Abstract
Background
Methylenetetrahydrofolate reductase gene (MTHFR C677T and A1298C) and methionine synthase gene (MS A2756G) polymorphisms have shown an association with male infertility risk in several ethnic populations. Although several studies have evaluated these associations in Chinese populations, their small sample sizes and inconsistent outcomes have prevented strong conclusions. Therefore, the present meta-analysis was performed with published studies to evaluate the associations of the three single nucleotide polymorphisms (SNPs) and male infertility in a Chinese population.
Methods
We conducted a search of PubMed, Embase, Web of Science, Chinese National Knowledge Infrastructure (CNKI), China biology medical literature (CBM), VIP, and Chinese literature (Wan Fang) databases up to May 31, 2016. Odds ratios (ORs) and 95% confidence intervals (95%CIs) were used to assess the strength of associations with a random-effect model or a fixed-effect model based on the heterogeneity analysis results. Sensitivity analysis was used to confirm the reliability and stability of the meta-analysis.
Results
A total of nine studies, including 1,713 cases and 1,104 controls, were included in the meta-analysis. The pooled results indicated that the MTHFR C667T polymorphism was significantly associated with increased risk of male infertility in the Chinese population in the allele model (T vs. C: OR = 1.47, 95%CI = 1.32–1.63), the dominant model (TT + CT vs. CC: OR = 1.51, 95%CI = 1.30–1.77), the additive model (TT vs. CC: OR = 2.08, 95%CI = 1.68–2.58) and the recessive model (TT vs. CT+CC: OR = 1.58, 95%CI = 1.31–1.90), whereas the MTHFR A1298C and MS A2756G polymorphisms were not risk factors. There was no significant heterogeneity in any genotype contrasts among the studies. The sensitivity analysis indicated that the results of this meta-analysis were relatively stable.
Conclusion
This study suggests that the MTHFR C667T polymorphism may contribute to the genetic susceptibility to male infertility in the Chinese population, whereas MTHFR A1298C and MS A2756G polymorphisms may be unrelated to male infertility. Studies with larger sample sizes and representative population-based cases and well-matched controls are needed to validate our results.
Introduction
Infertility is defined as the failure of a couple to achieve pregnancy after one year of unprotected, regular sexual intercourse, which affects approximately 15% of all couples attempting to conceive a child[1, 2]. In addition to environmental and lifestyle risk factors, genetic causes, such as chromosomal aberrations and single gene mutations, also play important roles in male infertility. Among the well-known genes that cause male infertility, such as FSHR[3], AR[4], PRM1[5], and GST[6], the folate-related enzyme genes are those most often involved.
Folate plays an important role in DNA synthesis, RNA synthesis, methylation reactions, and protein synthesis, which contribute to the maintenance of genome integrity[7, 8]. Several single-nucleotide polymorphisms (SNPs) of folate metabolism-related genes have been identified, including methylenetetrahydrofolate reductase (MTHFR; 607093) gene polymorphisms (MTHFR C677T, rs1801133 and MTHFR A1298C, rs1801131), a methionine synthase (MS; 156570) gene polymorphism (MS A2756G, rs1805087, also known as MTR A2756G), and a methionine synthase reductase (MTRR; 602568) gene polymorphism (MTRR A66G, rs1801394). These SNPs can affect the activity, stability, and level of folate metabolism-related enzymes, which may affect folate metabolism and DNA synthesis[9]. Folate metabolism disorder may lead to sperm DNA damage and spermatogenic failure[10].
To date, several studies have explored the associations between these SNPs and male infertility risk; however, their results are conflicting. As a result, several meta-analyses addressing these associations have been performed. Three recent meta-analyses consistently showed that the MTHFR C677T polymorphism was associated with a significantly increased male infertility risk in the Asian and overall populations but not the Caucasian population[11–13]. Two recent meta-analyses both showed that the MS A2756G polymorphism may be a genetic risk factor for idiopathic male infertility[13, 14]. Moreover, two recent meta-analyses were performed to examine the association between MTHFR A1298C and the risk of male infertility, the results were inconsistent[11, 13]. In the Chinese population, several studies have examined the associations between folate-related enzyme gene polymorphisms and the risk of male infertility; however, the results are inconclusive. Because the majority of relevant studies in the Chinese population were published in local Chinese journals, most international readers cannot access and/or read them. In addition, the recent meta-analyses do not include all relevant studies of Chinese populations[11–15]. Therefore, to evaluate the relationships between each of the three SNPs and male infertility risk within the Chinese population, we performed a meta-analysis including the most recent data in the literature. To our knowledge, this is the first meta-analysis performed on this topic in the Chinese population.
