Abstract
We investigated whether postextinction administration of methylene blue (MB) could enhance retention of an extinguished conditioned response. MB is a redox compound that at low doses elevates cytochrome oxidase activity, thereby improving brain energy production. Saline or MB (4 mg/kg intraperitoneally) were administered to rats for 5 d following extinction training of tone-footshock conditioning. Postextinction freezing was lower in rats receiving MB compared with saline, suggesting that MB improved retention of the extinction memory. The MB effect was specific to tone-evoked freezing because there were no differences in pretone freezing. Control subjects similarly injected with MB showed no evidence of nonspecific effects on measures of motor activity and fearfulness. MB-treated rats exhibited both greater retention of extinction and greater overall brain metabolic activity. Rats with higher retention of extinction also showed a relative increase in cytochrome oxidase activity in prefrontal cortical regions, especially anterior infralimbic cortex, dorsal and medial frontal cortex, and lateral orbital cortex. These regional metabolic increases were also correlated to the behavioral freezing index used to assess retention of extinction. It was concluded that MB administered postextinction could enhance retention of extinction memory through an increase in brain cytochrome oxidase activity.
The objective was to investigate whether postextinction administration of methylene blue (MB) could be used to enhance retention of an extinguished conditioned response. MB is a nonneuroleptic phenothiazine previously used safely in humans as a neuroprotective metabolic agent for treatment of dementia, depression, and drug-induced encephalopathy (Naylor 1986; Wainwright and Crossley 2002). MB serves as a redox compound that at low doses (1-5 mg/kg) improves mitochondrial respiration (Visarius et al. 1997) and prevents free radical damage (Salaris et al. 1991). Low-dose MB acts on the electron transport chain, and it increases cellular oxygen consumption by a well-known mechanism of action that involves accepting electrons from molecular oxygen (Lindahl and Oberg 1961). When MB acts as an alternative electron acceptor in mitochondria, it also inhibits the production of superoxide by competing with molecular oxygen (Salaris et al. 1991).
Low-dose MB increases brain cytochrome oxidase activity after intraperitoneal administration in rats (Callaway et al. 2004). Cytochrome oxidase is the terminal enzyme in the electron transport, and it catalyzes the utilization of molecular oxygen to form water and ATP in the process known as oxidative phosphorylation (Wong-Riley 1989). The brain is the organ most dependent on oxidative phosphorylation for the production of metabolic energy (Sokoloff 1992). Therefore, MB could be used to increase brain cytochrome oxidase activity and thereby improve oxidative energy metabolism in the brain.
Low doses of MB significantly enhance memory retention in both aversive and appetitive tasks. For example, posttraining MB administration improves memory retention tested 24 h after inhibitory avoidance training (Martinez Jr. et al. 1978). Corresponding low doses of MB also increase spatial memory retention in a holeboard food search task (Callaway et al. 2002, 2004). Intrigued by these findings, we have investigated MB as a possible metabolic enhancer that could improve retention of memory for extinction of a conditioned response. Specifically, we tested whether 4 mg/kg MB injected for 5 d postextinction could enhance the retention of extinction of a conditioned freezing response in rats.
Extinction is a behavioral phenomenon characterized by the reduction of the conditioned response that occurs as a consequence of nonreinforcement. Pavlov (1927) hypothesized that the neural mechanism of extinction involved the formation of cortical circuits that inhibited a conditioned response by counteracting the previously acquired excitatory associations between the conditioned stimulus (CS) and the unconditioned stimulus (US). Myers and Davis (2002) have recently reviewed the literature on the behavioral and neural mechanisms of extinction. Rats with lesions of the ventromedial prefrontal cortex have deficits in the retention of extinction across days (Morgan et al. 1993, 2003; Quirk et al. 2000). On the other hand, similar lesions of the ventromedial prefrontal cortex do not impair extinction across days (Gewirtz et al. 1997). Electrophysiological evidence provided by Milad and Quirk (2002) showed enhanced firing rates of neurons in the infralimbic cortex of rats with faster extinction of conditioned freezing. They also showed that electrical stimulation of the infralimbic cortex results in less conditioned freezing during extinction.
However, extinction effects are not likely to be limited to the infralimbic cortex. Likewise, the lesion literature is not conclusive because lesions assume that the mechanism of extinction is localized to one brain region or pathway. This is unlikely the case given our recently published study mapping mouse brain activity after extinction of conditioned freezing (Barrett et al. 2003). The findings of this study implicate multiple regions in the frontal cortex, such as medial frontal cortex, dorsal frontal cortex, and infralimbic cortex, in the retention of extinction memory. Brain-behavior correlations between metabolic activity and freezing behavior showed that subjects with higher activity in these frontal cortical regions were most successful at inhibiting conditioned freezing.
We hypothesized that if postextinction MB enhances extinction memory by increasing brain cytochrome oxidase activity, the prefrontal cortex regions identified in previous studies would be more activated in rats showing better retention of extinction. Therefore, in this study we investigated whether low-dose MB administered after extinction would enhance extinction memory through a mechanism involving increased cytochrome oxidase activity that may affect prefrontal cortical regions of the rat brain.
RESULTS
Acquisition and Extinction Behavior
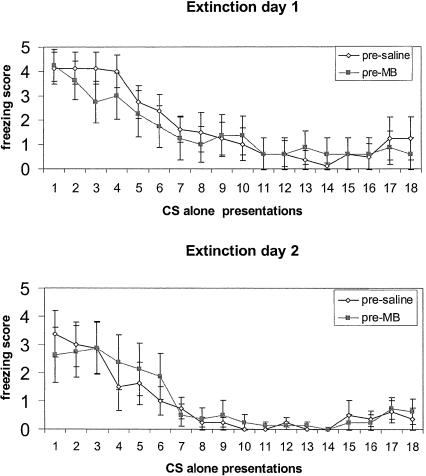
All subjects showed similar acquisition and extinction prior to the beginning of MB or saline administrations. On a zero-to-five scale, with zero behavioral score indicating no freezing and a score of five representing maximum possible freezing counts, mean presaline group CS freezing score was 4.7 ± 0.22 and mean pre-MB group CS freezing score was 4.29 ± 0.42. During the 2 d of extinction, all rats showed similar extinction curves (Fig. 1).
