Abstract
Objective:
We estimated the prevalence of hepatitis B surface antigen (HBsAg), a serologic marker of active hepatitis B virus (HBV) infection, among pregnant women, and estimated the proportion HBsAg-positive pregnant women who had received additional recommended testing.
Methods:
From 2008 through 2012, Perinatal Hepatitis B Prevention Programs (PHBPPs) in Florida, Michigan, Minnesota, New York City, and Texas prospectively collected data on demographic characteristics of HBsAg-positive pregnant women. We estimated the prevalence of HBsAg positivity among pregnant women by demographic characteristics using natality data. PHBPPs (excluding Texas) collected additional recommended testing (for hepatitis B e antigen [HBeAg] and/or HBV deoxyribonucleic acid [DNA]) among HBsAg-positive pregnant women to measure levels of viremia.
Results:
During the study period, 15,205 HBsAg-positive women were case-managed. The median age of HBsAg-positive women was 29 years; prenatal HBsAg screening was at a median of 27 weeks pre-delivery. Of 15,205 HBsAg-positive women, 11,293 (74.3%) were foreign-born. In four PHBPPs with 14,098 pregnancies among 12,214 HBsAg-positive women, HBeAg and/or HBV DNA testing was documented for 2,794 (19.8%) pregnancies. The estimated prevalence of HBsAg positivity among pregnant women was 0.38% (17,023 of 4,468,773). HBsAg prevalence was highest among foreign-born women from most regions in Asia (2.0% to 8.7%; with the exception of South Asia, 0.4%) and Africa (3.4%).
Conclusion:
One-fifth of HBsAg-positive pregnant women had documentation for HBeAg and/or HBV DNA, and about one-third reported receiving care for HBV infection during a case-managed pregnancy. Greater emphasis is needed on prenatal evaluation for HBV liver disease care and treatment among pregnant women with HBV infection.
Keywords: hepatitis B, pregnancy, women
Since 1988, the Advisory Committee on Immunization Practices (ACIP) has recommended that all pregnant women receive hepatitis B surface antigen (HBsAg) screening to identify infants at risk for mother-to-child transmission of hepatitis B virus (HBV) infection. Infants born to HBsAg-positive women require postexposure prophylaxis with hepatitis B vaccine and hepatitis B immune globulin within 12 hours of birth to prevent transmission of HBV infection, which has a 90% risk of becoming a chronic infection. Maternal screening for HBsAg is recommended at the first prenatal visit and again at delivery if a woman is at risk for HBV infection.1
In 2005, ACIP recommended that pregnant women with chronic HBV infection receive counseling, medical evaluation, and possibly antiviral treatment.2 Chronic HBV infection generally is asymptomatic until liver injury caused by infection results in cirrhosis, hepatocellular carcinoma, or liver-related death.3 Pregnant women with chronic HBV infection and high levels of viremia are at an increased risk for HBV-related complications (e.g., postpartum hepatic flare, a severe exacerbation of hepatitis characterized by large increases in measurable liver enzymes).4–6 Infants born to women with serologic markers of high levels of viremia (i.e., test positive for hepatitis B e antigen [HBeAg] or have ≥106 copies per milliliter [mL] of HBV deoxyribonucleic acid [DNA] [>200,000 international units (IU)/mL or the equivalent]) have a 10–15% risk for mother-to-child transmission of HBV infection despite receiving postexposure prophylaxis.7–9 Antiviral treatment can decrease levels of hepatitis B viremia.3 Although antiviral treatment is not currently the standard of care, evidence suggests that the risk of mother-to-child transmission of HBV infection decreases when pregnant women with high levels of viremia receive antiviral treatment during the third trimester.10–13 These findings increase the importance of prenatal evaluation of HBV-related liver disease among pregnant women with chronic HBV infection.
The most recent estimates of the prevalence of HBsAg positivity among pregnant women in the United States were from four urban centers during 1990–1993 and from New York City during 1995–2006.14,15 Widespread hepatitis B vaccination might have resulted in a change in demographic characteristics and prevalence of HBsAg-positive pregnant women.16,17 We are not aware of previous reports estimating the proportion of HBsAg-positive pregnant women in the United States who receive prenatal testing for level of HBV viremia according to the 2005 ACIP recommendations.2 The objective of this study was to describe the characteristics of HBsAg-positive pregnant women who were case-managed by five US Perinatal Hepatitis B Prevention Programs (PHBPPs) from 2008 through 2012 and estimate the proportion of these women who received additional testing and care for HBV infection.
Methods
Since 1990, the Centers for Disease Control and Prevention (CDC) has funded PHBPPs in 64 public health jurisdictions. These programs investigate positive HBsAg tests reported to public health departments to identify pregnant women with HBV infection. In collaboration with health-care providers, these programs educate, guide, and assist families to ensure that infants born to HBsAg-positive women complete timely postexposure prophylaxis.18 PHBPPs in Florida, Michigan, Minnesota, New York City, and Texas (excluding Houston and San Antonio) received additional funding to track characteristics of case-managed HBsAg-positive pregnant women and to encourage them to seek care for HBV infection. During a 5-year period from January 1, 2008, through December 31, 2012, a cumulative total of 17,951 pregnancies were enrolled for case management by the five PHBPP sites; 928 (5.2%) pregnancies were excluded after additional testing determined that the women were HBsAg negative. Because the project was defined as public health assessment, institutional review board review was waived.
