin method [24]. The total phenolic content was determined via the Folin-phenol method [25]. Lignin content was measured via concentrated sulfuric acid titration [26].
Following measurements, plants were harvested and divided into leaves, stems, and roots. Plant material was over-dried in an oven (at 105°C for 1 h and then at 80°C until a constant weight was reached). The leaf, stem, and root biomasses were weighed via balance with an accuracy of 0.1 mg. Total biomass and root/shoot ratios were calculated.
Statistical analysis
Paired t-test was used to analyze the difference in the rate of increase in the number of leaves, in plant height, and biomass traits between the shade and control treatment. Three-way ANOVA was conducted using treatment as fixed factor, population as random factor, and genotype as a random factor nested within population. Plant height at the onset of the experiment was used as a covariation to exclude the initial difference effect among different plants. All data is normally distributed, and satisfies homogeneity of variance.
The phenotypic plasticity index (PPI) was calculated as: (max(X0, Xi)—min(X0, Xi)) / max(X0, Xi), where X0 and Xi stand for the mean values of control and shade treatments, respectively. Max(X0, Xi) is the higher value of X0 and Xi, and min(X0, Xi) the lower value of X0 and Xi [26, 27]. The mean PPI per trait was calculated via the PPI value of 15 populations. The phenotypic differentiation coefficient (Vst) was calculated as: Vst = (δ2t/s) / (δ2t/s + δ2s), where δ2t/s and δ2s stand for the variance components within and among population, respectively [28].
To test whether phenotypic traits and plasticity of traits responded to the shade treatment increase the fitness of a genotype (j), we regressed the fitness value (biomass, Wj) of a genotype across both environments against the trait value (Xj) across both environments as well as the plasticity (Pj), using the following equation Wj = Constant + αXj + βPj [29,30].
All statistical analyses were conducted using SPSS 18.0 (IBM Co., USA) and all figures were plotted using Origin 9.0 (OriginLab Co., USA).
Results
Shade response of S. canadensis
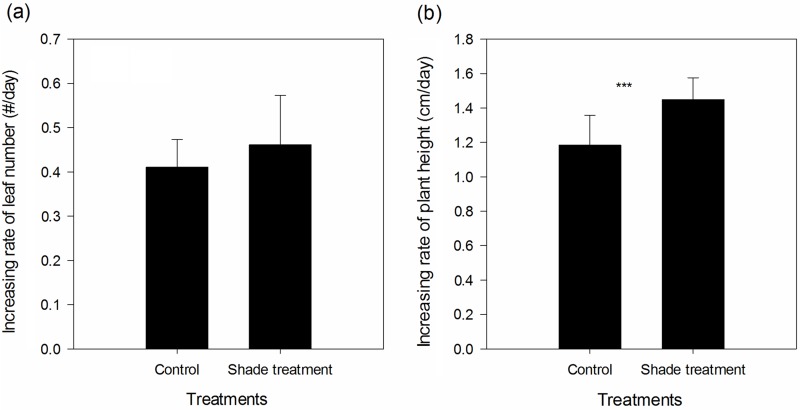
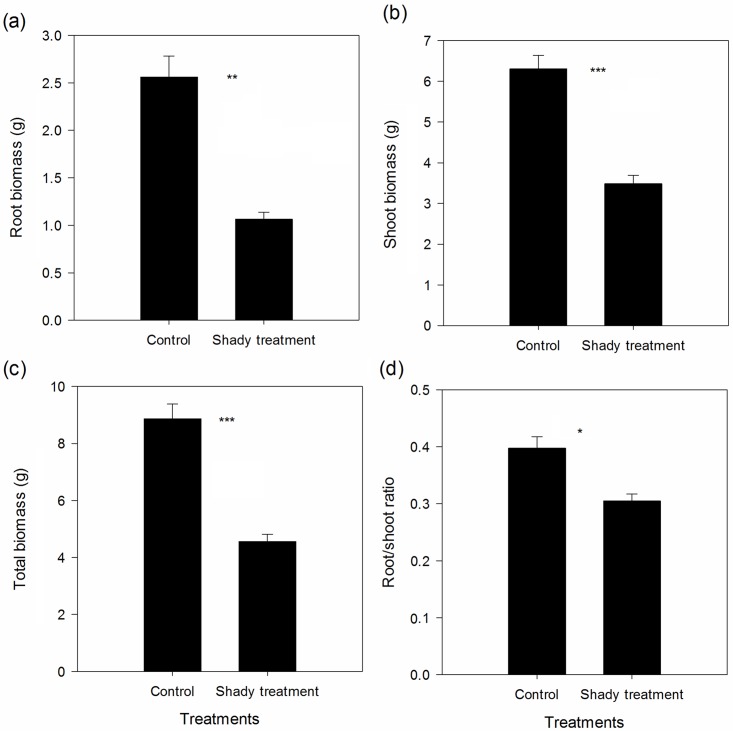
Shade treatment significantly elevated the rate of plant height increase (Fig 1B, paired t value = -5.059, p < 0.001); however, shade treatment had no significant effect on the rate of increase of the number of leaves (Fig 1A, paired t value = -1.867, p = 0.083). Shade treatment significantly reduced root biomass (Fig 2A, paired t value = 6.807, p < 0.001), shoot biomass (Fig 2B, paired t value = 10.785, p < 0.001), total biomass (Fig 2C, paired t value = 10.832, p < 0.001), and root/shoot ratio (Fig 2D, paired t value = 3.274, p = 0.006).
