Abstract
Background
Sepsis is usually accompanied by changes of body temperature (Tb), but whether fever and hypothermia predict mortality equally or differently is not fully clarified. We aimed to find an association between Tb and mortality in septic patients with meta-analysis of clinical trials.
Methods
We searched the PubMed, EMBASE, and Cochrane Controlled Trials Registry databases (from inception to February 2016). Human studies reporting Tb and mortality of patients with sepsis were included in the analyses. Average Tb with SEM and mortality rate of septic patient groups were extracted by two authors independently.
Results
Forty-two studies reported Tb and mortality ratios in septic patients (n = 10,834). Pearson correlation analysis revealed weak negative linear correlation (R2 = 0.2794) between Tb and mortality. With forest plot analysis, we found a 22.2% (CI, 19.2–25.5) mortality rate in septic patients with fever (Tb > 38.0°C), which was higher, 31.2% (CI, 25.7–37.3), in normothermic patients, and it was the highest, 47.3% (CI, 38.9–55.7), in hypothermic patients (Tb < 36.0°C). Meta-regression analysis showed strong negative linear correlation between Tb and mortality rate (regression coefficient: -0.4318; P < 0.001). Mean Tb of the patients was higher in the lowest mortality quartile than in the highest: 38.1°C (CI, 37.9–38.4) vs 37.1°C (CI, 36.7–37.4).
Conclusions
Deep Tb shows negative correlation with the clinical outcome in sepsis. Fever predicts lower, while hypothermia higher mortality rates compared with normal Tb. Septic patients with the lowest (< 25%) chance of mortality have higher Tb than those with the highest chance (> 75%).
Introduction
Sepsis constitutes a global burden for medical care with an estimated 31 million cases per year worldwide [1]. The incidence of sepsis has remained considerable [2–4] and it is associated with high mortality rates even nowadays [3]. It also underlies the importance and actuality of the topic for clinical praxis that the definitions of sepsis and associated illnesses have been updated recently [5].
As a systemic inflammation response, sepsis is often associated with changes of deep body temperature (Tb), which can be manifested as fever or hypothermia in experimental animals [6–8], as well as in human patients [8–10]. Not surprisingly, deep Tb is regularly measured as one of the vital signs in the clinical praxis. In fact, many scoring systems (e.g., APACHE II, PIRO, SAPS II, SIRS), which help in the diagnosis or in the assessment of the progress of sepsis, include an abnormal deviation of Tb from the normal range [5, 11–14]. Usually Tbs below 36.0°C or above 38.0°C are considered equally pathological [15], which values are in accordance with the criteria of the systemic inflammatory response syndrome [5, 11]. Based mainly on experimental data from animal studies Romanovksy and colleagues [6, 8] proposed that fever and hypothermia can both develop as two distinct adaptive mechanisms in sickness syndrome. The former characteristically occurs at the onset of an infection, representing an active fight against the pathogen, while the latter is usually associated with progressed stage or severity of the disease and it aims to secure the vital systems of the host [6, 8]. The two adaptive strategies can develop sequentially (e.g., early phase fever followed by late phase hypothermia) as the severity of the disease progresses [8], but hypothermia can be also one of the earliest developing events in animal models of endotoxin shock [16], moreover, septic patients admitted to ICU develop hypothermia more frequently in the early than in the late stages of their stay [17]. Despite the different pathological background of fever and hypothermia in systemic inflammation, both the increase and the decrease of Tb are evaluated commonly as equally severe signs in the clinical praxis [15]. This can be, at least in part, due to the standpoint that fever and hypothermia both represent an adaptive (though different) biological response to infection [8]. Accordingly, beneficial effects have been shown for elevated Tb on the clinical outcome of sepsis in clinical trials [18, 19], although no association between fever and disease severity was also reported [20]. Therapeutic (i.e., induced) hypothermia has been also shown to improve the outcome of sepsis in human studies [21, 22], but in case of spontaneously occurring hypothermia usually a positive association with mortality rate was found [20, 23, 24]. The definite association of Tb and mortality rate in a large study population has remained unknown.
We hypothesized that the deviation of Tb from the normal range predicts the clinical outcome in sepsis differently, and consequently septic patients with fever have lower chances for mortality than those who develop hypothermia. We performed an extensive literature search for human studies in septic patients and collected data on their Tb and mortality rate. The data were then analyzed with multiple statistical approaches, including Pearson regression, forest plot, and meta-regression analyses. Based on a high number of patients, we show a strong association between Tb and mortality ratio in sepsis across a wide temperature range.
Materials and Methods
Our meta-analysis was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols [25] (S1 Table). The analysis was based on the Participants, Intervention (prognostic factor), Comparison, Outcome (PICO) model: in septic population, we aimed to assess the predictive role of Tb deviations on the mortality ratio. No review protocol has been registered for the current meta-analysis.
Search strategy
A search of the PubMed, EMBASE, and Cochrane Controlled Trials Registry databases was performed with using the following Medical Subject Headings and search terms (from inception to February 2016): sepsis OR bacteremia OR "septic syndrome" AND ("body temperature" OR fever OR hypothermia OR normothermia OR hyperthermia) AND (mortality OR survival). We restricted our search to original human studies published in English without time period limitations. Publications reporting immunosuppressive conditions (e.g., cancer, transplantation, HIV infection) were not included in the analysis. As a specific example, in the EMBASE database, which identified the highest number of articles, the term “sepsis OR bacteremia OR "septic syndrome" AND ("body temperature" OR fever OR hypothermia OR normothermia OR hyperthermia) AND (mortality OR survival) NOT (cancer OR immunosuppressive OR aids OR hiv OR transplantation)” was entered, and then the following filters were selected: humans, English, article, article in press, conference abstract, conference paper, major clinical study, case control study, clinical trial, cohort analysis, comparative study, controlled clinical trial, controlled study, cross-sectional study, double blind procedure, medical record review, multicenter study, observational study, outcomes research, phase 3 clinical trial, prospective study, randomized controlled trial, retrospective study. The search was conducted separately by two authors (ZR, AG), who also assessed study eligibility and extracted data from the selected studies independently. Disagreements were resolved by consensus with the help of a third party (MR).