Figure 1.
Mean ± SE freezing counts during the 2 d of extinction before the beginning of MB and saline injections.
Effects of MB on the Retention of Extinction
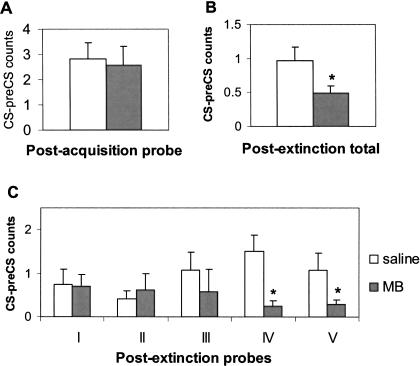
The eight rats assigned to the MB group were injected daily with MB (4 mg/kg, intraperitoneally) during 5 d after extinction, and the other eight rats were similarly injected with saline to serve as a control group (Table 1). The effects of MB and saline were evaluated in pre-CS (15 sec before tone) and CS periods (15-sec tone) in five postextinction probes (probes I through V, with three tones each). Each probe is reported as the mean of the three trials. There were no significant group differences in pre-CS freezing (saline, 0.31 ± 0.13; MB, 0.45 ± 0.23; t = -0.546, P = 0.593). Normalized freezing scores were computed as CS - preCS freezing counts for each trial for each subject, and mean group results are shown in Figure 2. Figure 2A shows that after 2 d of acquisition training with four tones (CS) coterminating with footshocks (US), all animals showed similar CS - preCS freezing scores in the postacquisition probe trial with three tones done before MB or saline administration.
Table 1.
Experimental Design
| Day | Procedure | Context | Treatment |
|---|---|---|---|
| 1-2 | Habituation | A | Context exposure |
| 3-4 | Acquisition | A | Tone-shock pairing |
| 5-6 | Extinction | B | Tone alone presentations |
| 7-12 | Probe with tone | B | Postextinction MB or saline |
Figure 2.
Behavioral effects of methylene blue on the extinction of conditioned freezing. (A) Mean ± SE freezing scores (CS - preCS freezing counts) after 2 d of acquisition training before the onset of MB/saline treatments. (B) Mean ± SE total postextinction freezing scores in the MB-treated and saline-treated groups. (C) Mean ± SE freezing scores in the MB-treated and saline-treated groups in postextinction probes I to V. Asterisks indicate significant differences at P < 0.05.
Figure 2B shows that the total postextinction freezing CS - preCS scores were significantly lower in animals receiving MB compared with the control group, suggesting that MB enhanced retention of the extinction memory. Overall, there was a group effect of MB administration (t = 2.328, P = 0.048), with saline-administered animals freezing about twice as much as MB-treated animals.
Figure 2C shows that the MB-treated animals had a longer-lasting extinction effect as seen in probes IV and V. On average, saline-treated rats showed freezing scores that were five times greater than those of MB-treated rats in the last two probes. But in postextinction probe I, there were no significant differences in the freezing scores between the two groups. Thus, repeated daily MB injections improved retention of extinction memory in MB subjects compared with the saline-injected subjects.
Effects of MB on General Motor Activity and Fearfulness
The subjects treated with saline or MB showed no group differences in pre-CS freezing behavior and average number of fecal boli during the postextinction probe sessions (saline, 1.85 ± 1.19; MB, 1.75 ± 1.21; t = -0.198, P = 0.847). To further evaluate whether there were nonspecific motor and fear-related effects of MB, a second experiment with separate groups of rats treated with saline (n = 11) or with one MB injection (n = 6) or five MB injections (n = 6) was done. Experiment 2 showed that there were similar motor activity measures in the open field after saline or repeated MB injections (Table 2). Experiment 2, together with the similar pre-CS freezing in MB and saline groups in experiment 1, served to rule out that repeated MB injections may simply increase levels of motor activity. Therefore, MB administration modified postextinction CS-evoked conditioned freezing, as opposed to general motor activity.
Table 2.
General Activity Measures (Mean ± SE) in Saline and MB-Treated Groups
| Measures | Saline | Five MB |
|---|---|---|
| Ambulatory distance (cm) | 2232 ± 349 | 1717 ± 276 |
| Ambulatory time (sec) | 40 ± 7 | 33 ± 6 |
| Ambulatory counts | 1014 ± 192 | 796 ± 155 |
| Stereotypic time (sec) | 125 ± 7 | 119 ± 9 |
| Stereotypic counts | 2286 ± 177 | 1979 ± 196 |
| Resting time (sec) | 428 ± 14 | 442 ± 16 |
| Vertical counts | 83 ± 7 | 76 ± 9 |
| Vertical time (sec) | 123 ± 14 | 144 ± 23 |
| Center zone entries | 421 ± 59 | 358 ± 64 |
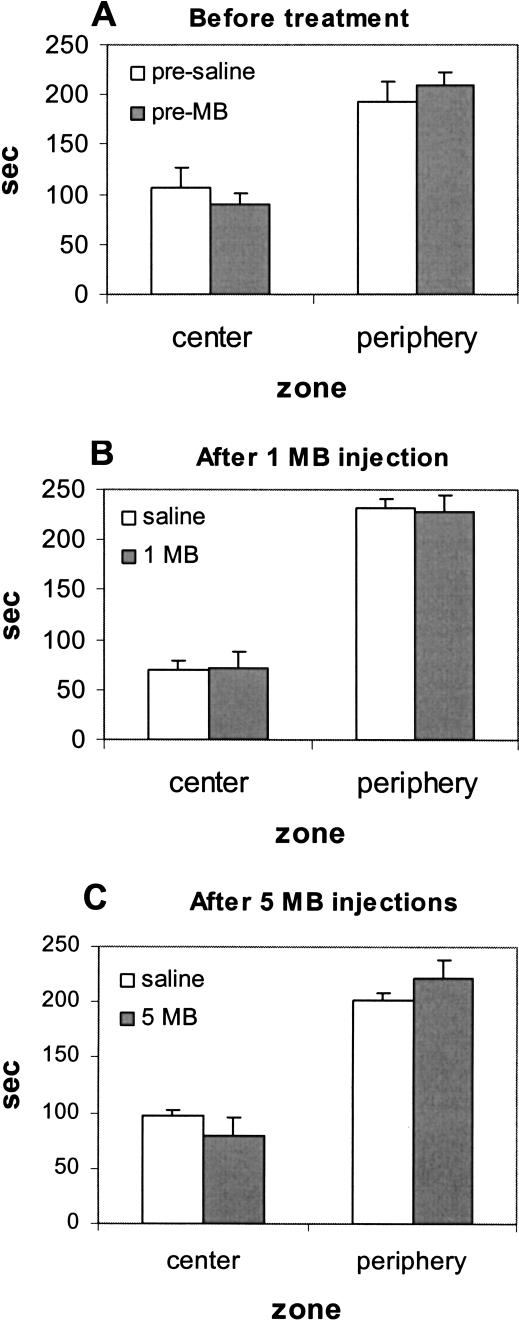
Fear-related behavior evaluated with center-avoidance/thigmotaxic behavior in experiment 2 also served to rule out that MB effects were simply due to a general decreased fearfulness as opposed to enhanced extinction memory. Subjects spent similar times in the center and periphery of the open field in each condition: before treatment (Fig. 3A), after one MB injection (Fig. 3B), or after five daily MB injections (Fig. 3C), indicating that there was no evidence of general decreased fearfulness with repeated MB injections.