Methods
Search strategy
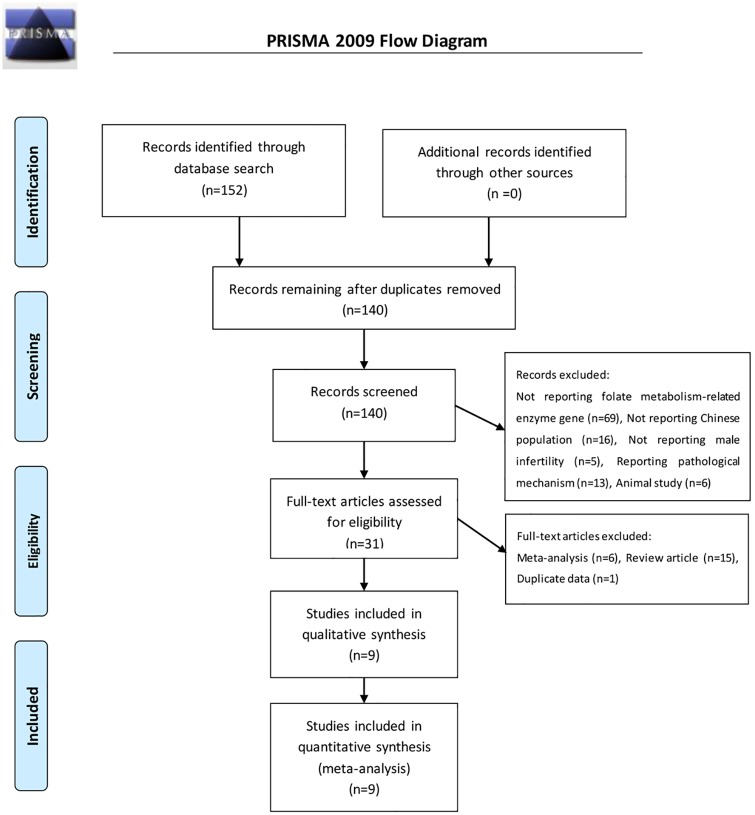
Two authors independently conducted a systematic literature search of the PubMed, Embase, Web of Science, Chinese National Knowledge Infrastructure (CNKI), China biology medical literature (CBM), VIP, and Chinese literature (Wan Fang) databases up to May 31, 2016. Search terms were as follows: “MTHFR or Methylenetetrahydrofolate reductase”, “MTR, MS or methionine synthase”, “SNP, polymorphism, mutation, or variant”, “male infertility”. In addition, the references of reviews and retrieved articles were reviewed to identify other eligible studies that were missed by the search. The search was limited to human subjects. The search strategy flowchart is shown in Fig 1.
Fig 1. Flowchart of the study selection procedure.
Inclusion and exclusion criteria
Only those studies meeting the following inclusive selection criteria were eligible: 1) The full text of the article was available. 2) The study was a case—control study evaluating at least one of the three SNPs. 3) The genotype distributions were available for both cases and controls. 4) There were no duplicate data. For studies that considered partially or fully duplicate data and that were by the same authors, we selected the study with the most subjects. 5) The published language was English or Chinese. 6) The study was of a Chinese population. 7) Genotypic distributions were available for the estimation of odds ratios (ORs) and 95% confidence intervals (CIs). Studies were excluded if any of the following criteria existed: 1) The study did not explore the association between any of the three SNPs and male infertility risk. 2) The article was an animal study, review article, meta-analysis, conference abstract or editorial article.
Quality assessment
The Newcastle-Ottawa Scale (NOS)[16] was used to assess the quality of the included studies. The NOS contains eight items for both cohort and case—control studies. The scale assesses the quality of case-control studies based on three areas: selection, comparability, and exposure. A “star” rating system is used to judge the methodological quality. Selection has a maximum of 4 stars, comparability has a maximum of 2 stars, and exposure has a maximum of 3 stars. The total scores ranged from 0 stars (worst) to 9 stars (best), and the quality of each study was graded as low (0–3), moderate (4–6), or high (7–9). Discrepant opinions were resolved by discussion and consensus.
Data extraction strategy
Two authors extracted the relevant data independently in compliance with the inclusion criteria. Extracted data were entered into a collection form and checked by a third author. Disagreement was solved by discussion and consensus. Data on the following variables for each study were extracted: 1) first author’s name, year of publication, region, and genotyping method; 2) sample sizes of the case and control groups; 3) genotype and allele frequencies; and 4) results of the Hardy—Weinberg equilibrium test.
Statistical analysis
The strength of the relationships between the MTHFR gene polymorphisms and male infertility risk were assessed using ORs and corresponding 95% CIs. The pooled ORs were calculated for the allele comparison model, dominant model, recessive model, and codominant model. The heterogeneity assumption was tested using the Chi-square-based Q test. Heterogeneity was considered significant at p<0.10, and I2 values of 25%, 50% and 75% corresponded to low, medium and high levels of heterogeneity, respectively. The significance of the pooled ORs were determined by the Z-test, and P<0.05 was considered statistically significant. The statistical analysis was performed with Reviewer Manager 5.3 and STATA 12.0. Potential publication bias was estimated using funnel plots and the Egger regression test. Sensitivity analysis was performed to evaluate the stability of the results. The pooled ORs were estimated by excluding one study each time to evaluate the influence of individual studies.
Results
Study characteristics
A total of 152 results were retrieved from the search of the PubMed, Embase, Web of Science, Chinese National Knowledge Infrastructure (CNKI), China biology medical literature (CBM), VIP, and Chinese literature (Wan Fang) databases. Three studies were excluded because they were meta-analyses as determined from reading the title and abstract. An additional two publications contained duplicate data and were published by the same author; the one with the most subjects was included in the present analysis. Nine case-control studies considering 1,713 cases and 1,104 controls met the inclusion criteria[17–25](Fig 1). Of these, all 9 studies addressed the MTHFR C667T polymorphism; 3 studies addressed MTHFR A1298C polymorphism, and 3 studies addressed the MS A2756G polymorphism. The year of publication ranged from 2007 to 2015. The Hardy-Weinberg test (HWE) was performed on all of the included studies, and HWE of the MTHFR C667T polymorphism was violated in one study[25]. The characteristics of each of the included studies are shown in Table 1. The quality of studies based on the NOS score is presented in Table 2.