Study selection and data extraction
The titles and abstracts of the publications from the literature search were screened and the full text of potentially eligible articles was obtained. We included studies in which both the Tb values and the mortality ratios were reported for the same group(s) of patients with systemic inflammation accompanied by suspected or confirmed blood infection. From all included articles we extracted the sample size, the reported mean Tb value of the patients with its standard error (SEM), and the mortality ratio within the group during 28–30 days in most cases. To analyze the influence of fever, normothermia, and hypothermia on the mortality ratio in sepsis we separated the collected data into three study groups based on the mean Tb of the patients.
Statistical analysis
We have used event rates (mortality rates) as effect size data. Studies were grouped by Tb as low (up to 36.0°C; n = 890), medium (36.1 to 38.0°C; n = 3,904) and high (above 38.0°C; n = 6,040) and forest plots in the three groups were used to describe mortality. Selection of the Tb groups was based on the SIRS criteria [5, 11]. Another grouping was conducted by mortalities, these were split into quartiles and the means of Tbs were compared by investigating the presence or absence of overlaps in the 95% confidence intervals (CI), just like in case of the grouping by Tb.
Between-study heterogeneity was tested with Q homogeneity test (P values of less than 0.05 were considered as indicators of significant heterogeneity) and with I2 statistical test, where I2 is the proportion of total variation attributable to between-study variability (an I2 value of more than 50 was considered as indicating considerable heterogeneity). These two values were used to model selection purposes as well (fixed vs random). The tests revealed considerable heterogeneity in the overall study population (Q = 809.509; I2 = 89.25) and also in all three Tb groups, in particular Q = 270.447; I2 = 85.58 in the high, Q = 373.357; I2 = 90.63 in the medium, and Q = 36.843; I2 = 70.14 in the low Tb group. Consequently, we applied the random effect model in our forest plot and meta-regression analyses.
Publication bias was tested by inspecting the funnel plot. Meta-regression was performed to assess the overall effect of Tb to mortality. Except for the Pearson correlation analysis for which Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) was used, all analyses were performed with the Comprehensive Meta-Analysis software (Biostat, Inc., Engelwood, MJ, USA).
Results
Study selection
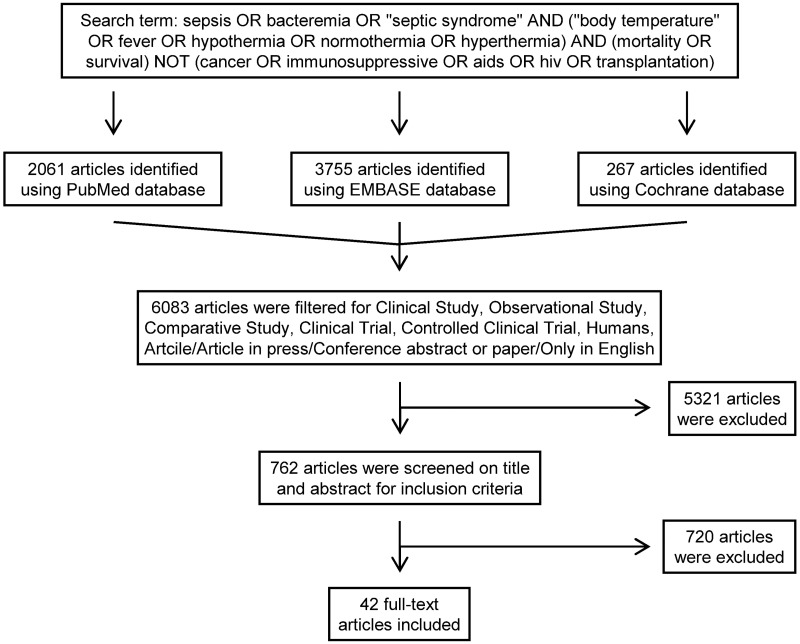
The flow chart of the study selection is presented in Fig 1. Until February 29, 2016 the electronic literature search identified altogether 6,083 studies from the PubMed, EMBASE, and Cochrane databases. After enabling filters for human studies and English language, 762 articles remained, which were screened on title and abstract for inclusion criteria. In 720 studies Tb or mortality rate was not suitably reported in the septic patients, these were also excluded, as a result 42 full-text publications were found eligible for statistical analysis which included data from a total of 10,834 septic patients.
Fig 1. Flowchart of study selection and inclusion.
Incidence of mortality in septic patients with fever, normothermia, and hypothermia
As a rude approach, first we performed a common (Pearson) correlation analysis between Tb and mortality rate of all septic patients. A weak negative linear correlation was found (y = -0.0909x + 3.6902; R2 = 0.2794), which suggests an association between Tb and mortality in sepsis. This method, however, did not allow us to weight the collected data according to the size of the studied populations, thus a detailed meta-analysis was needed.