Fig 1. Effect of shade treatment on the increase rate of the number of leaves (a) and plant height (b) of S. canadensis.
*** indicates a significant difference between control and the shade treatment at p < 0.001.
Fig 2. Effect of shade treatment on root biomass (a), shoot biomass (b), total biomass (c), and root/shoot ratio (d) of S. canadensis.
*, ***, indicate significant differences between control and shade treatment at p < 0.05 and p < 0.001, respectively.
Phenotypic plasticity of S. canadensis
The two-way ANOVA revealed that shade had a significant effect on the morphological, physiological, and defense traits of S. canadensis with the exception of Ci and Tr (Table 2). Shade significantly increased plant height, leaf length, leaf width, chlorophyll content, Pn, and Gs, whereas basal diameter, proline content, malondialdehyde, soluble sugar, total phenolic, total flavonoids, and lignin contents were significantly decreased. Geographical origin only significantly affected the basal diameter of S. canadensis (p > 0.05). Genotype significantly affected plant height, Ci, Tr, and proline content (p < 0.05 for each). Significant interactive effects between shade and geographic origin were revealed for plant height, leaf length, leaf width, basal diameter, chlorophyll content, Pn, total flavonoid content, total lignin content, and proline content (p < 0.05) (see Table 2).
Table 2. Phenotypic traits in control treatment and shade treatment as well as phenotypic plasticity indexes (PPI) of each measured trait of S. canadensis.
The data were shown as means ± standard error (SE). Two-way ANOVA results are listed.
| Trait | Mean±SE | Two-way ACNOVA | Plasticity | |||||
|---|---|---|---|---|---|---|---|---|
| Control | Shady | Fshady | Forigin | Fgenotype | Fshady×origin | Mean±SE | Vst | |
| Plant height (cm) | 66.81±1.87 | 79.74±1.77 | 25.86** | 0.7 | 3.25** | 2.16* | 0.20±0.02 | 0.04 |
| Leaf length(cm) | 9.26±0.22 | 11.05±0.31 | 14.47** | 0.76 | 1.65 | 2.66** | 0.19±0.02 | 0.02 |
| Leaf width(cm) | 1.61±0.05 | 2.17±0.07 | 26.42** | 0.64 | 1.29 | 2.17* | 0.27±0.02 | 0.02 |
| Basal diameter(cm) | 0.75±0.01 | 0.58±0.01 | 79.05** | 2.79* | 1.46 | 2.56** | 0.23±0.01 | 0.02 |
| Chlorophyll content (mg/g) | 8.51±0.26 | 27.61±0.51 | 670.45** | 2.01 | 1.04 | 2.59** | 0.68±0.01 | 0.09 |
| Photosythetic rate (μmol·m-2·s-1) | 9.56±0.39 | 12.61±0.25 | 37.09** | 1.04 | 1.76 | 2.09* | 0.27±0.03 | 0.21 |
| Stomatal conductance (mol·m-2·s-1) | 0.5±0.02 | 1.26±0.13 | 29.48** | 1.22 | 1.29 | 1.51 | 0.51±0.03 | 0.25 |
| Intercelluar CO2 concentration (μmol·mol-1) | 337.29±2.56 | 342.2±3.85 | 1.51 | 1.29 | 1.86* | 1.18 | 0.05±0.01 | 0.29 |
| Transpiration rate (mmol·m-2·s-1) | 9.74±0.33 | 9.42±0.32 | 0.76 | 1.96 | 2.67** | 1.73 | 0.17±0.02 | 0.31 |
| Total phenolic content (mg/g) | 36.31±0.84 | 8.83±0.43 | 734.56** | 1.54 | 1.36 | 1.97 | 0.75±0.01 | 0.36 |
| Total flavonoids content (mg/g) | 0.51±0.02 | 0.16±0.01 | 140.5** | 1.36 | 1.25 | 2.41* | 0.68±0.02 | 0.38 |