Table 1. Characteristics of the studies included in the meta-analysis and their genotype distributions of the MTHFR C677T, MTHFR A1298C and MS A2756G gene polymorphisms.
| Study | Region | Genotyping method | case | control | case | control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | C | T | CC | CT | TT | C | T | HWE | ||||||
| MTHFRC667T | Ni et al.2015 | Zhejiang | SNaPshot | 296 | 204 | 117 | 135 | 44 | 369 | 223 | 84 | 94 | 26 | 262 | 146 | 0.970 |
| Li et al.2015 | Sichuan | Sequencing | 162 | 120 | 61 | 77 | 24 | 199 | 125 | 48 | 54 | 18 | 150 | 90 | 0.661 | |
| Li et al.2014 | Beijing | PCR-RFLP | 82 | 133 | 14 | 36 | 32 | 64 | 100 | 36 | 61 | 36 | 133 | 133 | 0.340 | |
| Pei et al.2013 | Henan | PCR | 190 | 90 | 39 | 138 | 113 | 216 | 364 | 24 | 47 | 19 | 95 | 85 | 0.651 | |
| Liu et al.2012 | Shenzhen | PCR | 75 | 72 | 27 | 38 | 10 | 92 | 58 | 40 | 28 | 4 | 108 | 36 | 0.753 | |
| Qiu et al.2011 | Ningxia | PCR | 271 | 180 | 75 | 112 | 84 | 262 | 280 | 63 | 85 | 32 | 211 | 149 | 0.720 | |
| Sun et al.2007 | Jilin | PCR | 182 | 53 | 27 | 86 | 69 | 140 | 224 | 15 | 28 | 10 | 58 | 48 | 0.630 | |
| Yang et al.2010 | Anhui | PCR-RFLP | 131 | 293 | 34 | 55 | 42 | 123 | 139 | 98 | 142 | 53 | 338 | 248 | <0.05 | |
| A et al.2007 | Sichuan | PCR-RFLP | 355 | 252 | 130 | 160 | 65 | 420 | 290 | 128 | 95 | 29 | 351 | 153 | 0.085 | |
| AA | AC | CC | A | C | AA | AC | CC | A | C | |||||||
| MTHFRA1298C | Ni et al.2015 | Zhejiang | SNaPshot | 296 | 204 | 181 | 106 | 9 | 468 | 124 | 137 | 62 | 5 | 336 | 72 | 0.515 |
| Li et al.2015 | Sichuan | Sequencing | 162 | 120 | 101 | 54 | 7 | 256 | 68 | 80 | 38 | 2 | 198 | 42 | 0.290 | |
| Li et al.2014 | Beijing | PCR-RFLP | 82 | 133 | 49 | 29 | 4 | 127 | 37 | 88 | 36 | 9 | 212 | 54 | 0.060 | |
| AA | AG | GG | A | G | AA | AG | GG | A | G | |||||||
| MS A2756G | Li et al.2015 | Sichuan | Sequencing | 162 | 120 | 124 | 35 | 3 | 283 | 41 | 101 | 17 | 2 | 219 | 21 | 0.220 |
| Ni et al.2015 | Zhejiang | SNaPshot | 296 | 204 | 245 | 47 | 4 | 537 | 55 | 163 | 37 | 4 | 363 | 45 | 0.280 | |