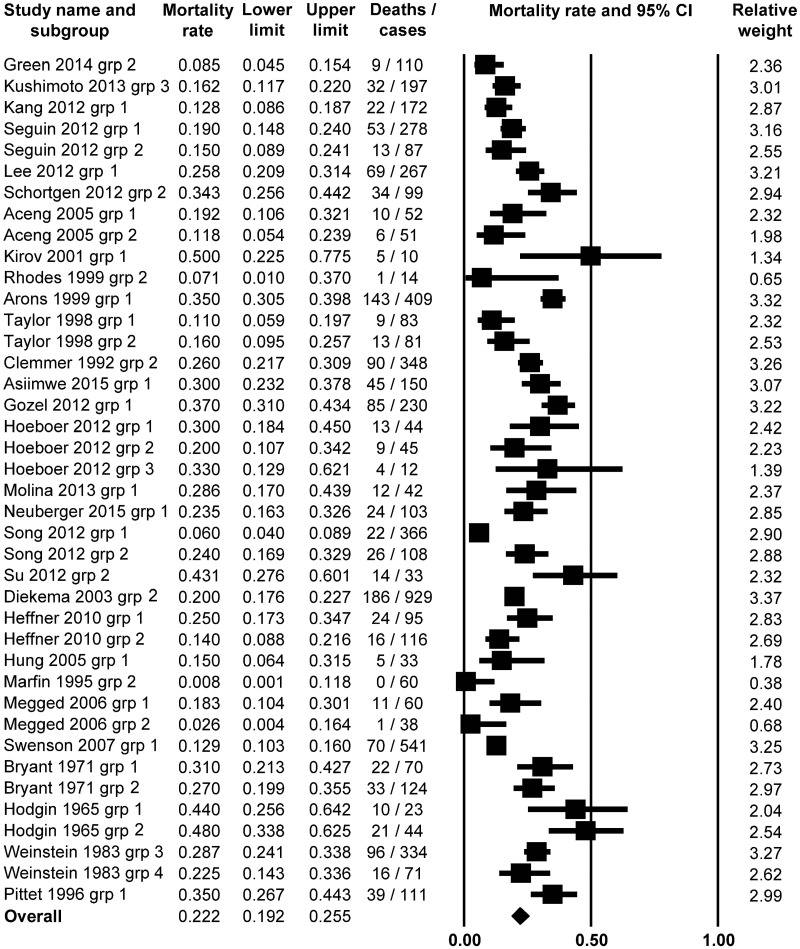
First, we investigated the incidence of mortality in fever associated with sepsis. We found 29 studies [18–20, 23, 26–50], in which the authors reported fever (defined as Tb > 38.0°C) in sepsis. From these studies, 40 groups of septic patients could be separated and included in the analysis with the random effect model. The meta-analysis of the mortality rates in the septic patients with fever revealed an average event rate of 22.2% (95% CI, 19.2–25.5%; Z = -13.4331) (Fig 2). This percentage was significantly (P = 0.000) lower than the 50% chance of mortality, which could be regarded as a random outcome.
Fig 2. Forest plot analysis of mortality rate using random-effects model in septic patients with fever (body temperature above 38.0°C; n = 6,040).
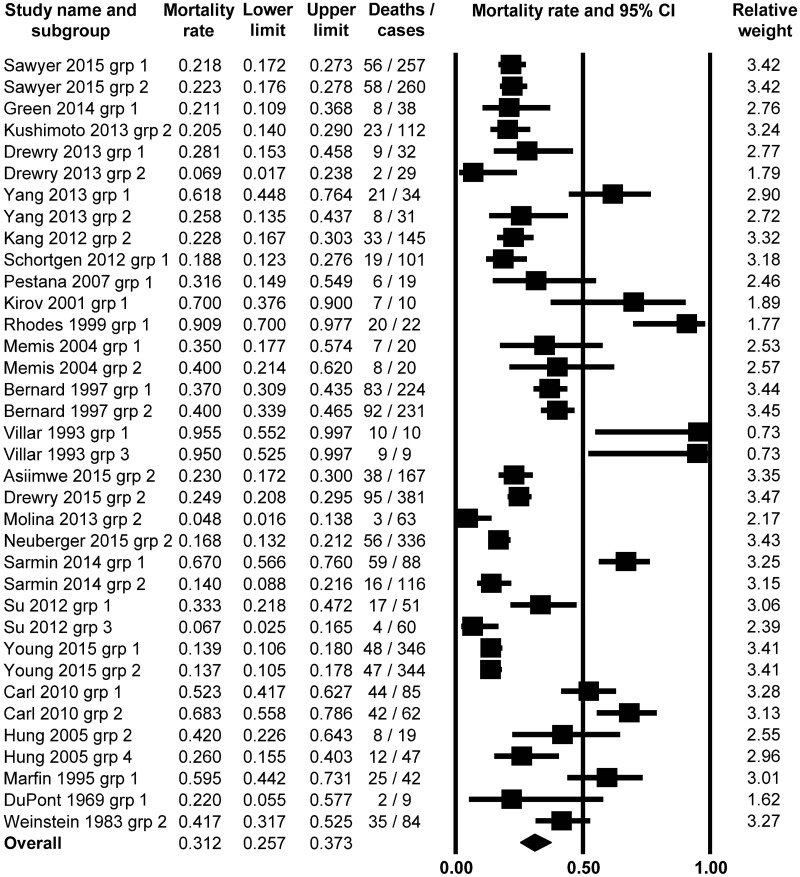
Next, we analyzed the mortality ratios of patients who developed neither fever nor hypothermia in association with sepsis, therefore this population could be regarded as normothermic (Tb = 36.0–38.0°C). From the 25 studies, in which normal Tb was reported in the septic patients [20, 22, 24, 28, 31, 35–37, 39–41, 43, 44, 47, 50–60], 36 subgroups of patients were separated, which were then analyzed with the random effect model. We found that the average mortality ratio was 31.2% (95% CI, 25.7–37.3%; Z = -5.7089) (Fig 3), which was higher than in the fever group. The mortality rate was significantly (P < 0.001) lower than 50% in this study population.
Fig 3. Forest plot analysis of mortality rate using random-effects model in septic patients with normothermia (body temperature between 36.1 and 38.0°C; n = 3,904).