| Total lignin content(mg/g) | 3.12±0.14 | 1.51±0.11 | 34.96** | 0.81 | 0.97 | 3.47** | 0.50±0.03 | 0.18 |
| Proline content (ug/g) | 79.79±3.57 | 62.41±1.93 | 13.26** | 1.29 | 1.7* | 2.33** | 0.26±0.02 | 0.13 |
| Malondialdehyde content (mmol/g) | 0.06±0 | 0.05±0 | 13.12** | 0.9 | 0.93 | 1.59 | 0.20±0.02 | 0.02 |
| Soluble sugar content (mg/g) | 61.05±3.26 | 44.59±2.52 | 12.65** | 1.59 | 0.95 | 1.72 | 0.34±0.03 | 0.13 |
Note:
*, ** indicate significant differences at p < 0.05 and p < 0.01 level.
The phenotypic plasticity indices of chlorophyll content, Ci, total phenolic content, total flavonoid content, and total lignin content were all above 0.4, while those of leaf length, Ci, and Tr were below 0.2 (Table 2). The phenotypic differentiation coefficiencies of the plasticity of all the traits were below 0.4, indicating that most of the variation of phenotypic plasticity existed within populations of different geographical origins, not among populations (Table 2).
Phenotypic selection of shade
Phenotypic selection analysis showed that fitness significantly and positively correlated with plant height, basal diameter, intercellular CO2 concentration, total flavonoid content, proline content, as well as the plasticities of plant height, leaf length, leaf width, stomatal conductance, intercellular CO2 concentration, total flavonoid content, and malondialdehyde content under normal condition. However, under shade treatment, significantly and positively correlations were only detected for plant height, basal diameter, and the plasticity of basal diameter (Table 3).
Table 3. Standardized regression coefficients of fitness metrics of traits (α) and plasticity of traits (β) under control and shade treatment.
All values are based on genotypic values and fitness was measured as biomass. Benefit of traits and plasticity of traits are indicated via positive regression coefficients.
| Traits | Control | Shade treatment | ||
|---|---|---|---|---|
| α value | β value | α value | β value | |
| Plant height | 0.967*** | 0.330*** | 0.789*** | -0.024 |
| Leaf length | 0.025 | 0.306** | 0.069 | 0.139 |
| Leaf width | 0.094 | 0.251* | 0.090 | 0.069 |
| Basal diameter | 0.381** | 0.008 | 0.496*** | 0.229* |
| Chlorophyll content | -0.069 | 0.050 | -0.170 | 0.109 |
| Photosythetic rate | 0.105 | 0.252 | 0.013 | -0.007 |
| Stomatal conductance | 0.258 | 0.371** | -0.035 | 0.055 |
| Intercelluar CO2 concentration | 0.271* | 0.529*** | 0.642 | 0.641 |
| Transpiration rate | 0.043 | 0.153 | 0.015 | -0.029 |
| Total phenolic content | 0.245 | 0.265 | 0.014 | -0.057 |
| Total flavonoids content | 0.368* | 0.398* | -0.008 | -0.004 |
| Total lignin content | 0.170 | -0.025 | 0.286 | 0.216 |
| Proline content | 0.777** | -0.044 | -0.010 | 0.187 |
| Malondialdehyde content | 0.036 | 0.321** | -0.040 | 0.093 |
| Soluble sugar content | -0.093 | 0.200 | 0.189 | 0.114 |
Note:
*, **, and *** indicate significant differences at p < 0.05, p < 0.01 and p < 0.001 level.