Figure 3.
Behavioral effects of methylene blue on fear-related behavior. (A) Mean ± SE time spent in center and periphery prior to any treatment. (B) Mean ± SE time spent in center and periphery 24 h following one MB injection. (C) Mean ± SE time spent in center and periphery 24 h after five daily MB injections.
Effect of MB on Absolute Brain Cytochrome Oxidase Activity
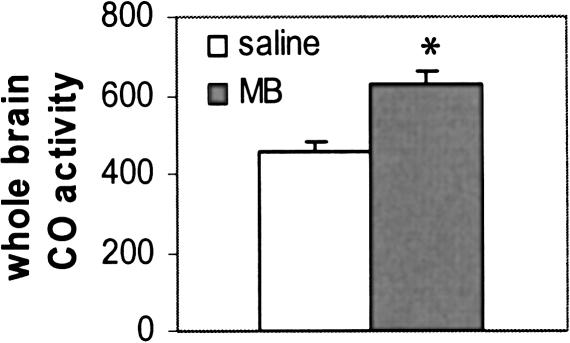
Animals receiving MB after extinction showed both greater extinction memory and greater absolute brain metabolic activity. After the last postextinction probe session, animals were decapitated, their brains were divided into two halves: One half was homogenized and used for spectrophotometry, and the other half was sectioned and used for histochemistry. Figure 4 shows the spectrophotometric analysis of the rate of cytochrome c oxidation in the brain homogenates of MB-treated and control rats.
Figure 4.
Overall absolute brain cytochrome oxidase (CO) activity units (μM/min/g) in the brains of MB-treated and saline-treated groups. Asterisk indicates significant group difference at P ≤ 0.001.
The brain homogenates of MB-treated animals showed an overall increase in absolute units of cytochrome oxidase activity that was 38% greater than the activity measured in brains from the saline-treated rats (t = -4.116, P = 0.001).
Effects of MB on Relative Cytochrome Oxidase Activity of Brain Regions
Independently of the absolute increase in brain metabolism produced by MB, in experiment 1 we investigated whether some regions of the brain were relatively more affected than others by using quantitative histochemistry of cytochrome oxidase. Because MB was administered in the postextinction period, it was hypothesized that MB metabolic-enhancing effects would be greater in regions that were more active in the postextinction period of testing for extinction memory retention. Data from 21 regions (Fig. 5) in the anterior half of the brain were normalized to the whole brain value of each subject.
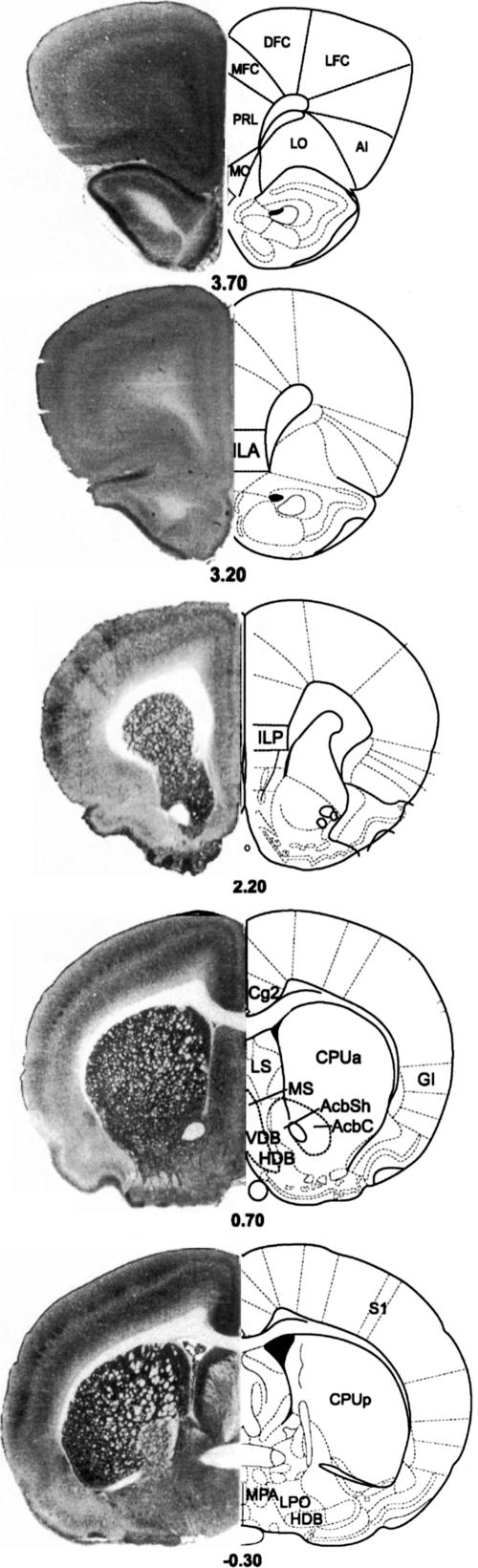
Figure 5.