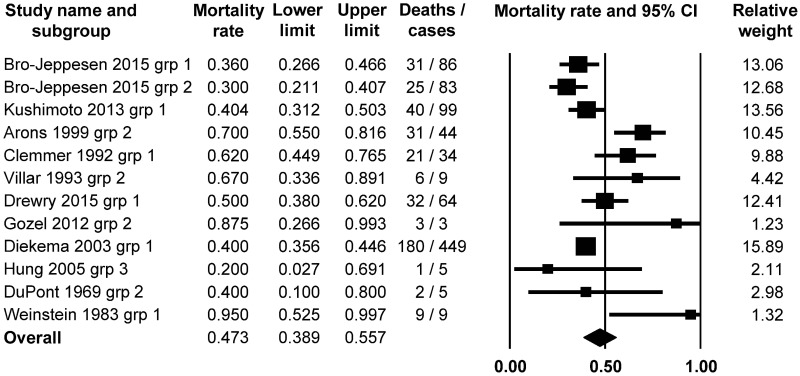
Then, we examined the incidence of mortality in hypothermic (Tb < 36.0°C) septic patients. We identified 11 studies [20, 22–24, 27, 29, 30, 35, 50, 54, 61], which included data on both Tb and mortality in septic patients. From these, the patients could be divided in 12 subgroups, which served as the basis of the meta-analysis. The random effect model revealed that the average mortality rate was the highest, 47.3% (95% CI, 38.9–55.7; Z = 0.520491) in the hypothermic patients (Fig 4), which did not significantly differ from the 50% random chance (P = 0.603).
Fig 4. Forest plot analysis of mortality rate using random-effects model in septic patients with hypothermia (body temperature up to 36.0°C; n = 890).
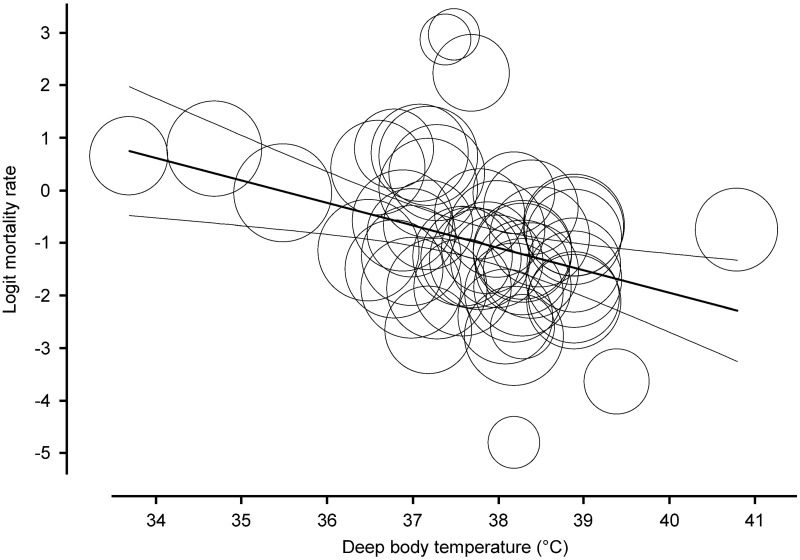
As a further statistical approach, we also performed a meta-regression analysis on the collected data. We found a significant (P < 0.001) negative linear correlation between Tb and mortality rate (regression coefficient: -0.4318; 95% CI, -0.6699 - -0.1938) based on 51 studies included in the analysis (Fig 5).
Fig 5. Meta-regression analysis of the association between body temperature and mortality ratio in septic patients (n = 10, 834).
Last, we divided the patients into quartiles (Q1-Q4) of mortality ratios (Q1: 0–25, Q2: 26–50, Q3: 51–75, and Q4: 76–100%) and calculated the average Tb for each mortality quartile. The weighted average Tbs were 38.1 (95% CI, 37.9–38.4°C), 37.8 (95% CI, 37.5–38.2°C), 37.6 (95% CI, 36.5–38.7°C), and 37.1°C (95% CI, 36.7–37.4°C) in the Q1, Q2, Q3, and Q4 groups, respectively. These results also indicate that in sepsis a higher Tb is associated with better outcome, while a lower Tb is related with higher risk of mortality. Of note, the Tbs in Q1 and Q4 (i.e., in the groups with lowest and highest mortality, respectively) are clearly distinct from each other, as the 95% CIs do not overlap.
Discussion
In the current analysis we revealed a clear association between Tb and mortality in septic patients by using a detailed statistical approach which was based on an extensive literature search of previous human studies. We found that the presence of fever reduces, while that of hypothermia promotes mortality in septic patients as compared to normothermic subjects.
From previous studies of septic patients only limited information is available to show an association between Tb and mortality in a wide temperature range. While a worse outcome was consistently found to be related to hypothermia [20, 23, 24, 27], those studies compared hypothermia with fever [23, 27] or with nonhypothermic (i.e., by merging febrile and normothermic) patients [20, 24]. In one study, no association was found between hypothermia and the clinical outcome of sepsis [19]. Regarding the role of fever, different clinical trials came to controversial results in sepsis. Several authors showed that a higher Tb was beneficial [18, 19, 50, 62], others that it was disadvantageous [44, 63], and a few found no association between fever and mortality [20, 60]. The discrepancy among the studies may result from the limited sample size used in the trials. Another explanation for not detecting significant association could be the insensitivity of the used statistical method. Indeed, when we first performed a commonly used regression analysis, the Pearson correlation, which did not allow us to weight the collected data for example for sample size, we found only a very weak negative correlation (P = 0.047) between Tb and mortality ratio in sepsis. Thus, we applied more precise statistical tools (forest plot and meta-regression analyses).
In our analyses, we used a sizeable, heterogeneous population of septic patients (n = 10,834) with a wide range of Tb (33.0–39.9°C). We showed that in sepsis mortality rates are lower if fever is present and higher in cases of hypothermia as compared to the normothermic group. In addition, we demonstrated a strong negative correlation (P = 0.0004) between Tb and mortality ratio with the help of meta-regression analysis. In our statistical approach, we also included a substantial number of septic patient groups with average Tbs within the normal range (n = 3,904). Furthermore, when we calculated the mean Tbs of septic patients in the mortality quartiles, we found that it gradually decreased from the lowest to the highest quartile and it was significantly higher in the lowest (0–25%) than in the highest quartile (75–100%) of mortality (38.1 ± 0.1 vs 37.1 ± 0.2°C for mean ± SEM, respectively). Taken together the results from all of our statistical approaches, our data strongly suggest a predictive role of Tb for the outcome of sepsis.