The five PHBPPs collected core data on the women, including date of birth, race/ethnicity, country of birth, primary spoken language, and HBsAg screening dates. Primary spoken language was classified by using US Census Bureau language codes.19 Program staff members asked women if they had been receiving care from a liver specialist and if they had been receiving antiviral treatment for HBV infection. If they were not receiving care, programs recommended they seek care from a provider with expertise in managing chronic HBV infection. Data on the indications for and timing of any treatment were not collected. To determine if additional recommended testing for chronic HBV infection had been performed, four of the programs (Florida, Michigan, Minnesota, and New York City) collected data on testing for HBeAg, a serologic marker of active HBV replication, and HBV DNA, tests not generally used for screening.20 Because level of HBV viremia can vary over time, we assumed each pregnancy was a unique event for which testing was indicated, including for each pregnancy among women with more than one pregnancy during the study period. HBeAg or HBV DNA tests performed before or while the pregnant women and their infants were case managed were accepted as tests received during that pregnancy. Results of liver function tests (e.g., alanine aminotransferase) were not collected because they are not specific to chronic HBV infection.21
The PHBPPs integrated data elements into existing interview forms and used several systems (e.g., Microsoft® ACCESS® databases, electronic disease reporting systems) to manage the data. De-identified data were sent to CDC quarterly for data cleaning and consolidation into a unified dataset.
For demographic and care characteristics, data were referenced to the date the women were enrolled for case management. Some women were enrolled for more than one pregnancy. For demographic analyses, each woman was counted only once regardless of the number of case-managed pregnancies she had during the 5-year study period. Because information on gestational age of newborn infants was not available, we calculated the time during pregnancy when women were screened for HBsAg as the number of weeks before the infant’s date of birth (or the expected date of delivery if infants had not been born by the end of the study period) to the HBsAg-positive test date. We calculated the age of an HBsAg-positive pregnant woman by subtracting her date of enrollment from her date of birth. We stratified age by 10-year intervals up to age 40 years (i.e., 10-19, 20-29, 30-39, and ≥40). We classified race/ethnicity as non-Hispanic white, non-Hispanic black, Asian American/Pacific Islander (AA/PI), non-Hispanic other (including women who self-identified as multiracial or American Indian/Alaska Native), and Hispanic. We classified women who identified as Hispanic as Hispanic regardless of race. We grouped women by maternal country of birth into regions using modified categories from the Global Burden of Disease 2004 Update.22
We estimated the prevalence of HBsAg positivity among pregnant women case-managed by PHBPPs as the percentage of all pregnant women who were HBsAg positive. We estimated prevalence overall for the five sites, by PHBPP site, by region of mother’s birth, and by race/ethnicity. The number of case-managed pregnancies among HBsAg-positive pregnant women was the numerator. If an HBsAg-positive pregnant woman had more than one pregnancy during the 5-year period, all of her pregnancies were counted in the numerator. Because data were not available to estimate site-specific and demographic characteristics of all pregnant women (HBsAg-positive and HBsAg-negative women) with ≥1 live birth (denominator), we used annual natality data provided by the National Center for Health Statistics to estimate the number of pregnant women by these characteristics. Natality data are for live births rather than pregnancies. They do not indicate the number of pregnancies with multiple births; therefore, no correction could be made for the number of pregnant women with multiple births, which has been estimated to account for approximately 3% of births annually in the United States.23
We used SAS® version 9.2 for all analyses,24 including calculation of point estimates and 95% confidence intervals (CIs) via exact binomial calculation. We used Mantel-Haenszel’s χ2 to test for significant associations between maternal characteristics (age, race/ethnicity, and place of birth) and the presence of hepatitis B serologic markers (HBeAg, HBV DNA). We set significance at α ≤ 0.05.
Results
The primary dataset consisted of 15,205 HBsAg-positive women and 17,023 pregnancies. During the study period, 1,621 (10.7%) women were enrolled for more than one pregnancy, and 197 (1.2%) pregnancies resulted in multiple births. By site, the number of women enrolled were as follows: 1,965 (12.9%) in Florida, 1,118 (7.4%) in Michigan, 1,440 (9.5%) in Minnesota, 7,791 (51.2%) in New York City, and 2,891 (19.0%) in Texas.
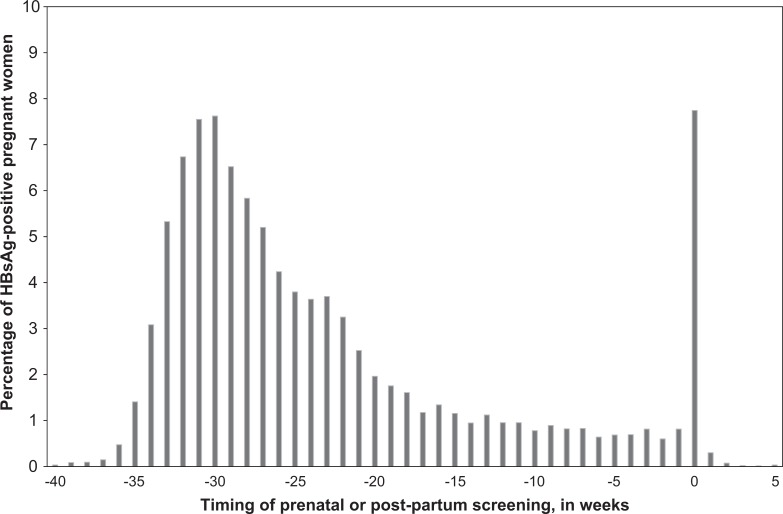
The date of HBsAg screening was available for 14,981 (98.5%) women. Of these, 13,316 (88.9%) women received prenatal screening during the current pregnancy and 1,160 (7.7%) women were screened during the week of or at delivery. Among the 1,160 women screened during the week of delivery, 304 (26.2%) had not previously been screened and 856 (73.8%) had been screened during a previous pregnancy but not during the current pregnancy (Figure 1). Fewer than 1% of women were screened ≥1 week postpartum, and 2.5% were screened during a prior pregnancy without rescreening. The median time of screening for the 14,981 HBsAg-positive pregnant women was 27 weeks before delivery; 12,030 (80.3%) women were screened at least 12 weeks before delivery or the expected date of delivery.