Coronal brain diagrams (right) and cytochrome oxidase stained sections (left) illustrating the location of the regions of interest by Bregma level according to the Paxinos and Watson (1997) atlas. Abbreviations are listed in Table 3.
Table 3 lists the relative cytochrome oxidase activity for each of the forebrain regions of interest for the two groups. Although there was a general relative metabolic increase in all the regions examined in the MB-treated group, five regions showed significant increases at P < 0.01: dorsal frontal cortex, medial frontal cortex, prelimbic cortex, lateral orbital cortex, and anterior infralimbic cortex. Furthermore, there was a high degree of positive interregional activity correlation (r ≥ 0.96, P ≤ 0.01) between these cortical regions. When a Bonferroni procedure was applied to all the P levels to correct for the multiple comparisons, only the anterior infralimbic cortex of rats given MB in the postextinction period showed a relatively greater activity at P < 0.05.
Table 3.
Regional Cytochrome Oxidase Activity (Mean ± SE) in Saline and MB-Treated Groups
| Region | Abb. | Saline | MB | t | P |
|---|---|---|---|---|---|
| Dorsal frontal cortexa | DFC | 215 ± 21 | 314 ± 21 | −3.564 | 0.003 |
| Medial frontal cortexa | MFC | 208 ± 20 | 303 ± 20 | −3.606 | 0.003 |
| Anterior infralimbic cortexa | ILA | 200 ± 10 | 275 ± 18 | −3.878 | 0.002 |
| Posterior infralimbic cortex | ILP | 198 ± 22 | 267 ± 27 | −2.113 | 0.053 |
| Prelimbic frontal cortexa | PRL | 188 ± 17 | 277 ± 19 | −3.776 | 0.002 |
| Lateral orbital cortexa | LO | 210 ± 21 | 301 ± 18 | −3.499 | 0.004 |
| Medial orbital cortex | MO | 194 ± 20 | 272 ± 20 | −2.882 | 0.012 |
| Lateral frontal cortex | LFC | 219 ± 24 | 310 ± 23 | −2.950 | 0.011 |
| Agranular insular cortex | AI | 206 ± 21 | 287 ± 22 | −2.789 | 0.014 |
| Granular insular cortex | GI | 205 ± 23 | 272 ± 18 | −2.429 | 0.029 |
| Anterior cingulate | CG2 | 232 ± 31 | 315 ± 23 | −2.307 | 0.037 |
| Nucleus accumbens | ACB | 195 ± 18 | 276 ± 25 | −2.808 | 0.014 |
| Medial septal nucleus | MS | 185 ± 22 | 251 ± 14 | −2.667 | 0.018 |
| Lateral septal nucleus | LS | 236 ± 30 | 297 ± 20 | −1.826 | 0.089 |
| Anterior caudate-putamen | CPUa | 218 ± 23 | 291 ± 19 | −2.612 | 0.021 |
| Posterior caudate-putamen | CPUp | 211 ± 25 | 273 ± 20 | −2.079 | 0.057 |
| Anterior parietal cortex | S1 | 227 ± 28 | 299 ± 26 | −2.022 | 0.063 |
| Medial preoptic area | MPO | 206 ± 28 | 246 ± 19 | −1.274 | 0.223 |
| Lateral preoptic area | LPO | 203 ± 24 | 256 ± 16 | −1.916 | 0.076 |
| Ventral diagonal band nucleus | VDB | 191 ± 23 | 250 ± 13 | −2.360 | 0.033 |
| Horizontal diagonal band nucleus | HDB | 201 ± 24 | 252 ± 19 | −1.782 | 0.097 |
P < 0.01.
Brain-Behavior Correlations
To further investigate whether the observed regional metabolic increases may be related to MB effects on postextinction freezing, correlations between brain activity and freezing behavior of each subject were computed. To avoid type I errors due to multiple pairwise comparisons, correlations between individual subjects' postextinction probe scores and metabolic activity were limited to the absolute brain measure and to the five regions meeting the criterion of a relative group difference at P < 0.01. Table 4 shows that there were highly significant negative correlations (r = 0.65 to 0.69, P < 0.01) between the postextinction freezing score and the individual subjects' metabolic activity in the dorsal frontal cortex, medial frontal cortex, lateral orbital cortex, and anterior infralimbic cortex. This analysis suggested that the activity in these four regions in the MB-treated group was better correlated with retention of the extinction memory than was the prelimbic frontal cortex and the whole brain activity.
Table 4.
Brain-Behavior Correlations Between Cytochrome Oxidase Activity and Postextinction Freezing Scores
| Region | r | P |
|---|---|---|
| Dorsal frontal cortexa (DFC) | −0.69 | 0.005 |
| Medial frontal cortexa (MFC) | −0.66 | 0.008 |
| Anterior infralimbic cortexa (ILA) | −0.65 | 0.009 |
| Prelimbic frontal cortex (PRL) | −0.60 | 0.018 |
| Lateral orbital cortexa (LO) | −0.68 | 0.006 |
| Whole brain homogenate (WB) | −0.4 | 0.125 |
P < 0.01.