Sepsis continues to constitute a major challenge in critical care medicine [1–4]. As a systemic inflammation process, sepsis is frequently accompanied by abnormalities of Tb, like fever and hypothermia. In animal experiments, lower doses of endotoxin usually cause fever, whilst higher doses lead to the development of hypothermia [64, 65], indicating that the severity of the disease determines the change in Tb and not the way around. Based on our statistical analyses of human studies fever seems beneficial, but hypothermia rather disadvantageous for the organism regarding the outcome. However, it has to be noted that the current analysis does not allow us to conclude that the change of Tb per se is responsible for the lower and higher mortality rates in fever and hypothermia, respectively. Instead of a cause-effect relationship, the abnormal Tb should be rather regarded as a prognostic vital parameter of the severity and progress of the inflammation, and as such, as a warning sign, which can help doctors to asses the outcome of to the infection.
With regard to the adaptive biological value of Tb alterations in mammals, the development of fever in systemic inflammation is considered to indicate the activation of defense mechanisms of the body to fight the intruding agent [6–8]. By enhancing immune functions and accelerating the elimination of the microorganism from the body, at the onset of the inflammation fever is an adaptive, beneficial thermoregulatory response, although it involves a higher energy cost [6–8]. Therefore, fever itself is assumed to have a direct, advantageous effect on the mortality ratio in systemic inflammation (e.g., sepsis), when it is affordable for the host. However, Tb regulation should be considered in the framework of complex energy balance [66], therefore, the beneficial value of fever as an energetically expensive defense response is doubtable when there is a risk of energy deficiency, which often develops as the severity of the disease further progresses. In support of that, the administration of antipyretics resulted in an increase of mortality rate of critically ill patients in prospective clinical trials [38, 67]. However, in severe sepsis or septic shock, the use of pharmacological antipyretics did not influence mortality [51, 68], while fever control with external cooling decreased early mortality in human studies [44].
Spontaneous hypothermia represents a distinct, adaptive mechanism to systemic inflammation in experimental animals [69] and in septic patients [17]. It characteristically develops in severe cases of already progressed diseases, when—instead of actively coping with the microorganism—the organism attempts to increase survival by saving its energy resources [6, 8]. A recent study by Fonseca et al. [17] revealed that spontaneous hypothermia is a transient, self-limiting, and nonterminal event in human sepsis, which underlies its biological value as an adaptive mechanism in the critically ill patients.
Although the results of our analysis showed that hypothermia is associated with higher mortality, it should be noted that we can not be sure how mortality ratio of the patients would have changed if hypothermia had not developed or if the patients were rewarmed. As of today, to our knowledge, the effect of rewarming vs non-rewarming on the mortality of septic patients with spontaneous hypothermia has not been compared in randomized controlled trials. Therefore, hypothermia in itself should not be regarded harmful for the body as the associated higher mortality rate of the septic patients is presumably due to their more severe clinical condition. We suggest that the difference between the mortality rates of febrile and hypothermic patients with sepsis is due to the different severity and progression of the inflammation and not due to Tb itself. As a consequence, Tb itself serves not as a detrimental factor, but instead, as an indicative predictor for the severity of the disease and as such for mortality in sepsis.
From a clinical perspective, our results highlight the importance of precise and regular measurements of deep Tb, since its abnormalities can help physicians—especially in critical care medicine—not only in the diagnosis, but also in the follow up of the progression, and in the prognosis of sepsis. Based on our findings, it would be worth to consider that hypothermia should be weighted differently than fever and not equally as currently used in many scoring systems (e.g., SIRS, APACHE, PIRO), since hypothermia indicates a more severe stage of sepsis, and, therefore it is associated with worse clinical outcome. Regarding therapeutic interventions, the Tb management of septic patients should be always carefully evaluated and perhaps guidelines could be established (e.g., for the initiation of antipyretic treatment) to improve the clinical outcome in sepsis.
Conclusions
The abnormalities of deep Tb are strongly associated with the clinical outcome in sepsis. The mortality ratio of febrile patients is lower, while in patients with hypothermia it is markedly higher than that of patients with normal Tb. In cases of sepsis, there is a strong negative correlation between the mortality ratio and deep Tb in a wide temperature range. Septic patients with the lowest (< 25%) chance of mortality have significantly higher deep Tb than those who belong to the highest mortality quartile (> 75%).
Strengths and Limitations
Our meta-analysis included data from a total of 10,834 septic patients with overall 2,724 mortality events. We believe that our search strategy was adequately broad and included the three main databases of human studies. As result, 42 full-text articles could be indentified and used in our analyses. Although the sample size and the overall event rate can be considered large enough to draw solid conclusions about the association of Tb and mortality rate in sepsis, our study has certain limitations.
First, due to the nature of the meta-analysis method, we have studied the reported mean Tbs in populations of patients, rather than the association between Tb and the outcome of sepsis in individual patients. The latter approach would certainly allow one to draw firmer conclusions about the association between Tb and mortality, but it would also necessitate access to the original data of the analyzed articles, which is not feasible. Alternatively, a well-designed clinical trial with a big sample size could also provide high-quality individual data and based on our results it can be warranted to conduct such trials.
Second, the studied population of patients is quite diverse, which diversity could also have its own impact on the results. For example, Tb measurements were performed in different ways and not at the same time points in the analyzed studies. Despite such differences, we believe that the size of the analyzed sample was big enough to mitigate the methodological differences among the studies and to allow for drawing conclusions about the association of Tb and mortality in the septic patients.
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This research has been supported by the Hungarian Scientific Research Fund (grant PD 105532 to AG), the Medical School, University of Pecs (grant KA-2016-15 to AG), and the New National Excellence Program of the Hungarian Ministry of Human Capacities (UNKP-16-4-III to AG).