Discussion
Previous studies have reported a detrimental effect of shade on plants, especially affecting anatomical structure, phenotypic, and physiological traits, hence reducing fitness traits related to biomass [1, 27]. We found similar results in our study. Shade treatment evidently decreased root biomass, shoot biomass, and total biomass of S. canadensis. These results suggest that shade treatment significantly reduced plant fitness, suggesting that low light intensity might not be a suitable habitat for S. canadensis. This may explain why the main distribution habitats of S. canadensis in China are open fields, rather than habitats with shady conditions [19].
Different light intensities significantly changed the morphological and physiological traits of plants [28]. In this study, we found S. canadensis to express the typical shade-avoidance syndrome [29], i.e. increased height and decreased basal diameter, reducing the mutual shade effect. Furthermore, increased width and length of leaves enable the interception of more light; increased chlorophyll content improves utilization efficiency, and stronger photosynthetic rate and stomatal conductance aid in the absorbance of light resources when exposed to shade. Similar results were reported enabling plants to functionally accommodate low light conditions and consequently, plants express developmental modifications that maximize light interception, e.g. via increasing specific leaf area, leading to large, thin leaves and elongating seedling internodes and stems, all of which facilitates the escape from a low-light environment for S. canadensis [27, 30–33]. Interestingly, an enhanced ability of light absorption and utilization efficiency, coupled with lower biomass was found in the shady treatment. This suggests that a complementary effect of the shady responses could not complement the inhibitory effect of shade on S. canadensis and 10% light intensity was a limiting factor for S. canadensis. We also found that shade significantly reduced the root/shoot ratio, indicating an adaptive strategy to allocate more energy to aboveground biomass to improve fitness and competitive ability [34]. In this study, we also found that shade significantly decreased the contents of soluble sugar, total phenolic compounds, total flavonoids, and lignin. In addition, the content of proline and malondialdehyde were also decreased, indicating that the anti-oxidant ability of S. canadensis reduced when exposed to shade. S. canadensis reduced resource allocation to defense mechanisms and anti-oxidant metabolites to allocate more energy to essential organs, thus increasing the growth input and retaining fitness in harsh condition. Shade tolerance is one of the most important ecological factors with respect to interspecific competition [35]. Thus, the shade tolerance ability of S. canadensis might play a key role for the successful invasion of this species.
The mean PPI of S. canadensis in response to shade was 0.36, with minimal intercelluar CO2 concentration (0.05) and maximal chlorophyll and total flavonoid contents (0.68). Matos et al. reported that plasticity of physiological and biochemical traits in Coffea arabica is more important for acclimation to intra-canopy light variations than morphological or anatomical trait plasticity [36]. In this study, we found that the mean PPI of defense traits was 0.455, which was above that of morphological traits (mean 0.223) and photosynthetic physiological traits (mean 0.336). These results suggest that photosynthetic physiological traits and defense traits were easier to change in response to shade and more important for the successful propagation of S. canadensis.
In this study, geographical origin only significantly affected the basal diameter of S. canadensis, not any other traits. However, we found significant interactive effect between shade and geographic origin for most of the traits of S. canadensis, indicating that the effect of shade varied among different populations. Si et al. reported that the effect of geographical origin and interactions between geographical origins and light treatments were all significant in traits of the invasive Wedelia trilobat and the authors thus concluded that both phenotypic plasticity and local adaptation occurred in W. trilobata in response to the light condition [9]. Adaptation to full sunlight intensity was also detected in W. trilobata populations from sunny sites, while this was not detected for those grown in shady sites [9]. All populations in our study were collected from typical S. canadensis habitats near the roadside, abandoned fields, and similar habitats (open field). All the populations grew in a full sunlight habitat without any shade and most of the accompanied species were grasses and small shrubs. Phenotypic selection analysis revealed only significantly positively relationships for plant height, basal diameter, and the plasticity of basal diameter under shade treatment. However, the phenotypic differentiation coefficient of plant height and basal diameter were only 0.04 and 0.02, respectively, indicating most of the variation existed among individuals within populations, rendering the existence of local adaption impossible. In addition, genotypes from all populations significantly affected the traits of height, Ci, Tr, and proline content, indicating individual plasticity rather than local adaption played central roles in the shade response of S. canadensis. The lack of local adaptation might be a result of a similarity of habitats of S. canadensis in China, without light heterogeneity. Further studies, investigating more populations from more variable habitats, should be conducted to reveal a general rule of the shade response of S. canadensis.