| Liu et al.2012 | Shenzhen | PCR | 75 | 72 | 60 | 14 | 1 | 134 | 16 | 61 | 11 | 0 | 133 | 11 | 0.480 | |
Table 2. Quality assessment for all of the included studies.
| First author | Publishing year | Selection | Comparability | Exposure | Total |
|---|---|---|---|---|---|
| Ni | 2015 | ★★★ | NA | ★★ | 5 |
| Li | 2015 | ★★★ | ★ | ★★ | 6 |
| Li | 2014 | ★★★ | ★★ | ★★ | 7 |
| Pei | 2013 | ★★★ | ★ | ★★ | 6 |
| Liu | 2012 | ★★★ | ★★ | ★★ | 7 |
| Qiu | 2011 | ★★ | ★ | ★★ | 5 |
| Sun | 2007 | ★★★ | ★ | ★★ | 6 |
| Yang | 2010 | ★★★ | NA | ★★ | 5 |
| A | 2007 | ★★★ | ★ | ★★ | 6 |
Association of the MTHFR C667T polymorphism with male infertility
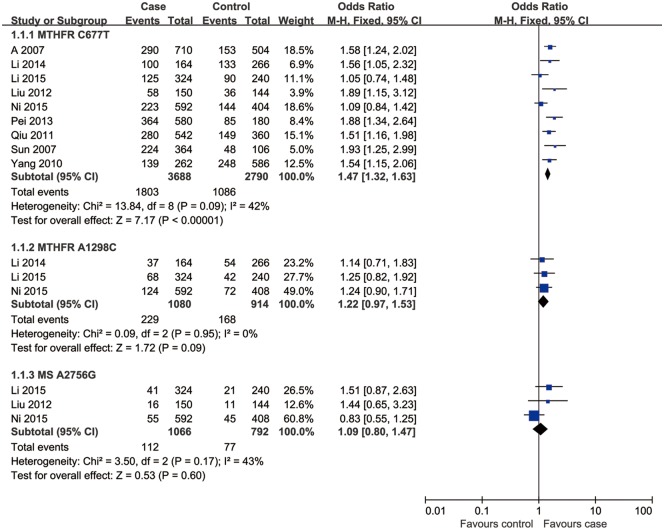
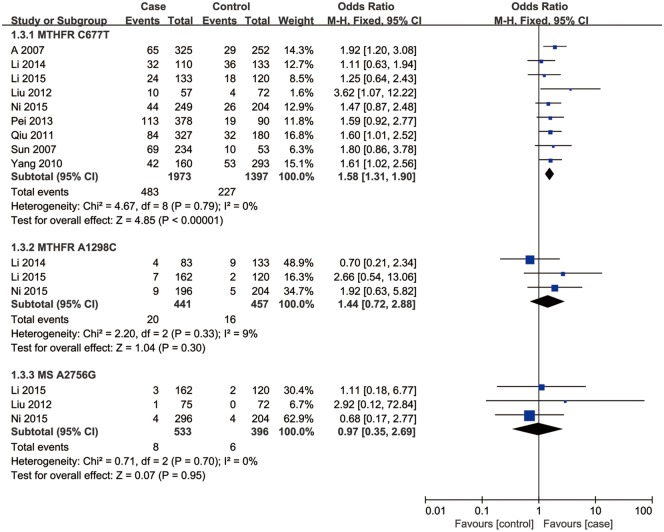
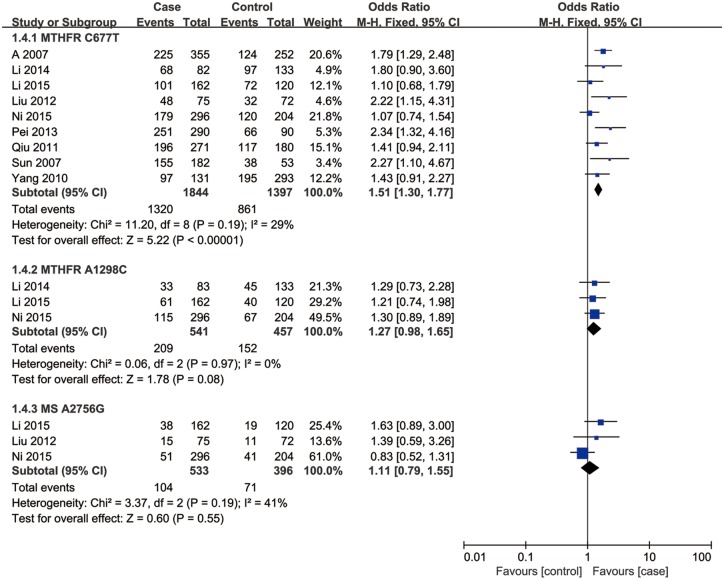
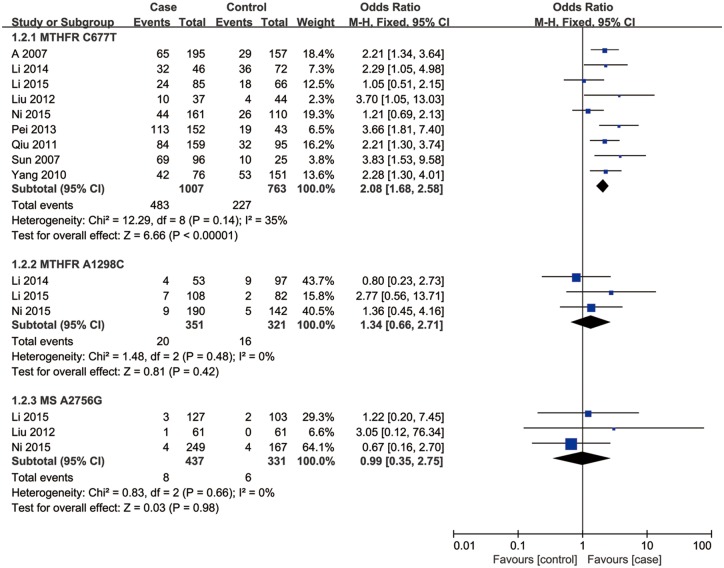
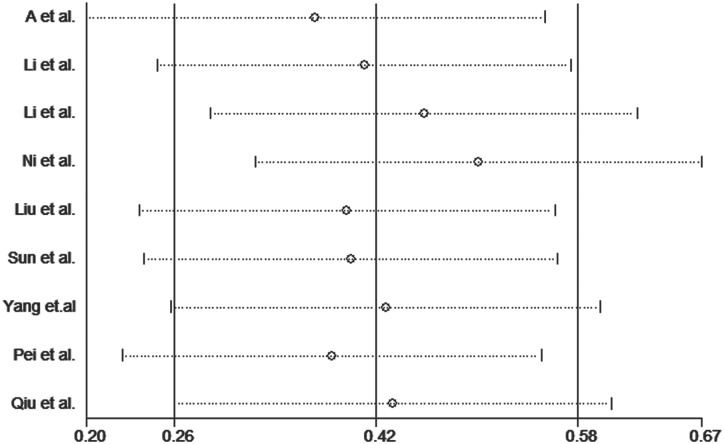
Nine studies involving a total of 2,817 individuals evaluated the influence of the MTHFR C667T polymorphism on the risk of male infertility. Figs 2–5 shows the meta-analysis results for the allele model (T/C), dominant model (TT+CT vs. CC), additive model (TT/CC) and recessive model (TT vs. CC+CT), for which the I2 value, representing the among-study heterogeneity, was 42%, 29%, 35%, and 0%, respectively. Thus, fixed-effects models were applied. Overall, the results revealed a significant association between the MTHFR C677T polymorphism and Chinese male infertility risk (T vs. C: OR = 1.47, 95%CI = 1.32–1.63; TT + CT vs. CC: OR = 1.51, 95%CI = 1.30–1.77; TT vs. CC: OR = 2.08, 95%CI = 1.68–2.58; TT vs. CT+CC: OR = 1.58, 95%CI = 1.31–1.90) (Figs 2–5).
Fig 2. Forest plot of the studies assessing the association between MTHFR C677T, MTHFR A1298C and MS A2756G polymorphisms and male infertility.
(allelic model: (a) T vs. C, (b) C vs. A, (c) G vs. A).
Fig 5. Forest plot of the studies assessing the association between MTHFR C677T, MTHFR A1298C and MS A2756G polymorphisms and male infertility.
(recessive model: (a) TT vs. CC+CT, (b) CC vs. AA+AC, (c) GG vs. AA+AG).