DISCUSSION
Experiment 1 showed that MB administered repeatedly in the postextinction period enhanced the retention of extinction of the CS-evoked conditioned freezing response. Rats receiving MB exhibited not only an enhanced extinction memory but also an absolute increase in brain energy metabolism, as revealed by cytochrome oxidase activity. This metabolic increase was relatively greater in prefrontal cortical regions previously related to the retention of extinction memory. Indeed, the freezing scores of rats showing better retention of the extinction memory were correlated with greater cytochrome oxidase activity in each of the more affected cortical regions. Experiment 2 showed that repeated MB injections had no significant effects on general motor activity or fear-related behavior.
MB Improves Memory Retention Without Affecting Motor Activity and Fearfulness
In Experiment 1, there was an acquisition phase and an extinction phase before MB or saline administration, in which subjects showed similar acquisition and extinction of conditioned freezing. MB was administered in the postextinction period to improve the retention of the extinction memory. The rationale was that MB administered postextinction would enhance brain metabolic processes underlying retention of extinction. Because MB was given after extinction, it could not have interfered with the acquisition trials. Instead, MB enhanced the retention of extinction memory as tested in the probe trials, suggesting that MB acted primarily on brain processes mediating the retention of extinction memory.
One alternative interpretation of Experiment 1 is that repeated MB injections could simply increase levels of motor activity and thereby reduce freezing behavior. However, postextinction pre-CS freezing behavior was not different between saline and MB-treated rats, suggesting that the effect of repeated MB administration was specific to the CS-evoked conditioned response. To further investigate the possibility of whether motor effects could account for the observed differences between saline and MB-treated rats, Experiment 2 was conducted with rats given repeated MB administration and tested for general motor activity in the open field. There were no significant differences in any activity measurements between saline and MB-treated rats. Experiment 2 served to rule out the possibility that repeated MB injections could simply increase levels of general motor activity.
Yet another possibility is that MB decreased fearfulness in the animals, as opposed to enhanced extinction memory. To test this possibility, we evaluated fear-related behavior in the open field. Increased time in the periphery (thigmotaxic time) and center avoidance (less time spent in center) is associated with fearfulness or anxiety in rats (Treit and Fundytus 1988). Experiment 2 showed that there were no differences in time spent in center or periphery in subjects in any condition tested: before treatment, after saline, after one MB injection or after five MB injections (Fig. 3). Therefore, the control experiments suggest that the observed MB effects on conditioned freezing are more likely the result of enhanced retention of extinction memory.
The observed improvement in memory retention following postextinction MB administration is consistent with the results of a number of previous animal MB studies supporting the conclusion that posttraining administration of low-dose (1-4 mg/kg) MB improves memory retention in different tasks (Martinez Jr. et al. 1978; Callaway et al. 2002, 2004). For example, successful memory retention is improved by posttraining MB administration in an inhibitory avoidance task (Martinez Jr. et al. 1978). Repeated posttraining MB administration has also enhanced spatial memory retention in probe trials in a holeboard food search task in rats without affecting general motor activity (Callaway et al. 2004). Large MB doses (50 to 100 mg/kg), however, impair memory because they lead to methemoglobin formation and impaired oxygen consumption (Martinez Jr. et al. 1978).
MB Increases Overall Brain Oxidative Metabolism
Cytochrome oxidase activity was used to quantify the long-term neural metabolic alterations that developed during the postextinction phase of the experiment. As neuronal activity (and consequent need for ATP) increases or decreases, cytochrome oxidase activity adjusts accordingly to meet neuronal demand for ATP. Our previous studies using different learning paradigms such as classical conditioning (Poremba et al. 1998) and the Morris water maze (Villarreal et al. 2002) indicate that changes in cytochrome oxidase activity require several days of training. Therefore, measuring cytochrome oxidase activity is well suited for evaluating the effects of MB on the brain over the postextinction phase.
The metabolic effect of low-dose MB on memory retention may be due to increased brain oxygen consumption because MB provides an alternate route of electron flow to oxygen (Visarius et al. 1997). This increase in brain oxidative metabolism is reflected by the overall increase in brain cytochrome oxidase activity observed in MB-treated animals. Our biochemical in vitro studies show a 25% increased rate of cytochrome oxidase activity in brain homogenates after introduction of low concentration of MB (Callaway et al. 2004). We also showed 30% increases in absolute brain cytochrome oxidase activity 24 h following in vivo administration of MB to rats (Callaway et al. 2004). In the present study, a 4 mg/kg intraperitoneal injection of MB given daily for 5 d resulted in a cumulative 38% increase in overall brain cytochrome oxidase activity measured 24 h after the last injection. MB progressively accumulates in the brain, reaching a concentration >10 times greater in the brain than in the blood 1 h after the administration (Peter et al. 2000).
If MB improves memory by increasing cytochrome oxidase activity, treatments that result in inhibition of cytochrome oxidase activity should lead to impaired memory. For example, it has been shown that sodium azide decreases cytochrome oxidase activity when chronically administered to rats (Cada et al. 1995; Berndt et al. 2001) and causes spatial memory deficits in rats tested in the Morris water maze (Bennett et al. 1996) and the holeboard maze (Callaway et al. 2002). Remarkably, when MB is administered to rats with reduced brain cytochrome oxidase activity, their memory retention scores are raised to the same level as that of control animals (Callaway et al. 2002). Administration of low-dose MB can also enhance spatial memory retention in normal rats by increasing brain cytochrome oxidase activity (Callaway et al. 2004). These findings, together with our present results, suggest that an increase in oxidative energy metabolism in the brain is a mechanism whereby MB enhances memory retention. An increase in cytochrome oxidase activity results in increased oxidative metabolic capacity of neurons because it allows more oxygen consumption and ATP formation in the brain (Gonzalez-Lima and Cada 1998).