Figure 1.
Time of HBsAg screening relative to date of infant’s birth or expected date of delivery among 14,981 HBsAg-positive pregnant women, Perinatal Hepatitis B Prevention Program sitesa, January 2008 through December 2012. Abbreviation: HBsAG, hepatitis B surface antigen. aSites were located in Florida, Minnesota, Michigan, New York City, and Texas. b0 indicates week of delivery; weeks below zero indicate prenatal period and weeks after zero indicate postpartum period.
The median age of the 15,205 HBsAg-positive pregnant women was 29 years (range: 14-51) (Table 1). AA/PIs were the largest single racial/ethnic group of HBsAg-positive women in Michigan (n = 508; 45.4%), Minnesota (n = 836; 58.1%), New York City (n = 5,384; 69.1%), and Texas (n = 1,471; 50.9%). In Florida, AA/PIs were the second-largest racial/ethnic group (n = 622; 31.7%) and non-Hispanic black women were the largest racial/ethnic group (n = 795; 40.5%). In other sites, non-Hispanic black HBsAg-positive pregnant women comprised 317 (28.4%) women in Michigan, 558 (38.8%) women in Minnesota, 1,273 (16.3%) women in New York City, and 633 (21.9%) women in Texas.
Table 1.
Characteristics of HBsAg-positive pregnant women case managed by Perinatal Hepatitis B Prevention programs in 5 US sites,a January 2008 through December 2012
| Maternal characteristics | No. of women testing HBsAg positive (percent)b |
|---|---|
| Total | 15,205 (100.0) |
| Age, in years | |
| 10-19 | 368 (2.4) |
| 20-29 | 7,266 (47.8) |
| 30-39 | 6,916 (45.5) |
| ≥40 | 655 (4.3) |
| Race/ethnicity | |
| Asian American/Pacific Islander | 8,821 (58.0) |
| Non-Hispanic black | 3,576 (23.5) |
| Hispanic | 658 (4.3) |
| Non-Hispanic white | 1,218 (8.0) |
| Non-Hispanic otherc | 249 (1.6) |
| Not reported | 683 (4.5) |
| Nativity | |
| US-born | 1,599 (10.5) |
| Foreign-born | 11,293 (74.3) |
| Not reported | 2,313 (15.2) |
| Primary languaged | |
| Chinese | 4,707 (31.0) |
| English | 3,959 (26.0) |
| Spanish | 415 (2.7) |
| Other Asian American/Pacific Islander languagee | 857 (5.6) |
| Other languagef | 2,224 (14.7) |
| Not reported | 3,043 (20.0) |
Abbreviation: HBsAg, hepatitis B surface antigen. aSites were located in Florida, Michigan, Minnesota, New York City, and Texas.
bPercentages may not sum to 100 because of rounding.
cDefined as American Indian/Alaska Native and multiracial.
dClassified using US Census Bureau codes. Census Bureau (US). Primary language code list [cited 2016 Jun 3]. Available from: http://www.census.gov/hhes/socdemo/language/about/02_primary_list.pdf
eOther Asian American/Pacific Islander languages designated by US Census Bureau as codes 684-695, 698-702, and 716-776.
fDefined as all other languages after excluding English, Spanish, Chinese, and other Asian.
US-born HBsAg-positive pregnant women had a median age of 27 years (range: 14-47) (Table 1). Most women were non-Hispanic black (n = 770; 48.2%), followed by non-Hispanic white (n = 343; 21.5%), AA/PI (n = 299; 18.7%), Hispanic (n = 118; 7.4%), and non-Hispanic other (n = 16; 1.0%).
Foreign-born HBsAg-positive women had a median age of 30 years (range: 14-51) (Table 1). Most foreign-born HBsAg-positive women were AA/PI (n = 890; 78.9%), followed by non-Hispanic black (n = 2,636; 23.3%), non-Hispanic white (n = 706; 6.3%), Hispanic (n = 431; 3.8%), and non-Hispanic other (n = 146; 1.3%). Foreign-born HBsAg-positive pregnant women were primarily from East Asia (n = 5,579; 49.4%), Southeast Asia (n = 1,897; 16.8%), and Africa (n = 1,671; 14.8%). The most common non-English spoken language was Chinese (multiple dialects).
The estimated prevalence of HBsAg-positive pregnant women was highest among women born in East, Southeast, and West/Central Asia and in Africa. Although the prevalence of HBsAg-positive pregnant women by race/ethnicity varied by site, the highest estimated prevalence was among AA/PIs in all sites except Florida, where the highest prevalence was among non-Hispanic other women (Table 2).
Table 2.