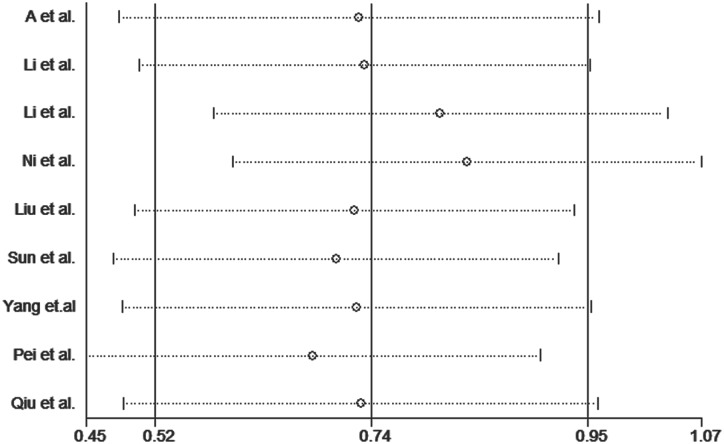
Fig 3. Forest plot of the studies assessing the association between MTHFR C677T, MTHFR A1298C and MS A2756G polymorphisms and male infertility.
(dominant model: (a) TT+CT vs. CC, (b) CC+AC vs. AA, (c) GG +AG vs. AA).
Fig 4. Forest plot of the studies assessing the association between MTHFR C677T, MTHFR A1298C and MS A2756G polymorphisms and male infertility.
(additive model: (a) TT vs. CC, (b) CC vs. AA, (c) GG vs. AA).
Association of MTHFR A1298C and MS A2756G polymorphisms with male infertility
Three studies including a total of 898 individuals evaluated the influence of the MTHFR A1298C polymorphism on the risk of male infertility. There was no significant heterogeneity in any genotype contrasts among the studies, and fixed-effects models were applied. Overall, the results revealed no association between the MTHFR A1298C polymorphism and Chinese male infertility risk in the allele model (C vs. A: OR = 1.22, 95%CI = 0.97–1.53, I2 = 0), dominant model (CC + AC vs. AA: OR = 1.27, 95%CI = 0.98–1.65, I2 = 0), additive model (CC vs. AA: OR = 1.34, 95%CI = 0.66–2.71, I2 = 0) or recessive model (CC vs. AC+AA: OR = 1.44, 95%CI = 0.72–2.88, I2 = 9) (Figs 2–5).
Three studies, including a total of 929 individuals, evaluated the influence of the MS A2756G polymorphism on the risk of male infertility. There was no significant heterogeneity in any genotype contrasts among the studies, and fixed-effects models were applied. Overall, the results revealed no association between the MS A2756G polymorphism and Chinese male infertility risk without heterogeneity in the additive model (GG vs. AA: OR = 0.99, 95%CI = 0.35–2.75, I2 = 0) or recessive model (GG vs. AG+AA: OR = 0.97, 95%CI = 0.35–2.69, I2 = 0) and no association between the polymorphism and infertility risk with low heterogeneity in the allele model (G vs. A: OR = 1.09, 95%CI = 0.80–1.47, I2 = 43) or dominant model (GG + AG vs. AA: OR = 1.11, 95%CI = 0.79–1.55, I2 = 41) (Figs 2–5).
Sensitivity and publication bias
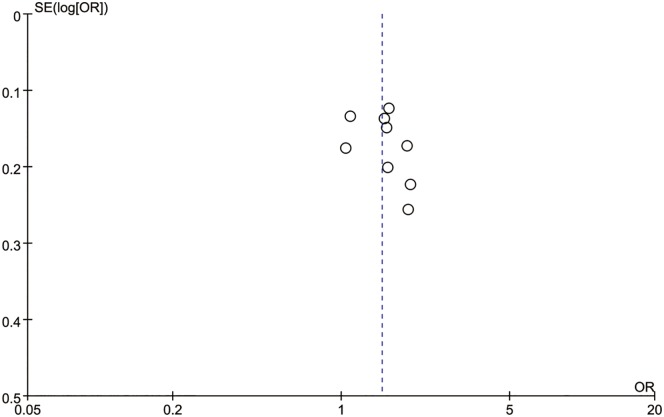
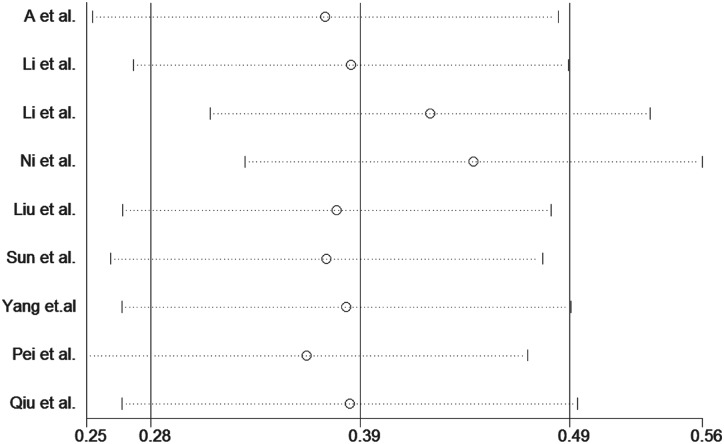
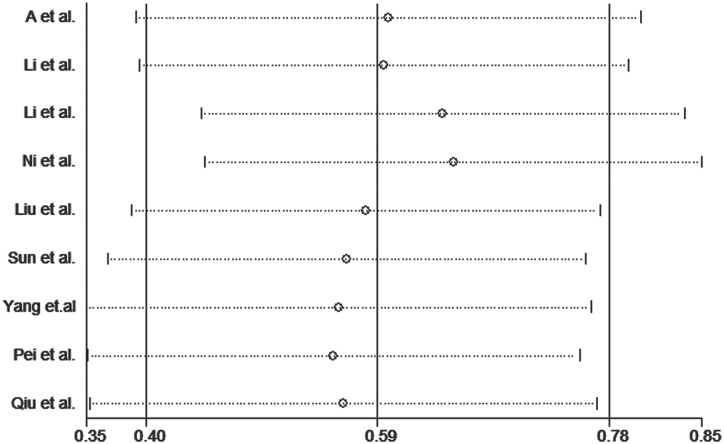
Publication bias was assessed for the MTHFR C667T polymorphism by funnel plots, Begg’s test and Egger’s test under all contrast models. The shape of the funnel plot did not indicate any evidence of obvious asymmetry in any contrast model for the MTHFR C667T polymorphism (Fig 6). In addition, Egger’s linear regression analysis suggested no evidence of publication bias (P = 0.99 for an allelic contrast model, P = 0.91 for a codominant model, P = 0.77 for a recessive model, and P = 0.51 for a dominant model) (Table 3). We did not produce funnel plots for the other two single nucleotide polymorphisms (SNPs) due to the limited number of studies on MTHFR A1298C and MS A2756G polymorphisms. The sensitivity analyses were conducted to calculate the pooled ORs by omitting one study each time. The results showed that no individual study influenced the overall pooled ORs (Figs 7–10), indicating that the results of this meta-analysis are relatively stable.