Conclusion
Shade evidently altered phenotypic and physiological traits, and furthermore reduced plant fitness, i.e. biomass of S. canadensis. However, typical shade-avoidance syndromes were also found in our study, such as increased height, increased width and length of leaves, increased chlorophyll content, and decreased root/shoot ratio, which all might play central roles in a successful invasion. Furthermore, photosynthetic physiological traits and defense traits changed in response to shade. Our study suggests that individual plasticity rather than local adaption played the key role in the shade response of S. canadensis. These adaptive strategies of S. canadensis to endure light deficiency provide a basic reference for the management and control of this invasive species, occurring in similar light conditions.
Acknowledgments
We thank Jianqing Ding and Zhengsheng He for their kind help with plant collection.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was financially support by the National Natural Science Foundation of China (No. 31270461) for JML, the National Key Research and Development Program (2016YFC1201100) for JSL and JML, and the Qianjiang Talents Project of Zhejiang Province (type D) (No. QJD1302021) for JML. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Puglielli G, Crescente MF, Frattaroli AR, Gratani L. Morphological, anatomical and physiological leaf trait plasticity of Sesleria nitida (Poaceae) in open vs shaded conditions. Polish Journal of Ecology. 2015;63(1):10–22. [Google Scholar]
- 2.Sultan SE. Promising directions in plant phenotypic plasticity. Perspectives in Plant Ecology Evolution & Systematics. 2004;6(4):227–233. [Google Scholar]
- 3.Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science. 2000;5(12):537–542. [DOI] [PubMed] [Google Scholar]
- 4.Williams DG, Mack RN, Black RA. Ecophysiology of Introduced Pennisetum setaceum on Hawaii: the role of phenotypic plasticity. Ecology. 1995;76(5):1569–1580. [Google Scholar]
- 5.Parker IM, Rodriguez J, Loik ME. An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus. Conservation Biology. 2003;17(17):59–72. [Google Scholar]
- 6.Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, et al. The population biology of invasive species. Annual Review of Ecology & Systematics. 2001;32(1):305–332. [Google Scholar]
- 7.Sexton JP, Mckay JK, Sala A. Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecological Applications. 2002;12(6):1652–1660. [Google Scholar]
- 8.Simberloff D. The role of propagule pressure in biological invasions. Annual Review of Ecology Evolution & Systematics. 2009;40:81–152. [Google Scholar]
- 9.Si CC, Dai ZC, Lin Y, Qi SS, Huang P, Miao SL, et al. Local adaptation and phenotypic plasticity both occurred in Wedelia trilobata invasion across a tropical island. Biological Invasions. 2014;16(11):2323–2337. [Google Scholar]
- 10.Matesanz S, Horgan-Kobelski T, Sultan SE. Phenotypic plasticity and population differentiation in an ongoing species invasion. Plos One. 2012;7(9):e44955–e. 10.1371/journal.pone.0044955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jesson L, Kelly D, Sparrow A. The importance of dispersal, disturbance, and competition for exotic plant invasions in Arthur's Pass National Park, New Zealand. New Zealand Journal of Botany. 2010;38(3):45–51. [Google Scholar]
- 12.Lake JC, Leishman MR. Invasion success of exotic plants in natural ecosystems: the role of disturbance, plant attributes and freedom from herbivores. Biological Conservation. 2004;117(2):215–226. [Google Scholar]
- 13.Legner N, Fleck S, Leuschner C. Within-canopy variation in photosynthetic capacity, SLA and foliar N in temperate broad-leaved trees with contrasting shade tolerance. Trees. 2014;28(1):263–280. [Google Scholar]
- 14.Liu Y, D Wayne; Prati Daniel; Haeuser Emily; Feng Yanhao; van Kleunen Mark. Does greater specific leaf area plasticity help plants to maintain a high performance when shaded? Annals of Botany. 2016: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozendaal DMA, Hurtado VH, Poorter L. Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Functional Ecology. 2006;20(2):207–216. [Google Scholar]