Prefrontal Cortex Was More Activated by the Postextinction MB Treatment
If MB increases overall brain metabolic activity, one may raise the question of why some brain regions may show relatively more effects than do others in this study. The hypothesis that regions more engaged in retention of extinction memory may be targeted by MB metabolic-enhancing effects is based on two premises. First, MB was not administered throughout the entire Experiment 1. MB was administered only during the postextinction period and thus could only affect brain metabolism during that period. Second, brain regions with more metabolic demand during the postextinction period, and the behavior they mediate, would be expected to benefit more from the metabolic-enhancing effects of MB than would regions engaged in other aspects of behavior prior to MB administration. Brain accumulation of MB during the postextinction phase of the experiment would selectively facilitate cytochrome oxidase activity during that period, as opposed to the initial acquisition and extinction phases of the experiment. On the other hand, if MB was administered in the acquisition phase, it would be expected that MB would facilitate retention of the acquired behavior, as has been found in previous studies (Martinez Jr. et al. 1978; Callaway et al. 2004). Therefore, it is not the case that MB preferentially affects the activity of only brain regions engaged in acquisition or extinction. Which regions are more affected would seem to depend on which regions have more metabolic energy demand during the period when MB is administered. Because postextinction MB administration increased oxidative energy metabolism in the brain during the period of extinction memory consolidation and retrieval, this effect may lead to enhanced extinction memory retention.
The findings also supported our hypothesis that the prefrontal regions identified in previous studies were more activated in rats showing better retention of extinction. Cytochrome oxidase activity revealed neural activity linked to the increased metabolic demand of certain brain regions engaged in the postextinction phase. There was a relatively greater metabolic effect of MB on cortical brain regions engaged in the retention of extinction memory, such as the dorsal frontal cortex, medial frontal cortex, anterior infralimbic cortex, and lateral orbital cortex. Furthermore, all of these regions showed brain-behavior correlations between their increased cytochrome oxidase activity and the subject's retention of extinction, suggesting that subjects with higher prefrontal activity were more successful at inhibiting conditioned freezing.
In addition, these regions appear to have strong functional coupling, as suggested by their high degree of intercorrelative activity. Because these prefrontal cortical regions participate in the retention of extinction memory as a functionally coupled network (Barrett et al. 2003), it is unlikely that any single one of these prefrontal regions exclusively mediates extinction memory in the intact rat brain.
Interestingly, there was a difference between the anterior and posterior parts of the infralimbic cortex. Although the posterior infralimbic cortex did not show a significant relative metabolic increase, the increase observed in the anterior infralimbic cortex corresponded to the most statistically reliable regional difference among all the brain regions investigated. In our previous study using fluorodeoxyglucose, the mouse infralimbic cortex showed the largest increase in metabolic activity among the regions with significantly elevated metabolism in the extinction group (Barrett et al. 2003). The involvement of the anterior infralimbic cortex in extinction memory supports the studies of Quirk and collaborators of the role of the ventromedial prefrontal cortex in the memory for fear extinction. The anterior infralimbic cortex reported in our study corresponds to the same infralimbic region (Bregma 3.2 mm), where there is strong coupling between neuronal electrical activity and retention of extinction (Milad and Quirk 2002) and where lesions impair the recovery of extinguished fear (Quirk et al. 2000). However, although infralimbic cortical activity was correlated with our behavioral extinction index, other prefrontal regions showed better brain-behavior correlations in the present study and in our previous mouse study (Barrett et al. 2003).
In conclusion, low-dose MB administered postextinction enhanced retention of extinction memory and overall brain cytochrome oxidase activity, especially within the prefrontal cortical regions of the rat brain. Metabolic enhancers such as MB, which improve brain energy production, may be successful at improving memory retention under many conditions depending on the period of administration. The metabolic enhancing effects of MB are not necessarily limited to one brain region or function; rather MB may help meet ongoing metabolic demands for any region engaged in a particular function, such as memory retention in the postextinction phase. MB is an Food and Drug Administration-approved drug that is readily available and that has already been used clinically for many years as an antidote for certain metabolic poisons (Kupfer et al. 1996) and as an inhibitor of oxygen free radical generation in reperfusion injury to the brain (Kelner et al. 1988; Salaris et al. 1991). Our results suggest that MB administered in conjunction with extinction behavioral therapy in humans may be a useful therapeutic agent to facilitate retention of extinction of conditioned fear or other traumatic memories.
MATERIALS AND METHODS
Animals
Subjects were 39 Long-Evans male rats (Experiment 1, n = 16; Experiment 2, n = 23; Harlan, Houston, TX) weighing an average of 135 g at the start of the experiment. Each rat was handled daily for 3 min for 7 d prior to the start of the experiment. Rats were given food and water ad libitum. They were housed three to four rats per cage and kept on a 12-h light/12-h dark cycle. All procedures were conducted in agreement with the American Association for the Accreditation of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committee.
Experiment 1
Behavioral Training
We used the same apparatus described previously by Barrett et al. (2003). Behavioral training lasted 12 d (Table 1). On days 1 and 2, animals were carried one at a time in a dark container to an operant chamber (MED Associates) illuminated with a red light bulb and enclosed in a sound-attenuated box. They were allowed to explore the chamber for 1 h, after which they were placed back in their home cage. On days 3 and 4, subjects underwent acquisition training, in which four CS tones (15-sec, 65 dB, 1- to 2-kHz frequency modulated tones, generated by two Wavetek Sweep/Modulation generators) coterminated with a 0.5-mA, 0.75-sec footshock (US). The acquisition sessions lasted 15 min each, with an average intertrial interval of 3 min. Animals were placed back in their home cages after the completion of each session. On day 5, one 10-min postacquisition probe trial was conducted with three 15-sec CS tones in a different context that consisted of a clear plastic cage (10 × 7.5 × 6 in.) with a speaker mounted on top.
Freezing was defined as a rat having all four feet on the floor; with shallow, rapid breathing and minimal head and vibrissae movement for at least 3 sec. Freezing was scored for 15 sec prior to the CS onset as well as during CS presentations in 3-sec bins. The normalized freezing score was computed as average CS freezing counts minus pre-CS freezing counts. Two hours after the postacquisition probe session (Fig. 2A), rats underwent extinction training in the probe context. On the first day of extinction training, rats were presented every 3 min with a nonreinforced CS tone (18 tones per session). On the second day of extinction training, animals received 18 nonreinforced CS presentations, and then 2 h later they were exposed to a series of 5-sec on/1-sec off CS presentations for another hour to simulate the testing session used in our previous studies with fluorodeoxyglucose (Barrett et al. 2003).