Estimated prevalencea of HBsAg among pregnant women identified by 5 Perinatal Hepatitis B Prevention programs, overall, by region of birth, by race/ethnicity, and by program site,b January 2008 through December 2012
| Characteristic | Estimated no. of women with pregnancyc | No. of HBsAg-positive women with pregnancyd | Estimated prevalence of HBsAg-positive women with pregnancy Percent (95% CI)d |
|---|---|---|---|
| Overall | 4,468,773 | 17,023 | 0.38 (0.38-0.39) |
| Region of birthe | |||
| Africa | 59,392 | 2,029 | 3.42 (3.27-3.56) |
| Australia/Oceania | 2,081 | 4 | 0.19 (0.00-0.38) |
| Caribbean, excluding Haiti | 136,714 | 425 | 0.31 (0.28-0.34) |
| East Asia | 73,593 | 6,422 | 8.73 (8.52-8.93) |
| Eastern Europe | 33,380 | 197 | 0.59 (0.51-0.67) |
| Haiti | 42,529 | 467 | 1.10 (1.00-1.20) |
| Mexico and Central America | 573,279 | 212 | 0.04 (0.03-0.04) |
| Middle East | 40,147 | 131 | 0.33 (0.27-0.38) |
| North America | 3,226,038 | 1,705 | 0.05 (0.05-0.06) |
| Pacific Islands | 1,268 | 20 | 1.58 (0.89-2.26) |
| South America | 100,187 | 167 | 0.17 (0.14-0.19) |
| South Asia | 69,228 | 293 | 0.42 (0.37-0.47) |
| Southeast Asia | 54,592 | 2,140 | 3.92 (3.76-4.08) |
| Southern Europe | 14,862 | 206 | 1.39 (1.20-1.57) |
| West/Central Asia | 8,372 | 169 | 2.02 (1.72-2.32) |
| Western and Northern Europe | 33,161 | 53 | 0.16 (0.12-0.20) |
| Overall, by race/ethnicity | |||
| Asian American/Pacific Islander | 254,679 | 10,014 | 3.93 (3.87-4.01) |
| Non-Hispanic black | 981,230 | 4,025 | 0.41 (0.40-0.42) |
| Hispanic | 1,423,790 | 691 | 0.05 (0.04-0.05) |
| Non-Hispanic white | 1,999,528 | 1,319 | 0.07 (0.06-0.07) |
| Non-Hispanic other | 29,649 | 261 | 0.88 (0.77-0.99) |
| PHBPP, by site and by race/ethnicity | |||
| Florida | 1,093,991 | 2,004 | 0.18 (0.18-0.19) |
| Asian American/Pacific Islander | 35,834 | 633 | 1.77 (1.63-1.90) |
| Non-Hispanic black | 250,810 | 807 | 0.32 (0.30-0.34) |
| Hispanic | 302,919 | 124 | 0.04 (0.03-0.05) |
| Non-Hispanic white | 501,290 | 243 | 0.05 (0.04-0.05) |
| Non-Hispanic other | 3,138 | 103 | 3.28 (2.66-3.91) |
| Michigan | 580,051 | 1,137 | 0.20 (0.18-0.21) |
| Asian American/Pacific Islander | 20,105 | 516 | 2.57 (2.35-2.79) |
| Non-Hispanic black | 110,169 | 320 | 0.29 (0.26-0.32) |
| Hispanic | 39,492 | 12 | 0.03 (0.01-0.05) |
| Non-Hispanic white | 405,901 | 275 | 0.07 (0.06-0.08) |
| Non-Hispanic other | 4,384 | 2 | 0.05 (0.00-0.11) |
| Minnesota | 348,858 | 1,948 | 0.55 (0.53-0.58) |
| Asian American/Pacific Islander | 26,131 | 1,115 | 4.27 (4.02-4.51) |
| Non-Hispanic black | 33,253 | 777 | 2.34 (2.17-2.50) |
| Hispanic | 25,182 | 0 | 0.00 |
| Non-Hispanic white | 255,838 | 44 | 0.02 (0.01-0.02) |
| Non-Hispanic other | 8,454 | 10 | 0.12 (0.05-0.19) |
| New York City | 604,151 | 9,009 | 1.49 (1.46-1.52) |
| Asian American/Pacific Islander | 91,877 | 6,268 | 6.82 (6.66-6.99) |
| Non-Hispanic black | 133,221 | 1,477 | 1.11 (1.05-1.16) |
| Hispanic | 192,277 | 274 | 0.14 (0.13-0.16) |
| Non-Hispanic white | 171,551 | 530 | 0.31 (0.28-0.34) |
| Non-Hispanic other | 8,746 | 50 | 0.57 (0.41-0.73) |
| Texas | 1,808,345 | 2,925 | 0.16 (0.16-0.17) |
| Asian American/Pacific Islander | 77,843 | 1,482 | 1.9 (1.81-2.00) |
| Non-Hispanic black | 200,297 | 644 | 0.32 (0.30-0.35) |
| Hispanic | 866,809 | 281 | 0.03 (0.03-0.04) |
| Non-Hispanic white | 658,469 | 227 | 0.03 (0.03-0.04) |
| Non-Hispanic other | 4,927 | 96 | 1.95 (1.56-2.33) |
Abbreviations: CI, confidence interval; HBsAg, hepatitis B surface antigen. aThe prevalence of HBsAg positivity, among pregnant women case-managed by PHBPPs, was calculated as the percentage of all pregnant women with a positive HBsAg test result. The number of case-managed pregnancies among HBsAg-positive pregnant women was the numerator and all pregnant women (HBsAg-positive and HBsAg-negative women) with ≥1 live birth was the denominator.
bSites were located in Florida, Michigan, Minnesota, New York City, and Texas.
cAnnual natality data for births were used as a proxy to estimate the total number of pregnant women by listed characteristics. Natality data were provided by the National Center for Health Statistics. We made no correction for pregnancies with multiple births, which were estimated to account for approximately 3% of births annually in the United States. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJK. Births: final data for 2008. Natl Vital Stat Rep 2010 Dec 8;59:1-72.
dMissing data accounted for differences in the total number of pregnancies for shown variables.
eClassification of regions was based on modified categories from: World Health Organization. The global burden of disease 2004, update 2008 [cited 2016 Jun 3]. Available from: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf.