Fig 6. Funnel plot for the MTHFR C677T polymorphism and male infertility risk in the Chinese population.
(allelic model: T vs. C).
Table 3. Publication bias test for the MTHFR C677T polymorphism.
| Comparisons | Egger test | Begg test | ||
|---|---|---|---|---|
| Coefficient | P value | 95% CI | P value | |
| T vs. C | -0.04 | 0.99 | -7.51 7.42 | 0.47 |
| TT vs. CC | -0.09 | 0.91 | -1.85 1.68 | 0.12 |
| TT vs. CC+CT | -0.05 | 0.77 | -0.44 0.34 | 0.75 |
| CT+TT vs. CC | -1.8 | 0.51 | -7.98 4.38 | 0.47 |
Fig 7. Sensitivity analysis diagram for each study used to assess the relative risk estimates for the MTHFR C677T polymorphism and male infertility in all of the included studies.
(allelic model: T vs. C).
Fig 10. Sensitivity analysis diagram for each study used to assess the relative risk estimates for the MTHFR C677T polymorphism and male infertility in all of the included studies.
(recessive model: TT vs. TC + CC).
Fig 8. Sensitivity analysis diagram for each study used to assess the relative risk estimates for the MTHFR C677T polymorphism and male infertility in all of the included studies.
(dominant model: TT + TC vs. CC).
Fig 9. Sensitivity analysis diagram for each study used to assess the relative risk estimates for the MTHFR C677T polymorphism and male infertility in all of the included studies.
(additive model: TT vs. CC).
Discussion
Folate-mediated one-carbon metabolism is essential for many reactions in human cells, such as DNA methylation, DNA repair and DNA synthesis[26, 27]. Abnormal folate metabolism has been proposed as a factor in male infertility. Methylenetetrahydrofolate reductase (MTHFR) and methionine synthase (MS) are the key enzymes implicated in the folate metabolic pathways and are crucial for DNA methylation and spermatogenesis. The single nucleotide polymorphisms (SNPs) of these folate-related enzymes gene can impair folate absorption or disturb the balance between folate derivatives by impacting the activity, stability, or level of the corresponding enzymes. The mechanisms of pathogenesis may involve changes of enzyme structure and mRNA properties that are due to these folate-related enzymes gene polymorphisms[28]. Recent studies have revealed that folate-related enzyme gene polymorphisms were associated with an increased risk of male infertility, particularly in the case of MTHFR gene polymorphisms[1, 29, 30]. Although many studies have reported associations between MTHFR and MS gene polymorphisms and male infertility risk[14, 31], no meta-analysis to date has comprehensively evaluated the relationships of MTHFR and MS gene polymorphisms with male infertility risk in the Chinese population. Hence, we performed such a meta-analysis.
In the present study, a meta-analysis was conducted of nine case-control studies to evaluate the association between three folate-related enzyme gene polymorphisms and male infertility in the Chinese population. Overall, we did not find the variant genotypes of the MTHFR A1298C and MS A2756G polymorphisms to be associated with male infertility risk. However, a significant association between the MTHFR C667T polymorphism and male infertility was detected (OR: 1.47, allelic genetic model; OR: 1.58, recessive genetic model; OR: 1.51, dominant genetic model; OR: 2.08, codominant genetic model). The results are consistent with recent meta-analysis studies that suggest a moderate to strong association between MTHFR C677T and male infertility, especially in Asian populations[11–13, 32].
Ni et al. reported that the MTHFR C667T polymorphism was not a risk factor for male infertility risk in a Chinese population, in contrast to the conclusions of a previous study. Similarly, Li et al. found no evidence an association of this polymorphism with male infertility risk. This difference among studies may be due to small sample sizes, study differences in genotyping method or population substructure, or other factors. The general Chinese population occupies a vast country such that cultures and habits, such as personality, diet, living environment, and customs, can vary greatly among regions, for example, between southern and northern China. In this meta-analysis, four of the included studies were from northern China, and the remaining five were from southern China. Xu et al. showed that the greatest genetic differentiation of the Chinese Han population occurred between the northern Han Chinese and the southern Han Chinese[33]. In addition, Yang et al. reported marked geographical variation in the prevalence of MTHFR C677T, A1298C and MTRR A66G gene polymorphisms among different Chinese Han populations[34]. Differences among studies regarding the relationship between the MTHFR C667T polymorphism and male infertility risk may also be associated with variation in the nutritional status of people among different regions of China; for example, a higher vitamin intake can mask the biological effects of the MTHFR C667T polymorphism[35]. Regarding the MTHFR A1298C and MS A2576G polymorphisms, our results provided no evidence of either's association with male infertility risk in any genetic model, which is consistent with previous studies. Only three studies addressing the MTHFR A1298C and MS A2576G polymorphisms were included in the present meta-analysis; thus, studies with larger sample sizes are needed to further investigate the potential relationships of MTHFR A1298C and MS A2576G polymorphisms with male infertility risk.