References
- 1.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016; 193(3):259–72. Epub 2015/09/29. 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- 2.Esteban A, Frutos-Vivar F, Ferguson ND, Penuelas O, Lorente JA, Gordo F, et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007; 35(5):1284–9. Epub 2007/04/07. 10.1097/01.CCM.0000260960.94300.DE [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Marshall JC, Namendys-Silva SA, Francois B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014; 2(5):380–6. Epub 2014/04/18. 10.1016/S2213-2600(14)70061-X [DOI] [PubMed] [Google Scholar]
- 4.Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States. A population-based study. Ann Am Thorac Soc. 2015; 12(2):216–20. Epub 2015/01/09. 10.1513/AnnalsATS.201411-498BC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016; 315(8):801–10. Epub 2016/02/24. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, et al. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci. 2005; 10:2193–216. Epub 2005/06/23. [DOI] [PubMed] [Google Scholar]
- 7.Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci. 2012; 15(8):1088–95. Epub 2012/07/28. 10.1038/nn.3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romanovsky AA, Szekely M. Fever and hypothermia: two adaptive thermoregulatory responses to systemic inflammation. Med Hypotheses. 1998; 50(3):219–26. Epub 1998/05/13. [DOI] [PubMed] [Google Scholar]
- 9.Cunha BA. With sepsis: If fever is good, then hypothermia is bad! Crit Care Med. 2012; 40(10):2926 Epub 2012/09/19. 10.1097/CCM.0b013e31825f78aa [DOI] [PubMed] [Google Scholar]
- 10.Kushimoto S, Yamanouchi S, Endo T, Sato T, Nomura R, Fujita M, et al. Body temperature abnormalities in non-neurological critically ill patients: a review of the literature. J Intensive Care. 2014; 2(1):14 Epub 2014/12/19. 10.1186/2052-0492-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992; 101(6):1644–55. Epub 1992/06/01. [DOI] [PubMed] [Google Scholar]
- 12.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985; 13(10):818–29. Epub 1985/10/01. [PubMed] [Google Scholar]
- 13.Opal SM. Concept of PIRO as a new conceptual framework to understand sepsis. Pediatr Crit Care Med. 2005; 6(3 Suppl):S55–60. Epub 2005/04/29. 10.1097/01.PCC.0000161580.79526.4C [DOI] [PubMed] [Google Scholar]
- 14.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993; 270(24):2957–63. Epub 1993/12/22. [DOI] [PubMed] [Google Scholar]
- 15.Beverly A, Walter E, Carraretto M. Management of hyperthermia and hypothermia in sepsis: A recent survey of current practice across UK intensive care units. J Intensive Care Soc. 2016; 17(1):88–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corrigan JJ, Fonseca MT, Flatow EA, Lewis K, Steiner AA. Hypometabolism and hypothermia in the rat model of endotoxic shock: independence of circulatory hypoxia. J Physiol. 2014; 592(17):3901–16. Epub 2014/06/22. 10.1113/jphysiol.2014.277277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fonseca MT, Rodrigues AC, Cezar LC, Fujita A, Soriano FG, Steiner AA. Spontaneous hypothermia in human sepsis is a transient, self-limiting, and nonterminal response. J Appl Physiol (1985). 2016; 120(12):1394–401. Epub 2016/03/19. [DOI] [PubMed] [Google Scholar]
- 18.Bryant RE, Hood AF, Hood CE, Koenig MG. Factors affecting mortality of gram-negative rod bacteremia. Arch Intern Med. 1971; 127(1):120–8. Epub 1971/01/01. [PubMed] [Google Scholar]
- 19.Megged O, Yinnon AM, Raveh D, Rudensky B, Schlesinger Y. Group A streptococcus bacteraemia: comparison of adults and children in a single medical centre. Clin Microbiol Infect. 2006; 12(2):156–62. Epub 2006/01/31. 10.1111/j.1469-0691.2005.01311.x [DOI] [PubMed] [Google Scholar]
- 20.Kushimoto S, Gando S, Saitoh D, Mayumi T, Ogura H, Fujishima S, et al. The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. Crit Care. 2013; 17(6):R271 Epub 2013/11/14. 10.1186/cc13106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blair E, Henning G, Hornick R, Cowley RA. Hypothermia in Bacteremic Shock. Arch Surg. 1964; 89:619–29. Epub 1964/10/01. [DOI] [PubMed] [Google Scholar]
- 22.Villar J, Slutsky AS. Effects of induced hypothermia in patients with septic adult respiratory distress syndrome. Resuscitation. 1993; 26(2):183–92. Epub 1993/10/01. [DOI] [PubMed] [Google Scholar]
- 23.Clemmer TP, Fisher CJ Jr., Bone RC, Slotman GJ, Metz CA, Thomas FO. Hypothermia in the sepsis syndrome and clinical outcome. The Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1992; 20(10):1395–401. Epub 1992/10/11. [DOI] [PubMed] [Google Scholar]
- 24.Drewry AM, Fuller BM, Skrupky LP, Hotchkiss RS. The presence of hypothermia within 24 hours of sepsis diagnosis predicts persistent lymphopenia. Crit Care Med. 2015; 43(6):1165–9. Epub 2015/03/21. 10.1097/CCM.0000000000000940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015; 349:g7647 Epub 2015/01/04. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 26.Aceng JR, Byarugaba JS, Tumwine JK. Rectal artemether versus intravenous quinine for the treatment of cerebral malaria in children in Uganda: randomised clinical trial. BMJ. 2005; 330(7487):334 Epub 2005/02/12. 10.1136/bmj.330.7487.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arons MM, Wheeler AP, Bernard GR, Christman BW, Russell JA, Schein R, et al. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Ibuprofen in Sepsis Study Group. Crit Care Med. 1999; 27(4):699–707. Epub 1999/05/13. [DOI] [PubMed] [Google Scholar]