- Africa: Algeria, Angola, Bassas Da India, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Comoros, Congo-Zaire, Congo, Cote d’ Ivoire, Djibouti, Egypt, Equatorial Guinea, Eritrea, Ethiopia, Europa Island, Gabon, The Gambia, Ghana, Glorioso Islands, Kenya, Lesotho, Liberia, Libya, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mayotte, Morocco, Mozambique, Namibia, Niger, Nigeria, Reunion, Rwanda, Sao Tome and Principe, Saint Helena, Senegal, Seychelles, Sierra Leone, Somalia, South Africa, Sudan, Swaziland, Tanzania, Togo, Tromelin Island, Tunisia, Uganda, Upper Volta, Western Sahara, Zaire, Zambia, Zimbabwe
- Australia/Oceania: Ashmore and Cartier Islands, Australia, Cocos (Keeling) Islands, Coral Sea Islands, New Hebrides, New Zealand, Norfolk Island
- Middle East: Bahrain, Cyprus, Gaza Strip, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Oman, Qatar, Saudi Arabia, Syria, Turkey, United Arab Emirates, West Bank, Yemen
- East Asia: China, Hong Kong, Japan, Macau, Mongolia, North Korea, South Korea, Taiwan
- South Asia: Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan, Sri Lanka
- Southeast Asia: Brunei, Burma, Cambodia, East Timor, Indonesia, Laos, Malaysia, Papua New Guinea, Paracel Islands, Philippines, Singapore, Spratly Islands, Thailand, Vietnam
- West/Central Asia: Afghanistan, Armenia, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, Uzbekistan, Tajikistan, Turkmenistan
- Pacific Islands: Christmas Island, Clipperton Island, Cook Islands, Faroe Islands, Fiji, French Polynesia, Heard Island and McDonald Islands, Howland Island, Jan Mayen, Jarvis Island, Johnston Island, Juan de Nova Island, Kiribati, Marshall Islands, Federated States of Micronesia, Midway Island
- Caribbean without Haiti: Anguilla, Antigua and Barbuda, Aruba, Bahamas, Barbados, Bermuda, British Virgin Islands, Cayman Islands, Cuba, Dominica, Dominican Republic, Grenada, Guadeloupe, Jamaica, Martinique, Montserrat, Netherlands Antilles, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadine, Trinidad and Tobago, Turks and Caicos Islands
- North America excluding the United States: Canada, Greenland, Saint Pierre, Miquelon
- Mexico and Central America: Belize, Costa Rica, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama
- South America: Argentina, Bolivia, Brazil, Chile, Colombia, Ecuador, Falkland Islands, French Guiana, Guyana, Paraguay, Peru, Suriname, Uruguay, Venezuela
- Eastern Europe: Belarus, Bulgaria, Czech Republic, Hungary, Moldova, Poland, Romania, Russia, Slovakia, Ukraine, Estonia, Latvia, Lithuania
- Northern and Western Europe: Austria, Belgium, Denmark, England, Finland, France, Germany, Great Britain, Guernsey, Iceland, Ireland, Isle of Man, Jersey, Liechtenstein, Luxembourg, Monaco, Netherlands, Norway, Svalbard, Sweden, Switzerland, United Kingdom
- Southern Europe: Albania, Andorra, Bosnia and Herzegovina, Croatia, Gibraltar, Greece, Holy See (Vatican City), Italy, Macedonia, Malta, Montenegro, Portugal, San Marino, Serbia, Slovenia, Spain, Yugoslavia
Testing for HBeAg and/or HBV DNA was documented for 2,794 of 14,098 (19.8%) pregnancies among 12,214 HBsAg-positive women in the four sites that provided information. HBeAg testing was most likely to be received at <30 years of age and by AA/PI, non-Hispanic black, or foreign-born women. Among foreign-born HBsAg-positive pregnant women, non-Hispanic black women were tested for HBeAg at a rate similar to AA/PIs (16.6% vs. 15.2%) and were more likely to receive HBV DNA testing than foreign-born women of other race/ethnicities (15.5% vs. 8.3%) (Table 3). Among US-born women, AA/PIs were substantially more likely than non-AA/PIs to be tested for HBeAg (25.6% vs. 8.3%) and HBV DNA (19.0% vs. 5.3%). AA/PIs also were more likely than non-AA/PIs to test positive for HBeAg (odds ratio [OR] = 4.2, 95% CI = 3.3, 5.4) or to have HBV DNA levels ≥2,000 IU/mL (OR = 2.2, 95% CI = 1.7, 2.8). Among 408 women who received both HBeAg and HBV DNA testing, US-born AA/PIs were more likely than US-born women of other races/ethnicities to receive both tests (8.8% vs. 0.2%), and foreign-born non-Hispanic black women were more likely than women of other race/ethnicities to receive both tests (6.3% vs. 2.8%).
Table 3.
Testing for hepatitis B serologic markers associated with 14,098 pregnancies among 12,414 HBsAg-positive pregnant women, by maternal characteristics, at 4 participating Perinatal Hepatitis B Prevention Program sites,a January 2008 through December 2012.