Some limitations of the present study should be considered when interpreting the results. First, only nine studies were included in the meta-analysis, and their sample sizes were small; therefore, limited data were available. Second, we did not estimate the potential gene—gene and gene—environment interactions due to the lack of information available in the original studies. Third, other clinical data, such as sources of control, subject age, and semen quality, were not considered here due to a lack of information. Finally, although the funnel plot and Egger’s test indicated no remarkable publication bias, some publication bias may exist in the results because only published studies were retrieved.
Conclusion
In summary, this meta-analysis provides evidence that the MTHFR C667T polymorphism may contribute to genetic susceptibility to the risk of male infertility in the Chinese population, whereas MTHFR A1298C and MS A2576G polymorphisms may have no impact. Nevertheless, large-scale, well-designed and population-based studies are needed to investigate the combined effects of these variants within the MTHFR gene or other folate-related enzyme genes in the Chinese population, which may lead to a comprehensive understanding of their potential roles in infertility.
Supporting Information
The PRISMA Checklist for our meta-analysis.
(DOC)
Meta-analysis on Genetic Association Studies Checklist.
(DOCX)
The PRISMA 2009 flow diagram for our meta-analysis.
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Technology Support Programs of Sichuan Department of Science and Technology, Grant ID: 2016FZ0103; and the central university basic business expenses, Grant ID: 2012015SCU2015C006.
References
- 1.Gurkan H, Tozkir H, Goncu E, Ulusal S, Yazar M. The relationship between methylenetetrahydrofolate reductase c.677TT genotype and oligozoospermia in infertile male patients living in the Trakya region of Turkey. Andrologia. 2015;47(9):1068–74. 10.1111/and.12380 [DOI] [PubMed] [Google Scholar]
- 2.Sharma RK, Said T, Agarwal A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian journal of andrology. 2004;6(2):139–48. [PubMed] [Google Scholar]
- 3.Wu W, Cai H, Sun H, Lu J, Zhao D, Qin Y, et al. Follicle stimulating hormone receptor G-29A, 919A>G, 2039A>G polymorphism and the risk of male infertility: a meta-analysis. Gene. 2012;505(2):388–92. 10.1016/j.gene.2012.02.023 [DOI] [PubMed] [Google Scholar]
- 4.Hiort O, Holterhus PM, Horter T, Schulze W, Kremke B, Bals-Pratsch M, et al. Significance of mutations in the androgen receptor gene in males with idiopathic infertility. The Journal of clinical endocrinology and metabolism. 2000;85(8):2810–5. 10.1210/jcem.85.8.6713 [DOI] [PubMed] [Google Scholar]
- 5.Ravel C, Chantot-Bastaraud S, El Houate B, Berthaut I, Verstraete L, De Larouziere V, et al. Mutations in the protamine 1 gene associated with male infertility. Molecular human reproduction. 2007;13(7):461–4. 10.1093/molehr/gam031 [DOI] [PubMed] [Google Scholar]
- 6.Safarinejad MR, Shafiei N, Safarinejad S. The association of glutathione-S-transferase gene polymorphisms (GSTM1, GSTT1, GSTP1) with idiopathic male infertility. J Hum Genet. 2010;55(9):565–70. 10.1038/jhg.2010.59 [DOI] [PubMed] [Google Scholar]
- 7.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Advances in nutrition. 2012;3(1):21–38. 10.3945/an.111.000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HC, Jeong YM, Lee SH, Cha KY, Song SH, Kim NK, et al. Association study of four polymorphisms in three folate-related enzyme genes with non-obstructive male infertility. Human reproduction. 2006;21(12):3162–70. 10.1093/humrep/del280 [DOI] [PubMed] [Google Scholar]
- 9.Weiner AS, Boyarskikh UA, Voronina EN, Tupikin AE, Korolkova OV, Morozov IV, et al. Polymorphisms in folate-metabolizing genes and risk of idiopathic male infertility: a study on a Russian population and a meta-analysis. Fertility and sterility. 2014;101(1):87–94 e3. 10.1016/j.fertnstert.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 10.Duthie SJ, Narayanan S, Blum S, Pirie L, Brand GM. Folate deficiency in vitro induces uracil misincorporation and DNA hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cells. Nutr Cancer. 2000;37(2):245–51. 10.1207/S15327914NC372_18 [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Luo YY, Wu S, Tang YD, Rao XD, Xiong L, et al. Association between C677T and A1298C polymorphisms of the MTHFR gene and risk of male infertility: a meta-analysis. Genetics and molecular research: GMR. 2016;15(2). [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Liu Z, Zhang M, Gong R, Xu Y, Wang B. Association of the methylenetetrahydrofolate reductase gene C677T polymorphism with the risk of male infertility: a meta-analysis. Renal failure. 2016;38(2):185–93. 10.3109/0886022X.2015.1111086 [DOI] [PubMed] [Google Scholar]
- 13.Liu K, Zhao R, Shen M, Ye J, Li X, Huang Y, et al. Role of genetic mutations in folate-related enzyme genes on Male Infertility. Scientific reports. 2015;5:15548 10.1038/srep15548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karimian M, Hosseinzadeh Colagar A. Methionine synthase A2756G transition might be a risk factor for male infertility: Evidences from seven case-control studies. Mol Cell Endocrinol. 2016;425:1–10. 10.1016/j.mce.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 15.Gong M, Dong W, He T, Shi Z, Huang G, Ren R, et al. MTHFR 677C>T polymorphism increases the male infertility risk: a meta-analysis involving 26 studies. PloS one. 2015;10(3):e0121147 10.1371/journal.pone.0121147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 17.Li SS, Li J, Xiao Z, Ren AG, Jin L. Prospective study of MTHFR genetic polymorphisms as a possible etiology of male infertility. Genetics and molecular research: GMR. 2014;13(3):6367–74. 10.4238/2014.March.24.26 [DOI] [PubMed] [Google Scholar]