- 28.Asiimwe SB, Abdallah A, Ssekitoleko R. A simple prognostic index based on admission vital signs data among patients with sepsis in a resource-limited setting. Crit Care. 2015; 19:86 Epub 2015/04/19. 10.1186/s13054-015-0826-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, Doern GV. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol. 2003; 41(8):3655–60. Epub 2003/08/09. 10.1128/JCM.41.8.3655-3660.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gozel MG, Erbay A, Bodur H, Eren SS. Risk factors for mortality in patients with nosocomial gram-negative bacteremia. Turkiye Klinikleri J Med Sci. 2012; 32(6):1641–7. [Google Scholar]
- 31.Green JE, Ariathianto Y, Wong SM, Aboltins C, Lim K. Clinical and inflammatory response to bloodstream infections in octogenarians. BMC Geriatr. 2014; 14:55 Epub 2014/04/24. 10.1186/1471-2318-14-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heffner AC, Horton JM, Marchick MR, Jones AE. Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clin Infect Dis. 2010; 50(6):814–20. Epub 2010/02/11. 10.1086/650580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodgin UG, Sanford JP. Gram-negative rod bacteremia. An analysis of 100 patients. Am J Med. 1965; 39(6):952–60. Epub 1965/12/01. [DOI] [PubMed] [Google Scholar]
- 34.Hoeboer SH, Alberts E, van den Hul I, Tacx AN, Debets-Ossenkopp YJ, Groeneveld AB. Old and new biomarkers for predicting high and low risk microbial infection in critically ill patients with new onset fever: a case for procalcitonin. J Infect. 2012; 64(5):484–93. Epub 2012/01/18. 10.1016/j.jinf.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 35.Hung MN, Chen SY, Wang JL, Chang SC, Hsueh PR, Liao CH, et al. Community-acquired anaerobic bacteremia in adults: one-year experience in a medical center. J Microbiol Immunol Infect. 2005; 38(6):436–43. Epub 2005/12/13. [PubMed] [Google Scholar]
- 36.Kang MJ, Shin TG, Jo IJ, Jeon K, Suh GY, Sim MS, et al. Factors influencing compliance with early resuscitation bundle in the management of severe sepsis and septic shock. Shock. 2012; 38(5):474–9. Epub 2012/10/09. 10.1097/SHK.0b013e31826eea2b [DOI] [PubMed] [Google Scholar]
- 37.Kirov MY, Evgenov OV, Evgenov NV, Egorina EM, Sovershaev MA, Sveinbjornsson B, et al. Infusion of methylene blue in human septic shock: a pilot, randomized, controlled study. Crit Care Med. 2001; 29(10):1860–7. Epub 2001/10/06. [DOI] [PubMed] [Google Scholar]
- 38.Lee BH, Inui D, Suh GY, Kim JY, Kwon JY, Park J, et al. Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: multi-centered prospective observational study. Crit Care. 2012; 16(1):R33 Epub 2012/03/01. 10.1186/cc11211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marfin AA, Sporrer J, Moore PS, Siefkin AD. Risk factors for adverse outcome in persons with pneumococcal pneumonia. Chest. 1995; 107(2):457–62. Epub 1995/02/01. [DOI] [PubMed] [Google Scholar]
- 40.Molina J, Penuela I, Lepe JA, Gutierrez-Pizarraya A, Gomez MJ, Garcia-Cabrera E, et al. Mortality and hospital stay related to coagulase-negative Staphylococci bacteremia in non-critical patients. J Infect. 2013; 66(2):155–62. Epub 2012/10/30. 10.1016/j.jinf.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 41.Neuberger A, Yahav D, Daitch V, Akayzen Y, Farbman L, Avni T, et al. The significance of persistent fever in the treatment of suspected bacterial infections among inpatients: a prospective cohort study. Eur J Clin Microbiol Infect Dis. 2015; 34(4):805–10. Epub 2014/12/17. 10.1007/s10096-014-2288-3 [DOI] [PubMed] [Google Scholar]
- 42.Pittet D, Thievent B, Wenzel RP, Li N, Auckenthaler R, Suter PM. Bedside prediction of mortality from bacteremic sepsis. A dynamic analysis of ICU patients. Am J Respir Crit Care Med. 1996; 153(2):684–93. Epub 1996/02/01. 10.1164/ajrccm.153.2.8564118 [DOI] [PubMed] [Google Scholar]
- 43.Rhodes A, Lamb FJ, Malagon I, Newman PJ, Grounds RM, Bennett ED. A prospective study of the use of a dobutamine stress test to identify outcome in patients with sepsis, severe sepsis, or septic shock. Crit Care Med. 1999; 27(11):2361–6. Epub 1999/12/01. [DOI] [PubMed] [Google Scholar]
- 44.Schortgen F, Clabault K, Katsahian S, Devaquet J, Mercat A, Deye N, et al. Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med. 2012; 185(10):1088–95. Epub 2012/03/01. 10.1164/rccm.201110-1820OC [DOI] [PubMed] [Google Scholar]
- 45.Seguin P, Roquilly A, Mimoz O, Le Maguet P, Asehnoune K, Biederman S, et al. Risk factors and outcomes for prolonged versus brief fever: a prospective cohort study. Crit Care. 2012; 16(4):R150 Epub 2012/08/15. 10.1186/cc11465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song YH, Shin TG, Kang MJ, Sim MS, Jo IJ, Song KJ, et al. Predicting factors associated with clinical deterioration of sepsis patients with intermediate levels of serum lactate. Shock. 2012; 38(3):249–54. Epub 2012/06/12. 10.1097/SHK.0b013e3182613e33 [DOI] [PubMed] [Google Scholar]
- 47.Su L, Han B, Liu C, Liang L, Jiang Z, Deng J, et al. Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: a prospective cohort study. BMC Infect Dis. 2012; 12:157 Epub 2012/07/20. 10.1186/1471-2334-12-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swenson BR, Hedrick TL, Popovsky K, Pruett TL, Sawyer RG. Is fever protective in surgical patients with bloodstream infection? J Am Coll Surg. 2007; 204(5):815–21; discussion 22–3. Epub 2007/05/08. 10.1016/j.jamcollsurg.2007.01.033 [DOI] [PubMed] [Google Scholar]