| Maternal characteristics | No. of pregnanciesb | HBeAg and/or HBV DNA testingc | HBeAgd | HBV DNA testinge | |||
|---|---|---|---|---|---|---|---|
| No. of women tested before or while case- managed (percent) | P valuef | No. of women tested before or while case- managed (percent) | P value | No. of women tested before or while case- managed (percent) | P value | ||
| Total | 14,098g | 2,794 (19.8)h | 1,936 (13.7) | 1,266 (9.0) | |||
| Age, in years | |||||||
| 10-19 | 315 | 65 (20.6) | .001 | 52 (16.5) | <.001 | 20 (6.3) | .06 |
| 20-29 | 7,102 | 1,482 (20.9) | 1,060 (14.9) | 623 (8.8) | |||
| 30-39 | 6,094 | 1,144 (18.8) | 763 (12.5) | 563 (9.2) | |||
| ≥40 | 587 | 103 (17.5) | 61 (10.4) | 60 (10.2) | |||
| Race/ethnicity | |||||||
| Asian American/Pacific Islander | 8,532 | 1,830 (21.4) | <.001 | 1,305 (15.3) | <.001 | 739 (8.7) | .18 |
| Non-Hispanic black | 3,381 | 719 (21.3) | 475 (14.0) | 412 (12.2) | |||
| Hispanic | 410 | 28 (6.8) | 23 (5.6) | 6 (1.5) | |||
| Non-Hispanic white | 1,092 | 157 (14.4) | 90 (8.2) | 87 (8.0) | |||
| Non-Hispanic otheri | 165 | 11 (6.7) | 9 (5.5) | 3 (1.8) | |||
| Place of birth | |||||||
| US-born | 1,131 | 198 (17.5) | .002 | 141 (12.5) | .04 | 95 (8.4) | .12 |
| Foreign-born | 11,424 | 2,450 (21.4) | 1,683 (14.7) | 1,124 (9.8) | |||
| Race/ethnicity of foreign-born | |||||||
| Asian American/Pacific Islander | 7,822 | 1,672 (21.4) | <.001 | 1,189 (15.2) | <.001 | 668 (8.5) | .21 |
| Non-Hispanic black | 2,399 | 620 (25.8) | 399 (16.6) | 373 (15.5) | |||
| Hispanic | 310 | 23 (7.4) | 19 (6.1) | 5 (1.6) | |||
| Non-Hispanic white | 669 | 116 (17.3) | 62 (9.3) | 72 (10.8) | |||
| Non-Hispanic otheri | 116 | 9 (7.8) | 7 (6.0) | 2 (1.7) | |||
| Race/ethnicity of US-born | |||||||
| Asian American/Pacific Islander | 273 | 98 (35.9) | <.001 | 70 (25.6) | <.001 | 52 (19.0) | <.001 |
| Non-Hispanic black | 559 | 67 (12.0) | 46 (8.2) | 33 (5.9) | |||
| Hispanic | 36 | 3 (8.3) | 2 (5.6) | 1 (2.8) | |||
| Non-Hispanic white | 204 | 25 (12.3) | 18 (8.9) | 8 (3.9) | |||
| Non-Hispanic otheri | 13 | 1 (7.7) | 1 (7.7) | 1 (7.7) | |||
Abbreviations: HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV DNA, hepatitis B virus deoxyribonucleic acid. aThe four program sites contributing data were in Florida, Michigan, Minnesota, and New York City. Texas did not provide these data.
bEach of the 14,098 pregnancies was considered a unique opportunity to test for HBeAg and/or HBV DNA. Testing included HBeAg and/or HBV DNA tests performed before or while the women and their infants were case-managed.
cHBeAg and/or HBV DNA testing was administered for 2,794 pregnancies; for 408 (2.9%) pregnancies, both tests were performed (number tested for HBeAg plus number tested for HBV DNA, minus number tested for HBeAg and/or HBV DNA, divided by the number of pregnancies).
dHBeAg testing only was administered in 1,528 (10.8%) pregnancies.
eHBV DNA testing only was administered in 858 (6.1%) pregnancies.
fBy Mantel-Haenszel’s χ2 test.
gMissing data accounted for differences from the total in some categories.
hPercentages are row percentages.
iDefined as American Indian/Alaska Native and multiracial.
Of 15,205 HBsAg-positive women, 8,664 (57.0%) provided information on whether or not they were receiving care for HBV disease; 3,156 (36.4%) reported being in care or being monitored for HBV disease. Among women in care, 192 (6.1%) reported receiving antiviral treatment, of whom 166 (86.8%) were AA/PI.
Discussion
Our analysis found that, in five PHBPPs, 80.3% of HBsAg-positive pregnant women were screened at least 12 weeks before delivery (or the expected date of delivery), in accordance with ACIP recommendations.1,2 However, despite screening relatively early in pregnancy to identify HBsAg-positive pregnant women, fewer than 20% of the identified HBsAg-positive pregnant women were documented as having additional recommended testing for levels of viremia. In addition, among HBsAg-positive pregnant women for whom information was available on receipt of care, only 36.4% reported receiving care for HBV infection. These findings suggest that the universally recommended evaluation for care and treatment of people with chronic HBV infection might not have been performed or might not have been optimally timed to benefit a large proportion of HBsAg-positive pregnant women.2–6 In interpreting these results, it is important to keep in mind the characteristics of the population we studied—15,205 HBsAg-positive women, of whom 74.3% were foreign-born. Of foreign-born women, 81.0% were from countries in Asia or Africa with intermediate (2-7%) or high (≥8%) HBsAg prevalence.2
HBsAg-positive women identified by the five PHBPPs likely reflect US patterns of immigration and settlement in recent years. Global childhood hepatitis B vaccination programs have decreased the burden of chronic HBV infection worldwide. Because of these programs, the number of women of childbearing age who are HBV infected is expected to decrease.16,17 In the United States, hepatitis B vaccination starting in infancy or at birth was introduced in the 1990s.2 In 2012, hepatitis B vaccine coverage among US adolescents aged 13-17 years was approximately 93%.16 Advances internationally in implementing hepatitis B vaccination in infancy have also been made since the 1990s.17 However, gaps remain worldwide in infant hepatitis B vaccination, including in administration of the first dose of hepatitis B vaccine at birth to prevent mother-to-child transmission of HBV infection.25 In 2010, people born in Asia and Africa accounted for 28% and 4%, respectively, of all foreign-born people in the United States.26 Until US and global hepatitis B vaccination programs eliminate chronic HBV infection among women of childbearing age, comprehensive prenatal screening will be needed to identify women who are HBV infected.