- 18.Li XY, Ye JZ, Ding XP, Zhang XH, Ma TJ, Zhong R, et al. Association between methionine synthase reductase A66G polymorphism and primary infertility in Chinese males. Genetics and molecular research: GMR. 2015;14(2):3491–500. 10.4238/2015.April.15.13 [DOI] [PubMed] [Google Scholar]
- 19.Ni W, Li H, Wu A, Zhang P, Yang H, Yang X, et al. Lack of association between genetic polymorphisms in three folate-related enzyme genes and male infertility in the Chinese population. Journal of assisted reproduction and genetics. 2015;32(3):369–74. 10.1007/s10815-014-0423-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A Z-C, Yang Y, Zhang S-Z, Li N, Zhang W. Single nucleotide polymorphism C677T in the methylenetetrahydrofolate reductase gene might be a genetic risk factor for infertility for Chinese men with azoospermia or severe oligozoospermia. Asian journal of andrology. 2007;9(1):57–62. 10.1111/j.1745-7262.2007.00225.x [DOI] [PubMed] [Google Scholar]
- 21.L L, C Z, L HM, Q WP. Association of MTHFR C677T and MS A2756G polymorphism with semen quality. J Cent South Univ. 2012;37(10):1054–9. [DOI] [PubMed] [Google Scholar]
- 22.Q XF, H XP, L YJ, D J, P L, X X, et al. Association between MethyIenterahydrofolate Reductase Gene C677 T Polymorphism and Male Infertility with Azoospermia or Severe oligozoospermia and Asthenospermia in Ningxia Han Population. Journal of Ningxia Medical University. 2011;33(7):625–7. [Google Scholar]
- 23.S HT, Z JY, L YJ. Associated of the Methylenetetrahydrofolate Reductase Gene C677T Polymorphism with Male Infertility. Reproduction & Contraception. 2007;27(7):443–5. [Google Scholar]
- 24.J P. Association between MTHFR C677T Polymorphism and Male Infertility in Han Population of He Nan China. China Hwalth Care & Nutrition. 2013;(7):629–30. [Google Scholar]
- 25.Y BH, P GF, P JP. Association of methylenetetrahydrofolate reductase gene C677T polymorphism with asthenospermia in Han population of South Anhui. J of Wannan Medical University. 2010;29(1):5–7. [Google Scholar]
- 26.Karimian M, Colagar AH. Association of C677T transition of the human methylenetetrahydrofolate reductase (MTHFR) gene with male infertility. Reproduction, Fertility and Development. 2016; 28(6):785–94. [DOI] [PubMed] [Google Scholar]
- 27.Xiong M, Gupta N, Gupta S, Dama M, David A, Khanna G, et al. Strong Association of 677 C>T Substitution in the MTHFR Gene with Male Infertility—A Study on an Indian Population and a Meta-Analysis. PloS one. 2011;6(7):e22277 10.1371/journal.pone.0022277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikzad H., Karimian M., Sareban K., Khoshsokhan M., Hosseinzadeh Colagar A. MTHFR-Ala222Val and male infertility: a study in Iranian men, an updated meta-analysis and an in silico-analysis. Reprod Biomed Online. 2015; 31:668–80. 10.1016/j.rbmo.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 29.Kurzawski M, Wajda A, Malinowski D, Kazienko A, Kurzawa R, Drozdzik M. Association study of folate-related enzymes (MTHFR, MTR, MTRR) genetic variants with non-obstructive male infertility in a Polish population. Genetics and molecular biology. 2015;38(1):42–7. 10.1590/S1415-475738120140179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mfady DS, Sadiq MF, Khabour OF, Fararjeh AS, Abu-Awad A, Khader Y. Associations of variants in MTHFR and MTRR genes with male infertility in the Jordanian population. Gene. 2014;536(1):40–4. 10.1016/j.gene.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 31.Shen O, Liu R, Wu W, Yu L, Wang X. Association of the methylenetetrahydrofolate reductase gene A1298C polymorphism with male infertility: a meta-analysis. Annals of human genetics. 2012;76(1):25–32. 10.1111/j.1469-1809.2011.00691.x [DOI] [PubMed] [Google Scholar]
- 32.Wei B, Xu Z, Ruan J, Zhu M, Jin K, Zhou D, et al. MTHFR 677C>T and 1298A>C polymorphisms and male infertility risk: a meta-analysis. Mol Biol Rep. 2012;39(2):1997–2002. 10.1007/s11033-011-0946-4 [DOI] [PubMed] [Google Scholar]
- 33.Xu S, Yin X, Li S, Jin W, Lou H, Yang L, et al. Genomic Dissection of Population Substructure of Han Chinese and Its Implication in Association Studies. The American Journal of Human Genetics. 2009;85(6):762–74. 10.1016/j.ajhg.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang B, Liu Y, Li Y, Fan S, Zhi X, Lu X, et al. Geographical distribution of MTHFR C677T, A1298C and MTRR A66G gene polymorphisms in China: findings from 15357 adults of Han nationality. PloS one. 2013;8(3):e57917 10.1371/journal.pone.0057917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forges T, Monnier-Barbarino P, Alberto JM, Gueant-Rodriguez RM, Daval JL, Gueant JL. Impact of folate and homocysteine metabolism on human reproductive health. Human reproduction update. 2007;13(3):225–38. 10.1093/humupd/dml063 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The PRISMA Checklist for our meta-analysis.
(DOC)
Meta-analysis on Genetic Association Studies Checklist.
(DOCX)
The PRISMA 2009 flow diagram for our meta-analysis.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.