Subjects were returned to their home cages following the extinction training, and then 30 min later, eight rats were injected intraperitoneally with saline, and another eight rats were injected with 4 mg/kg MB (Faulding Pharmaceuticals) dissolved in saline. This dose was selected because it is the same one given chronically to humans without side-effects (Naylor et al. 1986; Peter et al. 2000). Twenty-four hours after each injection, animals were tested for freezing responses during the pre-CS period of 15 sec, and during the CS presentation, using the same protocol as for the postacquisition freezing probe session. After each postextinction probe session, animals were transferred back into their home cage, left for 30 min, and then injected with saline or MB. This was repeated three more times, for a total of five drug administrations and five postextinction probe sessions (Fig. 2C). After the last probe session, rats were decapitated and their brains quickly extracted.
Brain Biochemistry
One hemisphere was rapidly frozen in isopentane and stored at -40°C. The other hemisphere was homogenized on ice by using Dounce glass tissue homogenizer and then frozen in isopentane. Our spectrophotometric procedure described in Callaway et al. (2004) was used to determine absolute units of cytochrome oxidase activity in brain homogenates.
Regions of Interest
Quantitative cytochrome oxidase histochemistry was performed by using our previously described procedures (Gonzalez-Lima and Cada 1994, 1998; Gonzalez-Lima and Jones 1994). The regions of interest were delineated by using our cytochrome oxidase histochemical atlas (Gonzalez-Lima and Cada 1998) and the Paxinos and Watson (1997) rat brain atlas. Optical density of all regions of interest was analyzed by using our image processing system (Valla et al. 2001). Two to four optical density readings for each region were collected from each of three different sections for each subject. These values were then converted into cytochrome oxidase activity by using a set of standards with a known cytochrome oxidase activity. Regional activity was then normalized to determine which of the brain regions were more affected independently of absolute activity. Relative values were normalized by using the whole brain value in every subject, with the formula R × WB/(mean activity of all subjects), where R represents the activity of a region of interest and WB represents whole brain activity as determined spectrophotometrically for that subject.
Statistical Analysis
Group differences in behavior, spectrophotometric cytochrome oxidase values, and densitometric cytochrome oxidase values were compared by using two-tailed Student's t tests between two independent groups. For interregional and brain-behavior correlations, we used Pearson's product-moment correlations as reported before (Barrett et al. 2003). The SPSS statistical program was also used to exclude one subject as an outlier from the brain-behavior correlational analysis because its Studentized residual value was found to be 3.17. The t and P values are reported in the Results section.
Experiment 2
A control experiment with separate groups of rats was conducted to rule out that the MB effects on postextinction freezing found in Experiment 1 could be due to nonspecific increases in motor activity or decreased fearfulness. One group of rats (n = 11) was injected with saline, a second group of rats (n = 6) was injected once with MB (4 mg/kg, intraperitoneally), and a third group of rats (n = 6) was given repeated MB injections as in Experiment 1 (one injection daily for 5 d).
All the parameters were the same as in Experiment 1 except that the three groups of rats were evaluated in an automated open field activity monitoring system from MED Associates (St. Albans). The open field chamber (17 × 17 × 12 in.) had clear plastic and a white Plexiglas floor. Four arrays of 16 parallel infrared motion detector beams spaced 1 in. apart and located 1 and 7 in. above the chamber floor were used to record behavioral measures. The measures recorded were ambulatory distance, ambulatory time, ambulatory counts, stereotypic time, stereotypic counts (defined as movement without displacement), resting time, vertical counts (defined as number of upper beam brakes), vertical time, and number of center zone entries (center defined as 38% of the area). Between animals, the chambers were cleaned with a mild detergent solution. After a 5-min habituation period in the room, rats were placed individually in the chamber facing one corner, and their motor behavior was automatically recorded for 10 min (Table 2).
Fear-related behavior was evaluated during the first 5 min of exposure to the open field, by testing for center avoidance (time in center, defined as 38% of the total area) and thigmotaxic behavior (time in periphery) before injections (Fig. 3A) or 24 h after one injection of MB or saline (Fig. 3B) or 24 h after the last of five MB injections (Fig. 3C). Data were analyzed by using Student t tests for paired or unpaired samples as appropriate, with significant group differences tested at two-tailed P < 0.05.
Acknowledgments
We thank Penny D. Riha for her assistance with brain extractions and spectrophotometry, and Douglas Barrett and Jason Shumake for their assistance with the figures. Supported by National Institutes of Health grant R01 NS37755 to FGL.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.82404.
References
- Barrett, D., Shumake, J., Jones, D., and Gonzalez-Lima, F. 2003. Metabolic mapping of mouse brain activity after extinction of a conditioned emotional response. J. Neurosci. 23: 5740-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, M.C., Mlady, G.W., Fleshner, M., and Rose, G.M. 1996. Synergy between chronic corticosterone and sodium azide treatments in producing a spatial learning deficit and inhibiting cytochrome oxidase activity. Proc. Natl. Acad. Sci. 93: 1330-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt, J.D., Callaway, N.L., and Gonzalez-Lima, F. 2001. Effects of chronic sodium azide on brain and muscle cytochrome oxidase activity: A potential model to investigate environmental contributions to neurodegenerative diseases. J. Toxicol. Environ. Health A 63: 67-77. [DOI] [PubMed] [Google Scholar]
- Cada, A., Gonzalez-Lima, F., Rose, G.M., and Bennett, M.C. 1995. Regional brain effects of sodium azide treatment on cytochrome oxidase activity: A quantitative histochemical study. Metab. Brain Dis. 10: 303-319. [DOI] [PubMed] [Google Scholar]
- Callaway, N.L., Riha, P.D., Wrubel, K.M., McCollum, D., and Gonzalez-Lima, F. 2002. Methylene blue restores spatial memory retention impaired by an inhibitor of cytochrome oxidase in rats. Neurosci. Lett. 332: 83-86. [DOI] [PubMed] [Google Scholar]
- Callaway, N.L., Riha, P.D., Bruchey, A.K., Munshi, Z., and Gonzalez-Lima, F. 2004. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol. Biochem. Behav. 77: 175-181. [DOI] [PubMed] [Google Scholar]
- Gewirtz, J.C., Falls, W.A., and Davis, M. 1997. Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medial prefrontal cortex in rats. Behav. Neurosci. 111: 712-726. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima, F. and Cada, A. 1994. Cytochrome oxidase activity in the auditory system of the mouse: A qualitative and quantitative histochemical study. Neuroscience 63: 559-578. [DOI] [PubMed] [Google Scholar]
- ____. 1998. Quantitative histochemistry of cytochrome oxidase activity: theory, methods and regional brain vulnerability. In Cytochrome oxidase in neuronal metabolism and Alzheimer's disease (ed. F. Gonzalez-Lima), pp. 55-90, Plenum Press, New York.