- 49.Taylor TE, Wills BA, Courval JM, Molyneux ME. Intramuscular artemether vs intravenous quinine: an open, randomized trial in Malawian children with cerebral malaria. Trop Med Int Health. 1998; 3(1):3–8. Epub 1998/03/04. [DOI] [PubMed] [Google Scholar]
- 50.Weinstein MP, Murphy JR, Reller LB, Lichtenstein KA. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. II. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis. 1983; 5(1):54–70. Epub 1983/01/01. [DOI] [PubMed] [Google Scholar]
- 51.Bernard GR, Wheeler AP, Russell JA, Schein R, Summer WR, Steinberg KP, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med. 1997; 336(13):912–8. Epub 1997/03/27. 10.1056/NEJM199703273361303 [DOI] [PubMed] [Google Scholar]
- 52.Carl DE, Grossman C, Behnke M, Sessler CN, Gehr TW. Effect of timing of dialysis on mortality in critically ill, septic patients with acute renal failure. Hemodial Int. 2010; 14(1):11–7. Epub 2010/04/10. 10.1111/j.1542-4758.2009.00407.x [DOI] [PubMed] [Google Scholar]
- 53.Drewry AM, Fuller BM, Bailey TC, Hotchkiss RS. Body temperature patterns as a predictor of hospital-acquired sepsis in afebrile adult intensive care unit patients: a case-control study. Crit Care. 2013; 17(5):R200 Epub 2013/09/14. 10.1186/cc12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DuPont HL, Spink WW. Infections due to gram-negative organisms: an analysis of 860 patients with bacteremia at the University of Minnesota Medical Center, 1958–1966. Medicine (Baltimore). 1969; 48(4):307–32. Epub 1969/07/01. [DOI] [PubMed] [Google Scholar]
- 55.Memis D, Karamanlioglu B, Turan A, Koyuncu O, Pamukcu Z. Effects of lornoxicam on the physiology of severe sepsis. Crit Care. 2004; 8(6):R474–82. Epub 2004/11/30. 10.1186/cc2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pestana D, Casanova E, Villagran MJ, Tormo C, Perez-Chrzanowska H, Redondo J, et al. Continuous hemofiltration in hyperthermic septic shock patients. J Trauma. 2007; 63(4):751–6. Epub 2007/12/20. 10.1097/TA.0b013e31802b9575 [DOI] [PubMed] [Google Scholar]
- 57.Sarmin M, Ahmed T, Bardhan PK, Chisti MJ. Specialist hospital study shows that septic shock and drowsiness predict mortality in children under five with diarrhoea. Acta Paediatr. 2014; 103(7):e306–11. Epub 2014/03/26. 10.1111/apa.12640 [DOI] [PubMed] [Google Scholar]
- 58.Sawyer RG, Claridge JA, Nathens AB, Rotstein OD, Duane TM, Evans HL, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015; 372(21):1996–2005. Epub 2015/05/21. 10.1056/NEJMoa1411162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang YL, Liu DW, Wang XT, Long Y, Zhou X, Chai WZ. Body temperature control in patients with refractory septic shock: too much may be harmful. Chin Med J (Engl). 2013; 126(10):1809–13. Epub 2013/05/16. [PubMed] [Google Scholar]
- 60.Young P, Saxena M, Bellomo R, Freebairn R, Hammond N, van Haren F, et al. Acetaminophen for Fever in Critically Ill Patients with Suspected Infection. N Engl J Med. 2015; 373(23):2215–24. Epub 2015/10/06. 10.1056/NEJMoa1508375 [DOI] [PubMed] [Google Scholar]
- 61.Bro-Jeppesen J, Kjaergaard J, Wanscher M, Nielsen N, Friberg H, Bjerre M, et al. Systemic Inflammatory Response and Potential Prognostic Implications After Out-of-Hospital Cardiac Arrest: A Substudy of the Target Temperature Management Trial. Crit Care Med. 2015; 43(6):1223–32. Epub 2015/03/11. 10.1097/CCM.0000000000000937 [DOI] [PubMed] [Google Scholar]
- 62.Mackowiak PA, Browne RH, Southern PM Jr., Smith JW. Polymicrobial sepsis: an analysis of 184 cases using log linear models. Am J Med Sci. 1980; 280(2):73–80. Epub 1980/09/01. [DOI] [PubMed] [Google Scholar]
- 63.Manthous CA, Hall JB, Olson D, Singh M, Chatila W, Pohlman A, et al. Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med. 1995; 151(1):10–4. Epub 1995/01/01. 10.1164/ajrccm.151.1.7812538 [DOI] [PubMed] [Google Scholar]
- 64.Pakai E, Garami A, Nucci TB, Ivanov AI, Romanovsky AA. Hyperbilirubinemia exaggerates endotoxin-induced hypothermia. Cell Cycle. 2015; 14(8):1260–7. Epub 2015/03/17. 10.1080/15384101.2015.1014150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005; 289(5):R1244–52. Epub 2005/08/06. 10.1152/ajpregu.00370.2005 [DOI] [PubMed] [Google Scholar]
- 66.Garami A, Szekely M. Body temperature: Its regulation in framework of energy balance. Temperature (Austin). 2014; 1(1):28–9. Epub 2014/04/01. [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schulman CI, Namias N, Doherty J, Manning RJ, Li P, Elhaddad A, et al. The effect of antipyretic therapy upon outcomes in critically ill patients: a randomized, prospective study. Surg Infect (Larchmt). 2005; 6(4):369–75. Epub 2006/01/26. [DOI] [PubMed] [Google Scholar]
- 68.Mohr N, Skrupky L, Fuller B, Moy H, Alunday R, Wallendorf M, et al. Early antipyretic exposure does not increase mortality in patients with gram-negative severe sepsis: a retrospective cohort study. Intern Emerg Med. 2012; 7(5):463–70. Epub 2012/08/29. 10.1007/s11739-012-0848-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu E, Lewis K, Al-Saffar H, Krall CM, Singh A, Kulchitsky VA, et al. Naturally occurring hypothermia is more advantageous than fever in severe forms of lipopolysaccharide- and Escherichia coli-induced systemic inflammation. Am J Physiol Regul Integr Comp Physiol. 2012; 302(12):R1372–83. Epub 2012/04/20. 10.1152/ajpregu.00023.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.