Evaluation of HBsAg-positive pregnant women for HBV-related liver disease is critically important for implementing care for these women and could lead to prevention of additional cases of mother-to-child transmission of HBV infection. Recommendations for initial evaluation of people with chronic HBV infection, which have been available for more than 15 years, include testing for HBeAg and/or HBV DNA.2,3,27–30 Simple algorithms for prenatal evaluation of pregnant women with HBV infection have been published since then.4,6,31 Lack of awareness of algorithms for prenatal evaluation in the obstetric community, or lack of access to health insurance providing for additional testing and specialty medical consultation, could have contributed to the lower rates of evaluation and referral observed in the jurisdictions examined in this study.32,33 In states where pregnant women receive comprehensive health insurance, providers can take advantage of prenatal coverage to obtain additional testing and specialty consultation.34
Prenatal evaluation of HBsAg-positive pregnant women creates new opportunities to prevent mother-to-child transmission of HBV infection. From 2012 to 2016, four professional organizations made recommendations to consider antiviral prophylaxis in the third trimester for pregnant women with high levels of viremia.29,35–37 Eighty percent of HBsAg-positive pregnant women in this study were identified sufficiently early in their pregnancy to obtain needed additional testing and consider antiviral treatment in the third trimester if indicated. In 2014, a health-care maintenance organization reported the results of a comprehensive protocol to screen pregnant women for HBsAg. Starting in 2006, HBsAg-positive pregnant women were referred for additional evaluation and selective use of prenatal antiviral prophylaxis as an adjunct to standard infant postexposure prophylaxis. Timely acceptance and application of this protocol eliminated mother-to-child transmission, including chronic HBV infections, among infants born to women with high levels of viremia.13
Limitations
This study had several limitations. A substantial number of missing values increased uncertainty for some variables. Programs were not able to locate and interview all of the identified HBsAg-positive pregnant women; language, culture, and finite program resources also were barriers to obtaining complete information. PHBPPs identified HBsAg-positive pregnant women through a variety of reports to public health. Although variation in the estimated completeness of identification among PHBPPs has been reported, up to half of HBsAg-positive pregnant women might not have been identified in some programs.18 Therefore, the estimated prevalence of HBsAg-positive pregnant women based on identified women case-managed by PHBPPs likely underestimated the true prevalence. The estimated prevalence of HBsAg-positive pregnant women was determined by using the site-specific number of births to capture data on the number of pregnancies and demographic characteristics, which, because of multiple births, could have contributed to underestimating the true prevalence of HBsAg positivity. HBeAg or HBV DNA results might not have been found, thereby underestimating the proportion of women who were evaluated for level of HBV viremia. Evaluation rates might have been different for women who were not identified by PHBPPs. The reason for antiviral treatment—whether for the women, for prevention of mother-to-child transmission of HBV infection, or both—was not determined when administered.
Conclusion
Our results and those of others suggest that prenatal evaluation of HBV liver disease should be emphasized for pregnant women with chronic HBV infection.1,2,13 In 2015, CDC and the American College of Obstetrics and Gynecology added guidance for prenatal evaluation of liver disease for HBsAg-positive pregnant women.31 Obstetric providers should be encouraged to perform prenatal evaluations according to this guidance and to consult with a provider who is experienced in managing chronic HBV infection, if necessary. This recent guidance and our findings might provide a baseline for encouraging evaluation of HBV liver disease among pregnant women with HBV infection and for measuring changes in evaluation rates with greater precision. Early prenatal identification of HBsAg-positive pregnant women might facilitate continuing care for women who have chronic HBV infection and accelerate progress toward eliminating mother-to-child transmission of HBV infection.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Centers for Disease Control and Prevention (US). Recommendations of the Immunization Practices Advisory Committee. Prevention of perinatal transmission of hepatitis B virus: prenatal screening of all pregnant women for hepatitis B surface antigen. MMWR Morb Mortal Wkly Rep 1988;37(22):341–6, 351. [PubMed] [Google Scholar]
- 2. Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP) part I: immunization of infants, children, and adolescents [published errata appear in MMWR Morb Mortal Wkly Rep 2006;55(06):158-9 and MMWR Morb Mortal Wkly Rep 2007;56(48):1267]. MMWR Recomm Rep 2005;54(RR-16):1–23. [PubMed] [Google Scholar]
- 3. Yapali S, Talaat N, Lok AS. Management of hepatitis B: our practice and how it relates to the guidelines. Clin Gastroenterol Hepatol 2014;12:16–26. [DOI] [PubMed] [Google Scholar]
- 4. Giles ML, Visvanathan K, Lewin SR, Sasadeusz J. Chronic hepatitis B infection and pregnancy. Obstet Gynecol Surv 2012;67:37–44. [DOI] [PubMed] [Google Scholar]
- 5. Bzowej NH. Optimal management of the hepatitis B patient who desires pregnancy or is pregnant. Curr Hepat Rep 2012;11:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan CQ, Duan ZP, Bhamidimarri KR, Zhou HB, Liang XF, Li J, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol 2012;10:452–459. [DOI] [PubMed] [Google Scholar]