- 16.Li Z, Xie Y. Alien invasive species in China. Beijing: China Forestry Publishing; 2002. [Google Scholar]
- 17.Weber E. The dynamics of plant invasions: a case study of three exotic goldenrod species (Solidago L.) in Europe. Journal of Biogeography. 1998;25(1):147–154. [Google Scholar]
- 18.Weber E. Current and Potential Ranges of Three Exotic Goldenrods (Solidago) in Europe. Conservational Biology. 2001;15(1):122–128. [Google Scholar]
- 19.Sun XF, He JQ, Huang XD, Ping J, Ge J. Growth characters and chlorophyll fluorescence of Goldenrod (Solidago canadensis) in different light intensities. Acta Botanica Boreali-Occidentalia Sinica. 2008;28:752–758. [Google Scholar]
- 20.Semchenko M, Lepik M, Götzenberger L, Zobel K. Positive effect of shade on plant growth: amelioration of stress or active regulation of growth rate? Journal of Ecology. 2012;100: 4590466. [Google Scholar]
- 21.Zhu GL, Deng XW, Zuo WN. The measurement of proline contentinplant. Journal of Plant Physiology. 1983; 35–7. [Google Scholar]
- 22.Lin ZF, Li SS, Lin GZ, Sun GC, Guo JY. Superoxide dismutase activity and lipid peroxidation in relation to senescence of rice leaves. Journal of Integrative Plant Biology. 1984;5(1):605–15. [Google Scholar]
- 23.Wintermans JFGM, Mots AD. Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. Biochimica et Biophysica Acta. 1965;109(2):448–53. [DOI] [PubMed] [Google Scholar]
- 24.Liang H, Pang W, Zhang W. The extract technique of flavonoid of Ginkgo biloba L. leaves. Journal of Plant Resource and Environment. 1999;(3):12–7. [Google Scholar]
- 25.Jin ZX, Li JM, Zhu XY. Analysis of the total phenols content in different organs of Calycanthus chinensis from different habitat. Journal of Anhui Agricultural University. 2006;33(4):454–7. [Google Scholar]
- 26.Xiong SM, Zuo XF, Zhu YY. Determination of cellulose, hemi-cellulose and ligin in rice hull. Cereal & Feed Industry. 2005;(8):40–1. [Google Scholar]
- 27.Schmitt J, Stinchcombe JR, Heschel MS, Huber H. The adaptive evolution of plasticity: phytochrome-mediated shade avoidance responses. Integrative & Comparative Biology. 2003;43(3):459–69. [DOI] [PubMed] [Google Scholar]
- 28.Droste T, Flory SL, Clay K. Variation for phenotypic plasticity among populations of an invasive exotic grass. Plant Ecology. 2010;207(2):297–306. [Google Scholar]
- 29.Franklin KA. Shade avoidance. New Phytologist. 2008;179(4):930–44. 10.1111/j.1469-8137.2008.02507.x [DOI] [PubMed] [Google Scholar]
- 30.Steinger T, Roy BA, Stanton ML. Evolution in stressful environments II: adaptive value and costs of plasticity in response to low light in Sinapis arvensis. Journal of Evolutionary Biology. 2003;16(2):313–23. [DOI] [PubMed] [Google Scholar]
- 31.Bell DL, Galloway LF. Population differentiation for plasticity to light in an annual herb: Adaptation and cost. American Journal of Botany. 2008;95(1):59–65. 10.3732/ajb.95.1.59 [DOI] [PubMed] [Google Scholar]
- 32.Poorter L. Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Functional Ecology. 1999;13(3):396–410. [Google Scholar]
- 33.Donohue K, Messiqua D, Pyle EH, Heschel MS, Schmitt J. Evidence of adaptive divergence in plasticity: density- and site dependent selection on shade-avoidance response in Impatiens capensis. Evolution. 2000;54(6):1956–68. [DOI] [PubMed] [Google Scholar]
- 34.Shipley B, Meziane D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Functional Ecology. 2002;16(3):326–31. [Google Scholar]
- 35.Gratani L, Crescente MF, D’Amato V, Ricotta C, Frattaroli AR, Puglielli G. Leaf traits variation in Sesleria nitida growing at different altitudes in the Central Apennines. Photosynthetica. 2014;54(3):2631–40. [Google Scholar]
- 36.Matos FS, Wolfgramm R. Phenotypic plasticity in response to light in the coffee tree. Environmental & Experimental Botany. 2009;67(2):421–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.