- Gonzalez-Lima, F. and Jones, D. 1994. Quantitative mapping of cytochrome oxidase activity in the central auditory system of the gerbil: A study with calibrated activity standards and metal-intensified histochemistry. Brain Res. 660: 34-39. [DOI] [PubMed] [Google Scholar]
- Kelner, M.J., Bagnell, R., Hale, B., and Alexander, N.M. 1988. Potential of methylene blue to block oxygen radical generation in reperfusion injury. In Oxygen radicals in biology and medicine (ed. C. Von Sonntag), pp. 895-898. Plenum Press, New York. [DOI] [PubMed]
- Kupfer, A., Aeschlimann, C., and Cerny, T. 1996. Methylene blue and the neurotoxic mechanisms of ifosfamide encephalopathy. Eur. J. Clin. Pharmacol. 50: 249-252. [DOI] [PubMed] [Google Scholar]
- Lindahl, P.E. and Öberg, K.E. 1961. The effect of rotenone on respiration and its point of attack. Exp. Cell Res. 23: 228-237. [DOI] [PubMed] [Google Scholar]
- Martinez Jr., J.L., Jensen, R.A., Vasquez, B.J., McGuinness, T., and McGaugh, J.L. 1978. Methylene blue alters retention of inhibitory avoidance responses. Physiol. Psychol. 6: 387-390. [Google Scholar]
- Milad, M. and Quirk, G.J. 2002. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420: 70-74. [DOI] [PubMed] [Google Scholar]
- Morgan, M.A., Romanski, L.M., and LeDoux, J.E. 1993. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci. Lett. 163: 109-113. [DOI] [PubMed] [Google Scholar]
- Morgan, M.A., Schulkin, J., and LeDoux, J.E. 2003. Ventral medial prefrontal cortex and emotional preservation: The memory for prior extinction training. Behav. Brain Res. 146: 121-130. [DOI] [PubMed] [Google Scholar]
- Myers, K.M. and Davis, M. 2002. Behavioral and neural analysis of extinction. Neuron 36: 567-584. [DOI] [PubMed] [Google Scholar]
- Naylor, G.J., Martin, B., Hopwood, S.E., and Watson Y. 1986. A two-year double-blind crossover trial of the prophylactic effect of methylene blue in manic-depressive psychosis. Biol. Psychiatry 21: 915-920. [DOI] [PubMed] [Google Scholar]
- Pavlov, I.P. 1927. Conditioned reflexes. Oxford UP, London.
- Paxinos, G. and Watson, C. 1997. The rat brain in stereotaxic coordinates. Academic Press, San Diego, CA.
- Peter, C., Hongwan, D., Küpfer, A., and Lauterburg, B.H. 2000. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 56: 247-250. [DOI] [PubMed] [Google Scholar]
- Poremba, A., Jones, D., and Gonzalez-Lima F. 1998. Classical conditioning modifies cytochrome oxidase activity in the auditory system. Eur. J. Neurosci. 709: 251-258. [DOI] [PubMed] [Google Scholar]
- Quirk, G.J., Russo, G.K., Barron, J.L., and Lebron, K. 2000. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J. Neurosci. 20: 6225-6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaris, S.C., Babbs, C.F., and Voorhees III, W.D. 1991. Methylene blue as an inhibitor of superoxide generation by xanthine oxidase. Biochem. Pharmacol. 42: 499-506. [DOI] [PubMed] [Google Scholar]
- Sokoloff, L. 1992. Imaging techniques in studies of neuronal functions. In Advances in metabolic mapping techniques for brain imaging of behavioral and learning functions (eds. F. Gonzalez-Lima et al.), pp. 1-37. Kluwer, Boston, MA.
- Treit, D. and Fundytus, M. 1988. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol. Biochem. Behav. 31: 959-962. [DOI] [PubMed] [Google Scholar]
- Valla, J., Berndt, J.D., and Gonzalez-Lima, F. 2001. Energy hypometabolism in posterior cingulate cortex of Alzheimer's patients: Superficial laminar cytochrome oxidase associated with disease duration. J. Neurosci. 21: 4923-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal, J.S., Gonzalez-Lima, F., Berndt, J., and Barea-Rodriguez, E.J. 2002. Water maze training in aged rats: Effects on brain metabolic capacity and behavior. Brain Res. 939: 43-51. [DOI] [PubMed] [Google Scholar]
- Visarius, T.M., Stucki, J.W., and Lauterburg, B.H. 1997. Stimulation of respiration by methylene blue in rat liver mitochondria. FEBS Lett. 412: 157-160. [DOI] [PubMed] [Google Scholar]
- Wainwright, M. and Crossley, K.B. 2002. Methylene blue: A therapeutic dye for all seasons? J. Chemother. 14: 431-443. [DOI] [PubMed] [Google Scholar]
- Wong-Riley, M.T.T. 1989. Cytochrome oxidase: An endogenous metabolic marker for neuronal activity. Trends Neurosci. 12: 94-101. [DOI] [PubMed] [Google Scholar]