- 7. Wen WH, Chang MH, Zhao LL, Ni YH, Hsu HY, Wu JF, et al. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol 2013;59:24–30. [DOI] [PubMed] [Google Scholar]
- 8. Cheung KW, Seto MT, Wong SF. Toward complete eradication of hepatitis B infection from perinatal transmission: review of the mechanisms of in utero infection and the use of antiviral treatment during pregnancy. Eur J Obstet Gynecol Reprod Biol 2013;169:17–23. [DOI] [PubMed] [Google Scholar]
- 9. Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAG-positive mothers. J Viral Hepat 2012;19:e18–25. [DOI] [PubMed] [Google Scholar]
- 10. Bleich LM, Swenson ES. Prevention of neonatal hepatitis B virus transmission. J Clin Gastroenterol 2014;48:765–772. [DOI] [PubMed] [Google Scholar]
- 11. Pan CQ, Lee HM. Antiviral therapy for chronic hepatitis B in pregnancy. Semin Liver Dis 2013;33:138–146. [DOI] [PubMed] [Google Scholar]
- 12. Kubo A, Shlager L, Marks AR, Lakritz D, Beaumont C, Gabellini K, et al. Prevention of vertical transmission of hepatitis B: an observational study. Ann Intern Med 2014;160:828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown RS, Jr, McMahon BJ, Lok AS, Wong JB, Ahmed AT, Mouchli MA, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatology 2016;63:319–333. [DOI] [PubMed] [Google Scholar]
- 14. Euler GL, Wooten KG, Baughman AL, Williams WW. Hepatitis B surface antigen prevalence among pregnant women in urban areas: implications for testing, reporting, and preventing perinatal transmission. Pediatrics 2003;111(5 Pt 2):1192–1197. [PubMed] [Google Scholar]
- 15. France AM, Bornschlegel K, Lazaroff J, Kennedy J, Balter S. Estimating the prevalence of chronic hepatitis B virus infection—New York City, 2008. J Urban Health 2012;89:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu PJ, Yankey D, Jeyarajah J, O’Halloran A, Elam-Evans L, Greby SM, et al. Hepatitis B vaccination among adolescents 13-17 years, United States, 2006–2012. Vaccine 2015;33:1855–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. Immunization coverage fact sheet. 2016. http://www.who.int/mediacentre/factsheets/fs378/en. Accessed May 26, 2016.
- 18. Smith EA, Jacques-Carroll L, Walker TY, Sirotkin B, Murphy TV. The national Perinatal Hepatitis B Prevention Program, 1994-2008. Pediatrics 2012;129:609–616. [DOI] [PubMed] [Google Scholar]
- 19. Census Bureau (US). Primary language code list. http://www.census.gov/hhes/socdemo/language/about/02_primary_list.pdf. Accessed March 23, 2013.
- 20. Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep 2008;57(RR-8):1–20. [PubMed] [Google Scholar]
- 21. Wong HY, Tan JY, Lim CC. Abnormal liver function tests in the symptomatic pregnant patient: the local experience in Singapore. Ann Acad Med Singapore 2004;33:204–208. [PubMed] [Google Scholar]
- 22. World Health Organization. The global burden of disease: 2004 update, annex C. Geneva: WHO; 2008. http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf. Accessed June 2, 2016.
- 23. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJK. Births: final data for 2008. Natl Vital Stat Rep 2010. December 8;59:1–72. [PubMed] [Google Scholar]
- 24. SAS Institute, Inc. SAS®: Version 9.2. Cary (NC): SAS Institute, Inc; 2008. [Google Scholar]
- 25. Howell J, Lemoine M, Thursz M. Prevention of materno-foetal transmission of hepatitis B in sub-Saharan Africa: the evidence, current practice and future challenges. J Viral Hepat 2014;21:381–396. [DOI] [PubMed] [Google Scholar]
- 26. Ioannou GN. Chronic hepatitis B infection: a global disease requiring global strategies. Hepatology 2013;58:839–843. [DOI] [PubMed] [Google Scholar]
- 27. Lok AS, McMahon BJ. Chronic hepatitis B [published erratum appears in Hepatology 2007;45:1347]. Hepatology 2007;45:507–539. [DOI] [PubMed] [Google Scholar]
- 28. Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, et al. National Institutes of Health Consensus Development Conference statement: management of hepatitis B. Ann Intern Med 2009;150:104–110. [DOI] [PubMed] [Google Scholar]
- 29. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD Guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McHugh JA, Cullison S, Apuzzio J, Block JM, Cohen C, Leong SL, et al. Chronic hepatitis B infection: a workshop consensus statement and algorithm. J Fam Pract 2011;60:e1–8. [PubMed] [Google Scholar]
- 31. CDC and the American College of Obstetricians and Gynecologists (ACOG). Screening and referral algorithm for hepatitis B virus (HBV) infection among pregnant women. http://www.cdc.gov/hepatitis/hbv/pdfs/prenatalhbsagtesting.pdf. Accessed June 2, 2016.
- 32. Yang EJ, Cheung CM, So SK, Chang ET, Chao SD. Education and counseling of pregnant patients with chronic hepatitis B: perspectives from obstetricians an perinatal nurses in Santa Clara County, California. Asian Pac J Cancer Prev 2013;14:1707–1713. [DOI] [PubMed] [Google Scholar]
- 33. Singh GK, Rodriguez-Lainz A, Kogan MD. Immigrant health inequalities in the United States: use of eight major national data systems. Scientific World J 2013. Article ID 512313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jarlenski MP, Bennett WL, Barry CL, Bleich SN. Insurance coverage and prenatal care among low-income pregnant women. Med Care 2014;52:10–19. [DOI] [PubMed] [Google Scholar]
- 35. European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012;57:167–185. [DOI] [PubMed] [Google Scholar]
- 36. Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 2012;5:531–561. [DOI] [PubMed] [Google Scholar]
- 37. National Institute for Health and Care Excellence (NICE). Hepatitis B (chronic): diagnosis and management of chronic hepatitis in children, young people and adults. London: National Institute for Health and Care Excellence; 2013. [PubMed] [Google Scholar]